Abstract
The appearance of animal colour signals depends jointly upon the ambient light spectrum and the signal's reflectance spectra. Light environment heterogeneity might, therefore, allow individuals to enhance their signal by signalling in an environment that increases signal efficacy. We tested this hypothesis by providing male guppies (Poecilia reticulata), a choice of three light environments in which to display their colour signal to females: green, lilac, and clear. We paired males with both receptive and non-receptive females to test whether female response might affect male behavioural decisions. Males preferred the clear environment in all trials and this environment also resulted in males having the highest average visual contrast. Sexual behaviour was influenced by complex interactions between female receptivity, light environment, and male colour pattern contrast. Males spent significantly more time in the environment in which their colour signal had the highest contrast, but only when paired with receptive females. Significant interactions between light environment and individual male colour components were also seen only in receptive trials. Our results suggest that males use light environment to enhance their colour pattern, but only in the presence of receptive females.
Keywords: light environment, colour signals, visual contrast, receptivity, sexual selection, vision
1. Introduction
Variation in light environments within a habitat causes associated changes in sensed colour signal parameters [1–5]. This results from interactions among the spectral composition of light (relative light intensity at each wavelength or ‘colour’), the total intensity of light, the medium through which light travels to the eye, and the surrounding visual backgrounds. When any of these components change, the spectral composition of the light arriving at the receiver's eyes will change [1]. Thus, the received signal can be different under different light environments, altering the perceived conspicuousness of a colour pattern. Visual spectral sensitivities [2,3] also change in response to the predominant light environment, causing changes in signal perception that can result in long-term changes in colour patterns [4]. These processes can lead to polymorphism, population divergence, or speciation [3,5,6].
In the context of mate choice, individuals with a more conspicuous or high contrast colour pattern are often more attractive [7–9]. For example, cichlid speciation is thought to have been driven by female preferences for conspicuous males in different light environments [10], male bowerbirds use ornaments in their sexual display that increase both the visual contrast with the background and the within-pattern contrast [11], and golden-collared manakins [12] and several species of bowerbirds [11] manipulate their display courts to increase the conspicuousness of their display. Increased contrast may allow faster decisions regarding mate choice (minimizing predation risk), and may be correlated with mate ‘quality’ measures such as lower parasite load [13,14] and increased immune function [15,16].
Habitat light transmission properties are related to colour signal conspicuousness at the species level [4,17]. However, because many species live in heterogeneous light environments we can expect individuals to seek out, and spend more time in, the environments that increase the efficacy (visual contrast) of their signal. Although animals alter individual courtship behaviour in response to light environments [9], we do not know whether individuals are able to actively choose environments that increase the efficacy of their colour signal. If individuals are able to use environmental variability then this may have important implications for the evolution of colour signals; signalling can be restricted to conditions which maximize fitness, reducing the trade-off between predation, courtship, and foraging. The degree and type of variation in light environments could also have effects on signal evolution that have never been considered before.
Guppies, Poecilia reticulata, are ideal for testing our hypothesis that individuals exploit the variability of the light environment to increase the efficacy of their sexual colour signals. They are small freshwater fish that live in streams with variable natural light environments caused by variation in vegetation cover and weather [18]. Guppy males alter both courtship mode (full displays versus ‘sneak’ copulations) and distance from females as light intensity changes [9,19]. Courtship mode also varies with light spectrum and relates to predation risk [20]. Furthermore, light environments directly affect the perceived conspicuousness of male guppy multi-component colour sexual signals [7,21,22]. Male guppies are able to use cues from conspecific females to adapt courtship behaviour, providing a mechanism by which they can receive feedback regarding the attractiveness of their signal [23].
To test the possibility of light environment choice to maximize conspicuousness, we gave guppies a choice of three different light environments in which to court females. These environments were chosen to produce among-environment variation in visual perception of male colouration and not necessarily to mimic natural environments. We paired males with highly receptive and less receptive females to determine whether female receptivity interacts with male environmental choice during sexual behaviour. This yields two groups of predictions: basic predictions about reproductive behaviour which, if verified, justify the experimental design, and predictions about light environment-related behaviour from the main hypothesis:
Reproductive behaviour
(1) more receptive females will show a higher number of posi-tive reactions to male courtship attempts.
(2) males will spend more time courting more receptive thanless receptive females.
Light environment-related behaviour
(1) females should respond more to males in the environmentthat results in higher male visual contrast.
(2) males will court receptive females more in the light environment that produces the highest male visual con-trast (where receptive females will provide more positive responses).
If these predictions are true, males can maximize reproductive output and minimize both energy expenditure and predation risk by choosing where to court and spending less time courting unreceptive females.
2. Material and methods
(a). Husbandry
The experiment was conducted under Deakin University's Animal Ethics Committee approval numbers A21-2010 and G01-2012. We used second to third generation wild caught guppies from Alligator Creek, a remote century-old feral population in Queensland, Australia (19°26′79″ S, 146°58′65″ E). We maintained the fish at 24°C and 12 light (L) : 12 dark (D) cycle on a combination of flake food fed once a day and brine shrimp twice a week. Prior to the experiment, we housed individuals in 196 l tanks with clear, unfiltered light environments, lit with high frequency fluorescent lamps. These tanks contained approximately 150 fish of both sexes (sex ratio approximately 1 : 1).
(b). Experimental treatments
We created three different light environments within a single 90 × 45 × 35 cm test tank by covering each third of the tank with a different coloured Rosco® filter sheet. The filters covered the top and the front of the tank, and the back and sides of the tank were covered with black cardboard. Incandescent lights over the test chamber provided light.
