Abstract
In the past two decades, neuroimaging investigations of stuttering have led to important discoveries of structural and functional brain differences in people who stutter, providing significant clues to the neurological basis of stuttering. One major limitation, however, has been that most studies so far have only examined adults who stutter, whose brain and behavior likely would have adopted compensatory reactions to their stuttering; these confounding factors have made interpretations of the findings difficult. Developmental stuttering is a neurodevelopmental condition, and like many other neurodevelopmental disorders, stuttering is associated with an early childhood onset of symptoms and greater incidence in males relative to females. More recent studies have begun to examine children who stutter using various neuroimaging techniques that allow examination of functional neuroanatomy and interaction of major brain areas that differentiate children who stutter compared with age-matched controls. In this article, I review these more recent neuroimaging investigations of children who stutter, in the context of what we know about typical brain development, neuroplasticity, and sex differences relevant to speech and language development. Although the picture is still far from complete, these studies have potential to provide information that can be used as early objective markers, or prognostic indicators, for persistent stuttering in the future. Furthermore, these studies are the first steps in finding potential neural targets for novel therapies that may involve modulating neuroplastic growth conducive to developing and maintaining fluent speech, which can be applied to treatment of young children who stutter.
Keywords: Childhood developmental stuttering, neuroimaging, MRI, fMRI, DTI
In the past two or so decades, neuroimaging techniques such as electroencephalography (EEG), functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and positron emission tomography (PET) have greatly contributed to our understanding of the neural bases of stuttering. Although we are still a ways from finding the “cause” of stuttering, these studies have nevertheless helped establish stuttering as a neurodevelopmental condition. It is well known that stuttering has eluded explanations of its nature, including characteristics of stuttered disfluencies, onset, natural recovery, and biological basis. Numerous and varied speculations about its etiology have been proposed, with at times very little evidence to support them. The recent decades of neuroimaging research, although with their own limitations, have provided a way to objectively examine the living brain at work and to examine where its function may go awry in stuttering. Neuroimaging research has thus provided a glimpse into the neurophysiological bases of this complex speech disorder. The brain differences found in stuttering speakers compared with typically speaking peers, which potentially reflect underlying deficits that are associated with stuttering, may serve as objective markers that can then be used in conjunction with behavioral assessments and therapies to improve clinical diagnosis and interventions in stuttering in the future.
For the speech-language pathologist (SLP), understanding the neural bases of stuttering may not be something that appears to be directly relevant to their clinical practice. It is true that, despite advances in understanding how brain anatomy and function differs in stuttering speakers, we are still perhaps years away from translating this knowledge into everyday clinical practice. For example, the differences we see between stuttering and control groups—based on even the most advanced neuroimaging techniques available at this time—are subtle, and to date these differences could only be determined at the group level and not at the individual level. Namely, if one were to look at any individual brain, we do not yet have the ability to discern whether that brain belongs to a person who stutters or not. Because the brains of most people with developmental stuttering who stutter do not exhibit gross abnormality, sufficient numbers of subjects are required to enable proper group comparisons that reveal the subtle differences between people who stutter compared with people who do not stutter.
The subtle differences in brain structure and function in people who stutter, although interesting, do not readily lend themselves to development of novel treatment that can be applied immediately. Another issue is that studies to date have mostly examined adults who stutter. Adults who stutter are likely those who have been stuttering for decades since stuttering onset, who probably have developed various emotional and motoric reactions associated with stuttering. To date, very few neuroimaging studies have examined children who stutter and fewer still children close to stuttering onset. Clearly, more research is needed, and careful and lengthy clinical trials likely will need to precede any therapies for stuttering that are neuroscience based.
Despite these limitations, and with the understanding that there are still clear gaps in the present knowledge base, it is important to remember that we do know much more than in the past about how the brain works differently in stuttering speakers. We also know much more about brain development, and the fact that brain plasticity can be greatly influenced by training and stimulation from the environment. SLPs are in a position to deliver such focused stimulation and training that can potentially have a major impact on neuroplasticity conducive to speech fluency, particularly during childhood. Given this, it is important that SLPs are updated on the recent research findings and continue to keep abreast with the development in research to provide their clients with the most updated and innovative therapies that become available for stuttering.
In this article, I review recent findings from neuroimaging research on the neural bases of stuttering, focused on the emerging literature examining children who stutter. These studies do not comprise a comprehensive list of research articles in the field, but rather those that can be discussed in the context of similarities and differences from what we know about typical brain development during childhood. From here, updated perspectives on the brain bases of stuttering are discussed, and their clinical implications.
