Examination of bone lesions is a compulsory part of baseline assessments in patients with multiple myeloma (MM). Low-dose computed tomography (CT) scan has recently been recommended as a replacement of conventional X-ray for the diagnosis of osteolytic lesions by the European Myeloma Network.1 In this prospective study we have compared the diagnostic performance of low-dose CT with conventional X-ray, and examined the added value of 18Flourodeoxyglucose (18FDG) positron emission tomography (PET), dual energy X-ray absorptiometry and serum markers of bone turnover in 35 previously untreated MM patients. Low-dose CT scan diagnosed significantly more patients with osteolytic lesions in both pelvis and spine compared with X-ray. 18FDG-PET of spine and pelvis was positive in 9% of CT scans without osteolysis.
The presence of osteolytic lesions is one of the CRAB criteria that determines whether a patient with MM requires anti-myeloma treatment.2 Skeletal X-ray is still widely used for the diagnosis of osteolytic lesions in MM, despite the limitations of 2-dimensional images for visualization of the complex anatomic structures of the spine and pelvis (Figure 1). Low-dose CT scan can visualize the bones in a 3-dimensional manner without the need for a major increase in radiation dose3 and, in addition, provides relevant information regarding extramedullary disease. 18FDG-PET scan can visualize increased metabolic activity of cells. Using 18FDG-PET, a diffuse or focal accumulation of metabolically active myeloma cells may possibly be identified prior to the development of osteolytic lesions.
Figure 1.
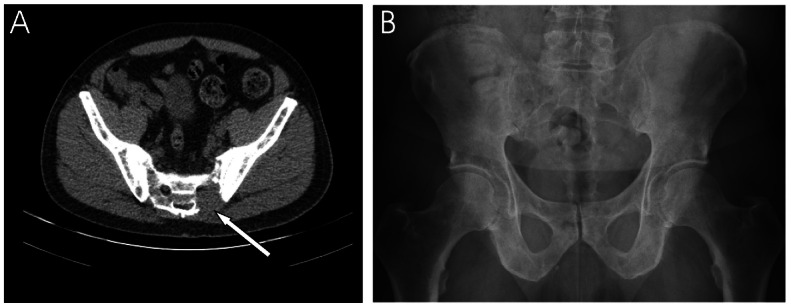
Low-dose CT versus conventional X-ray. Low-dose CT (A) and conventional X-ray (B) of a 67-year-old man with newly diagnosed multiple myeloma. X-ray performed 7 days after low-dose CT-scanning. The CT-scan shows a significant osteolytic lesion, not visualised at X-ray, of the pelvic area.
The bone assessments of this study serve as baseline for a set of secondary endpoints defined in a clinical trial testing an intensive 5-drug combination for first-line treatment of MM (ACVDL-trial). This clinical trial was approved by The Regional Scientific Ethical Committees for Southern Denmark (id: 2011-0123), registered at clinicaltrials.gov (identifier: 01481194) and by EUDRACT (number 2011-002751-34). The study was conducted in accordance with the Helsinki Declaration. All patients provided informed consent.
Thirty-five previously untreated MM patients in need of treatment according to the CRAB criteria2 were enrolled. X-ray and low-dose CT of spine and pelvis were assessed separately as either positive or negative for osteolytic lesions. The images were reviewed by a team of radiology experts. The patients fasted for at least six hours before injection of 18FDG with an activity of 4 MBq/kg (minimum 300 MBq), and then rested for one hour prior to the 18FDG/PET scan. PET images were reviewed by nuclear medicine specialists. The spine and pelvis were considered as either positive or negative for focal PET-activity based on a standardized uptake value (SUV) above 2.5. Dual energy absorptiometry was preformed of the hip and lumbar spine (L1-L4). Fasting blood tests were collected for measurement of the bone resorption marker C-terminal cross-linking telopeptide of type I collagen (CTX) and the bone formation marker N-terminal propeptide of procollagen I (P1NP).
The 35 patients consisted of 21 men and 14 women, and mean age was 64 (49–81) years. Autologous stem cell transplantation was planned in twenty-three of the patients. ISS-score (international staging system) was: I=17, II=10, III=8. In one case, the CT-scan revealed a paramedullary tumor growth in the vertebral canal; asymptomatic but with imminent medullary compression (Figure 2). The findings that were confirmed by MRI showed a tumor growth with compression of the spinal cord and nerve roots. Given that the patient was asymptomatic, emergency surgery was not required; however, anti-myeloma treatment was started immediately. The individual results of X-ray, CT and PET are illustrated in Figure 3.
