Inflammation and coagulation are closely linked through shared molecular components and crosstalk between the systems,1 and subjects with both acute and chronic inflammatory diseases have increased risk of venous thromboembolism (VTE).2 Biomarkers of low-grade inflammation have not displayed consistent associations with risk of first-lifetime VTE. Prospective studies on high-sensitivity C-reactive protein (CRP) have shown conflicting results.3,4 Neutrophil to lymphocyte ratio (NLR) is a predictor of all-cause5 and cardiovascular mortality6 among patients with coronary artery disease, and NLR was also found to be a long-term predictor of death from ischemic heart disease in a cohort without coronary heart disease at baseline.7 In contrast to CRP,8 NLR was associated with risk of first-ever stroke in patients with atrial fibrillation, and adding NLR to the CHA2DS2-VASc score improved the predictability of the score.9 The latter finding may suggest that NLR is superior to CRP to predict thromboembolic complications in low-grade inflammation. In cancer outpatients receiving chemotherapy, high NLR was reported to predict future symptomatic VTE.10 However, the role of NLR as a risk factor for VTE in the general population has not been explored, and no study has investigated whether NLR is associated with VTE recurrence. We aimed to investigate the association between NLR and future risk of incident and recurrent VTE, as well as all-cause mortality after VTE, in a large population-based cohort.
Participants were recruited from the fourth survey of the Tromsø Study conducted in 1994–95. A detailed description of the study design and population has been published previously.11 The Regional Committee of Medical and Health Research Ethics approved the study, and all 25,107 included subjects gave their written consent to participate. Baseline information was collected by physical examination, non-fasting blood samples, and self-administered questionnaires. NLR was calculated by dividing the total count of neutrophils by lymphocyte count. All first-lifetime and recurrent VTE events during follow up were identified by searching the discharge registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway, and thoroughly validated by medical records review, as previously described.12 The VTE events were classified as provoked or unprovoked depending on the presence of provoking factors [recent surgery or trauma, acute medical conditions (acute myocardial infarction, ischemic stroke or major infectious disease), active cancer, immobilization or any other factor particularly described to be provoking in the medical record] at the time of diagnosis. Information on deaths was obtained from the Population Registry of Norway.
Statistical analyses were carried out using STATA v.13.0 (Stata corporation, College Station, Texas, USA). For each participant, person-years of follow up were accrued from inclusion in 1994–1995 to the date of a VTE event, migration, death or to the end of the study period (31st December 2012). NLR was divided into quartiles based on the distribution of baseline NLR in the population (quartile 1: <1.30, quartile 2: 1.30–1.68, quartile 3: 1.68–2.19 and quartile 4: >2.19). An extra cut-off point was established at the 95th percentile (NLR >3.46). Cox proportional hazards regression models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for VTE across quartiles of NLR, using quartile 1 as reference. We additionally compared those with NLR above the 95th percentile to those with NLR in quartile 1. The analyses were adjusted for age and sex in one model, and for age, sex, body mass index, diabetes and smoking in a multivariable model. As fluctuation of NLR over time may lead to underestimation of risk, particularly with long follow up, we conducted additional analyses where the follow-up time was restricted to three years from study start. Separate analyses were performed to estimate the risk of unprovoked and provoked VTE, and the risk of deep vein thrombosis (DVT) and pulmonary embolism (PE). For analyses of VTE recurrence, subjects with a first VTE were followed from the date of their first event to the date of recurrence, death, migration or study end (31st December 2012). Finally, we calculated one-year and total mortality rates after VTE according to NLR. Since NLR was measured at baseline only, additional adjustment was made to account for differences in follow-up time between baseline and the first VTE event as a potential confounder in the analyses of recurrence and mortality.