NEUROANATOMY RELEVANT TO SPEECH PRODUCTION
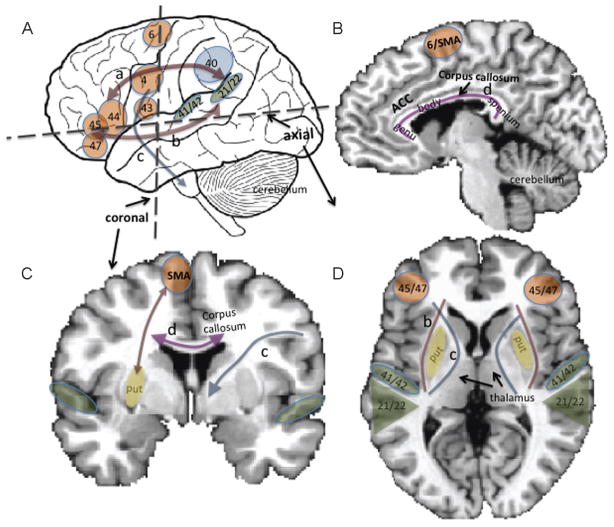
Speech production requires efficient communication among many different areas of the brain, encompassing cortical and subcortical regions in the central nervous system, as well as the peripheral nervous system that includes the many cranial nerves that innervate the respiratory, phonatory, resonance, and articulatory systems. These areas include the inferior frontal cortex/ventral premotor area (BA44/45/47, BA6), primary motor cortex (BA4, M1), supplementary motor area (SMA), pre-SMA, supramarginal gyrus (BA40), and superior temporal gyrus (STG) in the cortical areas (Fig. 1A, B). In deeper subcortical structures, the basal ganglia and thalamus have extensive connections with cortical regions, as does the cerebellum (Fig. 1C, D). These areas contribute to smooth speech motor control and adequate timing and rhythm of speech sound production. White matter tracts including the corticonuclear tracts that pass through the internal capsule interconnect the cortical areas with the cranial nerves that innervate speech musculature (Fig. 1).
Figure 1.
(A) Lateral view of the brain with perisylvian regions involved in speech and language function. These areas are focused around the sylvian fissure, a major fissure that encompasses the frontal, parietal, and temporal cortices. Orange blobs indicate those regions associated more with motor function, green blobs auditory, and blue, association areas. The numbers denote Brodmann areas: 4, primary motor cortex/M1; 21/22, posterior superior temporal gyrus (secondary and association auditory areas; overlaps with Wernicke area); 40, supramarginal gyrus; 41/42, Heschl gyrus (primary auditory cortex); 43, rolandic operculum; 44, pars opercularis; 45, pars triangularis (44 and 45 are often grouped to be considered Broca area); 47, pars triangularis (areas 44, 45, 47 are often grouped together as the inferior frontal gyrus). Arrows illustrate approximate position of major white matter tracts, including the superior longitudinal fasciculus (a), inferior longitudinal fasciculus/external capsule fiber system (b), and the corticonuclear tracts (c). (B) Midsagittal view of the brain, showing medial structures such as the supplementary motor area (SMA), anterior cingulate cortex (ACC), corpus callosum (C) Coronal section of the brain, showing structures and approximate areas of white matter tracts shown in A and B. (c) shows parts of the cortico-nuclear tracts that pass through the internal capsule, (d) corpus callosum. (D) Axial (horizontal) section of the brain (b) (shown left and right): external capsule, (c) (shown left and right): internal capsule.
Each of these regions have specialized functions; however, they form connections with spatially distant regions to enable complex functions such as speech and language production. For example, the inferior frontal gyrus (IFG) and posterior parietal-temporal regions, including the supramarginal gyrus and posterior superior temporal gyrus (pSTG), are interconnected via major white matter tracts such as the superior longitudinal fasciculus/arcuate fasciculus dorsally, and the inferior longitudinal fasciculus and external capsule fiber systems ventrally. These areas are not only structurally connected via white matter, but also functionally connected (as evidenced by temporally correlated brain activity among the areas), even during rest. The interconnections among the motor and auditory areas support speech imitation and development of speech motor control. This circuit also interacts with subcortical areas as well as the cerebellum to adjust movements in response to error. During early learning, the cerebellum may have a more active role, whereas when the skill is well learned the basal ganglia may play a greater role.1,2
It is generally understood that although both hemispheres support speech and language function, there is greater involvement and lateralization toward the left hemisphere regions that primarily support speech motor control and language processing.3,4 Asymmetric growth and increasing laterality occur with development3,5 and increasing speech-language skills.6,7
BRAIN DEVELOPMENT DURING CHILDHOOD AND SEX DIFFERENCES
During the first few years of life, there are many progressive and regressive changes across the brain, such as pruning of nerve cells, propagation of dendrites, increases in synaptic density, and white matter volume increases that are related to increased myelination. There is active pruning of less used areas, and strengthening of connections that are functionally active.8,9
According to large-scale studies of brain development, it has been shown that perisylvian brain areas supporting speech and language development, including the left IFG and bilateral pSTG, tend to have the most protracted growth pattern.10 In most other areas of the brain, cortical density had a linearly decreasing pattern with age, whereas in the IFG and pSTG regions, the developmental course was an inverted U shape, indicating that development and plasticity of these cortical regions were maintained for a longer period compared with other brain regions.11 In addition, the asymmetry of the sulcal pattern in the sylvian fissure increases with age,5 and thickening of IFG region was correlated with improvements in phonological processing.7
Developmental changes in cortical density and thickness, and increases in white matter development, underlie the strengthening of connectivity among brain areas that support important functions including speech and language and cognitive development. Structural and functional connectivity of long-range areas are also correlated with development, with many short-range connections being pruned out. These long-range fibers support later developing functions such as motor timing, cognitive control including inhibition that is needed for impulse control, and speech and language processing.12,13
