Abstract
Introduction
Breast cancer (BrCA) risk stratification using clinico-pathological biomarkers helps improve disease prognosis prediction. However, disease recurrence rates remain unfavorable and individualized clinical management strategies are needed. Consequently, we evaluated the influence of 14 sequence variants detected in IL-10, TGF-β1, VEGF, and their associated receptors as effective predictors of BrCA clinical outcomes.
Methods
Tumor DNA samples collected from 441 BrCA patients were genotyped using TaqMan-PCR. Most selected targets alter cytokine serum/plasma levels or signaling pathways. Relationships between genetic profiles and recurrence as well as disease-related mortality were evaluated using cumulative incidence curves and competing risk regression models.
Results
The VEGF−2578 C allele was associated with a 1.3-to 1.6-fold increase in BrCA recurrence (HRtrend = 1.28; 95% CI = 0.96–1.72) and disease-related mortality (HRtrend = 1.56; 95% CI = 0.93–2.56). Although this marker was marginally significant relative to BrCA outcomes, there were substantial gains in the 5- and 8-year predictive accuracy compared to standard prognostic indicators. Among ER+/PR+ status patients, there was a significant impact of the VEGF−2578 CC genotype on disease recurrence and predictive accuracy.
Conclusions
Our findings suggest inheritance of the VEGF−2578 C allele could serve as an independent prognostic indicator of BrCA prognosis. The VEGF−2578 marker may have clinical implications among a subset of ER+/ PR+ patients with an aggressive phenotype. Because the VEGF−2578 C allele is linked to high VEGF expression, this cytokine is a potential prognostic and targeted clinical management tool.
Keywords: Angiogenesis, Polymorphisms, Breast cancer, Disease recurrence, Overall survival
Introduction
Angiogenesis involves the division and migration of endothelial cells that form microvasculature. This process facilitates tumor growth by providing oxygenation through a series of interrelated steps, including endothelial cell proliferation, motility of endothelial cells through the extracellular matrix toward the angiogenic stimuli, and capillary differentiation. Tumor cells and their microenvironment mediate angiogenesis by altering the expression of angiostatic and angiogenic cytokines, which promote and inhibit angiogenesis, respectively. Recently, investigators reported poor survival among breast cancer (BrCA) patients exhibiting the interleukin (IL)-10−592A allele (linked with low IL-10 expression) after bone marrow transplantation [1]. IL-10 suppresses angiogenesis, tumor growth, and metastasis, presumably by inhibiting macrophage-derived angiogenic factors e.g., vascular endothelial growth factor (VEGF) [2–5]. In patients with breast- and other cancers, various cytokines such as IL-10, VEGF, and transforming growth factor (TGF)-β1 are frequently elevated and correlate with altered susceptibility to invasive disease, progression, metastasis, and poor clinical outcome [6–11].
Similar to IL-10, TGF-β1 can have divergent roles in cancer development. In normal epithelial, endothelial, and hematopoietic cells, TGF-β1 suppresses tumor growth by promoting differentiation and inhibiting proliferation. During later stages of tumorigenesis, defects in TGF-β1 signaling pathways cause many cancers to become resistant to TGF-β1-mediated growth inhibition, attributed to somatic mutations in downstream targets. Mechanisms supporting acquired resistance to inhibitory effects of TGF-β1 may involve reduced expression of TGF-β1 receptors, modulation of TGF-β1 binding partners, and loss or reduction of downstream mediators of cell survival and growth inhibition [6]. Once TGF-β1 cell signaling pathways are disrupted, TGF-β1 over-expression mediates tumor promotion through enhanced angiogenesis and immunosuppression, improving tumor invasion and metastatic potential. TGF-β1 may provide tumors with a growth advantage through immune suppression, promotion of angiogenesis, and extracellular matrix formation [12, 13]. Intriguingly, two TGF-β1 low-expressing alleles (29C and −509T) may be independently associated with lymph node metastases, advanced stage of BrCA, disease recurrence, and reduced disease-free survival (DFS) [14]. One report describes inhibiting somatic mutations in TGF-βR1(2) in recurrent and metastatic breast tumors [15].
VEGF, a potent angiogenic cytokine, serves a pivotal function during angiogenesis, promoting tumor growth and metastasis by binding to two major receptors, VEGF-R1 and VEGF-R2. Although VEGF-R1 has a greater affinity for VEGF, VEGF-R2 is tyrosine-phosphorylated more efficiently upon ligand binding, leading to mitogenesis, chemotaxis, and changes in cell morphology in endothelial cells. Once bound to its receptors, VEGF initiates a signal transduction pathway enhancing endothelial cell invasion, migration, and vascular permeability. Tumor cells, infiltrating T cells, and macrophages stimulate VEGF production mediated by various hormones, growth factors, and cytokines (e.g., IL-10). Elevated VEGF during early stages of BrCA associates with numerous dynamics, including increased tumor microvessel density, advanced stage of disease, poor responsiveness to therapy (e.g., tamoxifen and chemotherapy), and poor DFS [9, 10, 16–20]. Individuals inheriting the high-expressing VEGF alleles (e.g., −634C, −1154G, −2578C, 936C) are linked with increased risk of developing BrCA, tumor size[20 mm, tumor grade ≥2, and poor prognosis in some studies [21–23]. To our knowledge, no reports exist on the influence of functional single nucleotide polymorphisms (SNPs) detected in the vascular endothelial receptor gene on BrCA disease recurrence or survival.
Over the past decade, numerous observational studies suggest that functional variants associated with differential cytokine gene/protein expressions influence susceptibility to various cancers and poor disease prognosis. However, reports on genomic predictors of BrCA recurrence are limited in scope. There are no published reports on the impact of these six highly variant angiogenesis-related genes relative to BrCA disease-free (DFS) and overall survival (OS). This study (1) evaluated whether variations within regulatory or coding regions of selected angiogenesis biomarkers influence BrCA recurrence or OS, presumably from alterations in mRNA/protein expression critical to tumor vasculature-formation capacity; (2) assessed whether these markers add predictive value toward determining disease prognosis beyond standard demographic and clinico-pathological attributes; and (3) established a foundation for clinical studies to identify and validate markers as effective predictors of disease prognosis.
Materials & methods
Population
The study utilized de-identified information and specimens collected between 1989 and 1998 from 441 Caucasian women selected from the Hormone Receptor Laboratory (HRL) Biorepository and Tumor Marker Database (TMD). Human tissue specimens were collected from 235 node-negative and 206 node-positive patients having undergone mastectomy to remove primary infiltrating ductal or lobular BrCA. Tissue specimens were processed within an hour following surgery using stringent protocols to ensure specimen integrity for genomic and proteomic analyses. Specimens for this study were de-identified, with protected health information and linkers transferred to a third party. Study approval was obtained from the University of Louisville IRB.
Patient follow-up
The HRL Biorepository and the Microsoft, access-based TMD contain de-identified specimens of breast carcinoma with associated tumor marker/clinical outcome with up to 15 years of follow-up. Available clinico-pathological data include tumor-based properties (e.g., pathology, grade, stage, size, tumor marker status), patient-related characteristics (e.g., age, race, menopausal status, family history, nodal status), and clinical follow-up (e.g., treatment regimen, DFS, OS). Furthermore, the TMD has biochemical data for tissue specimens on select markers, including estrogen/progesterone receptor (ER/PR), epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor (HER)-2/neu status.
DNA Extraction & quality assessment
DNA was extracted from tissue sections using the AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Valencia, CA) or QIAamp DNA Mini Kit (Qiagen). DNA concentration was determined via NanoDrop® (Wilmington, DE) ND-1000 Spectrophotometer. Samples were diluted to 60 ng/µl and stored at −20°C until further analysis.
Selection of angiogenesis-related polymorphisms
We selected 14 angiogenesis-related SNPs spanning each gene with a minor allele frequency >0.05 and a location within the coding and non-coding [i.e., promoter, 3′ untranslated region (UTR), 5′UTR, and intronic] regions for further genomic and bioinformatic analysis.