We measured the total absolute spectral irradiance of the three light treatments 15 cm from the bottom of the tank (average guppy swimming depth), using a cosine-corrected receptor and a USB2000+ (Ocean Optics) spectrometer, calibrated to μmol photons m−2 s−1 nm−1 with a Li-Cor LI-1800 standard lamp. Electronic supplementary material, figure S1a shows the irradiance spectra resulting from the three filters and lamps, and electronic supplementary material, figure S1b shows the corresponding photoreceptor stimulations. Owing to differences in total transmission of the filters (the clear transmits more light), we illuminated the filters using 40 W incandescent spotlights connected to rheostats to equalize the total visible (300–700 nm) light intensity across the tank. We took intensity measurements with a calibrated Li-Cor LI-189 radiometer which measures PAR (total photosynthetically active radiation) over 400–700 nm, but the spectral measures indicate negligible UV making it possible to equalize irradiance with the rheostats easily. After rheostat adjustments, the light intensity (PAR) ranged between 6.0 and 6.6 µmol photons m−1 s−1 across all treatments; these values are slightly lower than average values of forest shade in the wild [18] and so make vigorous courtship more likely [9].
(c). Behavioural trials
We recorded behaviour by an observer (G.L.C.) 1.5 m away from the test tank and partially blocked by black cloth to minimize disturbance; movements made by the observer had no detectable effect on the fish. We recorded the amount of time that a fish spent in each light environment for each trial with ‘Sit and wait’ event recorder software [24].
We ran trials for males and females individually (SF, single-fish trials, N = 18 for each sex), to test for light environment preferences, and for pairs of males and females (PT, paired trials, N = 78), to test whether males adjusted this preference in the presence of females. In PT, we selected males and females from different tanks to avoid familiarity effects and fish were size matched among trials to within 3 mm within sexes.
Prior to PT, we moved males from the stock tanks to single sex 54 l tanks overnight. To start the trials we placed females into the observation tank 5 min before the males (females generally take longer to acclimate), and allowed 10 min for joint acclimation before recording behaviour. Behaviour observations ran for 15 min after acclimation. We excluded the trial if the fish did not traverse all light treatments or if they mated prior to the start of the trial. This only occurred in 15 of all 174 trials.
Guppy mating involves sigmoid displays in which the male curves his body into a distinct ‘S’ shape and jumps back and forth in front of the female [25]. We measured the sigmoid display time from when the male first created the ‘S’ shape to when he swam normally. In response to male courtship, receptive females glide towards the male to initiate copulation [25,26]. During PT, we used glide responses as an estimate of female responsiveness to a specific male. We also recorded the number of sigmoid display courtship attempts and the total time spent performing them to estimate the frequency and time during which a male could gain feedback from the female.
To test if female reproductive state influenced the use of light environment during PT, we used size-matched (within 3 mm) females which were either postpartum (within 3 days, PP, females) [26] (N = 38) or recently mated virgins (referred to as recent virgins, RV) (N = 40). We removed PP females from stock tanks and individually housed them in 3 l tanks until they had given birth. For RV females, we removed fry from stock tanks and allowed them to mature in 9 l tanks containing approximately 15 individuals. We removed any males prior to sexual maturation and housed the remaining RV females in a single sex 54 l tank containing approximately 50 fish. RV females were approximately nine months old. They could see an adjacent tank containing approximately 150 fish of both sexes. Prior to the trials, RV females spent 24 h in a 54 l tank with eight randomly chosen stock males (the same for all females) and allowed to mate. By contrast, PP females were isolated in the period leading up to the trials. There was no difference in the way RV and PP females behaved when placed in the tanks prior to adding the males so we are confident that the differences in behaviour during the trials was due to receptivity. We used RV females instead of virgin females because virgins are often indiscriminate in their mate choice [25]. RV females are also much more receptive to males than PP females and show much stronger colour pattern based male choice [25,27]. PP females were used instead of gravid females so that receptivity could be standardized within receptive types; our experimental design calls for a consistent difference in receptivity between female types which RV and PP females provide.
We conducted up to four 15 min trials per day between 08.30 and 10.30 h over eight weeks. We alternated PP trials and RV trials, both starting on alternative days. We used 40 pairs for each of the RV and PP females. Two PP trials were discarded due to unexplained mortality. We alternated the position of the light filters on the tank between trials in a Latin-Square block design to control for any effects of colour-location bias.
(d). Effect of development environment
Because all fish (SF, PP, and RV) had developed in an environment similar to the clear treatment, we tested whether development environment biased fish behaviour. We conducted SF trials exactly as before, but with fish that had spent their development (approx. six months) under one of the F89, F55, or unfiltered light environments. We used 10 size-matched fish of each sex from each environment. The receptivity level of these females was not known in the SF trials.
(e). Photography
To test whether male colour pattern had any association with their light environment use, we photographed males in the RV and PP trials using standard methods [7] (see electronic supplementary material). Following digital photography, we used Adobe Photoshop® CS5.1 to extract total area, tail area, and the areas of eight colour classes: orange, black, fuzzy black, silver, green, violet, yellow reticulation, and black reticulation. Black is identified by its solid continuous pigmented area, fuzzy black is represented by patchy black dots that look greyer at a distance (black and fuzzy black are controlled by different chromatophores). All eight colour classes [7,21,28] were measured individually (rather than combining them into black, orange, and ‘iridescent’ as in some published studies) because they are easily distinguishable and combining them into groups may eliminate important phenomena. Analysis was performed ‘blind’ to fish behaviour or origin and only the right side was analysed because 93% of all males were left–right symmetrical. We did not photograph males in the SF trials because they were naive to the light treatments and males are not able to determine their colour signal properties when isolated [23].