Given that there is a skewed sex ratio in the development and persistence in stuttering, it is highly relevant to examine sex differences in brain developmental trajectories in typically developing children. According to large-scale pediatric neuroimaging studies of brain development, robust sex differences have been reported in brain developmental trajectories in almost all brain structures, with gray matter volume (GMV) peaking ~1 to 2 years earlier in most brain areas in females than in males.14 In addition, there is evidence supporting increased interhemispheric involvement in females; namely, females seem to exhibit structural growth supporting increased interhemispheric connectivity and perhaps less laterality toward the left hemisphere.15–19 A recently well-publicized large-scale study examining brain connectivity differences between males and females similarly reported that females have greater connectivity between the left and right hemispheres, whereas males tend to have greater connectivity within hemispheres, especially among regions supporting visual motor and sensorimotor processing.20
There is also support for structural increases in perisylvian areas supporting language processing in females,21 and increased temporal parietal thickness in females than males, independent of brain or body size, starting in childhood.22 Sex differences in the temporo-parietal region has been reported in other studies,18,23 which may be associated with better structural support for language processing in females. Furthermore, it has been shown that sex hormones affect structural brain connectivity24 and interhemispheric inhibition differently in males and females,25 which has implications for functional brain organization for the two sexes. These sex differences in brain organization may underlie the sex differences seen in many neurodevelopmental disorders such as stuttering.
COMMONLY USED NEUROIMAGING-BASED STRUCTURAL MEASURES IN BRAIN DEVELOPMENT RESEARCH
Brain Structural Measures Derived from Magnetic Resonance Imaging Scans: Gray Matter
Brain structural differences can be best examined using high-resolution structural images collected with magnetic resonance imaging (MRI) scans. These images have 1 mm3 or better resolution across the whole brain. Using such images, gray matter (composed of nerve-cell bodies and dendrites; where information processing takes place) or white matter (composed of myelinated axons, which act like insulated cables that carry information between different brain regions) measurements can be obtained. In the past, these images were mostly analyzed using expert manual morphometric measurements, which derived measures such as volume, area, and thickness of selected brain areas of interest.26,27 On the other hand, more recently whole-brain based analyses were conducted using methods such as voxel-based morphometry (VBM)23,28,29 or surface-based analyses, which can also derive GMV, density, and thickness measurements.30,31
The relationships between the above-mentioned MRI-based measures and their cellular bases are not completely elucidated; however, it is thought that changes in GMV, density, and thickness reflect changes in the number of neuronal cell bodies, dendrites, synaptic density, as well as gliogenesis and changes in vasculature.32,33 As mentioned in a previous section, such measures can show an inverted U shape across development during childhood, possibly reflecting pruning as well as encroachment of white matter tracts that take up areas that used to be gray matter. Gray matter measures can change in a relatively short period of time in response to training: for instance, a period of intensive motor learning as associated with increases in gray matter in brain regions thought to support such learning, possibly reflecting increases in synaptic density and dendritic spine growth. In one study it was shown that learning to juggle for a 3-month period led to a transient increase in gray matter in regions specifically supporting complex visual motor learning.34 In another study, GMV increases were observed in response to reading intervention in dyslexic children after an 8-week training program.35 Many such examples exist in the literature, demonstrating neuroplasticity in both adults and children in response to learning and training.
Brain Structural Measures Derived from MRI Scans: White Matter
Measures of white matter can be examined with VBM; however, more detailed information can be derived with diffusion tensor imaging (DTI), which can be used to derive quantitative measures that reflect white matter integrity and can also be used to examine fiber pathways that flow between areas of interest.36,37 DTI-derived measures such as fractional anisotropy (FA) is thought to be influenced by factors such as the amount of myelin, integrity of axonal cell membranes, as well as the coherence in the organization of axonal tracts.38 Increases in these measures in certain areas might mean that there is better “white matter integrity” that may provide the structural support for efficient and rapid interaction among different brain areas, which supports the development of well-functioning and synchronized neural circuits that support complex functions such as speech production. Like gray matter, white matter measures have also been shown to change with development and in association with training and skill acquisition/practice.39–41
BRAIN ANATOMICAL DIFFERENCES IN CHILDREN WHO STUTTER
Compared with other neurodevelopmental disorders, very few neuroimaging studies to date have focused on examining children who stutter. Conducting neuroimaging studies with children presents many practical challenges. Information on various neuroimaging techniques that have been applied to brain development and stuttering research were reviewed in a previous publication.42 Any study that uses MRI or fMRI, for example, requires restriction of head movement; children must stay immobilized in a small space under loud noise during scanning. Other techniques, such as PET, involve injecting radioactive substances (this is not the case in MRI), which are inappropriate to use in children without a clinical justification. Perhaps reflecting these practical challenges, to date only a handful of studies have been conducted to examine childhood stuttering.
