Abstract
Background:
Since preterm premature rupture of membranes (PPROM) is one of the most important complications of pregnancy and its relationship with nutrition status have not been surveyed comprehensively, we decided to study the relationship of maternal received nutrients (36 macro- and micro-nutrients) in three trimesters and PPROM which could be considered as a unique study.
Materials and Methods:
In this prospective cohort study, data was collected by filling a questionnaire through interviews with 620 pregnant women who had no parameters to affect pregnancy outcome. 48-hr dietary recalls were completed for eligible women at 11th–15th, 26th, 34th–37th weeks of gestation. Physical activity was also assessed using a standard questionnaire. Also pregnant mother's reproductive and demographic characteristic and supplementation are considered.
Results:
The mean value of received saturated fatty acids, polyunsaturated fatty acids and energy, in the first trimester (P < 0.001, P = 0.007, and P < 0.001 respectively), the mean values of calcium, sodium intake in the second trimester (P = 0.045, P = 0.006, and P = 0.004 respectively), Vitamins C, A (mg), β-carotene, cartenoids intake in the second trimester (P = 0.03, P = 0.001, P = 0.007, and P = 0.01 respectively), and higher Vitamin C intake during the first trimester (P = 0.02) was significantly greater among subjects with PPROM compared to the others.
Conclusions:
The mean value of mentioned received nutrients in subjects who experienced PPROM later in pregnancy was higher than the others, which is independent of demographic and reproductive characteristic, estimated physical activity, and supplementation. Therefore, these findings could be considered in the nutritional programming for pregnant women to manage the risk of PPROM.
Keywords: Macro nutrients, minerals, pregnancy outcome, preterm premature rupture of membranes, vitamins
INTRODUCTION
Preterm premature rupture of membranes (PPROM) is defined as spontaneous rupture of the membranes at <37 weeks gestation at least 1 hour before the onset of contractions. It precedes delivery in approximately 25–30% of patients delivering prematurely.[1] Up to 4% of all pregnancies are complicated by PPROM.[2] It is associated with considerable increase in adverse maternal, fetal and neonatal risk.[3] The etiology of PPROM is believed to be multifactorial.[4] Maintenance of the chorioamniotic sac throughout normal pregnancy requires a balance between collagen synthesis by fibroblasts and collagenolytic activity by controlled responses of enzymes expressed in the fetal membranes or derived from inflammatory cells.[5,6] Reactive oxygen species (ROS) could be responsible for collagen damage in the chorioamniotic sac leading to tearing. Normally a balance exists between production and elimination of ROS. Oxidative stress (OS) occurs when pro-oxidants exceed antioxidants.[7,8,9] OS caused by increased ROS formation may modify the strength and the elasticity of collagen and cause PROM.[5,10] Therefore, PPROM may be an effect of collagen damage caused by increased ROS formation and/or antioxidant deletion, too.[10,11]
Micronutrient deficiencies that affect collagen formation have been shown to alter collagen structure and this has been associated with an increased risk of PPROM.[4,12,13] For instance, an association between low Vitamin C levels and PPROM has been reported.[14] Ferguson et al. looked at the association of PPROM to poor nutritional status using biochemical markers and dietary intake of micronutrients for the 1st time.[15]
On the other hand, Vitamins C and E supplementation at the recommended doses has been reported with an increased risk of PROM and PPROM.[16,17] Thus, we decided to survey the association between nutrition status of pregnant women and PPROM, especially it was urged by some of Cochrane reviews on this issue.[18] Nutritional status during pregnancy can be described by indicators of body size such as body mass index (BMI), nutritional intake, and serum assessments for various serum analytes.[4] In this study, the association between PPROM risk and intake of macro- and micro-nutrients during the first, second, and third trimesters was assessed. Our hypothesis is that the macro- and micro-nutrients are associated with PPROM.
MATERIALS AND METHODS
This prospective cohort study (approved by the Institutional Review Board of Isfahan University of Medical Sciences) was conducted in four stages (The first, second, and third trimester of pregnancy and postpartum) on a group of 620 Iranian pregnant women aged 15–49 years between 10 and 40 gestational weeks whose delivery in hospital led to the birth of alive and apparently healthy baby. In addition, they referred to 18 health centres and 12 private offices from April 2009 to August 2010. Considering the fact that 65% of women in Isfahan refer to health centres, 20% to private offices, and 15% to both, convenient sampling was performed. The women were in good general health, with no history of prior adverse pregnancy outcome. Exclusion criteria were conditions or factors causing PPROM, preterm delivery, low birth weight, and preeclampsia (placenta previa, placenta abruption, diabetes mellitus, gestational diabetes, uterine abnormalities, twine or multiple pregnancies, cervix incompetence, oligo-hydramnios, poly-hydramnios, <2 years pregnancies interval, trauma or surgery in the present pregnancy, urinary tract, genital system infections, abnormal presentations, and still birth). Other factors included smoking and drug addiction, digestive and metabolic diseases, hyper emesis gravid arum, gastro intestinal diseases, active hepatitis and hepatic disorders, hemoglobinopathies and anemia’s, eating disorders, allergies, mental diseases and neurologic disorders, malignancy, Gaucher's diseases, chronic inflammatory diseases, asthma and respiratory system diseases, and cardiovascular diseases.[19,20,21] Taking their consent, data was collected by a questionnaire which was completed through interviews with pregnant women and prenatal and obstetric care-related records considering demographic and reproductive characteristics and physical activity as important confounding variables. In addition to this, 48-h dietary recalls were completed for eligible pregnant women at the 11th–15th, 26th, and 34th–37th weeks of gestation.[22,23,24] Physical activity was considered as any physical movement due to skeletal muscles resulting in energy consumption. Data on physical activity was collected using a standard pregnancy physical activity questionnaire which consists of 4 parts including physical activity at home, exercise, leisure activities, and workplace activity.[25] Therefore, the performed physical activities within 48 h were also assessed at the 11th–15th, 26th, and 34th–37th weeks of gestation (Physical activity was measured in metabolic equivalent of task-hours [MET-hours] of each activity multiplied by the duration of the activity in the day. MET-hours is a unit for estimating the metabolic cost or oxygen consumption of a particular physical activity, according to a standard questionnaire). Total amount of activity was calculated by summing the activities in the 3 trimesters and was used for analysis. The subjects were then followed until the end of pregnancy. The risk of PPROM was collected through patient records. To increase reliability, all interviewers were trained in the same conditions. On the other hand, patient records were completed by experts who guaranteed their reliability. In addition, since the records are prepared in fixed and standardized forms, their reliability confidence has already been proven. Data obtained from the 48-hr dietary recalls was analyzed using Nutrition-IV software. Data analysis was performed in SPSS 18.0 (IBM Company, the United States) using t-test, binary logistic regression, and Chi-square analysis. P < 0.05 were considered significant in all tests.
