CC chemokine receptor 4 (CCR4), the receptor for macrophage-derived chemokine (MDC/CCL22) and thymus activation-regulated chemokine (TARC/CCL17), is expressed on tumor cells in approximately 30–65% of patients with peripheral T-cell lymphoma (PTCL).1 Mogamulizumab, a defucosylated, humanized, IgG1 monoclonal antibody directed against CCR4 has been approved in Japan for the treatment of CCR4-positive relapsed/refractory PTCL. Mogamulizumab demonstrated effectiveness [overall response rate (ORR) 34%] in a phase II study of 29 Japanese patients with relapsed CCR4-positive PTCL.2 We conducted a phase II study in patients with relapsed or refractory CCR4-positive PTCL at 15 European centers (clinicaltrials.gov identifier :01611142). All patients gave written informed consent prior to enrollment. The study was conducted in accordance with the Declaration of Helsinki and in compliance with Good Clinical Practice guidelines. The protocol was approved by the Ethics Committee at each participating institution. The primary objective was to determine the best ORR of mogamulizumab. Secondary objectives included the duration of response, progression-free survival (PFS), and overall survival (OS) as well as the safety and immunogenicity of mogamulizumab.
Adult patients of either sex with CCR4-positive, measurable PTCL who had failed prior therapy (relapsed or refractory) were recruited. Histologically confirmed diagnosis of PTCL according to the 2008 WHO classification3 had to be: PTCL-not otherwise specified (PTCL-NOS); angioimmunoblastic T-cell lymphoma (AITL); anaplastic large-cell lymphoma (ALCL), ALK-positive; ALCL, ALK-negative; or transformed mycosis fungoides. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) score had to be ≤2. Hematological, renal, and hepatic function had to be adequate.
Mogamulizumab 1.0 mg/kg was administered by intravenous infusion in 250 mL normal saline over at least 1 hour once weekly for 4 weeks, and every 2 weeks thereafter until progressive disease (PD), development of unacceptable toxicity, death, or withdrawal of consent. Dose modification was not permitted. Patients achieving a complete response (CR) could remain on treatment for up to 12 months after CR. No other systemic anticancer therapy was permissible while receiving mogamulizumab.
The International Working Group response criteria4 were used for the assessment of disease in lymph nodes, spleen, liver, and bone marrow, and a modified Severity Weighted Assessment Tool5 was used to assess for cutaneous disease, if present. Response classified as CR, partial response (PR), stable disease (SD), or PD was evaluated by the investigator every 8 weeks. Since the first assessment was at week 8, a patient who was off study due to PD prior to week 8 would be considered efficacy-evaluable with a best overall response of PD. Efficacy was determined in the efficacy-evaluable population, which included all patients who completed the first cycle of treatment and who had baseline and at least one on-study assessment of response. The Kaplan-Meier method was used to analyze PFS with exact two-sided 95% confidence interval (CI) calculated around the estimated proportion. PFS was defined as the time from the first dose of mogamulizumab to progression, relapse, or death by any cause.
The safety population included all patients who received at least one dose of mogamulizumab. AEs were graded by NCI-CTCAE, v4.0. Treatment-related AEs were those classified as possibly, probably, or definitely related to mogamulizumab. Serum samples were drawn regularly for the determination of anti-mogamulizumab antibodies.
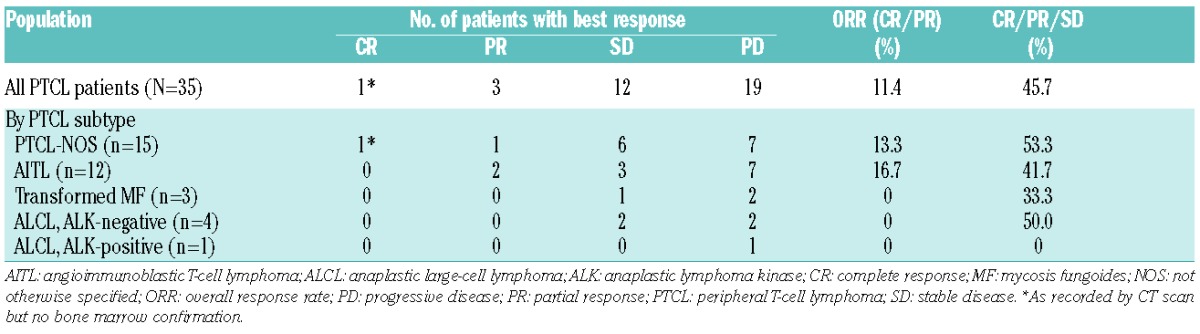
The baseline characteristics of the 38 recruited patients are summarized in Table 1. The median number of cycles administered was 2 (range: 1–22) with a mean of ~94% of the planned mogamulizumab dose administered. The mean (±SD) duration of therapy was 13.9±21.3 weeks. Thirty-five patients were evaluable for efficacy, as 3 patients did not have a post-baseline assessment for efficacy. ORR was 11.4% (95% CI: 3.2–26.7%) and SD or better rate was 45.7% (Table 2). Four patients achieved response (1 CR, 3 PR) at the first 8-week assessment (after two treatment cycles), who were treated for relapsed (n=3) or refractory (n=1) PTCL. The duration of response was 539+ (CR), 77 (PR), 43 (PR), and 1 (PR) days, respectively. The ECOG PS was 1, 2, 0, and 0 in these respective patients. The median PFS was 2.1 months (95% CI: 1.3–3.9 months). The median duration of SD or response was 2.8 months. OS was not analyzed due to inadequate follow-up for survival.