In the following section, I review the as yet small number of studies conducted to date that have examined children who stutter using neuroimaging techniques including MRI, EEG/event related potentials (ERP), MEG, functional near infrared spectroscopy (fNIRS), and fMRI methods.
Structural MRI Studies That Examined Children Who Stutter
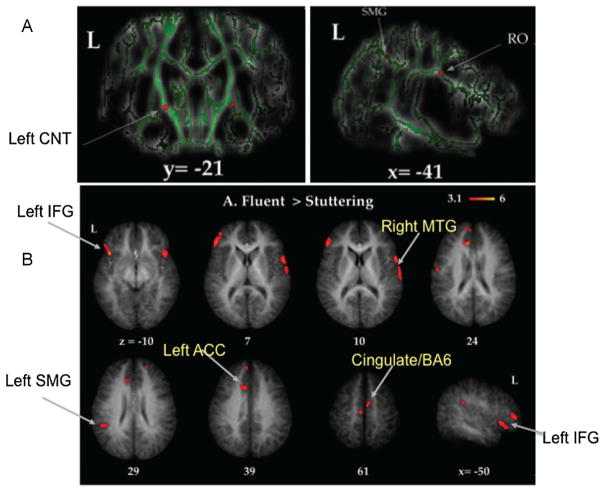
In the first neuroanatomical study examining children who stutter, Chang and colleagues compared children with persistent stuttering, children who recovered naturally from stuttering, and age-matched fluent controls on several different brain structure measures.29 All 21 children who participated were 8- to 12-year-old, right-handed boys. Group differences in white matter integrity and GMV were compared among the groups. The results supported evidence of decreased white matter integrity in the superior longitudinal fasciculus underlying the ventral sensorimotor cortex (rolandic operculum) in stuttering children relative to age-matched controls (Fig. 2A). A decrease in white matter integrity in this area may mean that signals among the movement planning, execution, and sensory brain areas may not be transmitted in a sufficiently rapid manner to allow for fluent speech production. This decrease was common for those who were persistent stutterers and those who had recovered from stuttering. In addition, this study reported significant differences in white matter integrity between children with a stuttering history (both persistent and recovered) versus fluent children in an area that contains thalamocortical and corticonuclear tracts (Fig. 2A, right). These tracts connect cortical brain regions with deep subcortical areas and cranial nerves that can directly control speech musculature. If these connections are affected, coordination of speech musculature allowing adequate timing, amplitude, and sequence manipulation that are typical of fluent speech could be affected as well.
Figure 2.
(A) Differences in white matter integrity as assessed through tract-based spatial statistics (TBSS) of diffusion tensor imaging data. TBSS allows whole-brain comparisons of measures of white matter integrity. Here, 8- to 12-year-old boys who stutter exhibited significantly decreased white matter integrity in the superior longitudinal fasciculus, underlying the rolandic operculum (RO), and the bilateral corticonuclear tracts (CNT). (B) Differences in gray matter volume between boys who do and boys who do not stutter. The same group of 8- to 12-year-old participants were examined for differences in gray matter volume (GMV) across the whole brain. Red blobs show areas where boys who stutter exhibited less GMV compared with age-matched peers. These regions included the left inferior frontal gyrus (IFG), right superior and middle temporal gyrus (MTG), left supramarginal gyrus (SMG), and anterior cingulate cortex (ACC)/supplementary motor area.29
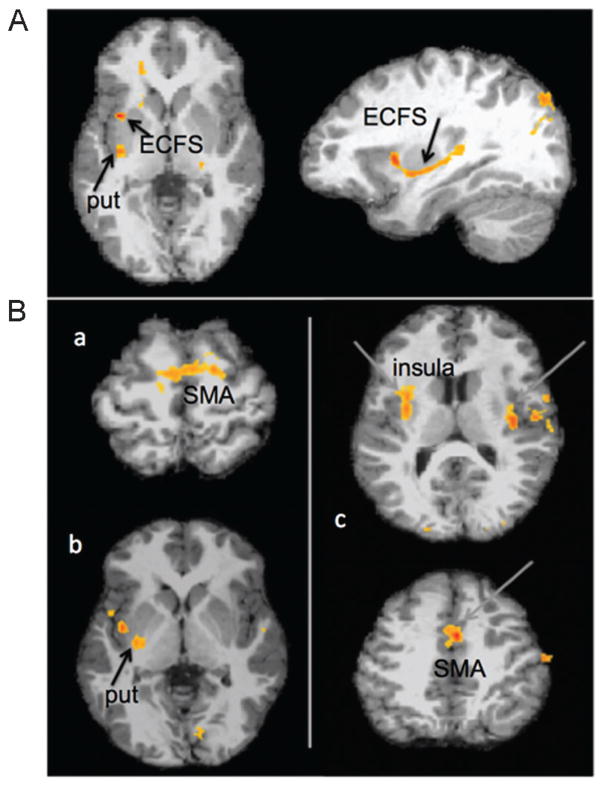
More recently, we conducted a study with a larger sample of children who stutter, that included both sexes and an extended age range that included children down to 3 years of age.43 In this study, the data supported decreased white matter connectivity in white matter tracts that interconnected the frontal motor areas with the auditory regions, and tracts that interconnected SMA and deeper structures such as the putamen (Fig. 3A). Currently, additional data are being collected to enable sex comparisons and to enable longitudinal analyses to examine changes in brain development as the child develops. These brain trajectories may differ between children who do and do not stutter and also show subtle differences between persistent and recovering children. These data would provide important information on the mechanisms of persistence and recovery during childhood.
