Abstract
Background
The PROMISE trial found that initial use of ≥64-slice multidetector computed tomographic angiography (CTA) versus functional diagnostic testing strategies did not improve clinical outcomes in stable symptomatic patients with suspected coronary artery disease (CAD) requiring noninvasive testing.
Objective
Economic analysis of PROMISE, a major secondary aim.
Design
Prospective economic study from the US perspective. Comparisons were made by intention-to-treat. Confidence intervals were calculated using bootstrap methods.
Setting
190 U.S. centers
Patients
9649 U.S. patients enrolled in PROMISE. Enrollment began July 2010 and completed September 2013. Median follow-up was 25 months.
Measurements
Technical costs of the initial (outpatient) testing strategy were estimated from Premier Research Database data. Hospital-based costs were estimated using hospital bills and Medicare cost-to-charge ratios. Physician fees were taken from the Medicare Fee Schedule. Costs were expressed in 2014 US dollars discounted at 3% and estimated out to 3 years using inverse probability weighting methods.
Results
The mean initial testing costs were: $174 for exercise ECG; $404 for CTA; $501 to $514 for (exercise, pharmacologic) stress echo; $946 to $1132 for (exercise, pharmacologic) stress nuclear. Mean costs at 90 days for the CTA strategy were $2494 versus $2240 for the functional strategy (mean difference $254, 95% CI −$634 to $906). The difference was associated with more revascularizations and catheterizations (4.25 per 100 patients) with CTA use. After 90 days, the mean cost difference between the arms out to 3 years remained small ($373).
Limitations
Cost weights for test strategies obtained from sources outside PROMISE.
Conclusions
CTA and functional diagnostic testing strategies in patients with suspected CAD have similar costs through three years of follow-up.
INTRODUCTION
Non-acute chest pain is a common reason for patients to seek medical care and a continuing challenge for the medical practitioners who must determine what those symptoms represent. Clinicians typically rely on the patient’s history and noninvasive tests to assess the possibility of obstructive coronary artery disease (CAD). To date, the evidence base on the effectiveness of the major testing alternatives has largely been limited to observational studies comparing diagnostic accuracy or prognostic significance (1–4). As a consequence, little consensus exists among clinicians and testing experts on which strategy provides the best outcomes for patients.
The PROspective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial examined the effect of different diagnostic testing strategies for CAD on patient outcomes (5) (see protocol, supplementary material). A detailed description of the overall PROMISE design has been published (5, 6). Briefly, between July 27, 2010 and September 19, 2013, PROMISE enrolled 10,003 symptomatic outpatients without known CAD at 193 North American sites (5). Patients were eligible if their physician believed that non-urgent noninvasive stress testing was indicated for evaluation of their symptoms (6). Patients were randomized to either initial computed tomographic angiography (CTA) (anatomic strategy) or initial prespecified functional testing using stress electrocardiogram (ECG), stress echo, or stress nuclear methods stratified by site (6). The choice of the functional test was left to the patient’s clinician as was the interpretation of the test and all subsequent management following the randomized test. Median follow-up was 25 months (interquartile range 18 to 34 months). The primary study outcome, a composite of all-cause mortality, myocardial infarction, hospitalization for unstable angina, or major 72-hour complications of diagnostic tests or cardiovascular procedures, occurred in 3.3% of the CTA arm and 3.0% of the functional testing arm (adjusted hazard ratio (HR) 1.04, 95% confidence interval (CI) 0.83 to 1.29, p=0.75) (5). None of the individual primary end point components, combinations of components, or subgroup analyses were significantly different between groups. Economic analysis of these strategies was a major secondary objective of the PROMISE research program (6).
METHODS
Overview of PROMISE Economic Study
The economic study design involved three major components: 1) collection of empirical resource use data and hospital cost data, 2) an intention-to-treat comparison of within-study medical costs, and 3) a lifetime cost-effectiveness analysis, which was not performed because the hypothesized primary clinical benefits for CTA were not found.
Patients enrolled in PROMISE and cared for in the fee-for-service sector of the US health care system (n=9649, 96.5%) were included in the economic study. Patients enrolled in the Military/VA system (n=39), an HMO (n=166), or in Canada (n=131) were excluded. Eighteen patients were excluded because their enrolling sites declined to participate in this study outcome. Costs for hospital-based care were assigned using prospectively collected hospital billing data along with the relevant charge-to-cost correction factors, as described below. For the initial office-based diagnostic tests, no method exists for converting billed charges to costs. Thus, for these costs, we used cost weights from a secondary source, as described below. We report total costs (fixed plus variable) discounted at 3%. Hospital-based costs were adjusted to 2014 US dollars using the Producer Price Index for hospital care. An overview of cost analysis methods and terminology is provided elsewhere (7).