Figure 2.
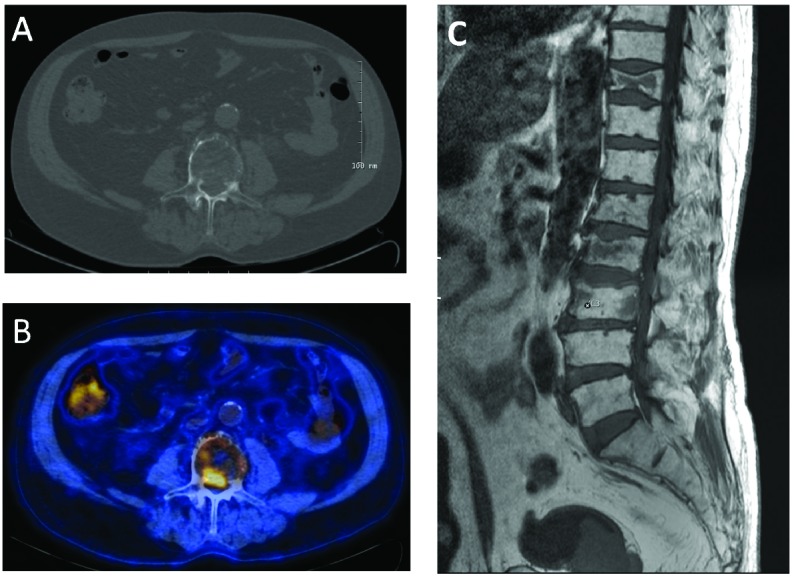
Imminent medullary compression revealed by low-dose CT. The low-dose CT-scan reveals tumor growth in the vertebral canal (A). This lesion was PET-positive (B). MR-scan confirmed the intra-spinal growth (C). The patient was asymptomatic.
Figure 3.
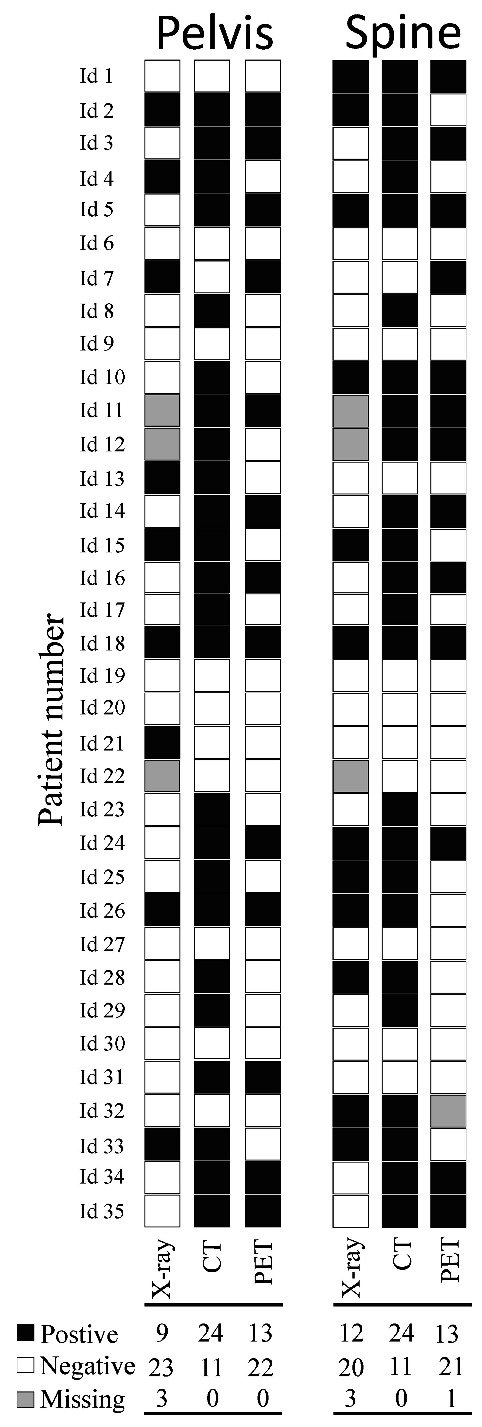
Results of X-ray, low-dose CT and PET. The individual results of the different imaging methods performed at baseline for the 35 previously untreated multiple myeloma patients. Below the columns are the numbers of positive, negative and missing results of each imaging modality shown. Low-dose CT identifies most patients with osteolysis. Black squares illustrate osteolysis at low-dose CT (CT), conventional X-rays (X-ray), and focal activity at 18FDG-PET (PET), while white squares illustrate imaging without osteolysis of focal activity. Grey squares represent missing data.