Figure 3.
(A) Areas where the probability of white matter tracts were decreased in children who stutter, when starting point of the white matter tracking was in the left posterior superior temporal gyrus (pSTG). Children who stutter showed decreased probability of white matter tracts in the putamen (put) and inferior frontal gyrus, via the external capsule fiber system (ECFS). (B) Differences in brain activity patterns in children who stutter when they were at rest. Resting state functional magnetic resonance imaging data were analyzed to compare correlated brain activity patterns (which indicate areas that “talk with each other,” or the presence of functional connectivity between those regions) between the stuttering and control groups. (a) When correlated brain activity with the left putamen was examined, the supplementary motor area (SMA) had significantly greater correlation with putamen in the typically speaking children compared with children who stutter. (b) When correlated activity with the left SMA was examined, the putamen insula showed significantly heightened correlated activity with the SMA in the typically speaking controls compared with children who stutter. (c) When correlated activity with the left pSTG was examined, the bilateral insula, STG, and SMA regions showed greater correlation in typically speaking controls compared with children who stutter.43
Compared with age-matched controls, children who stutter also showed decreases in GMV in areas that included the bilateral IFG, STG, SMA, and other areas (Fig. 2B). In a more recent investigation, Beal and colleagues examined GMV using the whole brain–based method VBM, to compare stuttering boys and their age-matched controls between 6 and 12 years of age. The authors found that there were decreased GMV in the bilateral IFG and in the left putamen in children who stutter. They also found increased GMV in stuttering children relative to controls in the right rolandic operculum, a sensorimotor region, and the right superior temporal gyrus.28
Decreased asymmetry/laterality toward the left hemisphere and greater interhemispheric involvement in the stuttering group relative to typically speaking controls warrant some discussion. Adults who stutter exhibited decreased asymmetry in the perisylvian regions,44,45 a right lateralized increase in perisylvian sulcal development,46 and greater area of the corpus callosum, particularly in the rostrum area that interconnects the two frontal lobe structures.47 Higher brain activity in the right hemisphere in adults who stutter have been captured with functional imaging.48–51 In children, the data so far show that in terms of GMV, there were no differences in asymmetry patterns between children who stutter and controls,29 but increased GMV in the right hemisphere structures such as the rolandic operculum and STG in children who stutter was reported in another study.28 Choo et al examined corpus callosum white matter measures in children who stutter and found no differences between the two groups.52 No differences in laterality during childhood might indicate that the greater right hemisphere involvement seen in adults who stutter relative to nonstuttering adults is something that may have developed as people continue to stutter, possibly as a reaction to stuttering, not reflecting the basis of stuttering. Namely, increased right-sided volume found in adults who stutter could be the result of compensation for aberrant left hemisphere connectivity. Because the above-mentioned studies involved children who were between 6 and 12, there is still a chance that we might be capturing reactions to stuttering. Examining children closer to stuttering onset might help elucidate some of these issues in the future.
In summary, children who stutter exhibited brain structural differences compared with age-matched, typically speaking peers, when examined with white and gray matter measures acquired with MRI. The differences suggested that structural support for dynamic and timely interactions among the left motor cortical and auditory areas might be affected in children who stutter, possibly contributing to development and maintenance of disfluent speech. Brain cortical volume differences were observed in the bilateral IFG and auditory areas and areas that support initiation and timing of speech motor control such as the SMA and putamen. There are some disagreements in terms of structural increases in the right hemisphere and laterality in children who stutter. These issues may be elucidated when conducting studies with children closer to stuttering onset.
BRAIN FUNCTION DIFFERENCES IN CHILDREN WHO STUTTER
Any anomalous brain structural growth reported in children who stutter may impact how brain regions interact when producing speech. In turn, sustained anomalous function could lead to further structural changes in the brain. To date, researchers have conducted only a few studies examining differences in brain function in young children who stutter. Following, I discuss some studies that have examined differences in brain function or brain activity patterns in children who stutter.
EEG/ERP and MEG Studies Examining Children Who Stutter
Most studies measuring brain function in stuttering children have so far used signals obtained from EEG.53–57 ERP, or stereotypical electrophysiological responses to a given stimulus (such as auditory presentation of a tone or a vowel), can be captured via an EEG. Using electrodes or very sensitive coils along the scalp, EEG or MEG can pick up electrical and magnetic field potentials, respectively, which are associated with neural activity. Both methods can capture the brain responses of interest almost as soon as they occur (excellent temporal resolution). However, the spatial resolution, which relates to localizing the brain activity to a certain region of the brain, is usually much less reliable than other neuroimaging methods such as fMRI.