All patients provided written informed consent and study protocol approval was obtained from each site’s or a central Institutional Review Board.
Index Diagnostic Testing Cost Estimation
Cost weights were derived for the index testing procedures from the Premier Research Database (www.premierinc.com), which contains discharge abstract and cost data for all inpatient and hospital-based outpatient encounters from over 800 geographically diverse hospitals. Two-thirds of these hospitals provide detailed, service-level data from resource-based cost-accounting systems, while the remainder provides itemized charges that are converted to costs using department level cost-to-charge ratios (RCCs). To estimate test costs, we extracted outpatient encounters for patients aged 45 years or older who were discharged between 1/1/2014 and 6/30/2014 with CPT codes for target non-invasive diagnostic tests and cardiac diagnoses.
We used testing cost data from Premier that was generated from bottom-up cost accounting methods, rather than RCCs, because those provide a detailed resource-based estimate of testing costs in a large group of US hospitals (7). The facility testing costs used in our base case analyses were derived from the distribution of these costs (data shown in Table 1).
Table 1.
| Estimated Costs of Non-Invasive Tests ($USD) Cost-Accounting Sites, Premier Research Database* |
||||||
|---|---|---|---|---|---|---|
| N | Mean | Standard deviation | Median | 25th– 75th percentile | Minimum – Maximum | |
| Pharm MPI | 3903 | $1132 | $416 | $1101 | $864 – $1356 | $432 – $4517 |
| Exercise MPI | 2396 | $946 | $420 | $898 | $649 – $1189 | $280 – $3052 |
| Pharm. Echo | 152 | $501 | $135 | $487 | $408 – $562 | $258 – $978 |
| Exercise Echo | 632 | $514 | $151 | $508 | $403 – $612 | $238 – $1261 |
| Exercise EKG | 455 | $174 | $80 | $152 | $117 – $196 | $61 – $465 |
| CTA w/Contrast | 489 | $404 | $122 | $401 | $307 – $486 | $167 – $878 |
| Estimated Costs of Non-Invasive Tests, by Cost Component ($USD) Cost-Accounting Sites, Premier Research Database* | ||||||
|---|---|---|---|---|---|---|
| Pharm Stress MPI (n=3903) |
Exercise Stress MPI (n=2396) |
Pharm Stress Echo (n=152) |
Exercise Stress Echo (n=632) |
Exercise EKG (n=455) |
CTA (n=489) |
|
| Primary test (imaging, treadmill or CTA) | $502 | $538 | $210 | $252 | $137 | $221 |
| Stress test | $137 | $134 | $157 | $160 | $0 | $0 |
| Stress agent | $214 | $0 | $14 | $0 | $0 | $0 |
| Isotope | $152 | $151 | $0 | $0 | $0 | $0 |
| Contrast | $0 | $0 | $26 | $15 | $0 | $48 |
| Lab | $1 | $1 | $1 | $1 | $0 | $6 |
| Other pharm | $3 | $2 | $7 | $0 | $0 | $10 |
| Other nuclear Medicine | $7 | $3 | $0 | $0 | $0 | $0 |
| Subtotal | $1015 | $829 | $415 | $428 | $137 | $285 |
| Physician fees** | $117 | $117 | $86 | $86 | $37 | $119 |
| Total | $1132 | $946 | $501 | $514 | $174 | $404 |
CTA= computed tomography angiogram, Pharm=pharmacological, echo=echocardiogram, EKG=electrocardiogram, MPI=myocardial perfusion imaging
Difference in totals due to rounding.
Outpatient isolated test encounters between 1/1/2014 – 6/30/2014 with admission diagnosis=chest pain, or primary/secondary diagnosis of chest pain/acute coronary syndrome/ischemic heart disease/valve/dysrhythmias. Excludes 6 sites with inconsistent data.