(f). Reflectance measurements
We took reflectance measurements of the eight colour classes from 20 non-experimental males housed in 196 l glass tanks without filters following methods used in [7] (see the electronic supplementary material). The number of colour classes measured per male was dependent on the size of the colour patch and whether the patch overlapped with other colours; not all colour classes could be measured per male. We used average spectra in further analyses.
(g). Contrast measurements
We calculated chromatic contrast to test whether the overall colour pattern contrast, rather than individual colours, predicted the time a male spent in a specific light environment. We used the mean reflectance spectra obtained for each colour class from the 20 males in combination with the colour class relative areas on each experimental male, following methods used in [29] (see the electronic supplementary material). The result is a contrast value for each male in each of the three environments.
(h). Statistical analysis
We performed permutation tests and compositional analysis when our data violated assumptions of ordinary statistical methods, and statistical methods such as generalized linear mixed models (GLMM) when data met the assumptions.
(i). Time spent in each light environment and receptivity of females
To identify any differences in the time spent in each light environment, for both the SF trials and the paired trials (PP and RV), we performed permutation tests for the three pairwise environmental comparisons: F89–clear, F55–clear, and F55–F89. We used permutation tests because the data violated all assumptions of GLMM and other statistical methods. First, we calculated the difference in means between two treatment groups of interest; this created the observed test statistic. We then pooled the data and randomly shuffled the data into two groups, recording the difference in the mean of these two groups. We repeated this 10 000 times and used the number of times the difference in the mean of the permuted data was equal or greater than that of the observed data to generate significance values. We used this to identify differences in the courtship behaviour of males when paired with PP and RV females (SF males could not court) and the glide response of RV and PP females in each light environment. We corrected for multiple comparisons before interpreting our results [30]. We carried out this analysis using R statistical software [31]. To estimate effect sizes for the permutation tests, we calculated requivalent which is an effect size estimate, taking values from 0 to 1, using the p-value, sample size, and associated t-value of the analysis [32].
We used a zero-inflated GLMM with Poisson distribution to identify factors determining the number of female glides. We treated female receptive state, male contrast, and number of courtship displays as fixed effects and trial number as a random factor. We used backward-stepwise reduction using the Akaike Information Criterion (AIC) to determine the best model. Model residuals showed approximate normality.
(j). The effects of development environment
To identify the effects of development on the time spent in each environment, we performed a multivariate linear regression. Because the response variable, fraction of time spent under each of the three light environments, comprises three non-independent fractions, the data violates assumptions of standard statistical tests. When variables are constrained in this way the data are called compositions and need to be transformed prior to use following the methods of compositional analysis [33–35]. Consequently, we applied the log-ratio transformation to the data: each composition is divided by its geometric mean before the logarithm is taken. This log-ratio transformation removes problems arising from the sum of all percentage components adding up to 100, and translates the data into multivariate sampling space where normal multivariate methods, such as multivariate linear regression, can be applied [33,35]. We used the irl function in the R [31] package compositions [36] to perform the transformation. The resulting data comprise the three log-ratio transformed time variables. After transformation, we checked data normality using a Monte Carlo analysis where each variable was permuted against a randomly generated normal distribution; there was no evidence for non-normality (p > 0.12).
Having satisfied normality, we used the data in a multivariate linear model [34] to test the effects of development environment. The data containing the three log-ratio transformed environment residence times formed the multivariate response variable and the development environment was the predictor variable. We performed the analysis using function lm in the R stats package [31].
(k). The effect of male colouration
We calculated the effect of male colouration for both the time spent in each environment and the number of courtship attempts in each light environment. We examined the effect of male colouration on the time spent in each environment using compositional multivariate analysis methods, as described above. The log-ratio transformed residence time formed the response variable and the eight colour classes (orange, black, fuzzy black, green, violet, silver, yellow reticulation, and black reticulation) formed the fixed predictor variables in the multivariate linear model. Owing to the large number of predictor variables, no colour class interactions were included. We used backward-stepwise reduction using AIC to assess the minimum adequate model; non-significant predictor terms were removed in turn until the minimum model was reached. The model residuals showed approximate normality.
We calculated the total number of males that spent the most time in the environment in which their pattern had the highest chromatic contrast. We then used a Pearson's χ2 test with Yates' continuity correction to test whether more males than expected spent time in their highest contrast environment. If males had no courtship-independent preferences then the expected proportions of best or other environments would be 0.33 : 0.67. Given that males had a preference for the clear environment and this environment yielded the highest average contrast we performed the test using expected proportions of 0.44 : 0.56; the percentage of time isolated males actually spent in their best and other environments. We repeated this using the total number of males that courted most in the environment in which their contrast was the highest. These time and courtship analyses were carried out for both PP and RV trials.
To identify the factors important in courtship behaviour, we performed a Poisson model GLMM with the number of courtships as the response variable, light environment, female type, and contrast value as fixed factors, and subject ID as a random factor. We conducted backward-stepwise reduction using AIC to assess the minimum adequate model; non-significant predictor terms were removed, in turn, until the minimum model for all three response variables was reached. We also used this procedure for the courtship rate (number of courtship attempts/time spent). The model residuals in both GLMMs were approximately normally distributed.