TaqMan allelic discrimination of angiogenesis sequence variants
Polymorphisms in six angiogenesis-related genes were ascertained using TaqMan Polymerase Chain Reaction (PCR) allelic discrimination assays [24]. Fourteen alleles were detected: (1) IL-10 (G−1082A, C−819T, C−592A); (2) IL-10R (Exon 7 A241G, Exon 7 G−109A); (3) VEGF (G−1154A, C−2578A, G−634C); (4) VEGFR2 (IVS6 A54G, G889A, T1416T, IVS25 G−92A); (5) TGF-β1 (T896C); and (6) TGF-βR1 (Exon 9 A195G). Discrimination assays contained approximately 60 ng of germ-line DNA, 1X Universal Master Mix [Applied Biosystems (ABI), Foster City, CA], 300 nM of each primer (forward and reverse), and 100 nM of each probe (FAM and VIC) to comprise a 10 µl reaction. The assay for the allelic discrimination of IL-10 (G−1082A) was obtained via custom-designed kit from ABI (C__1747360_10). Sequences for primers and probes for [IL-10R Exon 7 (G−109A), VEGF (G−634C), and TGF-βR1 (C915G)] were found in the NCI SNP500 database. These primers and probes were designed using PrimerExpress 3.0 software (ABI) to detect the following SNPs: (1) IL-10 (C−819T, C−592 A, A241G); (2) IL-10R (G109A); (3) VEGF (G−1154A); (4) VEGFR (IVS6+A54G, G889A, IVS25G−92A, T1416A); and (5) TGF-β1 (C−509T, A195G, T896C). Primer and probe concentrations varied per SNP.
PCRs were conducted in an ABI Prism 7900HT Sequence Detection System. Thermocycling settings consisted of two holds at 50°C for 2 min and 95°C for 10 min, followed by 40–42 cycles of 15 s at 95°C and 1 min at a specific temperature for each SNP. The fluorescent intensity emitted from probes was measured via ABI 7900 sequence detection system and assigned genotypes by SDS 2.2.1 software (ABI). Laboratory technicians were blinded to study participant case status to minimize misclassification bias. Based on 24 non-DNA template controls per batch analysis, percent cross-contamination during sample handling was minimal (≤4.2%). Duplicate genotyping was performed for 72 randomly selected samples for quality control purposes. The genotype call rates ranged between 83.5 and 97.3% across the fourteen SNPs. In addition, deviations from the Hardy–Weinberg equilibrium among controls were tested using a significance level of p<0.005.
Statistical analysis
Associations between candidate polymorphic genes and BrCA recurrence and OS, expressed as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs), were estimated using proportional hazard (PH) regression models for competing risks. For each SNP, the most frequent genotype was used as the baseline. Predicted impact for each SNP genotype on BrCA recurrence is detailed in Table 1. All-cause mortality was treated as a competing risk when evaluating time to recurrence; whereas, death from other causes was treated as a competing risk when evaluating disease-related mortality. Subjects with missing SNP values were excluded from statistical analyses. Cumulative incidence curves stratified by marker genotypes evaluated whether inheritance of high-expressing angiogenesis-associated alleles correlated with DFS and OS. Differences between the stratified curves were tested using chi-square statistics. Haplotypes for VEGF markers were estimated by fastPHASE [24]. Competing risk hazard models estimated effects for inheritance of one or more copies of each haplotype on patient survival. Predictive accuracy of the fitted hazard models corresponding to each biomarker were evaluated using time-dependent measures of sensitivity, specificity, and the associated receiver-operator characteristic (ROC) curve with estimates and 95% CIs for area underneath the curve (AUC). Gains in predictive ability over models including standard clinical diagnostic markers were assessed by differences in AUC values using the bootstrap bias-corrected and accelerated (BCA) method to calculate CIs. Multivariable hazard regression models evaluated single genes and reconstructed haplotype effects on the survival times after adjusting for potential confounders [e.g., age, tumor grade (aggressive vs. non-aggressive), tumor stage (advanced vs. non-advanced), tumor size, nodal status (+/−), estrogen receptor status (−), and progesterone status (+/−)].
Table 1.
Selected SNPs in the angiogenesis pathway and their potential role in breast cancer recurrence risk
| Gene | rs Number | Nucleotide change |
Amino- acid change |
Impact on mRNA/ protein stability/ expression |
Proposed influence on BrCA recurrence |
References |
|---|---|---|---|---|---|---|
| IL10-1082 | rs1800896 | G>A | Low expressor = AA | Increase | [48–50] | |
| IL10-819 | rs1800871 | C>T | Low expressor = TT | Increase | [48, 50] | |
| IL10-592 | rs1800872 | C>A | Low expressor = AA | Increase | [48, 50] | |
| IL-10R Ex7-109 | rs9610 | G>A | [48] | |||
| TGFB1 896 | rs1982073 | T>C | Leu10Pro | High expressor = CC | Increase | [48, 51, 52] |
| TGFB1-509 | rs1800469 | C>T | Promoter | Low expressor = CC | Decrease | [48, 52–54] |
| TGFB1 915 | rs1800471 | C>G | Pro25Arg | High expressor = GG | Increase | |
| TGFβR1 Ex9+195 | rs868 | A>G | 3′UTR | [48, 55, 56] | ||
| VEGF-2578 | rs699947 | C>A | High expressor = CC | Increase | [55–57] | |
| VEGF-1154 | rs1570360 | A>G | High expressor = GG | Increase | [48, 55, 56] | |
| VEGF-634 | Rs2010963 | G>C | High expressor = GG | Increase | ||
| VEGFR IVS6+54 | rs7692791 | A>G | [30, 48, 58, 59] | |||
| VEGFR+889 | rs2305948 | G>A | V297I | Essential for maintaining the association rate with VEGF and retention of the receptor; may alter VEGF signaling pathways |
[60] | |
| VEGFR+1416 | rs1870377 | T>A | H472Q | Essential for maintaining the association rate with VEGF and retention of the receptor; may alter VEGF signaling pathways |
[60] | |
| VEGFR IVS25-92 | rs1531289 | G>A | [60] |
All statistical analyses were conducted using R version 2.8.1 supplemented with survivalROC, cmprsk, and the bootstrap packages for time-dependent ROC curves and AUC values, calculation of cumulative incidence curves and PHs models, and bootstrap calculations, respectively. Statistical significance was assessed using an unadjusted p-value <0.05.
Results
Demographic and clinico-pathological data
The median age of the 441 Caucasian women BrCA patients in this study was 62 years (range: 26–89.5 years). Total patient follow-up was 2,414.4 person-years, with a median follow-up time per person of 67 months. There were 201 total deaths (45.6%); 122 (27.7%) patients died from disease and 79 (17.9%) died from other causes. There were 156 (35.4%) total recurrent BrCA cases among participants, with 52 (12%) patients listed as “never disease-free” and thus excluded from further analysis. The overall 5-year survival rate was 0.76 (0.72–0.80), with a 5-year recurrence-free survival rate of 0.75 (0.70–0.79). All patients underwent partial or complete (uni- or bilateral) mastectomy. Clinical and tumor characteristic information and association of these factors with overall patient survival are detailed in Table 2. As anticipated, tumor size larger than 2.0 cm, poor differentiation (tumor grade III, IV), advanced disease (tumor stage III, IV), positive nodal status, and negative hormone receptor (ER−/PR−) status were associated with decreased patient survival, shown in Table 2. Age did not reveal any significant effect on OS.
Table 2.