CT-scan was performed in all patients, whilst X-ray was carried out on 32 patients. The mean time between CT-scan and X-ray was 4 days (0–28 days). CT diagnosed significantly more patients with osteolysis than X-ray in both pelvis (P<0.01, n:32) and spine (P<0.05, n:32). However, only 6% of the patients would have been judged as asymptomatic if only skeletal X-ray had been performed, since osteolysis was diagnosed by X-ray in other parts of the skeleton. Two patients were diagnosed positive for osteolysis of the pelvis using X-ray, whilst their CT scans were evaluated as negative. The first case (id 7) was later judged to be a false positive following reevaluation of the initial X-ray and comparison with follow-up images. In the second case (id 21), re-evaluation of the CT-scan and retrospective comparison with follow-up CT-scans confirmed the presence of osteolysis. Overall, osteolytic lesions of either the pelvis or the spine were found in 50% of the patients examined by X-ray and in 74% by CT-scan.
PET imaging was carried out in all patients; however, in one of the cases the spine scan was considered inconclusive for technical reasons. The mean time between CT and PET was 3 days (0–48 days). PET positive foci were found in 37% of the patients in the pelvis and 38% of the patients in the spine, whilst 46% of the patients were PET positive in either the spine or the pelvis or both. PET positive foci were found in 9% of the negative CT-scans of the spine and the pelvis. PET was positive in 50% of the CT-scans showing osteolysis of the pelvis and positive in 52% of the CT-scans showing osteolysis of the spine.
Dual energy absorptiometry examinations were performed on all patients. The mean Z-score was 0.3 (−1.3 – 3.0) in the hip and 0.1 (−3.3 – 3.2) in the spine.
The serum levels of CTX and P1NP are shown in Online Supplementary Figure S1.
Correct evaluation of bone involvement is important in MM since the presence of osteolytic lesions is a “myeloma defining event”2 and an indication for treatment. In the last decade, several studies have highlighted the limitations of X-rays for identification of osteolytic lesions in MM when compared with newer imaging modalities.4 However, only a few studies (mainly retrospective) of mixed populations of previously treated and untreated MM patients have been made as a platform for claiming the superiority of CT-scan over X-ray for the detection of osteolytic lesions.5–8 With a special focus on challenging areas such as the spine and pelvis,3,6,8 our prospective study supports the superiority of CT over X-ray for detection of osteolytic lesions.
The higher sensitivity of CT-scan over conventional X-ray may lead to earlier detection of osteolytic lesions and, thereby, earlier initiation of treatment in patients with MM. Earlier detection of bone lesions by CT-scan followed by initiation of anti-myeloma treatment has not been formally proven to improve patient outcome. However, the improvement of survival seen after initiation of anti-myeloma treatment in patients with high-risk smoldering myeloma9 makes it likely that early detection of bone lesions in MM patients, leading to initiation of therapy, will also be beneficial.
We report one case where the CT-scan showed an imminent but still asymptomatic compression of the spinal cord not detected by X-ray. This finding was confirmed by MRI. Early diagnosis of such critical bone lesions may prevent morbidity by early intervention.
The combination of PET and CT-scan is efficient for the diagnosis of osteolytic lesions in MM,10 and may provide prognostic information.11 We identified only one patient out of 35 with a positive PET-scan and a CT-scan without osteolysis; this patient had foci both in the spine and the pelvis. In a recently published study, Zamagni et al. reported that 15% of newly diagnosed, symptomatic MM patients with a positive PET-scan had a negative CT-scan.12 The frequency of PET-positive patients in our study is lower than that reported by others.13 The reason for this is not clear but may be due to difference in methodology, the definitions of osteolysis and PET-positivity, the MM population studied, and/or small sample size. Patients with diffuse, rather than focal, lesions of the bone marrow may escape detection by PET. Standardization of the PET-CT methodology and reporting is clearly needed.10
In our study, the mean bone mineral density of newly diagnosed MM patients was similar to the normal reference and thus our results did not reproduce the finding of a generalized osteopenia in newly diagnosed MM which was reported in the 1990s.14
Forty percent of our patients had elevated levels of CTX, including patients without osteolysis by CT of the axial skeleton. Bone resorption markers have been reported to increase prior to progression of MM;15 however, more studies may be needed to clarify whether they have a role in clinical practice.