3. Results
(a). Female responsiveness
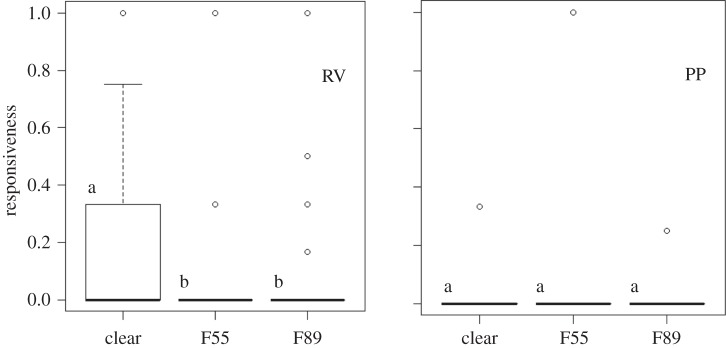
We calculated female responsiveness for each trial by dividing the number of female glide responses by the number of male courtship displays. In the paired trials (PT), RV females were more responsive to males than PP females (RVmean = 0.18, N = 40; PPmean = 0.01, N = 38; requivalent = 0.41, p < 0.0001), (requivalent is an effect size measure, hereafter referred to as re [32]). Females in the RV trials were most responsive in the clear light environment (mean = 0.16, N = 40, over both F55, mean = 0.033, re = 0.33, p = 0.02 and F89, mean = 0.075, re = 0.28, p = 0.038) and least responsive in F55, although no difference was detected between the F55 and F89 light environments (N = 40, re = 0.02, p = 0.463 over the F89) (figure 1; electronic supplementary material figure S2). PP trials showed no difference in female responsiveness in each of the light environments (clearmean = 0.0088, F55mean = 0.0088, F89mean = 0.0066, N = 38, re = 0.38, p ≈ 1). The GLMM of total female glide responses revealed significant effects of male courtship behaviour (Z = 2.52, N = 78, p < 0.0001) and female type (Z = −4.86, N = 78, p = 0.012) but no effects of male contrast (electronic supplementary material, table S1).
Figure 1.
Boxplots showing female responsiveness (the number of female glides per male courtship attempt) in each light environment for both RV (N = 40) and PP (N = 38) trials. Different letters denote significant differences on the basis of pairwise permutation tests.
(b). Time spent in each light environment
We found a significant differential use of light environments in the SF trials (electronic supplementary material, figure S3). The average proportion of time spent by single males in each environment was 44%, 30%, and 26% for clear, F55, and F89, respectively, and for females 44%, 35%, and 21%. Single males preferred the clear environment over the F89 environment (re = 0.43, N = 18, p = 0.036) but showed no preference between clear and F55 environments (re = 0.37, p = 0.066) or between F89 and F55 (re = 0.06, p = 0.6). Single females preferred clear over both F89 (re = 0.41, N = 18, p = 0.046) and F55 (re = 0.69, p < 0.001) environments, but none between F55 and F89 (re = 0.33, p = 0.088).
The significant, non-random use of the light environments was consistent across both the PP (N = 38) and RV (N = 40) paired trials (PT). The average proportions of time spent in each clear, F55, and F89, respectively, was 43%, 32%, and 25% for males and 38%, 23%, and 28% for females in the PP trials, and 42%, 22%, and 22% for males and 40%, 24%, and 22% for females in the RV trials. Permutation tests (electronic supplementary material, table S2) revealed that both males and females had a significant preference for the clear environment. A multivariate linear model revealed that there was no effect of trial type (SF, RV, or PP) on the environment preference (electronic supplementary material, table S3 and figure S3). Paired male and female times were correlated within trials for the PP (r = 0.66, N = 38, p < 0.0001) and RV trials (r = 0.47, N = 40, p < 0.0001); males and females spent a large amount of time together in their preferred environment. There was no difference in the strength of these correlations between RV and PP trials (z = 1.22, p = 0.223).
(c). Developmental environment
A multivariate linear model indicated an interaction between developmental environment and light environment preferences (electronic supplementary material, table S4) where males that had developed in the clear light environment spent least time in the F89 light environment (t = −2.17, N = 10, p = 0.04). However, fish did not spend more time in the same environment in which they had developed; developmental environment does not explain the overall preferences (electronic supplementary material, figure S4). Permutation tests revealed that males and females had a significant preference for the clear environment over at least one other light environment in all trials (electronic supplementary material, table S5).
(d). Overall courtship behaviour
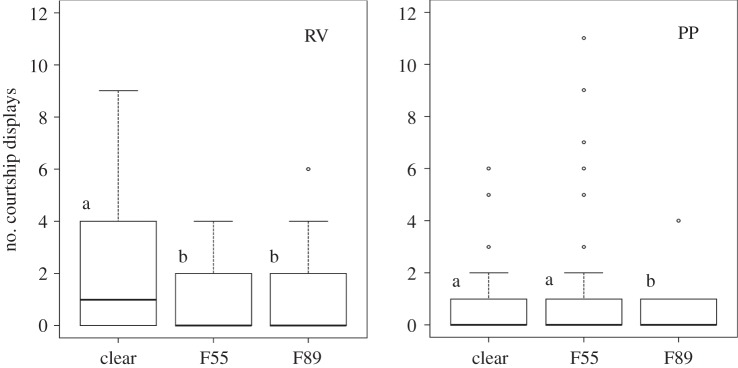
Not surprisingly, males showed significantly more courtship behaviour with females in the RV (receptive female) trials (electronic supplementary material, figure S5). Fifty-eight per cent of males attempted courtship in the RV trials and 42% of males attempted courtship in the PP trials. Permutation tests revealed that both the mean time spent courting (RV, 15.64 s; PP, 8.06 s) and the mean number of courtship attempts (RV, 4.28; PP, 2.42) was higher in the RV trials (N = 78; time courting, re = 0.24, p = 0.039; number of courtships, re = 0.20, p = 0.043). Permutation tests showed that the preferred courting environments differed between PP and RV trials (figure 2). The highest number of courtship displays was performed in the clear treatment in the RV trials (N = 38, re = 0.31, p = 0.028) with no difference between the F89 and F55 environments (N = 38, re = 0.08, p = 0.683). The highest number of courtship displays in the PP trials was performed in the F55 and clear environments jointly (re = 0.26, N = 40, p = 0.049).