Patient and tumor characteristics
| Demographic and tumor characteristics | Patients n (%) | No. of deaths | MST (months) | p-valuea |
|---|---|---|---|---|
| Age at diagnosis (years) (n = 441) | ||||
| >40 | 30 (7) | 11 | 99.7 | 0.76 |
| 40–49 | 71 (16) | 20 | 113.6 | |
| 50–59 | 97 (22) | 28 | 116.9 | |
| 60–69 | 103 (23) | 29 | 107.1 | |
| ≥70 | 140 (32) | 34 | 108.1 | |
| Missing | 0 (0) | – | – | |
| Family history (n = 253) | ||||
| No | 161 (37) | 53 | 108.0 | 0.05 |
| Yes | 92 (21) | 20 | 117.0 | |
| Missing | 188 (43) | – | – | |
| Tumor grade (n = 343) | ||||
| I | 43 (10) | 3 | 144.3 | <0.001 |
| II | 150 (34) | 35 | 116.3 | |
| III | 145 (33) | 54 | 97.4 | |
| IV | 5 (1) | 3 | 78.8 | |
| Missing | 98 (22) | – | – | |
| Tumor stage (n = 429) | ||||
| I | 122 (28) | 10 | 143.5 | <0.001 |
| II | 232 (53) | 67 | 109.7 | |
| III | 58 (13) | 27 | 75.8 | |
| IV | 17 (4) | 16 | 24.1 | |
| Missing | 12 (3) | – | – | |
| Tumor size (n = 420) | ||||
| <2.0 cm | 182 (41) | 27 | 132.7 | <0.001 |
| ≥2.0 cm | 238 (54) | 92 | 97.7 | |
| Missing | 21 (5) | – | – | |
| Nodal status (n = 441) | ||||
| Negative | 235 (53) | 33 | 135.0 | <0.001 |
| Positive | 206 (47) | 89 | 90.0 | |
| Missing | 0 (0) | – | – | |
| ER status (n = 438) | ||||
| Negative | 130 (29) | 53 | 91.6 | <0.001 |
| Positive | 308 (70) | 69 | 121.8 | |
| Missing | 3(1) | – | ||
| PR status (n = 437) | ||||
| Negative | 130 (29) | 51 | 94.3 | <0.001 |
| Positive | 307 (70) | 70 | 121.0 | |
| Missing | 4 (1) | – | – | |
| Cancer treatment | ||||
| Hormone therapy | ||||
| Yes (%) | 143 (33) | 39 | 116 | 0.68 |
| No (%) | 295 (67) | 83 | 111 | |
| Chemotherapy | ||||
| Yes (%) | 135 (31) | 57 | 93.8 | |
| No (%) | 306 (69) | 65 | 124.4 | <0.001 |
| Radiation therapy | ||||
| Yes (%) | 93 (21) | 39 | 93 | <0.001 |
| No (%) | 348 (79) | 83 | 120 |
Categorical entries are counts (%)
MST restricted mean survival time in months; ER estrogen receptor; PR progesterone receptor
Restricted mean is reported instead of the median, due to >50% survival in the majority of cases
Differences in survival tested by the chi-square test for comparing cumulative-incidence curves
Prevalence of Angiogeneis-related SNPs Relative to Patient and Tumor Characteristics
Thirteen of fourteen SNPs were successfully genotyped in 89–99.1% of patients with a relatively high percent concordance rate (median = 99%; range = 92–100%) between blind replicate samples. IL-10R 241 was not included for further analyses, because this marker had an unacceptable concordance rate between replicate samples (88%). Genotype frequencies did not deviate from the Hardy–Weinberg Equilibrium (p ≥ 0.01) at the 0.005 significance level. Within our study set, inheritance of at least one minor or “high-risk” allele (linked with increased mRNA/protein expression) was fairly common at loci IL-10−1082A (72.3%), IL-10−819T (32.8%), IL-10−592A (31.4%), IL-10R−109A (61.5%), TGF-β1−896C (59.5%), TGF-βR1+195G (35.2%), VEGF−2578C (78.6%), VEGF−1154G (93.2%), VEGF−634C (59.4%), VEGFR+889A (21.7%), VEGFR+1416A (33.3%), VEGFR IVS25−92A (42.0%), and VEGFR IVS6+54G (65.4%). With the exception of VEGFR+1416, genotype frequencies were in agreement with the NCBI database or published reports [25, 26].
Genotype frequencies and effects on BrCA clinical outcomes
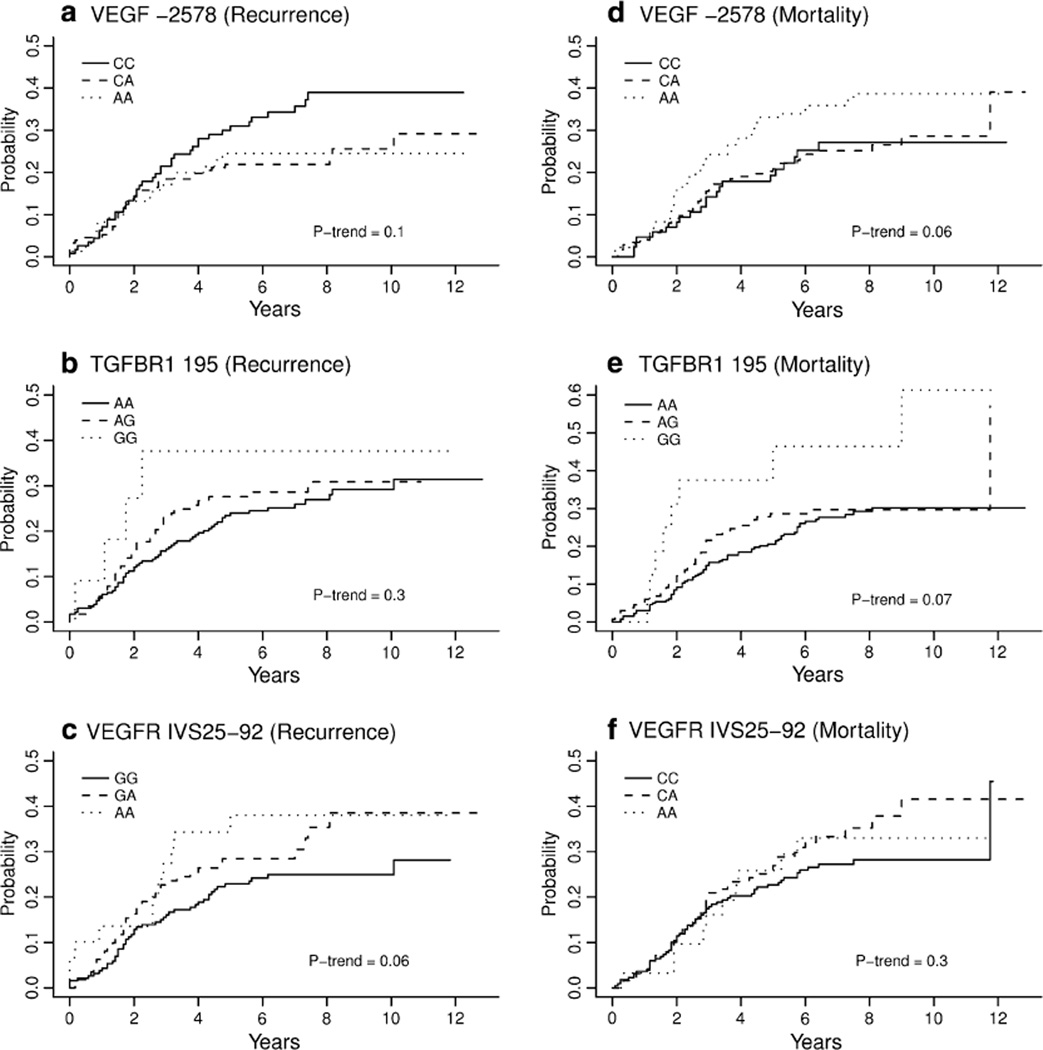
We observed marginal relationships between BrCA outcomes and angiogenesis-associated sequence variants summarized in Table 3. Univariate regression analysis revealed a decrease in BrCA DFS associated with homozygous VEGF−2578CC and VEGFR IVS25−92AA genotypes, with a near significant dosage effect for the VEGFR IVS25−92A minor allele (HRtrend = 1.32; 95% CI = 0.98–1.76; Ptrend = 0.06) and the VEGF−2578C allele (HRtrend = 1.28; 95% CI = 0.96–1.72; Ptrend = 0.10). After adjusting for age and tumor characteristics (i.e., tumor stage, nodal status, ER/ PR status), the magnitude of the aforementioned relationships persisted but the significance level decreased (VEGFR IVS25−92A: HRtrend = 1.28; 95% CI = 0.94–1.75; Ptrend = 0.12; VEGF−2578C: HRtrend = 1.25; 95% CI = 0.92–1.70; Ptrend = 0.15). For unadjusted OS, we observed a decrease in overall BrCA survival among those with the TFGβ-R1 195G (HRtrend = 2.45; 95% CI = 1.16–5.17; Ptrend = 0.07) and VEGF−2578C alleles (HRtrend = 1.56; 95% CI = 0.93–2.56; Ptrend = 0.06). Both SNPs remained borderline significant predictors of reduced OS after adjusting for age and other significant clinico-pathological characteristics. The TFGβ-R1195G allele was also associated with decreased DFS (HR for trend = 1.21; 95% CI 0.84–1.75; Ptrend = 0.30); however, many of the GG genotype patients were never disease-free, which likely contributed to the lack of statistical significance. Cumulative incidence curves for disease recurrence and mortality attributed to BrCA for VEGF C−2578A are depicted in Fig. 1a, b. The association between VEGF C−2578A, VEGR GIVS25−92A, and TFGβ-R1 A195G and patient and tumor characteristics revealed no significant findings, except for an increase in the percentage of VEGR IVS25−92 AA patients receiving chemotherapy (p ≤ 0.01). The remaining nine angiogenesis-associated SNPs rendered no significant associations with BrCA DFS or OS.