An ERP study conducted with school-aged children who stutter reported that stuttering children were significantly less accurate than controls when making rhyming judgments that required phonological rehearsal. The authors noted that the brain’s evoked responses related to the cognitive processes preceding this task were altered in children who stutter, and that the responses peaked earlier in the right hemisphere than in the left, whereas the brain responses peaked earlier in the left than the right in the controls. The authors noted that the “timing of the relative contributions of the left and right hemisphere functions may operate differently in [children who stutter].”56(p.333) The same research group conducted another ERP study on preschool-aged children who stutter and found that children who stutter lacked a characteristic waveform that is typically elicited in normal children in response to deviant auditory stimuli. This indicated aberrant cognitive mechanisms involved in processing auditory stimuli, even in the youngest stuttering children.54
Another study examining school-aged children who stutter used MEG to examine a well-known phenomenon that illustrates the interaction between speech motor and auditory areas: vocalization-induced suppression.58 Many parts of the auditory cortex is normally inhibited during vocalization, unlike when we listen to a recording of the same vocalization. According to scientists, this phenomenon underscores the tight collaboration between the auditory and motor regions of the brain to enable normal speech production. The researchers measured the brain’s evoked responses to listening to a tone, listening to a vowel, and producing a vowel in school-aged children who stutter. The children did not differ from age-matched controls in their evoked response to simply listening to the tone, but they did differ in their response to vowel perception and production. The amplitude of the evoked responses did not differ, but the latency of response was delayed in both hemispheres of children who stutter. These results indicate that children who stutter may have a less efficient auditory-motor connectivity relative to their peers, specifically for speech stimuli. Given that the latency of the response differed between the groups, it may be that timely and synchronized interactions among the auditory-motor regions are affected in children who stutter.
Studies Examining Hemodynamic Measurements of Brain Function (fNIRS, fMRI) in Children Who Stutter
In a functional near infrared spectroscopy (fNIRS) study, the extent of laterality (left versus right cerebral dominance) in brain function for phonological and prosodic contrast tasks was reported in adults, school-aged children, and preschool-aged children who stutter.59 The phonological contrasts involved perceiving differences in distinct units of speech sounds, and prosody contrasts involved perceiving differences in intonation. The authors expected that speech sounds, compared with intonation changes, would be perceived better in the left hemisphere compared with the right, as the former involves linguistic processing, which lateralizes to the left hemisphere in the vast majority of individuals. fNIRS is a method that allows noninvasive examination of brain function similar to fMRI but is less restrictive for young participants. The researchers found that age-matched nonstuttering speakers consistently exhibited greater left than right laterality of brain response when listening to auditory stimuli differing in phoneme versus prosody. In contrast, not even one subject among the stuttering group exhibited leftward laterality for the phoneme versus prosodic contrasts. This was true for all age groups, including the youngest preschool-aged children. The researchers speculated that due to left-sided anatomical deficiencies, both linguistic and prosodic functions may lateralize to the right hemisphere in stuttering children, and as this pattern is maintained, children may display right-sided structural increases, as have been reported in anatomical studies of adults who stutter.
In a recently published study, Chang and Zhu examined how different brain regions interact when the children were at rest.43 Such a study that uses resting state fMRI and functional connectivity analyses enables one to examine temporally correlated brain activity across different parts of the brain (i. e., examine which areas “talk to each other”). It has been found that such functional connectivity networks overlap with networks supporting specific functions such as motor performance and language and also reflect structural connections between areas. In this study, children who stutter had decreased functional connectivity relative to nonstuttering peers in two neural circuits in the left hemisphere: the inferior frontal-motor-auditory circuit (Fig. 3B(c)), and the SMA-putamen circuit (Fig. 3B(a, b)). Decreased functional connectivity in these circuits may suggest deficiencies in speech motor planning and execution, as well as timing of self-initiated speech production. Some preliminary sex differences were also found: both stuttering boys and girls had consistently lower functional connectivity in the SMA-putamen compared with sex-matched nonstuttering peers, but in the IFG-motor-STG circuit only stuttering boys but not stuttering girls showed decreased functional connectivity.43 Because more girls than boys grow out of stuttering naturally, it is possible that our stuttering girl group may have included those who will grow out of stuttering in the future. It may be that normalized patterns of connectivity in this circuit supports recovery, which may be confirmed in future longitudinal studies that track their brain growth as they age.
In summary, the functional studies reviewed in this section show that evidence is accruing for aberrant timing of activity between the two hemispheres for speech processing, possible inefficiencies in the interaction between auditory and motor areas, and decreased functional connectivity between cortical-subcortical structures that support self-initiated speech, including the SMA and putamen. There is emerging evidence of sex differences within the stuttering group: stuttering girls as a group seem to have more normalized or better than typical connectivity in the auditory-motor regions, which may indicate increased potential for later recovery in this group. Follow-up longitudinal studies are expected to elucidate some of the remaining questions in childhood stuttering.