Table 1.
Baseline clinical and demographic characteristics of the 38 patients enrolled in the trial.
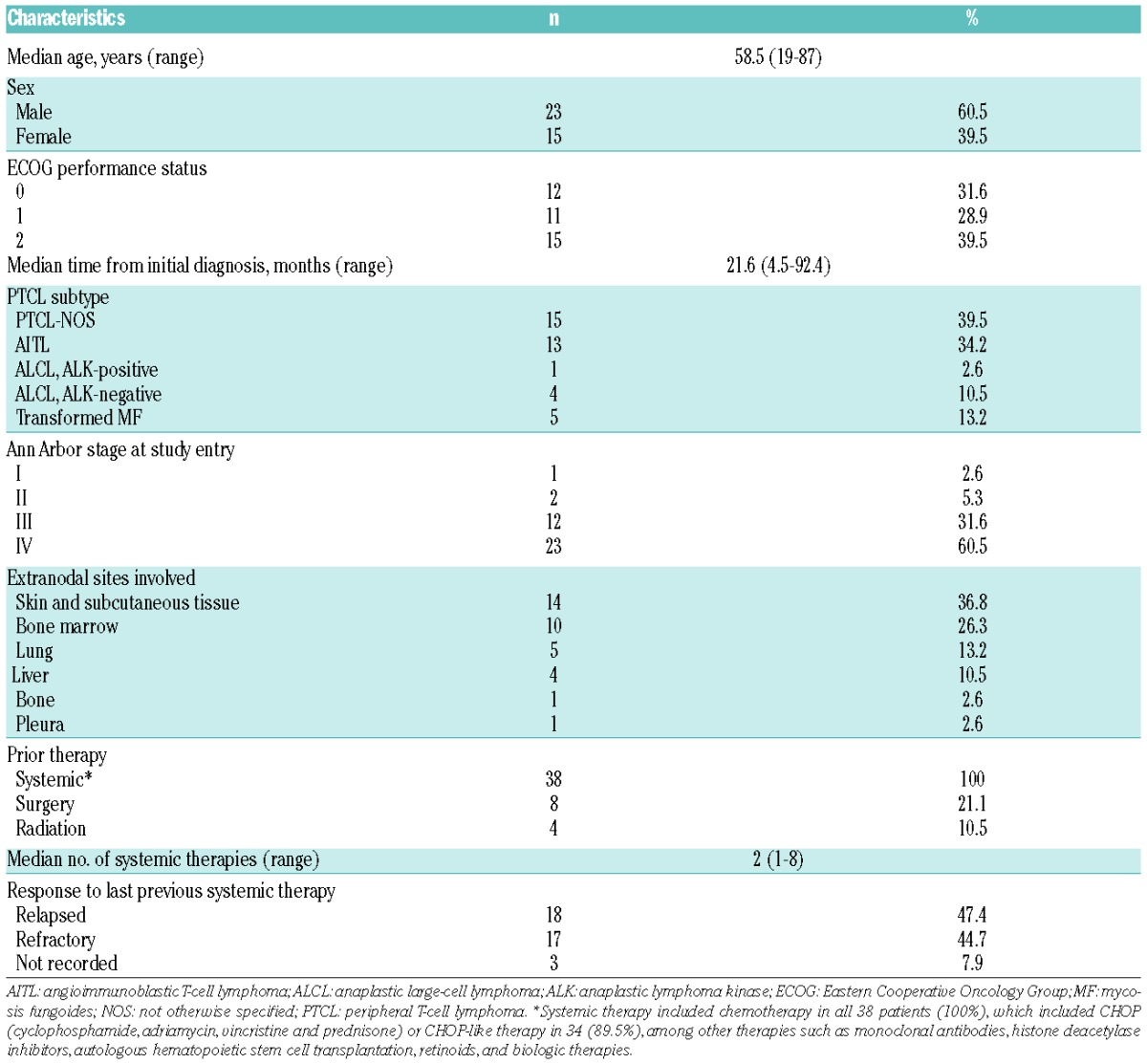
Table 2.