Table 3.
Univariate analysis for disease-free survival (DFS) and overall survival (OS)
| Gene | Genotype | Disease-free survival |
Overall Survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | NR | HRR (95% CI) | p-value (PTrend) | N | ND | HRD (95% CI) | p-value (PTrend) | ||
| IL10-1082 | GG | 104 | 27 | 1.00 | 0.77 (0.57) | 118 | 32 | 1.00 | 0.99 (0.91) |
| GA | 166 | 43 | 0.99 (0.62–1.6) | 191 | 53 | 1.03 (0.67–1.59) | |||
| AAlow expressor | 100 | 29 | 1.17 (0.69–2.0) | 116 | 32 | 1.03 (0.63–1.68) | |||
| IL10-819 | CC | 236 | 66 | 1.00 | 0.66 (0.57) | 267 | 74 | 1.00 | 0.71 (0.41) |
| CT | 96 | 28 | 1.01 (0.65–1.6) | 109 | 34 | 1.12 (0.75–1.68) | |||
| TTlow expressor | 17 | 3 | 0.59 (0.18–2.0) | 21 | 7 | 1.34 (0.60–3.01) | |||
| IL10-592 | CC | 258 | 68 | 1.00 | 0.89 (0.88) | 287 | 77 | 1.00 | 0.64 (0.33) |
| CA | 100 | 28 | 1.03 (0.66–1.6) | 118 | 37 | 1.18 (0.80–1.75) | |||
| AAlow expressor | 12 | 2 | 0.72 (0.16–3.2) | 13 | 4 | 1.39 (0.47–4.11) | |||
| IL-10R-109 | GG | 124 | 34 | 1.00 | 0.64 (0.86) | 145 | 48 | 1.00 | 0.14 (0.35) |
| GA | 142 | 35 | 0.86 (0.54–1.4) | 161 | 37 | 0.65 (0.43–1.00) | |||
| AA | 69 | 22 | 1.09 (0.65–1.8) | 74 | 23 | 0.86 (0.53–1.41) | |||
| TGFβ1 896 | TT | 144 | 43 | 1.00 | 0.45 (0.91) | 163 | 41 | 1.00 | 0.31 (0.12) |
| TC | 174 | 43 | 0.84 (0.56–1.3) | 204 | 61 | 1.29 (0.87–1.92) | |||
| CChigh expressor | 33 | 12 | 1.26 (0.66–2.4) | 35 | 13 | 1.49 (0.81–2.74) | |||
| TGFβR1 195 | AA | 235 | 61 | 1.00 | 0.49 (0.3) | 263 | 71 | 1.00 | 0.07 (0.07) |
| AG | 116 | 33 | 1.15 (0.75–1.8) | 133 | 39 | 1.16 (0.78–1.72) | |||
| GG | 11 | 4 | 1.77 (0.61–5.2) | 16 | 8 | 2.45 (1.16–5.17) | |||
| VEGF-2578 | AA | 76 | 18 | 1.00 | 0.12 (0.1) | 85 | 21 | 1.00 | 0.08 (0.06) |
| AC | 153 | 36 | 0.97 (0.55–1.7) | 176 | 45 | 1.02 (0.61–1.70) | |||
| CChigh expressor | 115 | 40 | 1.51 (0.87–2.6) | 135 | 48 | 1.56 (0.94–2.59) | |||
| VEGF-1154 | AA | 22 | 7 | 1.00 | 0.43 (0.76) | 24 | 7 | 1.00 | 0.16 (0.18) |
| AG | 138 | 33 | 0.64 (0.28–1.5) | 152 | 37 | 0.75 (0.33–1.70) | |||
| GGhigh expressor | 161 | 48 | 0.81 (0.36–1.8) | 187 | 62 | 1.10 (0.50–2.45) | |||
| VEGF-634 | GG | 146 | 42 | 1.00 | 0.53 (0.68) | 164 | 48 | 1.00 | 0.73 (0.88) |
| GC | 164 | 40 | 0.79 (0.51–1.2) | 189 | 50 | 0.88 (0.59–1.30) | |||
| CChigh expressor | 48 | 15 | 0.99 (0.56–1.7) | 54 | 17 | 1.04 (0.60–1.78) | |||
| VEGFR+889 | GG | 277 | 84 | 1.00 | 0.02 (0.07) | 310 | 93 | 1.00 | 0.44 (0.34) |
| GA | 68 | 9 | 0.40 (0.20–0.8) | 81 | 18 | 0.72 (0.43–1.20) | |||
| AA | 5 | 2 | 1.44 (0.33–6.3) | 5 | 2 | 1.18 (0.35–3.96) | |||
| VEGFR+1416 | TT | 244 | 72 | 1.00 | 0.15 (0.23) | 277 | 82 | 1.00 | 0.37 (0.61) |
| TA | 108 | 21 | 0.63 (0.39–1.0) | 121 | 28 | 0.77 (0.50–1.18) | |||
| AA | 15 | 5 | 1.09 (0.48–2.5) | 17 | 6 | 1.27 (0.56–2.86) | |||
| VEGFR IVS25-92 | GG | 191 | 46 | 1.00 | 0.19 (0.06) | 219 | 59 | 1.00 | 0.45 (0.3) |
| GA | 112 | 35 | 1.37 (0.89–2.1) | 127 | 42 | 1.29 (0.87–1.91) | |||
| AA | 30 | 11 | 1.67 (0.86–3.3) | 32 | 10 | 1.16 (0.60–2.24) | |||
| VEGFR IVS6+54 | AA | 118 | 38 | 1.00 | 0.38 (0.19) | 133 | 45 | 1.00 | 0.28 (0.34) |
| AG | 151 | 38 | 0.77 (0.49–1.2) | 167 | 41 | 0.71 (0.47–1.09) | |||
| GG | 72 | 18 | 0.71 (0.41–1.2) | 85 | 25 | 0.83 (0.51–1.33) | |||
NR number recurrent, ND number died, HRR hazard ratio for disease recurrence, HRD hazard ratio for disease-related mortality
Fig. 1.