Summary of Structural and Functional Neuroimaging Studies That Examined Children Who Stutter
Current neuroimaging data from children who stutter point to differences in brain function and anatomy, involving both auditory and motor areas of the brain, and in the cortical-subcortical circuits that include SMA and putamen, even in the earliest stages of stuttering. The functional brain differences in stuttering children, when sustained, could result in structural brain changes, in turn leading to further abnormal laterality of auditory-motor interaction for speech processing—which is reported in stuttering adults. Future studies that track both functional and structural brain growth as stuttering children develop are likely to give us more definitive answers on several still-unanswered issues, such as why some children naturally recover from stuttering and why many more girls grow out of stuttering than boys.
IMPLICATIONS FOR TREATMENT
The brain regions found to be different in stuttering children are primarily those that undergo active growth and are plastic during childhood and are thus potentially more likely to respond to treatment that stimulates brain development toward more normal growth patterns. It is probable that there is greater chance of lasting recovery if therapy is delivered during early childhood rather than after adolescence. Perhaps unlike in many cases of adults who stutter, the goal of treatment for children who stutter is normal fluency for most children. For these children, recovery may occur either because they adopt a compensatory neural growth pattern that successfully makes up for the deficient brain regions, or because they are able to adopt a pattern of development that resembles normally fluent children.
In the future, neuroimaging researchers may collaborate with clinicians to conduct treatment research to examine therapy’s effects on neuroplasticity that correlates with recovery in children. These studies could further help determine whether therapy-induced recovery during early childhood leads to similar brain function and structure as found in children who have recovered or children who have never stuttered. If the therapy-induced brain changes do not lead to brain structure and function that resemble normal brain growth in children who never stuttered, yet the children who once stuttered achieve full recovery without relapse, this may indicate a successful compensatory growth that may be a goal of future behavioral treatment for both children and adults.
With emerging brain stimulation techniques such as transcranial direct current stimulation,60 which is already being used in conjunction with behavioral therapy to treat aphasia61–64 and for motor rehabilitation,65 we may be able to enhance effects of behavioral treatment by stimulating neuroplastic growth that supports speech fluency in people who stutter, starting in childhood. With emerging research involving consortiums among many different groups of investigators, more research examining children closer to symptom onset, and before-and-after treatment studies, breakthroughs in novel stuttering treatment appear to be more attainable than ever before.
Learning Outcomes.
As a result of this activity, the reader will be able to (1) list major brain regions and/or neural circuits that have been reported to differ between children who do and children who do not stutter; (2) discuss how aberrant neural circuits as found in children who stutter may affect fluent speech production; (3) discuss how neuroimaging research could impact clinical practices, including diagnosis and therapy, in the future.
References
- 1.Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41(3):252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- 2.Penhune VB, Doyon J. Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage. 2005;26(3):801–812. doi: 10.1016/j.neuroimage.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Amunts K, Schleicher A, Ditterich A, Zilles K. Broca’s region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465(1):72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- 4.Foundas AL, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32(10):1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 5.Sowell ER, Thompson PM, Rex D, et al. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex. 2002;12(1):17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Holland SK, Vannest J, Mecoli M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu L, Leonard C, Thompson P, et al. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17(5):1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- 8.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubia K. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 2013;22(12):719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson P, Sowell E, Gogtay N, et al. Structural MRI and brain development. [Accessed on March 18, 2014];Neuroimaging, Pt B Int Rev Neurobiol. 2005 67:285–323. doi: 10.1016/S0074-7742(05)67009-2. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16291026. [DOI] [PubMed] [Google Scholar]
- 12.Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a “local to distributed” organization. PLOS Comput Biol. 2009;5(5):e1000381–e14. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RT, Yeatman JD, Wandell BA, Buonocore MH, Amaral DG, Nordahl CW. Diffusion properties of major white matter tracts in young, typically developing children. Neuroimage. 2013;88C:143–154. doi: 10.1016/j.neuroimage.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 18.Witelson SF. Neural sexual mosaicism: sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology. 1991;16(1–3):131–153. doi: 10.1016/0306-4530(91)90075-5. [DOI] [PubMed] [Google Scholar]
- 19.Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. J Neurosci. 2010;30(35):11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knaus TA, Corey DM, Bollich AM, Lemen LC, Foundas AL. Anatomical asymmetries of anterior perisylvian speech-language regions. Cortex. 2007;43(4):499–510. doi: 10.1016/s0010-9452(08)70244-2. [DOI] [PubMed] [Google Scholar]
- 22.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 24.Peper JS, van den Heuvel MP, Mandl RCW, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Weis S, Hausmann M. Sex hormones: modulators of interhemispheric inhibition in the human brain. Neuroscientist. 2010;16(2):132–138. doi: 10.1177/1073858409341481. [DOI] [PubMed] [Google Scholar]