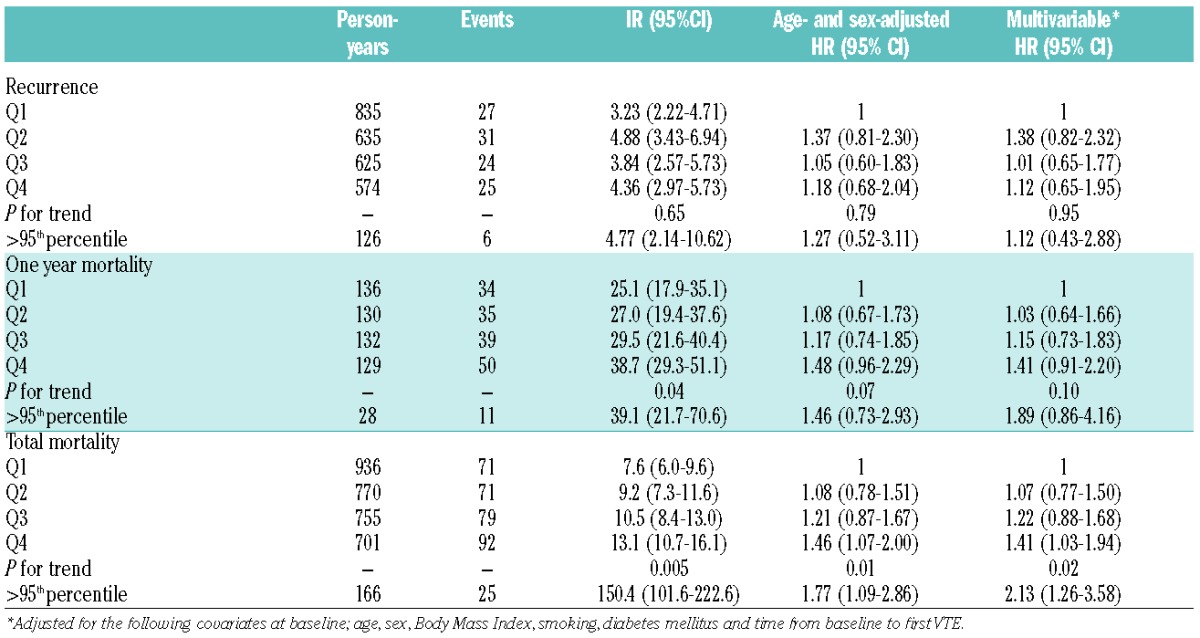
There were 664 VTE events during 367,233 person-years of follow up. The median follow up time was 17.7 years. Among those who developed VTE, the time from baseline to the VTE event ranged from 0.06–18.24 years (mean 9.95 years). The overall crude incidence rate (IR) of VTE was 1.81 (95%CI: 1.68–1.95) per 1000 person-years. Baseline characteristics across quartiles of NLR are shown in Table 1. Among the VTE patients, 58% had DVT and 42% had PE with or without concurrent DVT. In total, 273 (41%) of the VTE cases were classified as unprovoked. Active cancer, immobilization, surgery and acute medical conditions were the most frequent provoking factors among the provoked VTE cases. The risk of VTE remained unchanged across quartiles of NLR after multi-variable adjustment (HR quartile 4 vs. quartile 1: 1.07, 95%CI: 0.86–1.33, P for trend across quartiles: 0.36) (Table 2). NLR showed no significant association with either provoked or unprovoked VTE (Table 2), and no association was found between NLR and risk of DVT and PE separately (data not shown). There was still no association between quartiles of NLR and risk of VTE when follow-up time was restricted to three years. However, those with NLR above the 95th percentile had a 2.4-fold higher risk of VTE compared with quartile 1 (multivariable adjusted HR 2.36, 95%CI: 0.96–5.82). Out of 664 incident VTE-cases, 107 had a recurrent VTE event and 313 died during 2669 and 3162 person-years of follow up, respectively. There was no association between baseline NLR and risk of VTE recurrence (Table 3). The one-year mortality risk after VTE was 41% higher in quartile 4 versus quartile 1 of NLR (HR 1.41, 95%CI: 0.91–2.20). Similarly, the overall risk of mortality was 41% (HR 1.41, 95%CI: 1.03–1.94) higher in quartile 4 versus quartile 1 of NLR and 113% (HR 2.13, 95%CI: 1.26–3.58) higher in those with NLR above the 95th percentile. The relationship between NLR and VTE has not been extensively studied. Bakirci et al.13 investigated NLR in relation to anatomic extent of VTE, and found that NLR measured the first day of VTE diagnosis was higher compared to controls, and that NLR increased with the extent (distal DVT< proximal DVT< PE) of the thrombus. We found no association between NLR and future risk of VTE, and the risk estimates were essentially similar for DVT and PE. Previous studies have suggested that NLR is superior to CRP in predicting cardiovascular mortality,6 and that NLR may be a better predictor of thromboembolic complications in atrial fibrillation.8,9 In view of the diverging results from studies on CRP and VTE, we aimed to investigate whether NLR could be a more sensitive marker of inflammation related to VTE. However, in agreement with most prospective studies on the relation between CRP and VTE,4,14 we found no association between NLR and VTE. Although chronic inflammatory diseases carry an increased risk of VTE, the risk seems to be more pronounced during periods with high disease activity where acute inflammation predominates.2 When the follow-up time was restricted to the first three years in our study, subjects with NLR above the 95th percentile had a 2.4-fold higher risk of VTE compared to subjects in the lowest quartile. In these cases, high NLR could be a consequence of some other underlying condition that concomitantly increases the VTE-risk, and the observed risk could thereby reflect a more acute rather than a chronic inflammatory state. Regression dilution might also partly explain the diverging results in analyses with shorter and longer follow up.
Table 1.