Cumulative incidence plots for SNPs VEGF C2578A for disease recurrence and disease-related mortality
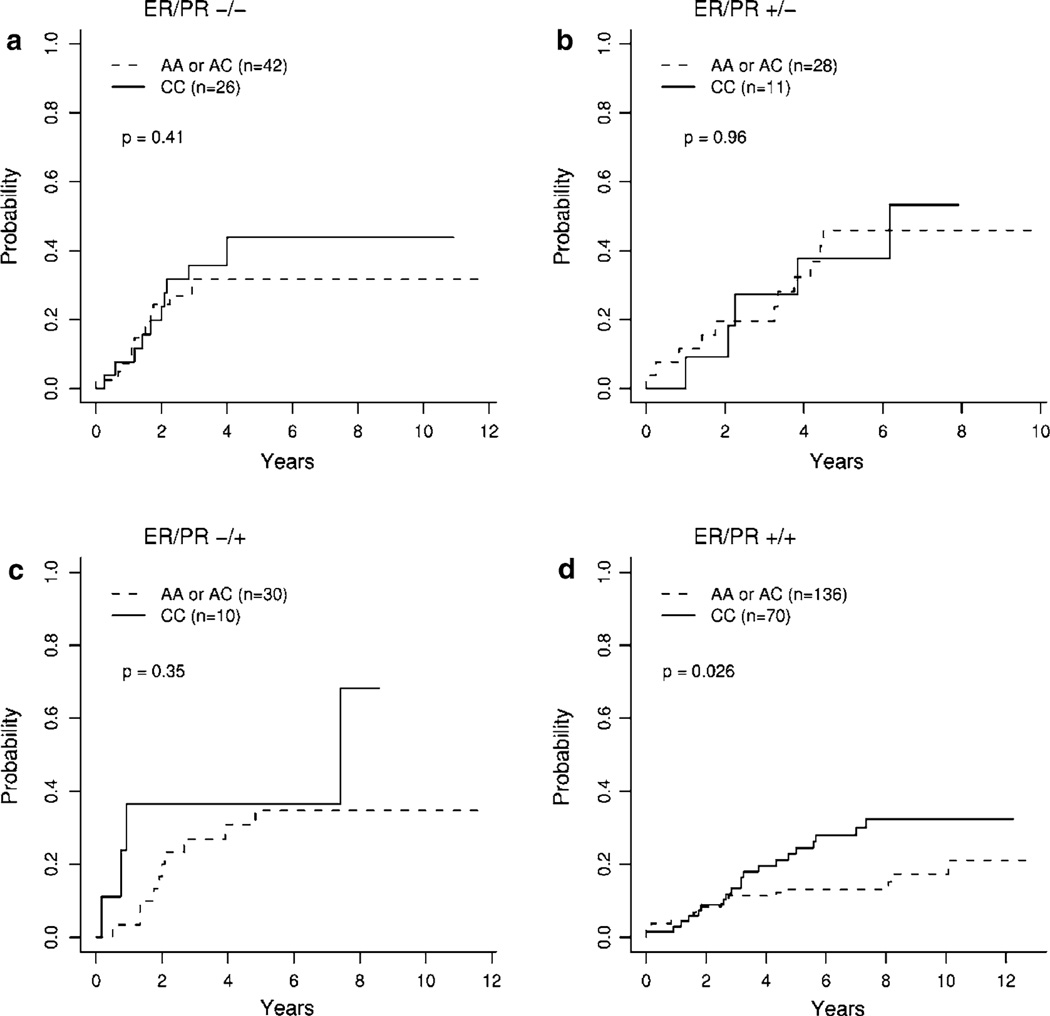
To visually assess whether significant SNPs enhanced predictive ability above standard diagnostic markers, we plotted cumulative incidence curves stratified by patient ER/PR status. Stratified cumulative incidence curves for disease recurrence for homozygous VEGF−2578CC patients relative to AA and CA patients (see Fig. 2). For ER+/PR+ patients, VEGF−2578CC patients had higher incidence of recurrence relative to individuals with at least one A allele (p = 0.026; Fig. 2d). For disease-related mortality, patients with the VEGF−2578CC genotype again had marginally higher incidence relative to the referent group for ER+/PR+ patients (p = 0.057).
Fig. 2.
Cumulative incidence plots for VEGF−2578CC versus VEGF−2578AA and AC genotypes for disease recurrence, stratified by ER/PR status
Haplotype effects
Given the association between VEGF C−2578A and survival, we further investigated haplotypes of VEGF SNPs C−2578A, A−1154G, and G−634C relative to DFS and OS patients were assigned their most likely haplotypes and carrier risk associated with individual VEGF haplotypes, which were evaluated and expressed as HRs for disease recurrence and disease-related mortality (see Table 4). Among the four most common haplotypes, the −2578A, −1154G, −634G haplotype offered a protective effect against disease recurrence (HR = 0.6; 95% CI 0.39–1.00; p = 0.05), whereas the −2578C, −1154G, −634G haplotype was deleterious (HR = 1.58, 95% CI 1.06–2.35; p = 0.03). This result coincides in expectation with the deleterious risk estimate associated with the VEGF−2578 CC genotype. However, the VEGF−2578C, −1154G, −634C haplotype was not associated with increased risk of disease recurrence, indicating that inheritance of the VEGF−2578C allele with this haplotype was not harmful. We observed no significant relationship between the VEGF AGG and CGG haplotypes and patient and tumor characteristics, except for a slight increase in advanced disease (stage III/IV) among AGG carriers (p = 0.02).
Table 4.
Carrier risk of VEGF haplotypes for disease recurrence and disease-related mortality
| VEGF Haplotype (−2578, −1154, −634) | Frequency | # Carriers (%) | HRR (95% CI) | p-value | HRD (95% CI) | p-value |
|---|---|---|---|---|---|---|
| AGGtwo low-expressing alleles | 0.165 | 128 (0.31) | 0.62 (0.39–1.00) | 0.05 | 0.70 (0.46–1.05) | 0.09 |
| CGCthree high-expressing alleles | 0.353 | 242 (0.58) | 0.91 (0.61–1.36) | 0.65 | 0.98 (0.68–1.42) | 0.93 |
| AAGtwo low-expressing alleles | 0.248 | 199 (0.48) | 0.88 (0.59–1.31) | 0.53 | 0.73 (0.51–1.05) | 0.09 |
| CGGtwo high-expressing alleles | 0.198 | 145 (0.35) | 1.58 (1.06–2.35) | 0.03 | 1.68 (1.16–2.42) | 0.01 |
HRR hazard ratio for disease recurrence, HRD hazard ratio for disease-related mortality
We also stratified haplotype cumulative incidence curves by ER/PR status for disease recurrence and disease-related mortality. Similar to the VEGF−2578CC genotype, the most detrimental effect of the VEGF CGG haplotype was for ER+/PR+ patients, although only disease-related mortality was statistically significant (p = 0.028). Conversely, the AGG haplotype had a protective effect against disease recurrence and disease-related mortality, although it never achieved statistical significance in any one ER/PR group. Patients with the CGC haplotype did not have significant differences in recurrence or disease-related mortality for any of the ER/PR groups.
Predictive accuracy of biomarker models
Predictive accuracy (AUC values) for disease recurrence of the multivariable PH model including ER, PR, nodal status, age, and disease stage (the ‘base’ model) was 0.717 (95% CI: 0.634–0.826) at 5 years and 0.706 (95% CI: 0.607–0.749) at 8 years. The addition of the VEGF−2578 marker to the base multivariable model (CC genotype vs. AA/AC genotypes) improved predictive accuracy at both 5 years (AUC = 0.772; 95% CI = 0.697–0.826) and 8 years (AUC = 0.760; 95% CI = 0.671–0.810). Improvements in AUC values relative to the base model were statistically significant based on a 95% CI for differences in AUC of 0.031–0.135 and 0.011–0.140 for years 5 and 8, respectively. Relative to a model with ER/PR status alone, the VEGF−2578 marker improved AUC at 5 years from 0.627 (95% CI = 0.56–0.682) to 0.671 (95% CI = 0.586– 0.721; 95% CI for difference = 0.001–0.080) and at 8 years from 0.613 (95% CI = 0.538–0.668) to 0.681 (95% CI = 0.587–0.725; 95% CI for difference = 0.012– 0.128). For OS, AUC of the base model at 5 years was 0.753 (95% CI 0.681–0.795). Adding the VEGF−2578 marker improved AUC slightly to 0.770 (95% CI = 0.683– 0.813); however, this difference was not statistically significant (95% CI for difference = −0.018–0.064).
Predictive accuracy for disease recurrence was improved by adding an ER/PR interaction term (5-year AUC = 0.741; 95% CI 0.665–0.799) to the base model. Inclusion of the VEGF−2578 marker and an ER/PR interaction term improved the AUC at 5 years to 0.779 (95% CI = 0.771–0.839); however, the difference in AUC compared to the base model combined with the interaction term was not quite statistically significant (95% CI for difference = −0.001–0.115). However, we observed significant improvement in predictive accuracy for 8-year disease recurrence when comparing the base model with the ER/PR interaction to the base model with ER/PR interaction and VEGF−2578 (AUC = 0.737; 95% CI 0.662–0.793 vs. 0.801; 95% CI 0.743–0.867; 95% CI for difference = 0.023–0.156).Gains in predictive accuracy were also checked for TFG-βR1 A195G and individual haplotypes of VEGF. However, they were either not significant or less substantial than gains seen with VEGF−2578 and hence not reported.
Discussion
Angiogenesis has a pivotal role in breast tumorigenesis as evidenced by preclinical and clinical studies. A remarkable piece of evidence supporting this role stems from substantial improvements in clinical outcome following angiogenesis inhibitor use among women diagnosed with BrCA [27]. Linderholm et al. (2009) demonstrated that BrCA patients with higher VEGF levels detected in tumor tissue had higher propensities for increased susceptibility to BrCA recurrence, metastatic disease, and poor OS/BrCA-corrected survival [28]. In addition, numerous published reports reveal individuals inheriting low IL-10 (−1082AA, −819TT, −592AA) and/or high VEGF (−2578CC, −1154GG, −634CC)—producing genotypes were significantly associated with BrCA risk, lymph node metastasis, poor DFS, high tumor grade, and large tumor size [14, 21–23, 29, 30]. For instance, Jin et al. (2005) demonstrate that carriers of the 634CC genotype and −2578/−634CC haplotype were linked with high VEGF expression and significantly associated with high tumor aggressiveness (large tumor size, high histologic grade, p < 0.01). In contrast, the putative low-expressing −2578AA genotype and −2578/−634AG haplotype corresponded with low histologic grade tumors (p = 0.04) [22]. Poor disease prognosis may be partially attributed to inheritance of variant cytokine/cytokine receptors linked to changes in mRNA/protein expression, mRNA stability, and protein structure/function, which may influence the tumor’s capacity to form vasculature essential to growth and metastasis. This study assessed the role of angiogenesis-related polymorphisms and their haplotypes relative to BrCA recurrence and OS among 441 Caucasian women diagnosed with primary BrCA with a median of 5.6 years (range = 0–12.8 years) of clinical follow-up data. We proposed an elevated risk of BrCA recurrence and poor OS for individuals possessing high-risk alleles.