Best response in the efficacy-evaluable population (N=35).
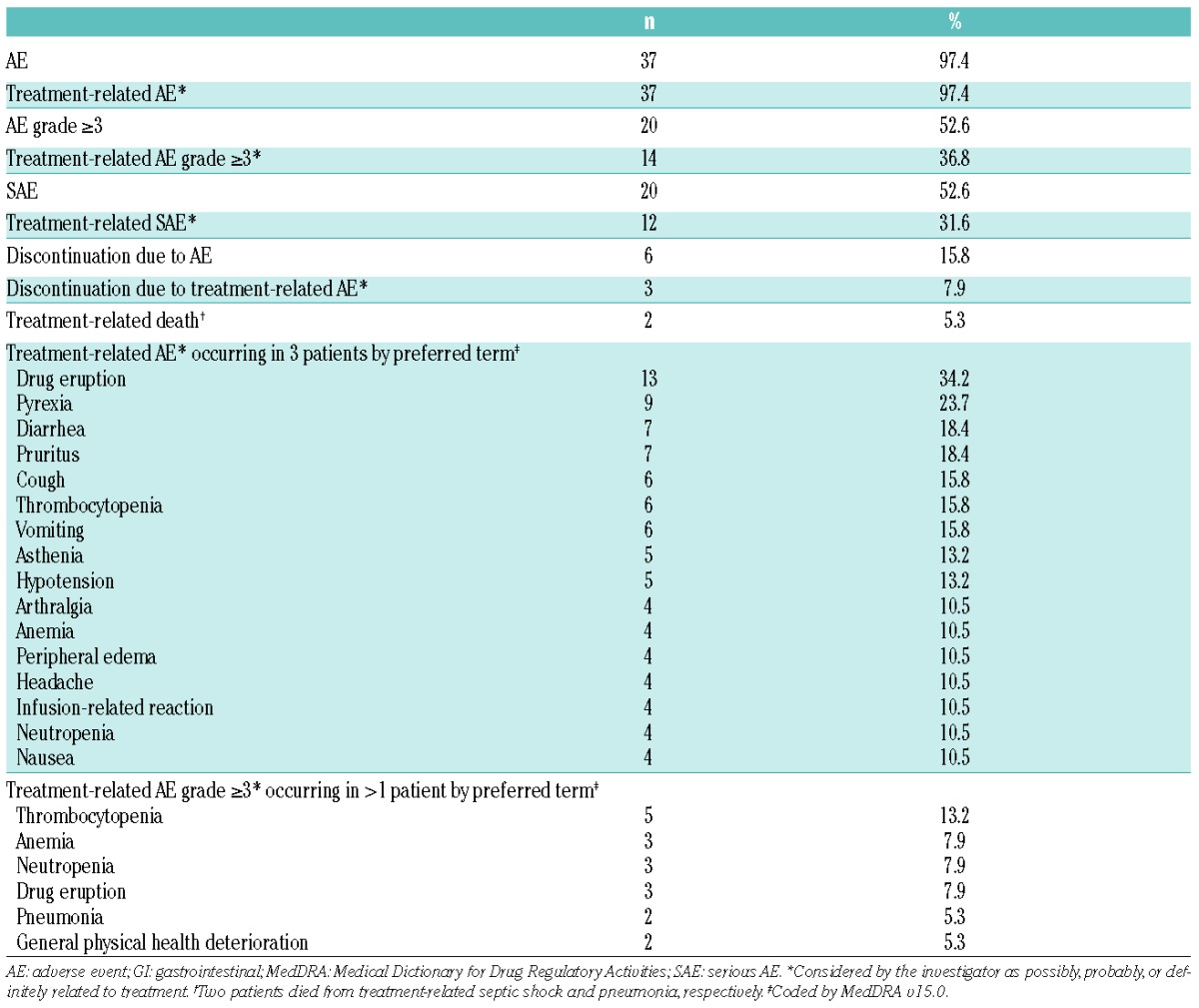
Treatment-related AEs of any grade occurred in 37 patients (97.4%), and treatment-related AEs grade ≥3 in 14 patients (36.8%) (Table 3). The most common treatment-related AEs of any grade were drug eruption (n=13, 34.2%), pyrexia (n=9, 23.7%), diarrhea (n=7, 18.4%), and pruritus (n=7, 18.4%). The only treatment-related AEs grade ≥3 occurring in more than 2 patients were thrombocytopenia (n=5, 13.2%), anemia (n=3, 7.9%), neutropenia (n=3, 7.9%), and drug eruption (n=3, 7.9%). Twelve patients (31.6%) experienced treatment-related SAEs, including 2 patients who died from septic shock and pneumonia, respectively. No unexpected trends or safety concerns were identified from laboratory parameters, vital signs, or ECG assessments. No patients developed anti-mogamulizumab antibodies.