Kolmogorov–Smirnov test was used to determine if the data followed Gaussian distribution. It has been shown that the distribution of macro- and micro-nutrients intake variables followed Gaussian distribution in groups with and without PPROM. So Student's t-test was used to compare the received macro- and micro-nutrients means between two groups.
RESULTS
According to our findings, 17 patients out of the 620 studied pregnant women (2.74%) were diagnosed with PPROM. Their demographic and reproductive characteristics are shown in Table 1.
Table 1.
Demographic and reproductive characteristic of the participants
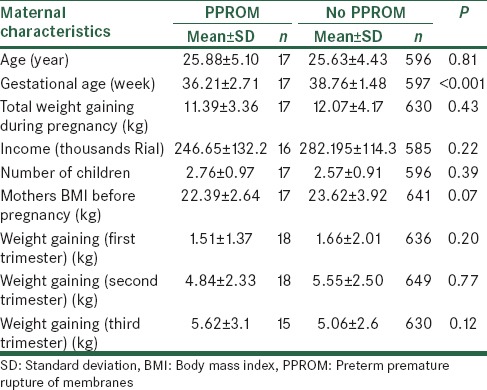
None of personal and reproductive characteristics which are shown in Table 1 weren’t significantly associated with PPROM.
Mean values of micro- and macro-nutrient were calculated in the first, second and third trimesters [Table 2a–e]. The mean of received saturated fat (26.20 ± 14.35 in compare of 68.96 ± 154.58, P < 0.001), polyunsaturated fat (33.77 ± 11.82 in compare of 41.77 ± 15.71, P = 0.007) and energy (2299.59 ± 729.57 in compare of 3134.75 ± 2234.06, P < 0.001), in the second trimester in subjects who experienced PPROM later in pregnancy was higher than the other pregnant women. In addition, the mean values of iron (20.47 ± 8.79 in compare of 24.87 ± 11.59, P = 0.04), calcium (943.26 ± 425.01 in compare of 1234.20 ± 426.14, P = 0.006), and sodium (3080.73 ± 1621.73 in compare of 4252.58 ± 2845.20, P = 0.004) intake in the second trimester among women with PPROM were significantly more than healthy pregnant women. Vitamins C (152.70 ± 105.76 in compare of 208.66 ± 193.10, P = 0.03), A (mg) (780.95 ± 1067.45 in compare of 1707.11 ± 2929.92, P = 0.001), β-carotene (371.80 ± 569.39 in compare of 783.31 ± 525.75, P = 0.007), carotenoids (371.80 ± 569.39 in compare of 783.32 ± 1975.79, P = 0.01) intake in the second trimester was significantly greater among subjects with PPROM compared to the others.
Table 2a.
Maternal intake of macronutrients in the first, second, and third trimesters of pregnancy
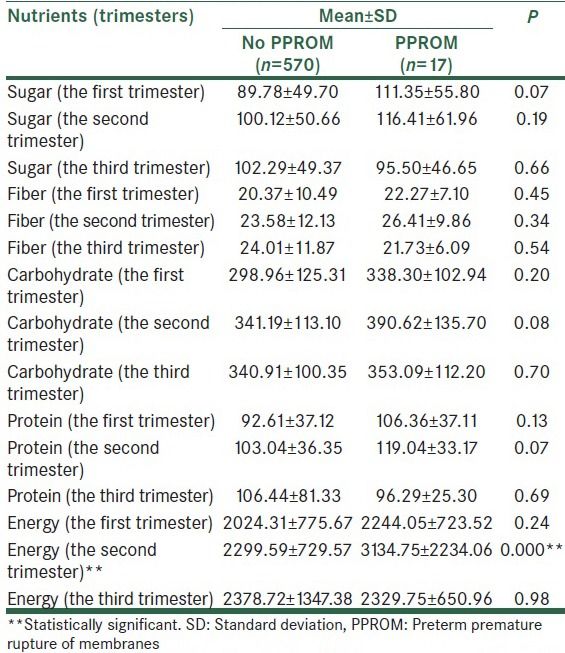
Table 2e.