Commensurate with our a priori hypothesis, we observed modest increases in BrCA recurrence and poorer OS among patients with the VEGF−2578CC genotype and VEGF−2578/−1154/−634CGG haplotype. Although the VEGF−2578 marker was only marginally significant when considered singly in the PH model, there were substantial gains in predictive accuracy for disease recurrence when including this marker with current prognostic indicators for BrCA patients. This suggests that the VEGF−2578 SNP provides additional information regarding BrCA clinical outcomes beyond conventional clinico-pathological factors such as hormone receptor status, nodal status, age, and disease stage. In addition, upon stratification of recurrence risk by ER/PR status, the marker offered additional separation of risk curves and improved predictive accuracy relative to ER/PR alone. Given the relatively high frequency of patients with the VEGF−2578 CC genotype, the impact on BrCA patient outcomes could be substantial, especially for ER+/PR+ patients.
The hypothesis that polymorphisms in the promoter region of VEGF are related to BrCA recurrence and poor OS partially stems from the notion that functional and commonly studied SNPs are associated with increased VEGF expression. Higher levels of VEGF may increase the capacity to form blood vessels enabling BrCA tumorigenesis [31, 32]. Tumor neovascularization is required by most solid tumors to meet metabolic demand and provide potential routes for tumor dissemination and metastasis [33]. This phenomenon is partially supported by a report demonstrating shorter DFS among BrCA patients with high VEGF expression and microvessel density than those with lower expression of these preclinical biomarkers [32]. Some epidemiological evidence supports the role of the high-expressing VEGF−2578C allele alone relative to aggressive BrCA [22].
Unfortunately, the VEGF−2578C allele was not significantly related to various clinico-pathological factors such as tumor grade, stage, size, nodal status, hormone receptor status, and cancer treatment in this study. However, these findings are commensurate with two other null reports relative to lymph node involvement, metastases, tumor size, and histological grade. Despite the null relationship between VEGF−2578 and standard clinico-pathological factors, this marker seemingly serves as an important predictor of BrCA recurrence and OS. Notably, after adjusting for demographic (age) and clinical parameters [i.e., tumor grade, disease stage, tumor size, nodal status, hormone receptor status, chemotherapy], the hazard risk estimates did not vary substantially. Confounders are only considered in Cox regression models if such factors significantly modify the risk estimates by 20%, which was not the case in this study.
We considered the durability and shortcomings of this study on the impact of genomic tools critical to predicting BrCA recurrence risk using high-throughput genomic and statistical strategies. As these markers were specifically selected for their biological relevance and inclusion in published reports, we opted not to adjust for multiple comparisons when reporting the statistical significance of our findings. Given the number of markers evaluated, falsely significant findings are a possibility. Consequently, our results require further substantiation and validation in subsequent studies. A positive aspect of this study was access to de-identified clinical follow-up data (e.g., DFS, OS) and tumor/patient characteristics (e.g., pathology, nodal status, tumor grade, stage).
Numerous quality control measures were maintained to insure accurate and highly reproducible allelic discrimination assays. For SNP analysis of selected angiogenesis-related genes, random genotype errors are unlikely to be significant in our study because of the following factors: (a) a rigorous high-quality control protocol is followed in our laboratory, including repeat analysis if the percent contamination or genotype failure rate exceeds 5% among 72 replicate and 24 non-DNA template controls within each 384-well plate; (b) allele determination is standardized by a computer program guidance; and (c) utilization of validated allelic discrimination assays. If genotyping errors exist after these steps, this misclassification is evenly distributed between patients with non-recurrent and recurrent disease.
There is some concern that DNA extracted from tumors/ lymph nodes with evidence of metastatic disease may represent germ-line combined with somatic mutations. SNP analysis in this study was restricted to variations present in cancerous tissue, rather than biospecimens representative of germ-line mutations, including peripheral blood, uninvolved lymph node, or non-cancerous adjacent tissue. This limited our ability to assess whether occurrence of selected angiogenesis-associated SNPs in DNA extracted from cancerous tissue may serve as a proxy for variations in uninvolved lymph nodes or peripheral blood samples. However, evidence suggests the occurrence of an angiogenic cytokine genotype profile present in DNA sequestered from cancerous tissue is synonymous to DNA obtained from uninvolved lymph nodes. For instance, a recent pilot study observed a 100% concordance comparing the genotype status of a VEGF 936C>T polymorphism among DNA samples isolated from primary tumors and uninvolved lymph nodes [34]. Future studies will allow us to compare the VEGF−2578 SNP profile extracted from whole blood relative to BrCA biopsies.
We cannot rule out the possibility that other angiogenesis-related markers may influence vascular growth and ultimately disease prognosis. Although we included several angiogenesis-related markers known to influence tumorigenesis, the list was not exhaustive. It is plausible that other genetic markers of tumor neovascualarization may involve other targets, such as hypoxia inducible factor 1 and VEGF receptor 2/FMS-related tyrosine kinase 1. In addition, cytokines related to lymph-angiogenesis may also impact BrCA clinical outcomes, including VEGFC, VEGFD, VEGFR-3, chemokines, chemokine receptors, integrins, and downstream signaling targets [35–37]. Thus, future studies will consider whether the aforementioned markers may be used to predict BrCA recurrence and OS within a large retrospective case series study set.
Another concern is the failure to adjust hazard and predictive models for well-known clinico-pathological factors of BrCA risk (e.g., menopausal status, number of first degree relatives with a history of BrCA, age at men-arche, age at first menstruation, number of biopsies). However, this is the first report that demonstrates VEGF−2578 alone or combined with ER/PR status may serve as a significant predictor of BrCA clinical outcomes, even after adjusting for factors having an important role as significant predictors of disease prognosis such as age, ER/PR status, and tumor stage.
Prior studies have demonstrated impaired disease-free survival among BrCA patients with high VEGF protein levels following adjuvant tamoxifen treatment with various degrees of duration [38–42]. However, prolonged therapy (i.e., 5 years) is associated with a more favorable prognosis for those patients with high intratumoural VEGF levels when compared to those on a 2-year adjuvant regimen [42]. Presumably, tamoxifen blocks estrogen-induced transcription and secretion of VEGF resulting in reduced vascularization, as demonstrated in MCF-7 BrCA cells [43]. Unfortunately, our database contained limited information on the actual length of adjuvant endocrine therapy. Nevertheless, in a post hoc exploratory analysis, we investigated the association of the high-expressing VEGF−2578CC genotype and −2578/−1154/−634CGG haplotype with disease-free and overall survival in the subset of patients who received adjuvant tamoxifen therapy (n = 138). Relative to those with the VEGF−2578AA genotype, neither the risk estimates for tumor recurrence (HR = 1.30; 95% CI = 0.51–3.32, p = 0.6) nor mortality (HR = 1.06, 95% CI = 0.42–2.66; p = 0.9) was elevated among patients on adjuvant tamoxifen who possessed the VEGF−2578CC genotype, whereas patients who received no adjuvant therapy did have elevated risk associated with VEGF−2578CC (n = 278; HRrecurrence = 1.64, 95% CI = 0.82–3.26, p = 0.16 and HRmortality = 1.81, 95% CI = 0.97–3.39, p = 0.06). However, the hazard ratios for disease recurrence (HR = 1.77, 95% CI = 0.90–3.49, p = 0.1) and patient mortality (HR = 2.15, 95% CI 1.10–4.24, p = 0.03) were higher among carriers of the VEGF−2578/−1154/−634CGG haplotype who were treated with tamoxifen, compared to the non-adjuvant group (HRrecurrence = 1.49, 95% CI = 0.91–2.44, p = 0.11 and HRmortality = 1.52, 95% CI = 0.98–2.35, p = 0.06). These risk estimates remained unchanged even after adjusting for ER/PR status. Collectively, our findings indicate the importance of considering the VEGF haplotypes in relation to breast cancer recurrence and mortality; evaluation of VEGF genotypes in isolation may only tell part of the story concerning recurrence risk.