Baseline characteristics of study participants by quartiles (Q) of neutrophil to lymphocyte ratio (NLR): the Tromsø Study 1994–2012.
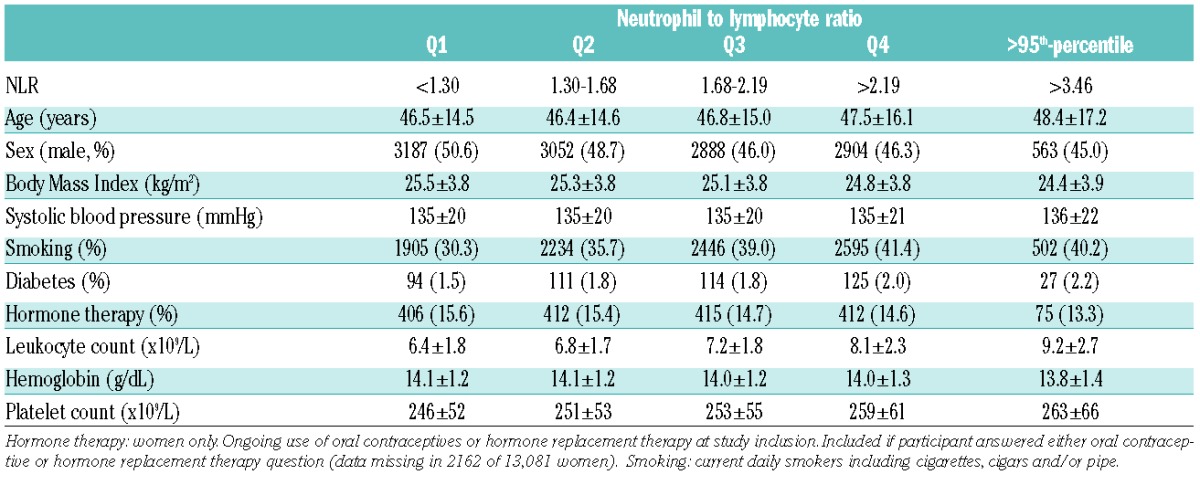
Table 2.
Crude incidence rates (IR), age- and sex-adjusted and multivariable-adjusted hazard ratios (HR) for total venous thromboembolism (VTE), unprovoked and provoked VTE by quartiles (Q) of neutrophil to lymphocyte ratio (NLR): the Tromsø Study 1994–2012.
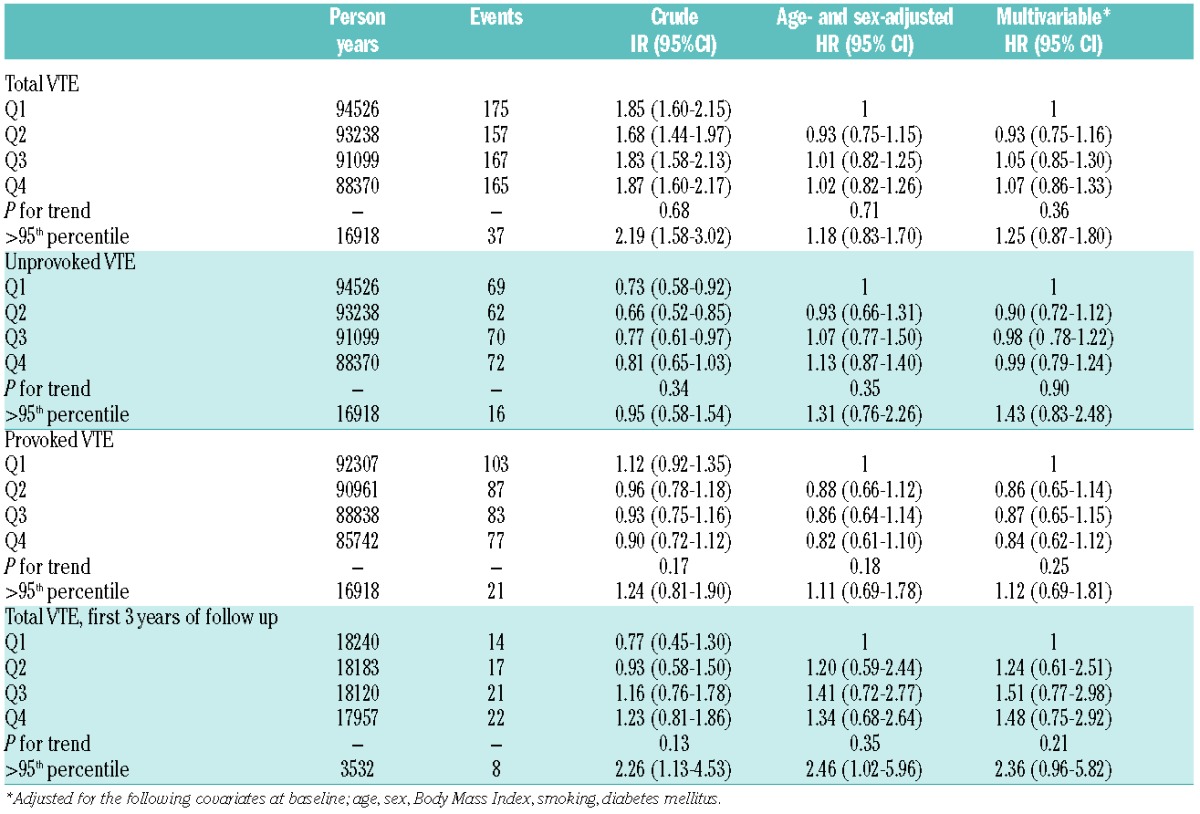
Table 3.