Maternal intake of micronutrients in the first, second, and third trimesters of pregnancy
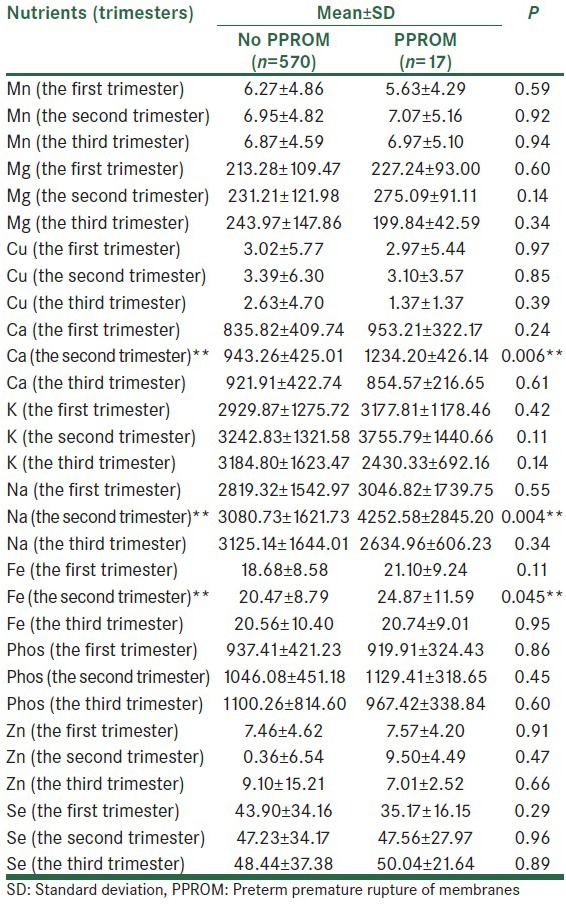
Table 2b.
Maternal Intake of fatty acids and cholesterol in the first, second, and third trimesters of pregnancy
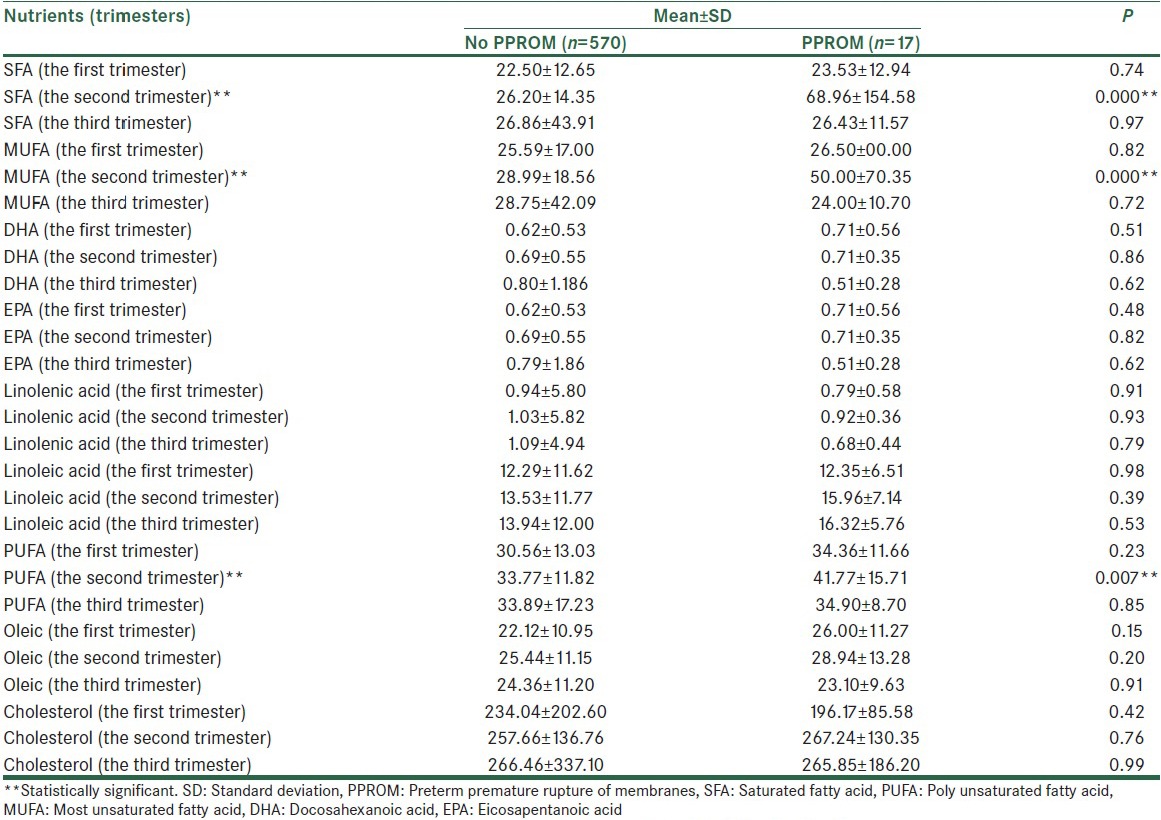
Table 2c.