Figure 2.
Boxplots showing the number of courtship displays for both RV and PP trials, N = 40 and 38, respectively, across the three different light environments. Permutation tests reveal pairwise differences in across trials for both (denoted by different letters).
(e). Male colouration
In the RV trials, male colouration was significantly associated with the time spent in each environment (electronic supplementary material, table S6): males with a larger area of violet spent more time in the F55 environment (t = 2.12, N = 40, p = 0.04) and less time in the clear environment (t = −3.81, N = 40, p < 0.001). Males with larger silver areas spent more time in the clear environment (t = 3.071, N = 15, p < 0.01). There were no significant interactions between light environment and the remaining male colour classes: green, orange, black, fuzzy black, yellow reticulation, or black reticulation. No significant interactions were detected between male colour class and light environment in the PP trials.
The average chromatic contrast values (figure 3) for the three light environments were 0.077, 0.053, and 0.047 for clear, F55, and F89, respectively, consistent with times spent in the three environments. Differences were significant between clear and F55 (W = 176.5, N = 78, p < 0.001) and between clear and F89 (W = 302.5, N = 78, p < 0.001). No difference was observed between RV and PP trials (re = 0.012, N = 78, p = 0.46).
Figure 3.
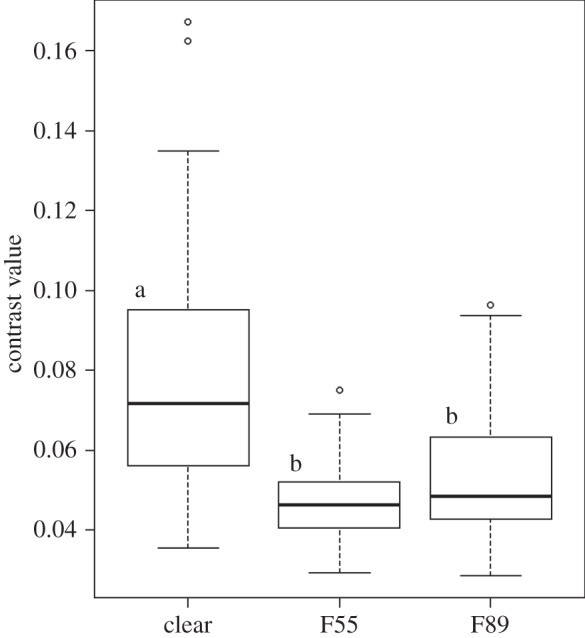
Boxplots showing the distribution of chromatic contrast in the three light environments, N = 78. The greater the contrast value the greater the perceived conspicuousness in a given environment. Letters denote significant differences between light environments. No difference was detected between the distributions of chromatic contrast between RV and PP trials.
In RV trials, more males than expected spent most time in the light environment in which their colour patterns had the highest contrast (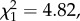 N = 40, p = 0.014, expected = 0.44). Similarly, more males than expected performed the most courtship displays in the environment in which his colour signal had the highest contrast, and again only in the RV trials (
N = 40, p = 0.014, expected = 0.44). Similarly, more males than expected performed the most courtship displays in the environment in which his colour signal had the highest contrast, and again only in the RV trials (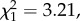 N = 40, p = 0.037, expected = 0.44). Neither pattern was seen in the PP trials (time spent,
N = 40, p = 0.037, expected = 0.44). Neither pattern was seen in the PP trials (time spent, 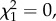 N = 38, p = 0.5, expected = 0.44; courtship displays,
N = 38, p = 0.5, expected = 0.44; courtship displays, 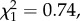 N = 38, p = 0.805). There was no difference in the rate of courtship (number of courtships/time courting,
N = 38, p = 0.805). There was no difference in the rate of courtship (number of courtships/time courting, 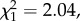 N = 38, p = 0.078), probably due to the confound between the preference for the clear environment and the average highest contrast there.
N = 38, p = 0.078), probably due to the confound between the preference for the clear environment and the average highest contrast there.
We found a negative effect of the F55 environment (Z = −2.184, N = 234, p = 0.029), and a positive effect of contrast (Z = 2.176, N = 234, p = 0.03), on the number of courtship displays as indicated by the GLMM exploring the effects of female type, contrast value, and light environment (electronic supplementary material, table S7) on the number of courtship displays. RV females elicited more courtship displays in the F89 environment compared with PP females as shown by an interaction between the F89 environment and receptive (RV) female type (Z = −2.340, N = 234, p = 0.019). An interaction between the F55 environment and contrast (Z = 2.97, p = 0.003, N = 234), arose because the relationship between male courtship and contrast was much stronger in F55 compared with the other two light environments. Finally, an interaction between the F89 environment, RV female type, and contrast (Z = 2.13, N = 234, p = 0.033) indicates that the relationship between male courtship and contrast depends on both light environment and female type. To explore this three-way interaction (electronic supplementary material, figure S6), we calculated the regression of contrast on courtship displays in each environment for each trial type. The standard deviation of these slopes was higher in the PP (s.d. = 41.05) compared with the RV trials (s.d. = 14.05); unreceptive females respond more erratically than receptive females, resulting in more variation in behaviour, consequently, much higher slope variation, which yields a significant interaction.
When the same GLMM was run with courting rate as the response variable, only visual contrast was found to have a significant effect (Z = 1.99, N = 234, p = 0.046) indicating that light environment had no effect on courtship rate. We removed light environment to see whether the positive relationship between the number of courtship displays and contrast depended on female type. We found a significant, positive interaction between RV trials and contrast indicating higher contrast males courted more in the presence of more receptive females, regardless of the light environment in which they displayed. This is confounded by the fact that visual contrast directly depends upon light environment, so it enters twice in that analysis. There was no effect of individual identity.