- 26.Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci U S A. 1996;93(2):719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto LA, Zatorre RJ. The morphometry of auditory cortex in the congenitally deaf measured using MRI. Neuroimage. 2003;20(2):1215–1225. doi: 10.1016/S1053-8119(03)00373-2. [DOI] [PubMed] [Google Scholar]
- 28.Beal DS, Gracco VL, Brettschneider J, Kroll RM, De Nil LF. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. 2013;49(8):2151–2161. doi: 10.1016/j.cortex.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson PM, Lee AD, Dutton RA, et al. Abnormal cortical complexity and thickness profiles mapped in Williams syndrome. J Neurosci. 2005;25(16):4146–4158. doi: 10.1523/JNEUROSCI.0165-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 35.Krafnick AJ, Flowers DL, Napoliello EM, Eden GF. Gray matter volume changes following reading intervention in dyslexic children. Neuroimage. 2011;57(3):733–741. doi: 10.1016/j.neuroimage.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 37.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 38.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage. 2005;26(4):1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 39.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansen-Berg H. Structural plasticity: rewiring the brain. Curr Biol. 2007;17(4):R141–R144. doi: 10.1016/j.cub.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8(9):1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 42.Chang S, Ludlow C. Brain imaging in children. In: Maassen Ben, van Lieshout Pascal., editors. Speech motor control: New developments in basic and applied research. Oxford, England: Oxford University Press; 2010. [Google Scholar]
- 43.Chang SE, Zhu DC. Neural network connectivity differences in children who stutter. Brain. 2013;136(Pt 12):3709–3726. doi: 10.1093/brain/awt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foundas AL, Corey DM, Angeles V, Bollich AM, Crabtree-Hartman E, Heilman KM. Atypical cerebral laterality in adults with persistent developmental stuttering. Neurology. 2003;61(10):1378–1385. doi: 10.1212/01.wnl.0000094320.44334.86. [DOI] [PubMed] [Google Scholar]
- 45.Jäncke L, Hänggi J, Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BMC Neurol. 2004;4(1):23. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cykowski MD, Kochunov PV, Ingham RJ, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cereb Cortex. 2008;18(3):571–583. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- 47.Choo AL, Kraft SJ, Olivero W, et al. Corpus callosum differences associated with persistent stuttering in adults. J Commun Disord. 2011;44(4):470–477. doi: 10.1016/j.jcomdis.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun AR, Varga M, Stager S, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120(Pt 5):761–784. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- 49.Fox PT, Ingham RJ, Ingham JC, et al. A PET study of the neural systems of stuttering. Nature. 1996;382(6587):158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- 50.De Nil LF, Kroll RM, Lafaille SJ, Houle S. A positron emission tomography study of short- and long-term treatment effects on functional brain activation in adults who stutter. J Fluency Disord. 2003;28(4):357–379. doi: 10.1016/j.jfludis.2003.07.002. quiz 379–380. [DOI] [PubMed] [Google Scholar]
- 51.Chang SE, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal-premotor structural and functional connectivity deficits in adults who stutter. Cereb Cortex. 2011;21(11):2507–2518. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choo AL, Chang SE, Zengin-Bolatkale H, Ambrose NG, Loucks TM. Corpus callosum morphology in children who stutter. J Commun Disord. 2012;45(4):279–289. doi: 10.1016/j.jcomdis.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold HS, Conture EG, Key APF, Walden T. Emotional reactivity, regulation and childhood stuttering: a behavioral and electrophysiological study. J Commun Disord. 2011;44(3):276–293. doi: 10.1016/j.jcomdis.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaganovich N, Wray AH, Weber-Fox C. Non-linguistic auditory processing and working memory update in pre-school children who stutter: an electrophysiological study. Dev Neuropsychol. 2010;35(6):712–736. doi: 10.1080/87565641.2010.508549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozge A, Toros F, Cümelekoğlu U. The role of hemispheral asymmetry and regional activity of quantitative EEG in children with stuttering. Child Psychiatry Hum Dev. 2004;34(4):269–280. doi: 10.1023/B:CHUD.0000020679.15106.a4. [DOI] [PubMed] [Google Scholar]
- 56.Weber-Fox C, Spruill JE, III, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Dev Sci. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber-Fox C, Hampton Wray A, Arnold H. Early childhood stuttering and electrophysiological indices of language processing. J Fluency Disord. 2013;38(2):206–221. doi: 10.1016/j.jfludis.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beal DS, Quraan MA, Cheyne DO, Taylor MJ, Gracco VL, De Nil LF. Speech-induced suppression of evoked auditory fields in children who stutter. Neuroimage. 2011;54(4):2994–3003. doi: 10.1016/j.neuroimage.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato Y, Mori K, Koizumi T, et al. Functional lateralization of speech processing in adults and children who stutter. Front Psychol. 2011;2:70. doi: 10.3389/fpsyg.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flöel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage. 2014;85(Pt 3):934–947. doi: 10.1016/j.neuroimage.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 61.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41(6):1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42(3):819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monti A, Ferrucci R, Fumagalli M, et al. Transcranial direct current stimulation (tDCS) and language. J Neurol Neurosurg Psychiatry. 2013;84(8):832–842. doi: 10.1136/jnnp-2012-302825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira JB, Junqué C, Bartrés-Faz D, et al. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson’s disease. Brain Stimulat. 2013;6(1):16–24. doi: 10.1016/j.brs.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Reis J, Schambra HM, Cohen LG, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]