Maternal intake of micronutrients (vitamins) in the first, second, and third trimesters of pregnancy
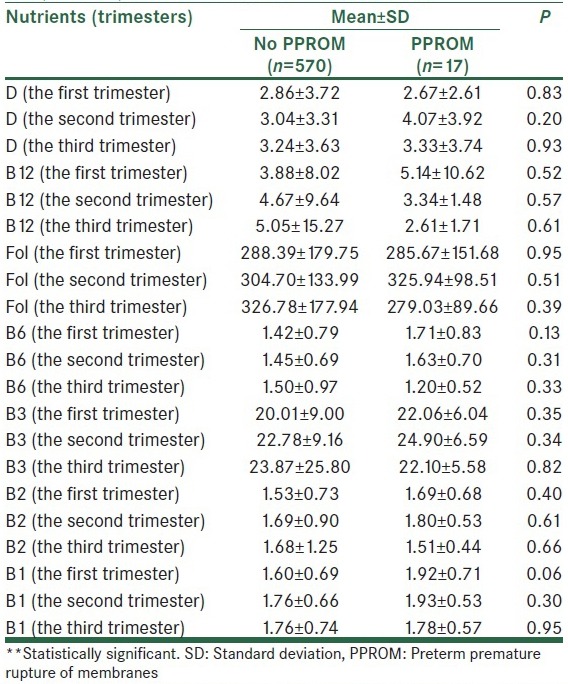
Table 2d.
Maternal intake of antioxidants in the first, second, and third trimesters of pregnancy
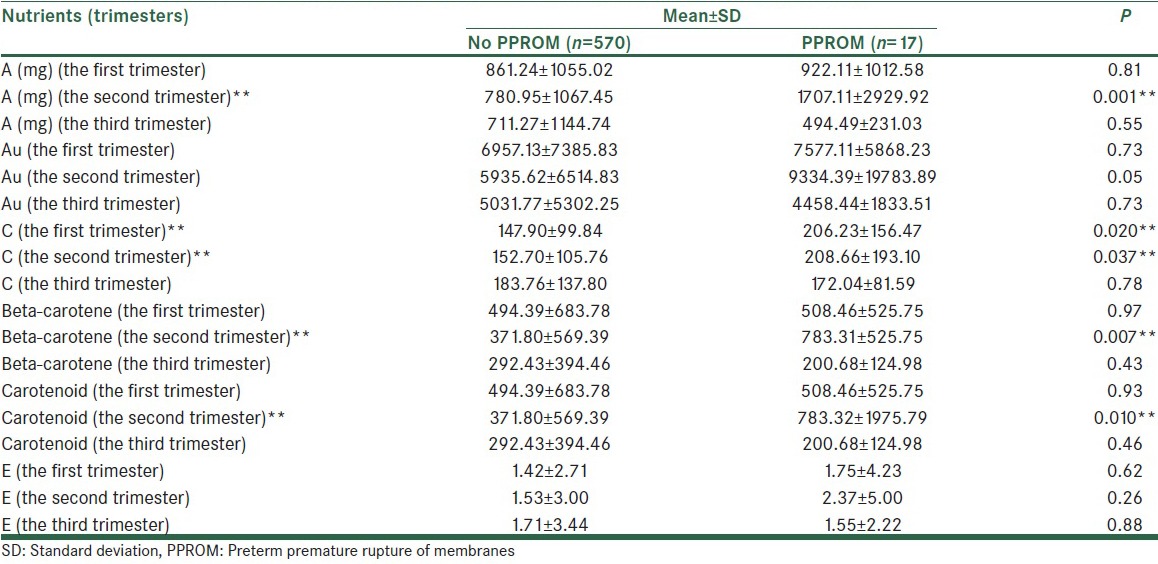
Also significant relation between higher Vitamin C intake during the first trimester and PPROM risk was found (147.90 ± 99.84 in compare of 206.23 ± 156.47, P = 0.02).
Except for the mentioned variables, there were no significant differences between other micro- and macro-nutrients and the risk of PPROM [Table 2a–e].
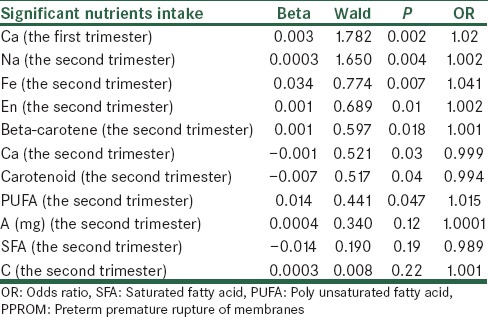
In this study, all variables associated with PPROM were also analyzed by logistic regression analysis. This analysis method showed that these variables could predict PPROM affection in 97.4% of the cases. The most significant risk factor for PPROM was Vitamin C intake in the first trimester. Others have been shown respectively in Table 3 (The more Wald statistic, the stronger significant relationship).
Table 3.
The association of significant nutrients intake with PPROM by logistic regression
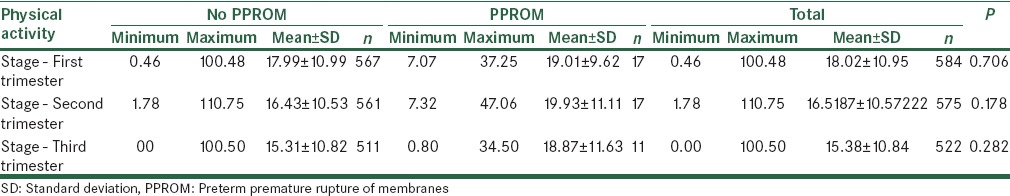
Based on the calculated values of physical activity per day, no significant relations were found between physical activity during the first, second, and third trimesters and risk of PPROM [Table 4].