Table 3.
Treatment-emergent adverse events (N=38).
Our study of European patients with relapsed or refractory PTCL showed an ORR of 11.4% (95% CI: 3.2–26.7%), which did not reach the target ORR of 35% set by the protocol, and appeared lower when compared to the previous study2 of Japanese patients with relapsed PTCL (34%) and other key studies of approved agents on relapsed/refractory PTCL: pralatrexate, romidepsin, and belinostat of 29%,6 25%,7 and 26%,8 respectively. The median PFS was similar in both the Japanese and our studies (2.1 and 2.0 months, respectively). The safety profile of mogamulizumab was similar in both studies. Drug eruption was relatively common, occurring in approximately one-third of patients. It has been hypothesized as being related to the antitumor mechanism of mogamulizumab, as CCR4 contributes to skin-specific lymphocyte homing.9 There is no immediately clear explanation for the lower ORR in our study compared to the previous Japanese study.2 Different ethnicity is an unlikely explanation. Histopathological PTCL subtypes (predominantly PTCL-NOS and AITL in similar proportions) and the number of previous systemic therapies were similar. Nonetheless, there were some differences that might impact ORR. Our study included patients with either relapsed or refractory disease whereas the Japanese study only included patients with relapsed disease. It has been shown that refractory PTCL has been associated with poorer outcome, with a median OS of 2.5 compared to 6 months for those with relapsed PTCL.10 It is therefore likely that the relapsed disease status in the Japanese study would have biased toward a better outcome. Responders in the current study had relapsed (n=3) or refractory (n=1) disease. ECOG PS was worse in our study; 39% of patients scored 2 compared to none in the Japanese study. It has recently been demonstrated that PS is the most predictive prognostic factor with respect to OS and PFS in PTCL patients.10 This raises the possibility that PS may have impacted outcome and may partially account for the difference in ORR between the studies. Only 1 patient with ECOG PS 2 responded in our study, and was also the only responder with refractory disease. Furthermore, dose intensity appeared to be higher in the Japanese study; mogamulizumab 1.0 mg/kg once weekly to a maximum of 8 doses being permitted (68% of patients completing this course). In our study, the same dose was administered once weekly for 4 weeks followed by every 2 weeks continuously until PD (mean (±SD) duration of therapy was 13.9±21.3 weeks with a median of 6 doses administered). Finally, treatment response was assessed by the investigators in our study whereas an Independent Review Committee (IRC) was used in the Japanese study. It has been shown that there may be a difference in ORR estimation between investigators and IRCs.11 However, a retrospective review of data from the Japanese study showed that there was no difference between investigator and IRC evaluation of ORR (data on file, Kyowa Kirin Pharmaceutical Development, Inc.).
Although mogamulizumab therapy demonstrated an ORR of 11.4% in our study, disease control (SD or better) rate was 45.7%. In order to enhance the efficacy of relapsed/refractory PTCL, a combined therapeutic approach might be considered for the future development of mogamulizumab in relapsed/refractory PTCL.
Acknowledgments
The authors thank Peter Todd, PhD, (Tajut Ltd, Kaiapoi, New Zealand) for medical editorial assistance with this article, for which he received financial compensation from Kyowa Kirin Pharmaceutical Development, Inc. (Princeton, New Jersey, USA).
Footnotes
Funding: this work was supported by Kyowa Kirin Pharmaceutical Development, Inc. (Princeton, New Jersey, USA).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res 2004;10(16):5494–5500. [DOI] [PubMed] [Google Scholar]
- 2.Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-CC chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol 2014;32(11):1157–1163. [DOI] [PubMed] [Google Scholar]
- 3.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117(19):5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 5.Stevens SR, Ke MS, Parry EJ, Mark J, Cooper KD. Quantifying skin disease burden in mycosis fungoides-type cutaneous T-cell lymphomas. Arch Dermatol 2002;138(1):42–48. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol 2011;29(9):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 2012;30(6):631–636. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol 2015;33(23):2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell JJ, Haraldsen G, Pan J, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999;400(6746):776–780. [DOI] [PubMed] [Google Scholar]
- 10.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 2014;124(10):1570–1577. [DOI] [PubMed] [Google Scholar]
- 11.Tang PA, Pond GR, Chen EX. Influence of an independent review committee on assessment of response rate and progression-free survival in phase III clinical trials. Ann Oncol 2010;21(1):19–26. [DOI] [PubMed] [Google Scholar]