Clinically, these results may facilitate future studies to determine whether the VEGF sequence variant at position −2578 is an important predictor of clinical response to tamoxifen and angiogenesis inhibitors (e.g., bevacizumab or carboxyamidotriazole) [44, 45]. This locus, along with other angiogenesis-associated markers, may guide dosing regimens of selective therapies based on the genetic profile of angiogenic targets, resulting in more appropriate and individualized treatment for BrCA patients. In fact, commercially available and FDA-approved gene expression profiles consisting of up to 70 genes are in use to help physicians and patients make informed treatment decisions [46, 47]. Motivated in part by these prognostic signatures, these study findings establish the foundation for future studies to identify and validate SNP profiles capable of predicting clinical outcomes and improving clinical management.
In closing, our findings suggest the inheritance of the VEGF−2578C allele may serve as an effective predictor of BrCA recurrence and OS among women of European descent. However, these findings must undergo substantiation in larger observational studies. Such studies will analyze a comprehensive panel of genes involved in angiogenesis. Our findings combined with ongoing research will help clarify the role of polymorphisms in VEGF and other angiogenesis-associated genes as effective prognostic and diagnostic indicators of BrCA clinical outcomes. These efforts can also support studies to identify SNP signatures that indicate disease progression and regression within diverse sub-populations.
Acknowledgments
Special thanks are extended to Andrew Marsh for editing services. The authors appreciate access to the CGeMM DNA Core Facility at the UofL, directed by Dr. Ron Gregg, for the use of their high-throughput genotyping facilities. Grant Support: This project was supported in part by a JGBCC Pilot Project Initiative Grant, a Prostate Cancer Foundation Award, and the JGBCC Bucks for Brains “Our Highest Potential” in Cancer Research Endowment to LRK and the Phi Beta Psi Charity Trust to JLW. GNB was partially supported by the National Institute of Health grants P30-ES014443 and P20-RR/DE177702, and DOE grant 10EM00542.
Contributor Information
LaCreis R. Kidd, Department of Pharmacology and Toxicology, University of Louisville, 505 South Hancock Street, Clinical & Translational Research Building, Room 306, Louisville, KY 40202, USA, lrkidd01@louisville.edu
Guy N. Brock, Department of Bioinformatics and Biostatistics, School, of Public Health Information Sciences (SPHIS), UofL, Louisville, KY, USA
Tiva T. VanCleave, Department of Pharmacology and Toxicology, University of Louisville, 505 South Hancock Street, Clinical & Translational Research Building, Room 306, Louisville, KY 40202, USA
Marnita L. Benford, Department of Pharmacology and Toxicology, University of Louisville, 505 South Hancock Street, Clinical & Translational Research Building, Room 306, Louisville, KY 40202, USA
Nicole A. Lavender, Department of Pharmacology and Toxicology, University of Louisville, 505 South Hancock Street, Clinical & Translational Research Building, Room 306, Louisville, KY 40202, USA
Traci L. Kruer, Department of Biochemistry and Molecular Biology, University of Louisville, 319 Abraham Flexner Way, Louisville, KY 40202, USA, tlkrue01@louisville.edu
James L. Wittliff, Department of Biochemistry and Molecular Biology, University of Louisville, 319 Abraham Flexner Way, Louisville, KY 40202, USA, jlwitt01@louisville.edu
References
- 1.Wu JM, Bensen-Kennedy D, Miura Y, et al. The effects of interleukin 10 and interferon gamma cytokine gene polymorphisms on survival after autologous bone marrow transplantation for patients with breast cancer. Biol Blood Marrow Transplant. 2005;11(6):455–464. doi: 10.1016/j.bbmt.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Stearns ME, Fudge K, Garcia F, Wang M. IL-10 inhibition of human prostate PC-3 ML cell metastases in SCID mice: IL-10 stimulation of TIMP-1 and inhibition of MMP-2/MMP-9 expression. Invasion Metastasis. 1997;17(2):62–74. [PubMed] [Google Scholar]
- 3.Stearns ME, Rhim J, Wang M. Interleukin 10 (IL-10) inhibition of primary human prostate cell-induced angiogenesis: IL-10 stimulation of tissue inhibitor of metalloproteinase-1 and inhibition of matrix metalloproteinase (MMP)-2/MMP-9 secretion. Clin Cancer Res. 1999;5(1):189–196. [PubMed] [Google Scholar]
- 4.Stearns ME, Wang M. Antimestatic and antitumor activities of interleukin 10 in transfected human prostate PC-3 ML clones: orthotopic growth in severe combined immunodeficient mice. Clin Cancer Res. 1998;4(9):2257–2263. [PubMed] [Google Scholar]
- 5.Williams FM, Cherkas LF, Spector TD, MacGregor AJ. A common genetic factor underlies hypertension and other cardiovascular disorders. BMC Cardiovasc Disord. 2004;4(1):20. doi: 10.1186/1471-2261-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bello-DeOcampo D, Tindall DJ. TGF-betal/Smad signaling in prostate cancer. Curr Drug Targets. 2003;4(3):197–207. doi: 10.2174/1389450033491118. [DOI] [PubMed] [Google Scholar]
- 7.Chan LW, Moses MA, Goley E, et al. Urinary VEGF and MMP levels as predictive markers of 1-year progression-free survival in cancer patients treated with radiation therapy: a longitudinal study of protein kinetics throughout tumor progression and therapy. J Clin Oncol. 2004;22(3):499–506. doi: 10.1200/JCO.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Dummer W, Becker JC, Schwaaf A, et al. Elevated serum levels of interleukin-10 in patients with metastatic malignant melanoma. Melanoma Res. 1995;5(1):67–68. doi: 10.1097/00008390-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61(14):5407–5414. [PubMed] [Google Scholar]
- 10.Gasparini G, Toi M, Gion M, et al. Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst. 1997;89(2):139–147. doi: 10.1093/jnci/89.2.139. [DOI] [PubMed] [Google Scholar]
- 11.Hasegawa Y, Takanashi S, Kanehira Y, Tsushima T, Imai T, Okumura K. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer. 2001;91(5):964–971. [PubMed] [Google Scholar]
- 12.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development. 1995;121(6):1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Letterio JJ, Lechleider RJ, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 1999;18(5):1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu XO, Gao YT, Cai Q, et al. Genetic polymorphisms in the TGF-beta 1 gene and breast cancer survival: a report from the Shanghai breast cancer study. Cancer Res. 2004;64(3):836–839. doi: 10.1158/0008-5472.can-03-3492. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Carter D, Garrigue-Antar L, Reiss M. Transforming growth factor beta type I receptor kinase mutant associated with metastatic breast cancer. Cancer Res. 1998;58(21):4805–4810. [PubMed] [Google Scholar]
- 16.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 17.Guidi AJ, Schnitt SJ, Fischer L, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer. 1997;80(10):1945–1953. doi: 10.1002/(sici)1097-0142(19971115)80:10<1945::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Kushlinskii NE, Gershtein ES. Role of vascular endothelial growth factor during breast cancer. Bull Exp Biol Med. 2002;133(6):521–528. doi: 10.1023/a:1020259702427. [DOI] [PubMed] [Google Scholar]
- 19.Linderholm B, Lindh B, Tavelin B, Grankvist K, Henriksson R. p53 and vascular-endothelial-growth-factor (VEGF) expression predicts outcome in 833 patients with primary breast carcinoma. Int J Cancer. 2000;89(1):51–62. [PubMed] [Google Scholar]
- 20.Linderholm BK, Lindahl T, Holmberg L, et al. The expression of vascular endothelial growth factor correlates with mutant p53 and poor prognosis in human breast cancer. Cancer Res. 2001;61(5):2256–2260. [PubMed] [Google Scholar]