Table 4.
Physical activity during the first, second, and third trimesters and risk of PPROM
DISCUSSION
In this prospective cohort study of pregnancies, we found increased risk for PPROM among women reporting higher dietary intake of saturated fatty acids (SFA), polyunsaturated fatty acids (PUFAs), energy, iron, calcium, sodium, Vitamins C, A, β-carotene, Carotenoid, in the second trimester (26th weeks of gestation) and Vitamin C intake in the first trimester (11–15th week of gestation).
Women with normal pregnancies experience increased OS compared with nonpregnant women. Elevations appear by the second trimester and gradually diminish later in gestation, decreasing further after delivery.[26] Overproduction or inadequate control of OS may be involved in the etiology of obstetric complications such as PPROM.[27] Macronutrients are either targets of oxidative modifications after absorption or are present in a pro-oxidant form in the diet.[28] For instance, the effect of unsaturated fat intake on lipoprotein oxidation and lipoprotein oxidizability are documented along with the widely accepted increased antioxidant demand associated with the intake of diets rich in PUFA.[29]
According to our results, the mean of saturated fat intake during the second trimester in pregnant women who developed PPROM in later weeks of pregnancy was more than the other subjects (P < 0.001). Detrimental effects attributable to saturated fat consumption have been shown by increased inflammatory bioactivity,[30] increased lipid per oxidation,[31] decreased antioxidant activity,[32] and enhanced cytokine levels[33] which can be involved in the pathophysiology of PPROM.
Our data showed that higher intake of poly unsaturated fat (both omega-3 and omega-6) is associated with the risk of PPROM (P = 0.007).
Although there seems to be a general agreement that moderate intake of long-chain PUFAs (LCPUFA) reduce the risk of some diseases, there are indications that too high intake of polyunsaturated fat may be harmful. These harmful effects ascribed to their pro-oxidative role.[22,32]
Nutrients like LCPUFA are precursors of important bioactive compounds such as the prostacyclins, prostaglandins, thromboxanes and leukotriens, too.[33]
Fetal membrane production of these eicosanoids may stimulate uterine contractions in late preterm delivery and eicosanoide activated type IV collagenase activity may play a role in PPROM.[34]
We also found that mean of energy intake in the second trimester of women with PPROM was more than women without PPROM (P < 0.001).
Fat provides energy, indeed it is the most energy dense of all the macronutrients, with 1 g providing 37 kj (9 kcal).[33,35]
Considering higher intake of SFA and PUFAs among women who experienced PPROM, the relationship between higher intake of energy and PPROM is reasonable. Specifically, the association between energy intake and risk for PPROM in the current study was independent of BMI, estimates of physical activity, and weight gaining (in the second trimesters).
Moreover, researchers have shown that energy-restricted diet increases plasma antioxidant capacity and is able to decrease lipid per oxidation together with the benefits related to weight loss.[36]
Another result obtained in this study was higher iron and calcium intake in women who experienced PPROM (P = 0.045 and P = 0.006 respectively). Although energy production is an important function of mitochondria, these organelles also participate in initiating and executing both apoptotic and necrotic cell death as well as in maintaining calcium and iron homeostasis.[37] Thus, it appears that mitochondria serve as a central barometer for assessing changes in cellular viability. Mitochondrial–related events such as increased concentrations of free Ca2+ and bioavailable ferrous iron appear to be important contributors to the demise of the cell and OS, either collectively or individually.[38,39]
In another study, researchers demonstrated that the burden of ROS can be further amplified by the presence of free metals such as iron, copper and manganese that are released from metalloprotein complexes.[40] Also Brion et al. reported that ROS production is enhanced by the presence of free iron.[41] A growing body of literature indicates that PPROM may result from ROS-induced damage to amnion epithelium or collagen in the chorioamnion.[26]
As mentioned above, mitochondrial matrix ca2+ overload can lead to enhanced generation of reaction oxygen species (ROS).[42] In conformity with our results about calcium, Iams et al. showed that although agents such as calcium or antioxidants were able to reduce preeclampsia, a resulting decrease in preterm birth was not observed and nor did the rate if PPROM in some studies.[43]
We also found that mean of sodium intake in the second trimester of women with PPROM was more than women without PPROM (P = 0.004).
There are two sodium-dependent membrane transporters encoded by SLC23A1 and SLC23A2, which have key roles in human Vitamin C metabolism and which control dietary uptake, re-absorption and tissue distribution of Vitamin C.[44] On this background, Erichsen et al. provided new data to link Vitamin C transport mechanisms, albeit indirectly, to preterm birth.[45] The mechanisms for Vitamin C uptake, re-absorption and distribution are dependent on sodium. Therefore, the relationship between higher sodium intake and the risk of PPROM can be attributed to Vitamin C absorption and distribution. Increasing Vitamin C and PPROM is also seen in the current study. However, these findings need to investigate biologically and cellularly, that was not possible in this study.
In accordance to the data, higher intake of Vitamin C, Vitamin A (mg), carotenoids, and β-carotene intakes were significantly associated to the risk of PPROM (P < 0.05).