4. Discussion
We confirmed that the more receptive (RV) females performed more glide responses than PP females and that males courted RV females more than PP females, validating our experimental design.
Our study showed that, on average, males sought out, and spent more time in, the environment in which their colour pattern had the highest contrast, but only in the RV trials. Males also courted RV females more in the light environment that produces the highest contrast of male colour patterns as predicted. RV, but not PP females, performed more glide responses in the environment that yielded the highest average male contrast. We found effects of individual male colours on the amount of time spent in each environment, but again only with RV females. These results indicate complex interactions between light environment, male sexual behaviour, female receptivity, and male colouration that have strong implications for the evolution of species that inhabit areas with varying light environments.
We found a general clear environment preference across all trials except the single male trials where F55 and clear were preferred equally. It is interesting to note that the pattern of preference for the light environments follows that of the contrast measures. The development experiment shows that these light environment preferences were not due to development environment. Food colour is known to be important in foraging behaviour [37], and it is possible that these colour-based environmental preferences may relate to foraging. Guppies feed on algae and small invertebrates [25] which are more abundant in photosynthetically active light (400–500 nm and 600–700 nm). If guppies use the light environment as a cue for food abundance, as has been shown in studies using both guppies [38] and other animals [39], they should avoid the F89 (green) light environment, as they do here. Furthermore, because these fish spend much time foraging, male colouration may have evolved for maximum conspicuousness in environments that have the greatest food abundance.
Colouration often renders males conspicuous to predators [28]. If light environment preferences evolve under natural selection from predators, we expect individuals to avoid environments in which they were most conspicuous to predators, or spend more time in an intermediate environment as a trade-off between predation and courtship. Guppies may avoid bright light due to increased predation risk [9], however, we equalized the irradiance in each environment, and made the intensity low, so this should not be a factor here. Any trade-off between foraging and predator avoidance may be managed through the avoidance of environments that increase conspicuousness at given times (such as midday when predation is high) although guppies are not necessarily most visible to predators under these light conditions [28]. Preferentially courting receptive (RV) females in environments that yield higher contrast (such as our clear environment) might be important in minimizing predation risk. Males should only court more receptive females (RV) in environments that maximize visual contrast in order to minimize both predation risk and time costs.
As expected, we have shown that RV females were more likely to perform glide responses than PP females. We also found that RV female glide responses were highest in the clear light environment, where the average male contrast was the highest, and lowest in F55, where the average male contrast was lowest. These results contradict a previous study that found females to be more responsive under F55 filters [20], however, that study used the same filters but a spectrally different light source which produced different irradiance spectra than ours. Furthermore, fish in that study were not given an environmental choice. We did not find a relationship between the number of female glide responses and male pattern contrast, which suggests females do not perform these responses based on male colour pattern contrast alone, nor did we find a significant interaction between contrast and the number of courtship displays on the number of female glides. We did, however, find a positive effect of the number of courtship displays on glide response suggesting that the number of courtship displays is important in eliciting positive female responses. This result supports other studies that have found female preferences to be correlated with male courtship effort [40–43]; effort may be a reflection of overall fitness because courtship may increase the probability of predation [9].
We found that male courtship is influenced by female receptivity, light environment, and male colour pattern contrast: (i) contrast was positively related to the number of male courtship attempts, (ii) males increased their courtship behaviour when paired with RV (more receptive) females, and (iii) males courted RV females more in the clear environment. Our results suggest that males with more highly contrasting colour patterns that are more attractive [7], court more frequently. This relationship is also stronger in the presence of more receptive females which implies that this is a reaction to positive feedback from females, as has been suggested by previous studies [44,45]. If males are able to determine female receptivity through subtle cues such as behaviour and pheromones [46], and if receptive females vary these cues as a function of how attractive she perceives a male to be, males may increase their courtship in the environment in which his contrast is the highest (assuming male attractiveness is determined by contrast). This helps to explain why males vary their courtship behaviour in relation to both female receptivity and light environment, and why there is a correlation between courtship behaviour and male colour pattern contrast. If light environment or male contrast alone were responsible for this result, we would expect to see an increase in courtship behaviour in the clear environment in the PP trials. That this did not occur suggests that female receptivity is important in mediating male sexual behaviour and enabling decision-making.
Males courted more in the environment in which their contrast was the highest. The light environment preference did not change between the trial types but this does not mean that, on an individual scale, males did not court more in the environment that produced the highest contrast. The number of courtship attempts and male contrast were more strongly positively correlated in the F55 environment, this may be because on average males were less conspicuous and thus needed to increase courtship effort in order to attract females. The three-way interaction between female type, contrast, and the F89 environment is very likely due to the high variability of the strength as well as the sign of the relationship between contrast and courtship attempts in the PP trials, the low variation in RV trials, and the complete disappearance of this relationship in the F89 environment in the PP trials. This is expected if females are less responsive and less discriminating in the PP compared with the RV trials.
In addition to overall colour pattern contrast, we found that individual male colours were a significant predictor of the time spent in each light environment and the number of their courtship displays (for RV trials only). We found that males with larger violet patches spent more time in the F55 (lilac) environment and males with larger silver patches spent more time in the clear environment. This is an exciting result that supports other studies that have shown that females prefer combinations of male colour patches that include colours that ‘match’, and therefore, reflect most intensively, the light environment [7]; the more light a colour patch can reflect, the more conspicuous the patch should be. For example, a violet spot in light containing little short or medium wavelength light (such as F55 and clear) will appear dark because it does not reflect much of the available light. Conversely, an orange spot will look brighter because it reflects more of the same available light [47]. The results for violet and silver indicate that males can place themselves in the environment that best enhances aspects of their colour signal, but may do so only in the presence of receptive females.