Based on Medicare Fee Schedule
Hospital Cost Estimation
Medical resource use through the first 60 days was recorded on the study case report form. Further follow-up information was collected from patients using mail (46%) or telephone-based (54%) methods. Resource consumption data covered emergency department visits, days in the hospital, use of intensive care unit (ICU) services, catheterization and coronary revascularization procedures, and major complications related to procedures. Hospitalizations and hospital-based testing and procedures were verified using medical billing information. Patient reports of hospitalizations that could not be verified were not counted in the analysis.
Hospital-based costs were calculated using hospital billing data (UB-04 forms), with charges converted to costs using the departmental charge to cost conversion factors available from each hospital’s annual Medicare Cost Report. Of the 5863 hospital-based care episodes, we collected billing information for 4876 (83.2%). In all cases, uncollectible billing information from verified hospitalizations was missing due to administrative reasons (e.g., hospital refusal to provide requested bills) unconnected with the randomized diagnostic strategy, actual tests received, subsequent care, or patient outcomes. Therefore, we assumed these data were missing at random and used linear regression methods to impute cost weights for these records from the portion of the study cohort with complete cost data. The imputation models included reason for hospitalization, type of hospital visit (inpatient vs emergency department), length of stay, ICU admission during stay, coronary artery bypass grafting surgery (CABG), percutaneous coronary intervention (PCI), and peripheral vascular bypass/stenting. Some catheterization and revascularization procedures were performed on an outpatient basis or were captured as a clinical event but not as a hospitalization; these services were imputed using average prices from services with billing records, as summarized in eTable 1.
Physician Cost Estimation
Physician costs for both the index testing strategy as well as for any follow-up care on the case report form or identified through billing data were estimated by mapping major procedures and physician services to appropriate CPT/HCPCS codes in the 2014 Medicare national reimbursement schedule. The physician fees assigned to the initial randomized tests in this study are shown in Table 1.
Statistical Analyses
Descriptive Analyses and General Considerations
All primary comparisons were performed according to the principle of intention-to-treat. Descriptive statistics included percentages for discrete variables, and medians with 25th to 75th percentiles plus means with standard deviations for continuous variables. The chi-square test was used for discrete variable comparisons, and nonparametric tests such as the Wilcoxon Mann Whitney test were used for continuous variable comparisons. Follow-up cost data on surviving patients lost to follow-up were assumed to be missing at random and were imputed using linear regression models developed on patients with complete cost data.
Estimation and Comparison of Cumulative Within Trial Costs
A nonparametric partitioned estimator was used to estimate diagnostic strategy-specific, 3-year medical costs with 12 partitions corresponding to 3-month intervals following randomization (8). Comparisons between the two testing strategies were made using a normal approximation with standard errors estimated using the bootstrap approach. To account for uncertainty in test prices, in each bootstrap repetition, we randomly sampled (with replacement) a price weight from the Premier database distribution for each test type and used these price weights to calculate costs during that bootstrap repetition. Bootstrapping was performed using 10,000 repetitions, with percentile-based confidence intervals reported. The primary effect size was the mean cost difference between the two arms with 95% confidence intervals. P values were calculated for selective comparisons, with a “significant” p value equivalent to a 95% confidence interval that excludes 0. No adjustment in significance levels for multiple comparisons was planned or used. Differences in cost by diagnostic testing group were interpreted in the context of the trial clinical results, looking for both consistency and plausibility with respect to end points as well as measures of resource use.
In addition, we used bootstrap methods to plot the probability of differences in cost greater than arbitrary thresholds of interest (such as $500, $750, or $1000).
Subgroups
Prespecified subgroup analyses were: age, sex, race, site-generated pre-test probability of CAD assessment, CAD risk equivalent (history of diabetes, peripheral vascular disease, or cerebrovascular disease), Diamond and Forrester/CASS pre-test probability of CAD (9), and pre-randomization physician choice for functional stress test (5).
Sensitivity Analyses
To examine the sensitivity of our results to the specific testing cost weights chosen, we performed two sensitivity analyses. First, we replaced the (bottom up) cost accounting-based test costs with the top-down estimates of test costs from the Premier database using the sample of hospitals that provided data in terms of charges and cost-to-charge correction ratios (eTable 2a). Second, we used the 2014 Medicare reimbursements for hospital-based testing in place of the Premier cost weights (eTable 2b). This latter analysis did not substitute Medicare reimbursements for all follow-up tests and was not intended to represent a Medicare perspective cost analysis.