Incidence rates (IR) per 100 person-years, hazard ratios (HR) of recurrent venous thromboembolism (VTE), and one year and total mortality rates after VTE by quartiles (Q) of neutrophil to lymphocyte (NLR): the Tromsø study 1994–2012.
Case studies of PE-patients have found NLR on admission to be associated with mortality.15 We found that high NLR at baseline was associated with increased risk of mortality among the subjects who later experienced a VTE. The mechanism for the increased risk of mortality by high NLR in VTE patients is unclear. Several studies have shown that NLR is associated with worse outcome in patients with established cardiovascular disease,5,6 and high baseline NLR values were associated with increased cardiovascular mortality in a cohort of initially disease-free subjects.7 Potentially, NLR may reflect cardiovascular disease or some other underlying condition contributing to a worse prognosis after a VTE event. Unfortunately, we did not have information on the causes of death among VTE patients in our study.
Strengths of this study include the temporal sequences between exposures and outcomes, the large cohort recruited from the general population with a long follow-up time and high attendance rate, and the thorough adjudication of VTE events. A potential limitation is that our analyses are based on one single measurement of NLR at baseline, which may lead to underestimation of associations due to regression dilution.
In conclusion, single measurement of NLR, as a marker of inflammatory status, was not associated with future risk of first or recurrent VTE in this prospective, population-based cohort with long follow-up time. However, high NLR was associated with increased risk of mortality among those who experienced a VTE.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tichelaar YI, Kluin-Nelemans HJ, Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012;107(5):827–837. [DOI] [PubMed] [Google Scholar]
- 3.Quist-Paulsen P, Naess IA, Cannegieter SC, et al. Arterial cardiovascular risk factors and venous thrombosis: results from a population-based, prospective study (the HUNT 2). Haematologica. 2010;95(1):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hald EM, Braekkan SK, Mathiesen EB, et al. High-sensitivity C-reactive protein is not a risk factor for venous thromboembolism: the Tromso study. Haematologica. 2011;96(8):1189–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657. [DOI] [PubMed] [Google Scholar]
- 6.O Hartaigh B, Bosch JA, Thomas GN, et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis. 2012;224(1):161–169. [DOI] [PubMed] [Google Scholar]
- 7.Shah N, Parikh V, Patel N, et al. Neutrophil lymphocyte ratio significantly improves the Framingham risk score in prediction of coronary heart disease mortality: insights from the National Health and Nutrition Examination Survey-III. Int J Cardiol. 2014;171(3):390–397. [DOI] [PubMed] [Google Scholar]
- 8.Wu N, Chen X, Cai T, et al. Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2015;31(3):278–286. [DOI] [PubMed] [Google Scholar]
- 9.Saliba W, Barnett-Griness O, Elias M, Rennert G. Neutrophil to lymphocyte ratio and risk of a first episode of stroke in patients with atrial fibrillation: a cohort study. J Thromb Haemost. 2015;13(11):1971–1979. [DOI] [PubMed] [Google Scholar]
- 10.Ferroni P, Riondino S, Formica V, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. 2015;136(5):1234–1240. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso Study. Int J Epidemiol. 2012;41(4):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braekkan SK, Borch KH, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Body height and risk of venous thromboembolism: The Tromso Study. Am J Epidemiol. 2010;171(10):1109–1115. [DOI] [PubMed] [Google Scholar]
- 13.Bakirci EM, Topcu S, Kalkan K, et al. The role of the nonspecific inflammatory markers in determining the anatomic extent of venous thromboembolism. Clin Appl Thromb Hemost. 2015;21(2):181–185. [DOI] [PubMed] [Google Scholar]
- 14.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE). Am J Med. 2002;113(8):636–642. [DOI] [PubMed] [Google Scholar]
- 15.Akgullu C, Omurlu IK, Eryilmaz U, et al. Predictors of early death in patients with acute pulmonary embolism. Am J Emerg Med. 2015;33(2):214–221. [DOI] [PubMed] [Google Scholar]