- 21.Jacobs EJ, Feigelson HS, Bain EB, et al. Polymorphisms in the vascular endothelial growth factor gene and breast cancer in the Cancer prevention study II cohort. Breast Cancer Res. 2006;8(2):R22. doi: 10.1186/bcr1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Q, Hemminki K, Enquist K, et al. Vascular endothelial growth factor polymorphisms in relation to breast cancer development and prognosis. Clin Cancer Res. 2005;11(10):3647–3653. doi: 10.1158/1078-0432.CCR-04-1803. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka N, Cai Q, Wen W, et al. Population-based case-control study of VEGF gene polymorphisms and breast cancer risk among Chinese women. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1148–1152. doi: 10.1158/1055-9965.EPI-05-0871. [DOI] [PubMed] [Google Scholar]
- 24.Scheet P, Stephens M. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am J Hum Genet. 2006;78(4):629–644. doi: 10.1086/502802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. http://www.ncbi.nlm.nih.gov/
- 26.McGuigan FE, Macdonald HM, Bassiti A, et al. Large-scale population-based study shows no association between common polymorphisms of the TGFB1 gene and BMD in women. J Bone Miner Res. 2007;22(2):195–202. doi: 10.1359/jbmr.061016. [DOI] [PubMed] [Google Scholar]
- 27.Schneider BP, Sledge GW., Jr Drug insight: VEGF as a therapeutic target for breast cancer. Nat Clin Pract Oncol. 2007;4(3):181–189. doi: 10.1038/ncponc0740. [DOI] [PubMed] [Google Scholar]
- 28.Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20(10):1639–1646. doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- 29.Faupel-Badger J, Kidd LR, Albanes D, Virtamo J, Woodson K, Tangrea JA. Association of IL-10 polymorphisms with prostate risk and grade of disease. Cancer Causes Control. 2008;19(2):119–124. doi: 10.1007/s10552-007-9077-6. [DOI] [PubMed] [Google Scholar]
- 30.Krippl P, Langsenlehner U, Renner W, et al. A common 936 C/T gene polymorphism of vascular endothelial growth factor is associated with decreased breast cancer risk. Int J Cancer. 2003;106(4):468–471. doi: 10.1002/ijc.11238. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura M, Abe Y, Tokunaga T. Pathological significance of vascular endothelial growth factor A isoform expression in human cancer. Pathol Int. 2002;52(5–6):331–339. doi: 10.1046/j.1440-1827.2002.01367.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang F, Wei L, Chen L. The relationship between vascular endothelial growth factor, microvascular density, lymph node metastasis and prognosis of breast carcinoma. Zhonghua BingLi XueZa Zhi. 2000;29(3):172–175. [PubMed] [Google Scholar]
- 33.Ferrara N, vis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Wang J, Fraig MM, et al. Defects of DNA mismatch repair in human prostate cancer 1. Cancer Res. 2001;61(10):4112–4121. [PubMed] [Google Scholar]
- 35.Eccles S, Paon L, Sleeman J. Lymphatic metastasis in breast cancer: importance and new insights into cellular and molecular mechanisms. Clin Exp Metastasis. 2007;24(8):619–636. doi: 10.1007/s10585-007-9123-5. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita J, Kitamura K, Kabashima A, Saeki H, Tanaka S, Sugimachi K. Clinical significance of vascular endothelial growth factor-C (VEGF-C) in breast cancer. Breast Cancer Res Treat. 2001;66(2):159–164. doi: 10.1023/a:1010692132669. [DOI] [PubMed] [Google Scholar]
- 37.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Coradini D, Biganzoli E, Pellizzaro C, et al. Vascular endothelial growth factor in node-positive breast cancer patients treated with adjuvant tamoxifen. Br J Cancer. 2003;89(2):268–270. doi: 10.1038/sj.bjc.6601060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linderholm B, Bergqvist J, Hellborg H, et al. Shorter survival-times following adjuvant endocrine therapy in oestrogen- and progesterone-receptor positive breast cancer over-expressing HER2 and/or with an increased expression of vascular endothelial growth factor. Med Oncol. 2009;26(4):480–490. doi: 10.1007/s12032-008-9157-9. [DOI] [PubMed] [Google Scholar]
- 40.Linderholm B, Grankvist K, Wilking N, Johansson M, Tavelin B, Henriksson R. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18(7):1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 41.Ryden L, Stendahl M, Jonsson H, Emdin S, Bengtsson NO, Landberg G. Tumor-specific VEGF-A and VEGFR2 in postmenopausal breast cancer patients with long-term follow-up. Implication of a link between VEGF pathway and tamoxifen response. Breast Cancer Res Treat. 2005;89(2):135–143. doi: 10.1007/s10549-004-1655-7. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez BC, Sundqvist M, Fohlin H, et al. Prolonged tamoxifen treatment increases relapse-free survival for patients with primary breast cancer expressing high levels of VEGF. Eur J Cancer. 2010 doi: 10.1016/j.ejca.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Garvin S, Nilsson UW, Dabrosin C. Effects of oestradiol and tamoxifen on VEGF, soluble VEGFR-1, and VEGFR-2 in breast cancer and endothelial cells. Br J Cancer. 2005;93(9):1005–1010. doi: 10.1038/sj.bjc.6602824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Figg WD, Kruger EA, Price DK, Kim S, Dahut WD. Inhibition of angiogenesis: treatment options for patients with metastatic prostate cancer. Invest New Drugs. 2002;20(2):183–194. doi: 10.1023/a:1015626410273. [DOI] [PubMed] [Google Scholar]
- 45.Tan WW. Novel agents and targets in managing patients with metastatic prostate cancer. Cancer Control. 2006;13:194–198. doi: 10.1177/107327480601300306. [DOI] [PubMed] [Google Scholar]
- 46.Esteva FJ, Sahin AA, Cristofanilli M, et al. Prognostic role of a multigene reverse transcriptase-PCR assay in patients with node-negative breast cancer not receiving adjuvant systemic therapy. Clin Cancer Res. 2005;11(9):3315–3319. doi: 10.1158/1078-0432.CCR-04-1707. [DOI] [PubMed] [Google Scholar]
- 47.van ‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 48. http://snp500cancer.nci.nih.gov/home_1.cfm.
- 49.Howell WM, Turner SJ, Bateman AC, Theaker JM. IL-10 promoter polymorphisms influence tumour development in cutaneous malignant melanoma. Genes Immun. 2001;2(1):25–31. doi: 10.1038/sj.gene.6363726. [DOI] [PubMed] [Google Scholar]
- 50.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immuno Genet. 1997;24(1):1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 51.Ewart-Toland A, Chan JM, Yuan J, Balmain A, Ma J. A gain of function TGFB1 polymorphism may be associated with late stage prostate cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(5):759–764. [PubMed] [Google Scholar]
- 52.Yokota M, Ichihara S, Lin TL, Nakashima N, Yamada Y. Association of a T29->C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation. 2000;101(24):2783–2787. doi: 10.1161/01.cir.101.24.2783. [DOI] [PubMed] [Google Scholar]
- 53.Dunning AM, Ellis PD, McBride S, et al. A transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res. 2003;63(10):2610–2615. [PubMed] [Google Scholar]
- 54.Grainger DJ, Heathcote K, Chiano M, et al. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet. 1999;8(1):93–97. doi: 10.1093/hmg/8.1.93. [DOI] [PubMed] [Google Scholar]
- 55.Lambrechts D, Storkebaum E, Morimoto M, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34(4):383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 56.Shahbazi M, Fryer AA, Pravica V, et al. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 2002;13(1):260–264. doi: 10.1681/ASN.V131260. [DOI] [PubMed] [Google Scholar]
- 57.Sfar S, Hassen E, Saad H, Mosbah F, Chouchane L. Association of VEGF genetic polymorphisms with prostate carcinoma risk and clinical outcome. Cytokine. 2006;35(1–2):21–28. doi: 10.1016/j.cyto.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Krippl P, Langsenlehner U, Renner W, et al. The L10P polymorphism of the transforming growth factor-beta 1 gene is not associated with breast cancer risk. Cancer Lett. 2003;201(2):181–184. doi: 10.1016/s0304-3835(03)00468-3. [DOI] [PubMed] [Google Scholar]
- 59.Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res. 2000;37(6):443–448. doi: 10.1159/000054076. [DOI] [PubMed] [Google Scholar]
- 60.Park HW, Lee JE, Shin ES, et al. Association between genetic variations of vascular endothelial growth factor receptor 2 and atopy in the Korean population. J Allergy Clin Immunol. 2006;117(4):774–779. doi: 10.1016/j.jaci.2005.12.1328. [DOI] [PubMed] [Google Scholar]