Role of Funding Source
The PROMISE economic study was a prospectively designed part of the PROMISE trial and was funded through a separate R01 grant by the National Heart, Lung, and Blood Institute (NHLBI) along with the parent trial. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health.
RESULTS
Baseline Characteristics
Baseline characteristics for the economic study cohort were well balanced by randomized assignment (eTable 3). The mean age was 60.8 years, 53.1% were female, 87.8% had a primary symptom of chest pain or dyspnea, and the Diamond-Forrester/CASS pretest probability of CAD averaged 53.4%. Patients excluded from the economic study were about 1 year younger, less often female, more often minority, more often had non-cardiac chest pain, and averaged a slightly lower pretest probability of CAD (eTable4).
Medical Costs Analyzed by Intention-to-Treat
Among patients in the economic study cohort who were randomized to the CTA arm, 4523 (93.9%) had CTA as their initial test, 134 (2.8%) had an initial functional study, 9 (0.2%) had invasive angiography, and 152 (3.2%) had no diagnostic test. Among patients randomized to functional testing, 4523 (93.6%) had a functional test as their initial test, 46 (1.0%) had CTA, 20 (0.4%) had invasive angiography, and 240 (5.0%) had no test.
Costs to 3 Years
Within 90 days after randomization, 12.2% of CTA patients and 8.1% of functional testing patients had follow-up invasive catheterization, and 6.2% of the CTA arm versus 3.2% of the functional testing arm had undergone coronary revascularization. At 90 days, the mean cost for the CTA arm was $2494 versus $2240 for the functional testing arm, with a mean difference of $254 (95% CI −$634 to $906).
Between 91 days and 365 days, the mean cost difference between the two arms was $99 (95% CI −$136 to $342). Thus, the cumulative 1-year cost difference was $353 (95% CI −$568 to $1071). In year 2, the mean cost difference was $26 (95% CI −$252 to $297) and in year 3, the mean difference was $249 (95% CI −$227 to $811). The 3-year cumulative costs were $7213 for CTA and $6586 for functional testing, with a mean difference of $627 (95% CI −$463 to $1609) (Figure 1). None of the 95% confidence intervals excluded the null value (no cost difference).
Figure 1.
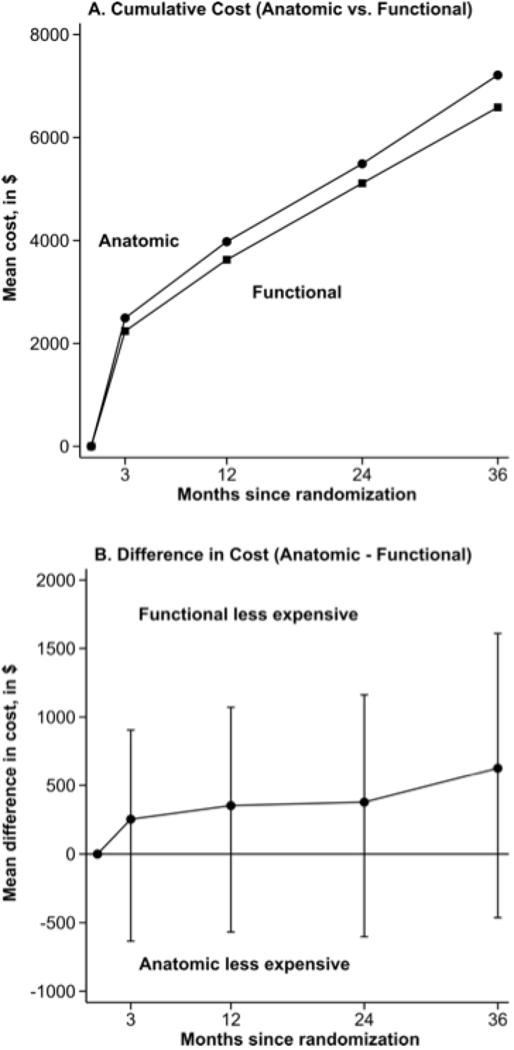
Cumulative Total Costs by Randomized Assignment. Cumulative total costs by randomized assignment (panel A) and mean cost differences with 95% confidence intervals (panel B).