There are two types of antioxidant in the human body: Enzymatic antioxidants and nonenzymatic antioxidants. The latter include glutathione, Vitamins A, C and E. In agreement with our findings, Mathews and Neil have been able to demonstrate no protective effect of increased levels of antioxidant nutrients against PPROM.[46] The possibility of a pro-oxidative effect of β-carotene, Vitamins C, E and lipoid acid as contributory factors to the observed negative effects have been also suggested.[47,48,49]
At the following, we will explain the above mentioned association of antioxidants and the risk of PPROM in detail.
An important finding of the present study are the significant differences in mean values if Vitamin C intake in patients with PPROM in the first and second trimester compared to other mothers (P = 0.02, P = 0.03, respectively). In fact, Vitamin C intake in mothers with PPROM was significantly more than healthy mothers.
The elevated risk of preterm premature rapture of the membranes has been shown for women with a low Vitamin C intake in some studies.[50]
A Cochrane review on the effects of Vitamin C supplementation in pregnancy concluded that there was too little data to determine whether Vitamin C supplementation is beneficial and that it may be associated with PPROM.[50]
Also other studies reported an increased frequency of PPROM among women randomized to vitamin supplementation.[17,51]
Kumar et al., s results indicate that hydrogen peroxide (HP) induces apoptosis in amnion-derived WISH cells (WISH cells have long been utilized as a model for human amnion epithelial cells from which they were thought to have been derived, the strability of this cell line makes it usefull for studies that primary culture of amnion cells will not folerate). WISH cells have long been utilized as a model for human amnion epithelial cells from which they were thought to have been derived, the strability of this cell line makes it usefull for studies that primary culture of amnion cells will not folerate. Vitamin C preincubation does not inhibit, and may accelerate and exacerbate, HP induced apoptosis. It is imperative that detailed characterization of time and dose effects of Vitamin C and Vitamin E upon human chorio-amnion be completed before such agents are recommended routinely as a means to prevent preterm labor.[52]
Therefore, the association between higher intake of Vitamin C and PPROM may be explained by dose dependent pro-oxidant effects.
The last result showed the significant difference between the two groups in Vitamin A (mg), carotenoids, and β-carotene intake in the second trimester (P = 0.001, P = 0.01, and P = 0.007 respectively).
Vitamin A is considered to have antioxidant properties.[53] While not necessarily a casual relationship, some researchers have also observed an increased risk of PPROM among women with high serum levels of lutiein.[46]
It is now becoming clear that there is an optimal dose of carotenoids that results in maximum antioxdidant effectiveness in human cells, but it is still not evident why higher doses of carotenoid should be less effective than lower doses, or actually result in increased damage to the cell.[54,55]
Also, authors using cells in culture have shown not only loss of antioxidant effectiveness, but also pro-oxidant effects of carotenoids at high carotenoid concentrations.[56] Of the carotenoids, β-carotene has the highest provitamin A activity. The efficiency of β-carotene conversion to Vitamin A decrease, with higher β-carotene intake.[57]
Burton and Ingold more over than 30 years ago proposed that β-carotene may act as a pro-oxidant under conditions of high carotenoid and oxygen concentrations.[57] Also recently Deoliveira et al. concluded that vitamins such as β-carotene can exert an antioxidant effect and a pro-oxidant effect according to their concentrations, and could be an indicator of an inflammatory process in vitro generating severe complications to the body in cellular levels.[58]
The study has a large sample size and prospective design. The work is unique in having collected data on a wide range of nutrients intake, and in measuring nutrients intake at three stages in pregnancy, which allowed investigating the possible changes in nutrients during gestation. Particular care was taken to assess potential confounding variables accurately, especially physical activity, demographic and reproductive characteristics.
We did not measure serum levels of nutrients, because it was not economically beneficial. Therefore, we recommend to assess the relationship between serum levels of macro- and micro-nutrients (demonstrated in the present study) and the risk of PPROM.
CONCLUSION
Based on the results of this study, the mean value of received SFAs, poly unsaturated fatty acids, energy, iron, calcium, sodium, Vitamin C, A, β-carotene, Carotenoids in the second trimester and Vitamin C intake in the first trimester in subjects who experienced PPROM later in pregnancy was higher than the other pregnant women. Therefore, these findings could be considered in the nutritional programming for pregnant women to manage the risk of PPROM.