Our results show that light environment influences the relationship between male colour contrast and courtship behaviour in both RV and PP trials and that light environment, male contrast, and female receptivity interact to influence courtship behaviour and environmental choice in a more complex manner than anticipated. That males have the potential to enhance their colour signal is an important finding and one that has novel and general evolutionary implications. The reproductive fitness of a male in the wild is not only related to his sexual signal, but also with his ability to acquire and interpret information from conspecifics. If a male uses the response of females as an indicator of how his signal is perceived, then he can use the interaction with light environment to enhance his signal at any given time. Associating specific colour pattern components with certain light environments may also help maintain polymorphisms and enhance behaviour syndromes. It may well be that the degree to which a male can successfully adapt his signal is dependent on the quality of information provided by conspecifics. Associating with receptive females may not just be a method of readily obtaining matings but also of improving signalling opportunities in the future. Additionally, although not directly shown in this study, this may lead to complicated interactions between cognitive ability, learning opportunities provided by conspecifics, environment, and sexual signals that have not been fully considered previously.
Supplementary Material
Acknowledgement
We thank Priti Singh and Xandy Kranz for their assistance with the animal husbandry. We would also like to thank three anonymous reviewers whose comments and suggestions greatly improved the manuscript.
Ethics
The experiment was approved by the Deakin University Animal Ethics Committee, approval numbers A21-2010 and G01-2012.
Data accessibility
The raw data for this study can be found in the Dryad data repository doi:10.5061/dryad.cd1sf [48].
Authors' contribution
The experiment was designed by G.L.C. The data were collected by G.L.C. The data analysis was carried out by G.L.C and J.A.E and the manuscript was prepared by G.L.C and was critically reviewed by J.A.E.
Competing interests
The authors do not have any competing interests.
Funding
The authors thank ARC grants DP110101421 and DP150102817 for financial support.
References
- 1.Endler JA. 1990. On the measurement and classification of color in studies of animal color patterns. Biol. J. Linn. Soc. 41, 315–352. ( 10.1111/j.1095-8312.1990.tb00839.x) [DOI] [Google Scholar]
- 2.Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J. 2005. Genetic and environmental variation in the visual properties of bluefin killifish, Lucania goodei. J. Evol. Biol. 18, 516–523. ( 10.1111/j.1420-9101.2005.00886.x) [DOI] [PubMed] [Google Scholar]
- 3.Carleton KL, Parry JW, Bowmaker JK, Hunt DM, Seehausen O. 2005. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol. Ecol. 14, 4341–4353. ( 10.1111/j.1365-294X.2005.02735.x) [DOI] [PubMed] [Google Scholar]
- 4.Fuller RC. 2002. Lighting environment predicts the relative abundance of male colour morphs in bluefin killifish (Lucania goodei) populations. Proc. R. Soc. Lond. B 269, 1457–1465. ( 10.1098/rspb.2002.2042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boughman JW. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948. ( 10.1038/35082064) [DOI] [PubMed] [Google Scholar]
- 6.Macedonia JM. 2001. Habitat light, colour variation, and ultraviolet reflectance in the Grand Cayman anole, Anolis conspersus. Biol. J. Linn. Soc. 73, 299–320. ( 10.1111/j.1095-8312.2001.tb01365.x) [DOI] [Google Scholar]
- 7.Cole GL, Endler JA. 2015. Variable environmental effects on a multicomponent sexually selected trait. Am. Nat. 185, 452–468. ( 10.1086/680022) [DOI] [PubMed] [Google Scholar]
- 8.Cole GL. 2013. Lost in translation: adaptation of mating signals in changing environments. Springer Sci. Rev. 1, 25–40. ( 10.1007/s40362-013-0009-4) [DOI] [Google Scholar]
- 9.Endler JA. 1987. Predation, light-intensity and courtship behavior in Poecilia reticulata (Pisces, Poeciliidae). Anim. Behav. 35, 1376–1385. ( 10.1016/S0003-3472(87)80010-6) [DOI] [Google Scholar]
- 10.Maan ME, Hofker KD, van Alphen JJ, Seehausen O. 2006. Sensory drive in cichlid speciation. Am. Nat. 167, 947–954. ( 10.1086/503532) [DOI] [PubMed] [Google Scholar]
- 11.Endler JA, Westcott DA, Madden JR, Robson T. 2005. Animal visual systems and the evolution of color patterns: sensory processing illuminates signal evolution. Evolution 59, 1795–1818. ( 10.1111/j.0014-3820.2005.tb01827.x) [DOI] [PubMed] [Google Scholar]
- 12.Uy JAC, Endler JA. 2004. Modification of the visual background increases the conspicuousness of golden-collared manakin displays. Behav. Ecol. 15, 1003–1010. ( 10.1093/beheco/arh106) [DOI] [Google Scholar]
- 13.Figuerola J, Domènech J, Senar JC. 2003. Plumage colour is related to ectosymbiont load during moult in the serin, Serinus serinus: an experimental study. Anim. Behav. 65, 551–557. ( 10.1006/anbe.2003.2072) [DOI] [Google Scholar]