Year 3 results were skewed by a high cost outlier in the CTA arm involving a >$300,000 hospitalization for orthopedic care unrelated to the PROMISE testing question. With this one observation removed, the mean difference between the arms in year 3 was reduced to $91 and the cumulative difference to $469. Other approaches to addressing outliers reduced the mean difference at three years to $539, while winsorizing all outlier observations at less than the 1st or greater than the 99th percentiles to the 1st and 99th percentiles, respectively, reduced the mean difference at three years to $493.
As shown in eTable 5, CTA testing costs were $332 lower than the functional testing costs. However, downstream costs following testing were about $600 higher for the CTA arm by 90 days.
Sensitivity Analyses
The difference in cost between anatomic and functional testing was robust to various alternative price specifications. Using Medicare Fee Schedule-based cost weights for non-invasive tests, the 90-day cost difference favored CTA slightly (mean difference −$32 [95% CI −314 to 256]), while use of the RCC-derived price weights created a larger difference (mean difference −$401 [95% CI −2131 to 619]).
Cost Components
Analysis of costs according to the type of care (Figure 2A) showed that during the initial 90 days, the CTA arm saved an average of $378 in additional noninvasive testing costs but had an excess cost of $423 due to invasive catheterization and revascularization as well as $166 in other cardiovascular inpatient care. After 90 days, costs were very low in all categories and equivalent between the two strategies. Most of the excess $249 cost for CTA in year 3 (Figure 2D) was due to an average of $256 in extra non-cardiovascular inpatient care.
Figure 2.
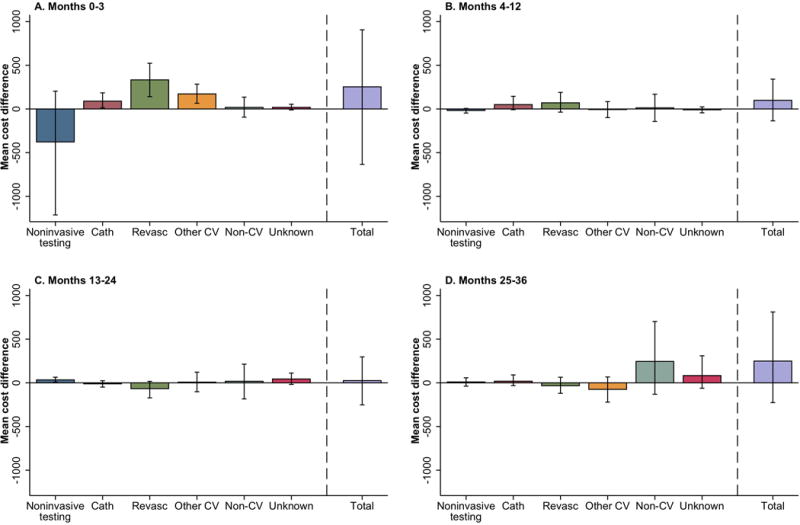
Mean Cost Differences by Cost Categories. Mean cost differences by cost categories A) baseline to 3 months, B) 4 months to 12 months, C) 13 months to 24 months, and D) 25 months to 36 months. Above 0 means more cost for CTA and below 0 means more cost for functional testing. Cath=cardiac catheterization; revasc=revascularization; CV=cardiovascular
In our base case analysis, the CTA patients had their cost distribution shifted upwards relative to the functional testing patients (Figure 3). Because of the outlier effects in year 3, year 2 data were felt to best represent the relevant probabilities. Specifically, our analysis indicated that the CTA arm costs would exceed functional testing strategy arm by no more than $500 in 58.6% of 1000 bootstrap samples, by no more than $750 in 79.7% of samples, and by no more than $1000 in 93.4% of samples. When we substituted the Premier RCC cost testing weights in sensitivity analysis, the CTA arm costs exceeded the functional testing arm costs by no more than $500 in 89.9% of bootstrap samples and 63.4% of samples had a cost difference ≤$0 (i.e., CTA costs lower than functional testing arm) (eFigure 1a). With Medicare hospital-based testing cost weights, a cost difference ≤$500 was seen in 94.7% of samples while 38% of samples had a cost difference ≤$0 (eFigure 1b).
Figure 3.
