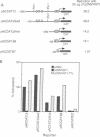
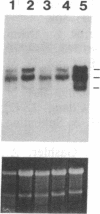
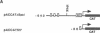Abstract
Wilms tumor, an embryonic kidney malignancy, accounts for approximately 6% of all pediatric neoplasms. A gene implicated in the genesis of this tumor, the Wilms tumor suppressor gene (WT1), encodes a zinc-finger DNA-binding protein (WT1) that functions as a transcriptional repressor. In certain Wilms tumors, the platelet-derived growth factor A chain (PDGF-A) is overexpressed; it has therefore been suggested that it may play an autocrine role in development of these neoplasms. Since the PDGF-A promoter contains putative binding sites for WT1, we explored the role of WT1 in regulating A-chain expression. The major PDGF-A promoter activity was localized in transient transfection assays to a region spanning from -643 to + 8 relative to the transcription start site. WT1 bound to several sites in this region of the promoter, as demonstrated by gel-shift analysis and DNase I footprinting, and functioned as a powerful repressor of PDGF-A transcription in vivo. Maximal repression (> 50-fold) of the PDGF-A promoter was dependent on the presence of multiple WT1 binding sites in transient transfection assays. Our observations suggest a mechanism for normal downregulation of a growth factor gene and of an autocrine growth process of import in kidney development and other biological systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckwith J. B., Kiviat N. B., Bonadio J. F. Nephrogenic rests, nephroblastomatosis, and the pathogenesis of Wilms' tumor. Pediatr Pathol. 1990;10(1-2):1–36. doi: 10.3109/15513819009067094. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Bonthron D. T., Morton C. C., Orkin S. H., Collins T. Platelet-derived growth factor A chain: gene structure, chromosomal location, and basis for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1492–1496. doi: 10.1073/pnas.85.5.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D., Collins T., Grzeschik K. H., van Roy N., Speleman F. Platelet-derived growth factor A chain: confirmation of localization of PDGFA to chromosome 7p22 and description of an unusual minisatellite. Genomics. 1992 Jun;13(2):257–263. doi: 10.1016/0888-7543(92)90240-s. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., van Koppen A., Schatteman G. Is PDGF really important? Testing the hypotheses. Trends Genet. 1991 Nov-Dec;7(11-12):413–418. [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Cao X. M., Koski R. A., Gashler A., McKiernan M., Morris C. F., Gaffney R., Hay R. V., Sukhatme V. P. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol. 1990 May;10(5):1931–1939. doi: 10.1128/mcb.10.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Collins T., Bonthron D. T., Orkin S. H. Alternative RNA splicing affects function of encoded platelet-derived growth factor A chain. Nature. 1987 Aug 13;328(6131):621–624. doi: 10.1038/328621a0. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Tadaro G. J. A human fibrosarcoma cell line producing multiplication stimulating activity (MSA)-related peptides. Nature. 1978 Mar 23;272(5651):356–358. doi: 10.1038/272356a0. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Fleming T. P., Matsui T., Heidaran M. A., Molloy C. J., Artrip J., Aaronson S. A. Demonstration of an activated platelet-derived growth factor autocrine pathway and its role in human tumor cell proliferation in vitro. Oncogene. 1992 Jul;7(7):1355–1359. [PubMed] [Google Scholar]
- Fraizer G. E., Bowen-Pope D. F., Vogel A. M. Production of platelet-derived growth factor by cultured Wilms' tumor cells and fetal kidney cells. J Cell Physiol. 1987 Oct;133(1):169–174. doi: 10.1002/jcp.1041330122. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Halper J., Moses H. L. Epithelial tissue-derived growth factor-like polypeptides. Cancer Res. 1983 May;43(5):1972–1979. [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Hoheisel J., Pohl F. M. Simplified preparation of unidirectional deletion clones. Nucleic Acids Res. 1986 Apr 25;14(8):3605–3605. doi: 10.1093/nar/14.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Little M. H., Prosser J., Condie A., Smith P. J., Van Heyningen V., Hastie N. D. Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4791–4795. doi: 10.1073/pnas.89.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Nistér M., Claesson-Welsh L., Eriksson A., Heldin C. H., Westermark B. Differential expression of platelet-derived growth factor receptors in human malignant glioma cell lines. J Biol Chem. 1991 Sep 5;266(25):16755–16763. [PubMed] [Google Scholar]
- Patwardhan S., Gashler A., Siegel M. G., Chang L. C., Joseph L. J., Shows T. B., Le Beau M. M., Sukhatme V. P. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991 Jun;6(6):917–928. [PubMed] [Google Scholar]
- Paulsson Y., Hammacher A., Heldin C. H., Westermark B. Possible positive autocrine feedback in the prereplicative phase of human fibroblasts. Nature. 1987 Aug 20;328(6132):715–717. doi: 10.1038/328715a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Ratner L., Thielan B., Collins T. Sequences of the 5' portion of the human c-sis gene: characterization of the transcriptional promoter and regulation of expression of the protein product by 5' untranslated mRNA sequences. Nucleic Acids Res. 1987 Aug 11;15(15):6017–6036. doi: 10.1093/nar/15.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Morris J. F., Tournay O. E., Cook D. M., Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990 Nov 30;250(4985):1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman F., Bywater M., Knott T. J., Scott J., Betsholtz C. Structural characterization of the human platelet-derived growth factor A-chain cDNA and gene: alternative exon usage predicts two different precursor proteins. Mol Cell Biol. 1988 Feb;8(2):571–577. doi: 10.1128/mcb.8.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose E. A., Glaser T., Jones C., Smith C. L., Lewis W. H., Call K. M., Minden M., Champagne E., Bonetta L., Yeger H. Complete physical map of the WAGR region of 11p13 localizes a candidate Wilms' tumor gene. Cell. 1990 Feb 9;60(3):495–508. doi: 10.1016/0092-8674(90)90600-j. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol. 1990 Dec;1(6):859–866. doi: 10.1681/ASN.V16859. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. F., Volkenant M. E., Branum E. L., Moses H. L. Comparison of intra- and extracellular transforming growth factors from nontransformed and chemically transformed mouse embryo cells. Cancer Res. 1983 Apr;43(4):1581–1586. [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]