We found that low-dose CT of the axial skeleton diagnoses significantly more patients with osteolysis than X-ray. In this study, 18FDG-PET provided only little extra information regarding bone disease compared with low-dose CT. However, PET-CT may carry prognostic information and constitute a valuable parameter for the assessment of response.
We recommend low-dose CT rather than X-ray of the axial skeleton as a standard procedure for detection of osteolytic lesions at baseline assessments of MM patients. Low-dose CT may also provide information regarding paramedullary and extramedullary involvement, and risk of spinal cord compression. However, in cases with clinical symptoms of medullary cord or cauda equina compression, we recommend MRI for optimal visualization of the lesion. Thus, CT scan may be regarded as a powerful screening procedure to alert the clinicians to the existence of extramedullary manifestations in need of attention.
Acknowledgments
The authors would like to thank the study nurses at the Clinical Research Unit of Hematology, Vejle Hospital, for their work regarding the ACVDL-protocol.
Footnotes
Funding: this study received financial support from Janssen. MH received a study grant from the University of Southern Denmark; Lillebaelt Hospital, Denmark; and The Danish Cancer Society.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Terpos E, Kleber M, Engelhardt M, et al. European Myeloma Network Guidelines for the Management of Multiple Myeloma-related Complications. Haematologica. 2015;100(10):1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. [DOI] [PubMed] [Google Scholar]
- 3.Horger M, Claussen CD, Bross-Bach U, et al. Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol. 2005;54(2):289–297. [DOI] [PubMed] [Google Scholar]
- 4.Regelink JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162(1):50–61. [DOI] [PubMed] [Google Scholar]
- 5.Gleeson TG, Moriarty J, Shortt CP, et al. Accuracy of whole-body low-dose multidetector CT (WBLDCT) versus skeletal survey in the detection of myelomatous lesions, and correlation of disease distribution with whole-body MRI (WBMRI). Skeletal Radiol. 2009;38(3):225–236. [DOI] [PubMed] [Google Scholar]
- 6.Kröpil P, Fenk R, Fritz LB, et al. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol. 2008;18(1):51–58. [DOI] [PubMed] [Google Scholar]
- 7.Princewill K, Kyere S, Awan O, Mulligan M. Multiple myeloma lesion detection with whole body CT versus radiographic skeletal survey. Cancer Invest. 2013;31(3):206–211. [DOI] [PubMed] [Google Scholar]
- 8.Wolf MB, Murray F, Kilk K, et al. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur J Radiol. 2014;83(7):1222–1230. [DOI] [PubMed] [Google Scholar]
- 9.Mateos MV, Hernandez MT, Giraldo P, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med. 2013;369(5):438–447. [DOI] [PubMed] [Google Scholar]
- 10.van Lammeren-Venema D, Regelink JC, Riphagen II, Zweegman S, Hoekstra OS, Zijlstra JM. (1)(8)F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: a systematic review. Cancer. 2012;118(8):1971–1981. [DOI] [PubMed] [Google Scholar]
- 11.Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114(10):2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamagni E, Nanni C, Mancuso K, et al. PET/CT Improves the Definition of Complete Response and Allows to Detect Otherwise Unidentifiable Skeletal Progression in Multiple Myeloma. Clin Cancer Res. 2015;21(19):4384–4390. [DOI] [PubMed] [Google Scholar]
- 13.Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica. 2007;92(1):50–55. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar MV, Weinstein R, Tricot G, et al. Biologic and therapeutic determinants of bone mineral density in multiple myeloma. Leuk Lymphoma. 1998;32(1–2):121–127. [DOI] [PubMed] [Google Scholar]
- 15.Lund T, Abildgaard N, Andersen TL, Delaisse JM, Plesner T. Multiple myeloma: changes in serum C-terminal telopeptide of collagen type I and bone-specific alkaline phosphatase can be used in daily practice to detect imminent osteolysis. Eur J Haematol. 2010;84(5):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]