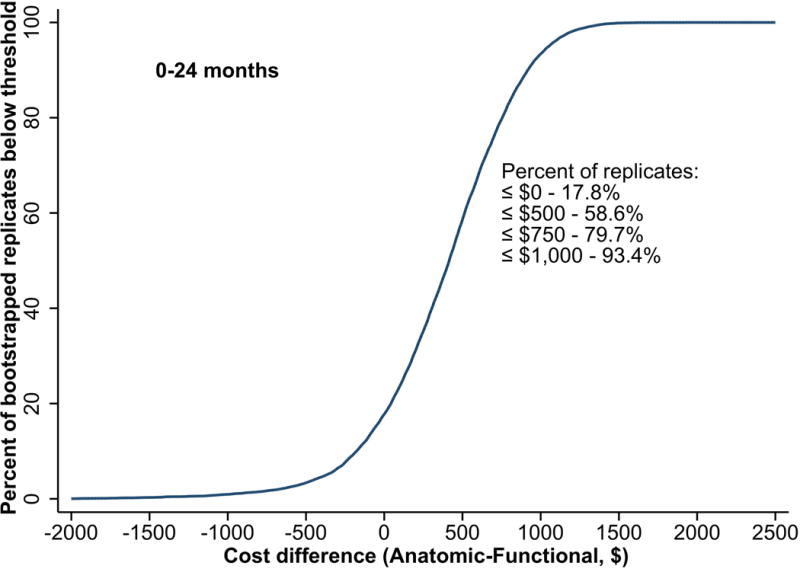
Two-year Cost Threshold Differences from Bootstrap Analysis. The curve shows the cumulative distribution function of the mean cost difference (CTA − functional testing) from 1000 bootstrap replications out to 24 months. A cost difference ≤ $500 was seen in 58.6% of samples, a difference ≤$750 in 79.7% of samples, and ≤$1000 in 93.4% of samples.
Prespecified Subgroup Analysis
Five pre-specified subgroups did not differ importantly from the overall result (Figure 4). For patients with high pretest probability of CAD (n=431) or who had a high Diamond and Forrester pre-test probability of CAD (N=482), the CTA arm had a mean cost difference in excess of $2000 at 90 days (Figure 4). The primary cost drivers for these differences were extra revascularization procedures, but costs were also higher for the category of other cardiovascular care and for non-cardiovascular care (eTable 6).
Figure 4.
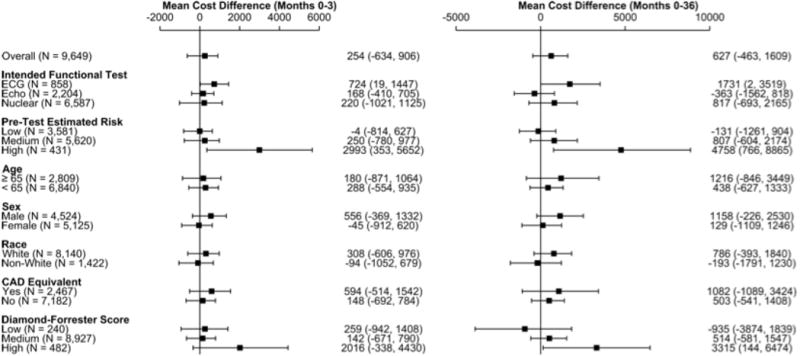
Three-year Costs in Subgroups. Forest plot for mean differences and 95% confidence interval of CTA minus functional testing 3-year costs in prespecified subgroups.
ECG=electrocardiogram, Echo=echocardiogram, CAD=coronary artery disease
DISCUSSION
In this economic analysis of PROMISE, we have shown that an initial CTA strategy had similar costs to a functional stress testing strategy, although the patterns of care were somewhat different. Specifically, the CTA arm had less follow-up noninvasive testing and more invasive catheterization and revascularization. After 90 days, our data suggest that very little happened to these patients out to 3 years that was driven specifically by which testing strategy they received.