Financial support and sponsorship
Research Bureau of Medical Sciences, University of Isfahan.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schleußner E. The prevention, diagnosis and treatment of premature labor. Dtsch Arztebl Int. 2013;110:227–35. doi: 10.3238/arztebl.2013.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health UNLoMNIo. ACOG practice bulletin no.80: Premature rupture of membranes. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2007;109:1007–19. doi: 10.1097/01.AOG.0000263888.69178.1f. [DOI] [PubMed] [Google Scholar]
- 3.Menon R. Spontaneous preterm birth, a clinical dilemma: Etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods JR., Jr Reactive oxygen species and preterm premature rupture of membranes-a review. Placenta. 2001;22(Suppl A):S38–44. doi: 10.1053/plac.2001.0638. [DOI] [PubMed] [Google Scholar]
- 6.Woods JR, Jr, Plessinger MA, Miller RK. Vitamins C and E: Missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001;185:5–10. doi: 10.1067/mob.2001.115868. [DOI] [PubMed] [Google Scholar]
- 7.Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res. 2000;47:221–4. doi: 10.1203/00006450-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Buonocore G, Perrone S, Longini M, Vezzosi P, Marzocchi B, Paffetti P, et al. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res. 2002;52:46–9. doi: 10.1203/00006450-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Buonocore G, Zani S, Perrone S, Caciotti B, Bracci R. Intraerythrocyte nonprotein-bound iron and plasma malondialdehyde in the hypoxic newborn. Free Radic Biol Med. 1998;25:766–70. doi: 10.1016/s0891-5849(98)00126-9. [DOI] [PubMed] [Google Scholar]
- 10.Wall PD, Pressman EK, Woods JR., Jr Preterm premature rupture of the membranes and antioxidants: The free radical connection. J Perinat Med. 2002;30:447–57. doi: 10.1515/JPM.2002.071. [DOI] [PubMed] [Google Scholar]
- 11.Curran SF, Amoruso MA, Goldstein BD, Berg RA. Degradation of soluble collagen by ozone or hydroxyl radicals. FEBS Lett. 1984;176:155–60. doi: 10.1016/0014-5793(84)80931-x. [DOI] [PubMed] [Google Scholar]
- 12.McParland PC, Taylor DJ. Preterm prelabour rupture of the membranes. In: Bonnar J, Dunlop W, editors. Recent Advances in Obstetric and Gynaecology. Vol. 23. Oxford University Press; 2005. pp. 27–38. [Google Scholar]
- 13.Oluwafemi O. The biochemical, physiological and therapeutic roles of ascorbic acid. Afr J Biotechnol. 2008;7:4700–5. [Google Scholar]
- 14.Shen TT, DeFranco EA, Stamilio DM, Chang JJ, Muglia LJ. A population-based study of race-specific risk for preterm premature rupture of membranes. Am J Obstet Gynecol. 2008;199:373.e1–7. doi: 10.1016/j.ajog.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SE, Smith GN, Salenieks ME, Windrim R, Walker MC. Preterm premature rupture of membranes. Nutritional and socioeconomic factors. Obstet Gynecol. 2002;100:1250–6. doi: 10.1016/s0029-7844(02)02380-3. [DOI] [PubMed] [Google Scholar]
- 16.Hauth JC, Clifton RG, Roberts JM, Spong CY, Myatt L, Leveno KJ, et al. Vitamin C and E supplementation to prevent spontaneous preterm birth: A randomized controlled trial. Obstet Gynecol. 2010;116:653–8. doi: 10.1097/AOG.0b013e3181ed721d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinnato JA, 2nd, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, Koch MA, et al. Antioxidant supplementation and premature rupture of the membranes: A planned secondary analysis. Am J Obstet Gynecol. 2008;199:433.e1–8. doi: 10.1016/j.ajog.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori R, Ota E, Middleton P, Tobe-Gai R, Mahomed K, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database System Rev. 2012 Jul 11;7:CD000230. doi: 10.1002/14651858.CD000230.pub4. [DOI] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Ludvigsson J. Milk consumption during pregnancy and infant birthweight. Acta Paediatr. 2004;93:1474–8. doi: 10.1080/08035250410018319. [DOI] [PubMed] [Google Scholar]
- 20.Lee BE, Hong YC, Lee KH, Kim YJ, Kim WK, Chang NS, et al. Influence of maternal serum levels of vitamins C and E during the second trimester on birth weight and length. Eur J Clin Nutr. 2004;58:1365–71. doi: 10.1038/sj.ejcn.1601976. [DOI] [PubMed] [Google Scholar]
- 21.Kac G, Benício MH, Velásquez-Meléndez G, Valente JG, Struchiner CJ. Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of brazilIan women. J Nutr. 2004;134:661–6. doi: 10.1093/jn/134.3.661. [DOI] [PubMed] [Google Scholar]
- 22.McGowan CA, McAuliffe FM. Maternal nutrient intakes and levels of energy underreporting during early pregnancy. Eur J Clin Nutr. 2012;66:906–13. doi: 10.1038/ejcn.2012.15. [DOI] [PubMed] [Google Scholar]
- 23.Arija V, Cucó G, Vila J, Iranzo R, Fernández-Ballart J. Food consumption, dietary habits and nutritional status of the population of Reus: Follow-up from preconception throughout pregnancy and after birth. Med Clin (Barc) 2004;123:5–11. doi: 10.1016/s0025-7753(04)74395-x. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan U, González-Cossío T, Neufeld LM, Rivera J, Martorell R. Effect of prenatal multiple micronutrient supplements on maternal weight and skinfold changes: A randomized double-blind clinical trial in Mexico. Food Nutr Bull. 2005;26:273–80. doi: 10.1177/156482650502600304. [DOI] [PubMed] [Google Scholar]
- 25.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36:1750–60. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 26.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 27.Connors N, Merrill D. Antioxidants for prevention of preterm delivery. Clin Obstet Gynecol. 2004;47:822–32. doi: 10.1097/01.grf.0000141431.23642.fc. [DOI] [PubMed] [Google Scholar]