Interpretation of empirical cost comparisons in which both the clinical and cost outcomes are similar poses important challenges. One possible interpretation of our results is that the CTA arm was modestly more costly than the functional testing arm and our study had insufficient precision to exclude the null case (no cost difference). No criteria have been established for defining how small a numerical difference in costs must be to qualify as “similar” (a notion akin to “noninferior” in the comparison of clinical outcomes). Instead of using fixed decision rules to interpret cost data, as is typical for clinical outcome comparisons, health economics has developed methods that recognize that policy makers vary both in their willingness to tolerate uncertainty about the “true” cost difference and in the threshold cost difference they consider important. Four lines of evidence support the case that the costs for CTA and functional testing strategies are “similar.” First, the mean cost difference at 90 days was $254 higher for CTA, with an additional $99 extra costs from 91 days to 1 year. Because the cumulative 1-year total difference ($353) represents a shift equivalent to about 4% of a standard deviation of the 0–12 month partitioned cost estimator ($9436), it provides a useful, if imperfect, reference point for assessing the magnitude of the differences we observed. Second, the mean cost difference in year 2 was $26 and in year 3 was $249 which fell to $91 after removal of one very high cost (non-cardiovascular) outlier. These data provide strong evidence that the randomized diagnostic testing strategy had no discernable net effect on costs after the first year. Third, our bootstrap analysis of the 2-year cost difference (Figure 3) showed that 59% of bootstrap repetitions had a cost difference of $500 or less ($500 is 4% of the 2-year partitioned estimator SD of $12,242) and 80% of repetitions had a difference of $750 or less (6% of the 2-year cost SD). Finally, in sensitivity analyses where we used either Medicare Fee Schedule cost weights or the top down RCC method, costs for the CTA arm came out numerically lower than the functional testing arm.
To date, there have been few large multi-center empirical studies of the long-term costs and resource consumption effects of coronary CTA relative to alternative diagnostic imaging strategies. In a consecutive cohort study (2005–2007) from one academic institution in Ottawa, Canada, Chow and colleagues found that implementation of a cardiac CT program (February 2006) was associated with a decrease in the frequency of normal invasive coronary angiograms (from 31.5% to 26.8%) (10). A retrospective cohort study (2005–2008) from the US Medicare population (age ≥66 years) compared the effects of coronary CTA with functional stress testing options on subsequent catheterization, revascularization, and costs out to 180 days (11). CTA was associated with about a 2-fold increase in referral to invasive catheterization, a 2.5-fold increase in PCI, and a 3-fold increase in CABG after adjustment. CAD-related costs after CTA were almost 40% higher compared with stress perfusion imaging at 180 days ($14,943 vs $10,626). Finally, in a 41 center prospective cohort study (2006–2008) involving 1703 patients with suspected CAD, 2-year unadjusted costs for CAD management were about $1000 higher for CTA than for stress perfusion imaging (12). After adjustment for baseline differences, CTA costs remained 15% higher. The extra costs of CTA were driven by more frequent use of invasive catheterization (13% vs 4% for stress nuclear at 90 days), as well as more PCI (6% vs 1% at 90 days) and more CABG (2% vs 0.4%).
Thus, observational studies reflecting data from 2005 to 2008 suggested CTA would modestly reduce referral to catheterization for patients with normal coronary arteries but would increase use of invasive catheterization and revascularization overall with an associated net increase in medical costs. These patterns were also seen in PROMISE (enrollment 2010–2013) but the magnitude of the effect on incremental medical costs compared with functional testing was much less than these earlier studies predicted.
The Prospective Longitudinal Trial of FFRCT Outcomes and Resource Impacts (PLATFORM) Study recently reported an economic comparison of 204 stable chest patient patients from Europe with intermediate likelihood for CAD managed either with stress testing/usual care or fractional flow reserve (FFR) CTA imaging (13). Using U.S. Medicare reimbursement rates to estimate costs, Hlatky and colleagues found the 90 day costs were not significantly different between the two strategies ($542 mean difference, 95% CI −$1,153 to +$2,237). No difference was seen in the use of invasive catheterization but the FFRCT strategy had about 6 per 100 more PCIs and 1 per 100 fewer CABGs. Although a much smaller sample from a different geographic region (Europe versus the US), using a different CTA strategy (with FFR in 60%) and a different usual care strategy (60% had CTA without FFR), these results are reasonably concordant with our findings.
Of the seven prespecified subgroup comparisons in PROMISE, five showed consistencies in cost results with the overall comparison. Both measures of high pre-test probability of CAD were associated with significantly higher cost at 90 days for CTA (Figure 4) from higher use of invasive catheterization and revascularization in that arm. These high probability subgroups are much too small to allow any reliable assessment of outcome differences produced by these different treatment patterns. However, this finding does show that the modest trend toward higher costs for CTA in our base case cost comparison comes mostly from differences in management created in the high pretest probability subgroup, the patient group in whom a higher rate of catheterization and revascularization would be most clinically justified.
Caveats
The initial diagnostic test costs used in our analysis were derived from data outside the trial, necessitated by the lack of suitable cost weights from the trial patients. There are no standard methods for converting the charges for an office-based test in the US to the relevant resource-based cost. Thus, we selected data from the Premier database to provide these cost weights, since it reflects the input of many hospital-based testing laboratories and is provided in a cost accounting format that shows not only the total facility cost of testing but also the key component costs. Also, we did not include the costs of outpatient medications, routine outpatient medical care, or patient and caregiver time because of budget constraints. Given how close the costs are between the two diagnostic strategies compared in PROMISE, regional variation in the relative costs of functional versus anatomic testing and in the type of functional testing preferred may result in different net cost positions from those reported for the trial overall.
In summary, in a large cohort of outpatients with stable chest pain referred for stress testing, we found similar net costs for the CTA and functional stress testing strategies out to 90 days. After 90 days, the choice of test had little effect on differential costs.
Supplementary Material
Acknowledgments
We are particularly indebted to the coordinators at the PROMISE sites who enrolled the study subjects and collected the study data and to the patients who agreed to participate in this trial.
GRANT SUPPORT
Supported by grants R01HL098237, R01HL098236, R01HL098305, and R01HL098235 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Primary Funding Source: NHLBI
DISCLOSURES
Dr. Mark has received grant funding from Eli Lilly & Company, Medtronic, Gilead, AstraZeneca, AGA Medical, and Bristol Myers Squibb and he has received consulting fees from Medtronic, CardioDx, Gilead, and St Jude Medical. Dr. Anstrom has received research support from AstraZeneca, Eli Lilly & Company, and Medtronic; has served as a consultant for Abbott Vascular, AstraZeneca, Bristol-Meyers Squibb, Gilead, Pfizer, GlaxoSmithKline, Promedior, and Ikaria; and has served on data monitoring committees for NIH, University of North Carolina, University of Miami, Forest, Pfizer, GlaxoSmithKline, and Vertex. Dr. Anstrom has an equity interest in Biscardia. Dr. Douglas has received research support from HeartFlow and has served as a consultant for CardioDx.
Trial Registration: clinicaltrials.gov identifier:NCT01174550
DATA SHARING
Protocol: provided as supplementary material.
Code: not available.
Data: to be made available according to NIH requirements.
References
- 1.Nielsen LH, Ortner N, Norgaard BL, Achenbach S, Leipsic J, Abdulla J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15(9):961–71. doi: 10.1093/ehjci/jeu027. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan N, Polavaram L, Vankayala H, Ference B, Wang Y, Ager J, et al. Diagnostic accuracy of myocardial perfusion imaging and stress echocardiography for the diagnosis of left main and triple vessel coronary artery disease: a comparative meta-analysis. Heart. 2010;96(12):956–66. doi: 10.1136/hrt.2009.182295. [DOI] [PubMed] [Google Scholar]
- 3.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49(2):227–37. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction. Health Technol Assess. 2004;8(30):iii–iv. 1–207. doi: 10.3310/hta8300. [DOI] [PubMed] [Google Scholar]
- 5.Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372(14):1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167(6):796–803 e1. doi: 10.1016/j.ahj.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mark DB, Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part I. Circulation. 2002;106(4):516–20. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]
- 8.Bang H, Tsiatis AA. Estimating medical costs with censored data. Biometrika. 2000;87:329–43. [Google Scholar]
- 9.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Chow BJ, Abraham A, Wells GA, Chen L, Ruddy TD, Yam Y, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging. 2009;2(1):16–23. doi: 10.1161/CIRCIMAGING.108.792572. [DOI] [PubMed] [Google Scholar]
- 11.Shreibati JB, Baker LC, Hlatky MA. Association of coronary CT angiography or stress testing with subsequent utilization and spending among Medicare beneficiaries. JAMA. 2011;306(19):2128–36. doi: 10.1001/jama.2011.1652. [DOI] [PubMed] [Google Scholar]
- 12.Hlatky MA, Shilane D, Hachamovitch R, Dicarli MF, Investigators S Economic outcomes in the Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease registry: the SPARC Study. J Am Coll Cardiol. 2014;63(10):1002–8. doi: 10.1016/j.jacc.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 13.Hlatky MA, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, et al. Quality-of-life and economic outcomes of assessing fractional flow reserve with computed tomography angiography: PLATFORM. J Am Coll Cardiol. 2015;66(21):2315–23. doi: 10.1016/j.jacc.2015.09.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