- 28.Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155:517–23. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- 29.Olsen SF, Secher NJ, Björnsson S, Weber T, Atke A. The potential benefits of using fish oil in relation to preterm labor: The case for a randomized controlled trial? Acta Obstet Gynecol Scand. 2003;82:978–82. doi: 10.1034/j.1600-0412.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- 30.Catov JM, Bodnar LM, Kip KE, Hubel C, Ness RB, Harger G, et al. Early pregnancy lipid concentrations and spontaneous preterm birth. Am J Obstet Gynecol. 2007;197:610.e1–7. doi: 10.1016/j.ajog.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Mozurkewich E, Berman DR, Chilimigras J. Role of omega-3 fatty acids in maternal, fetal, infant and child wellbeing. Exp Rev Obstet Gynecol. 2010;5:125–38. [Google Scholar]
- 32.Williams C. Dietary fatty acids and human health. Ann Zootech. 2000;49:165–80. [Google Scholar]
- 33.Haggarty P. Placental regulation of fatty acid delivery and its effect on fetal growth – A review. Placenta. 2002;23(Suppl A):S28–38. doi: 10.1053/plac.2002.0791. [DOI] [PubMed] [Google Scholar]
- 34.Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am J Obstet Gynecol. 2005;193:1292–301. doi: 10.1016/j.ajog.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Lunn J, Theobald H. The health effects of dietary unsaturated fatty acids. Nutr Bull. 2006;31:174–228. [Google Scholar]
- 36.Parra D, Bandarra NM, Kiely M, Thorsdottir I, Martínez JA. Impact of fish intake on oxidative stress when included into a moderate energy-restricted program to treat obesity. Eur J Nutr. 2007;46:460–7. doi: 10.1007/s00394-007-0686-3. [DOI] [PubMed] [Google Scholar]
- 37.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 38.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–65. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 39.Napier I, Ponka P, Richardson DR. Iron trafficking in the mitochondrion: Novel pathways revealed by disease. Blood. 2005;105:1867–74. doi: 10.1182/blood-2004-10-3856. [DOI] [PubMed] [Google Scholar]
- 40.van der Vliet A. NADPH oxidases in lung biology and pathology: Host defense enzymes, and more. Free Radic Biol Med. 2008;44:938–55. doi: 10.1016/j.freeradbiomed.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 43.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 44.Stratakis CA, Taymans SE, Daruwala R, Song J, Levine M. Mapping of the human genes (SLC23A2 and SLC23A1) coding for Vitamin C transporters 1 and 2 (SVCT1 and SVCT2) to 5q23 and 20p12, respectively. J Med Genet. 2000;37:E20. doi: 10.1136/jmg.37.9.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erichsen HC, Peters U, Eck P, Welch R, Schoen RE, Yeager M, et al. Genetic variation in sodium-dependent Vitamin C transporters SLC23A1 and SLC23A2 and risk of advanced colorectal adenoma. Nutr Cancer. 2008;60:652–9. doi: 10.1080/01635580802033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathews F, Neil A. Antioxidants and preterm prelabour rupture of the membranes. BJOG. 2005;112:588–94. doi: 10.1111/j.1471-0528.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang P, Omaye ST. DNA strand breakage and oxygen tension: Effects of beta-carotene, alpha-tocopherol and ascorbic acid. Food Chem Toxicol. 2001;39:239–46. doi: 10.1016/s0278-6915(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Omaye ST. Antioxidant and prooxidant roles for beta-carotene, alpha-tocopherol and ascorbic acid in human lung cells. Toxicol In Vitro. 2001;15:13–24. doi: 10.1016/s0887-2333(00)00054-0. [DOI] [PubMed] [Google Scholar]
- 49.Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 50.Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Editorial Group: Cochrane Pregnancy and Childbirth Group, Published Online: 24 JAN 2005, Published Online: 24 JAN 2005, Assessed as up-to-date: 4 SEP 2004. DOI: 10.1002/14651858.CD004072. pub2. [Google Scholar]
- 51.Steyn PS, Odendaal HJ, Schoeman J, Stander C, Fanie N, Grové D. A randomised, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labour. J Obstet Gynaecol. 2003;23:150–5. doi: 10.1080/014436103000074673. [DOI] [PubMed] [Google Scholar]
- 52.Kumar D, Lundgren DW, Moore RM, Silver RJ, Moore JJ. Hydrogen peroxide induced apoptosis in amnion-derived WISH cells is not inhibited by vitamin C. Placenta. 2004;25:266–72. doi: 10.1016/j.placenta.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Knuppel RA, Hassan MI, McDermott J, Tucker JM, Morrison J. Oxidative Stress and Antioxidants: Preterm Birth and Preterm Infants. Preterm Birth-Mother and Child, Dr. John Morrison (Ed) ISBN: 978-953-307-828-1, InTech. [Google Scholar]
- 54.Lowe GM, Vlismas K, Young AJ. Carotenoids as prooxidants? Mol Aspects Med. 2003;24:363–9. doi: 10.1016/s0098-2997(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 55.El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, et al. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48. doi: 10.1016/j.abb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Lockwood CJ, Kuczynski E. Markers of risk for preterm delivery. J Perinat Med. 1999;27:5–20. doi: 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- 57.Burton GW, Ingold KU. beta-Carotene: An unusual type of lipid antioxidant. Science. 1984;224:569–73. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- 58.DeOliveira BF, Veloso CA, Nogueira-Machado JA, Martins Chaves M. High doses of in vitro beta-carotene, alpha-tocopherol and ascorbic acid induce oxidative stress and secretion of IL-6 in peripheral blood mononuclear cells from healthy donors. Curr Aging Sci. 2012;5:148–56. doi: 10.2174/1874609811205020148. [DOI] [PubMed] [Google Scholar]