- 14.Thompson CW, Hillgarth N, Leu M, McClure HE. 1997. High parasite load in house finches (Carpodacus mexicanus) is correlated with reduced expression of a sexually selected trait. Am. Nat. 149, 270–294. ( 10.1086/285990) [DOI] [Google Scholar]
- 15.Grether GF, Kasahara S, Kolluru GR, Cooper EL. 2004. Sex–specific effects of carotenoid intake on the immunological response to allografts in guppies (Poecilia reticulata). Proc. R. Soc. Lond. B 271, 45–49. ( 10.1098/rspb.2003.2526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. 2003. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300, 125–127. ( 10.1126/science.1082142) [DOI] [PubMed] [Google Scholar]
- 17.Douglas JM, Cronin TW, Chiou TH, Dominy NJ. 2007. Light habitats and the role of polarized iridescence in the sensory ecology of neotropical nymphalid butterflies (Lepidoptera: Nymphalidae). J. Exp. Biol. 210, 788–799. ( 10.1242/jeb.02713) [DOI] [PubMed] [Google Scholar]
- 18.Endler JA. 1993. The color of light in forests and its implications. Ecol. Monogr. 63, 1–27. ( 10.2307/2937121) [DOI] [Google Scholar]
- 19.Long KD, Rosenqvist G. 1998. Changes in male guppy courting distance in response to a fluctuating light environment. Behav. Ecol. Sociobiol. 44, 77–83. ( 10.1007/s002650050518) [DOI] [Google Scholar]
- 20.Gamble S, Lindholm AK, Endler JA, Brooks R. 2003. Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol. Lett. 6, 463–472. ( 10.1046/j.1461-0248.2003.00449.x) [DOI] [Google Scholar]
- 21.Endler JA. 1983. Natural and sexual selection on color patterns in Poeciliid fishes. Environ. Biol. Fishes 9, 173–190. ( 10.1007/BF00690861) [DOI] [Google Scholar]
- 22.Blows MW, Brooks R, Kraft PG. 2003. Exploring complex fitness surfaces: multiple ornamentation and polymorphism in male guppies. Evolution 57, 1622–1630. ( 10.1111/j.0014-3820.2003.tb00369.x) [DOI] [PubMed] [Google Scholar]
- 23.Gross MR, Suk HY, Robertson CT. 2007. Courtship and genetic quality: asymmetric males show their best side. Proc. R. Soc. B 274, 2115–2122. ( 10.1098/rspb.2007.0432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabo P, Janos K. 2013. Sit and wait. See https://sourceforge.net/projects/sitwait/.
- 25.Houde A. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 26.Liley NR. 1966. Ethological isolating mechanisms in four sympatric species of Poeciliid fishes. Behaviour, suppl. 14: III, p. 197.
- 27.Kodric-Brown A, Nicoletto PF. 2001. Age and experience affect female choice in the guppy Poecilia reticulata. Am. Nat. 157, 316–323. ( 10.1086/319191) [DOI] [PubMed] [Google Scholar]
- 28.Endler JA. 1991. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vis. Res. 31, 587–608. ( 10.1016/0042-6989(91)90109-I) [DOI] [PubMed] [Google Scholar]
- 29.Endler JA, Mielke PW. 2005. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 86, 405–431. ( 10.1111/j.1095-8312.2005.00540.x) [DOI] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- 31.R Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 32.Rosenthal R, Rubin DB. 2003. r(equivalent): A simple effect size indicator. Psychol. Methods 8, 492–496. ( 10.1037/1082-989x.8.4.492) [DOI] [PubMed] [Google Scholar]
- 33.Aitchison J. 2003. The statistical analysis of compositional data, 2nd edn Caldwell, NJ: The Blackburn Press. [Google Scholar]
- 34.Aitchison J, Egozcue JJ. 2005. Compositional data analysis: where are we and where should we be heading? Math. Geol. 37, 829–850. ( 10.1007/s11004-005-7383-7) [DOI] [Google Scholar]
- 35.Egozcue JJ, Pawlowsky-Glahn V, Mateu-Figueras G, Barceló-Vidal C. 2003. Isometric logratio transformations for compositional data analysis. Math. Geol. 35, 279–300. ( 10.1023/A:1023818214614) [DOI] [Google Scholar]
- 36.van den Boogaart GK, Tolosana R, Bren M.2009. Compositions: compositional data analysis. http://www.r-project.org , R package version 1.01-1.
- 37.Cole GL, Endler JA. 2015. Artificial selection for food colour preferences. Proc. R. Soc. B 282, 20143108 ( 10.1098/rspb.2014.3108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White EM, Church SC, Willoughby LJ, Hudson SJ, Partridge JC. 2005. Spectral irradiance and foraging efficiency in the guppy, Poecilia reticulata. Anim. Behav. 69, 519–527. ( 10.1016/j.anbehav.2004.05.011) [DOI] [Google Scholar]
- 39.Maddocks SA, Church SC, Cuthill IC. 2001. The effects of the light environment on prey choice by zebra finches. J. Exp. Biol. 204, 2509–2515. [DOI] [PubMed] [Google Scholar]
- 40.Kodric-Brown A. 1993. Female choice of multiple male criteria in guppies: interacting effects of dominance, coloration and courtship. Behav. Ecol. Sociobiol. 32, 415–420. ( 10.1007/BF00168825) [DOI] [Google Scholar]
- 41.Shamble PS, Wilgers DJ, Swoboda KA, Hebets EA. 2009. Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20, 1242–1251. ( 10.1093/beheco/arp116) [DOI] [Google Scholar]
- 42.Stoltz JA, Elias DO, Andrade MCB. 2009. Male courtship effort determines female response to competing rivals in redback spiders. Anim. Behav. 77, 79–85. ( 10.1016/j.anbehav.2008.09.012) [DOI] [Google Scholar]
- 43.Patricelli GL, Uy JAC, Walsh G, Borgia G. 2002. Sexual selection: male displays adjusted to female's response. Nature 415, 279–280. ( 10.1038/415279a) [DOI] [PubMed] [Google Scholar]
- 44.Farr JA. 1980. The effects of sexual experience and female receptivity on courtship-rape decisions in male guppies, Poecilia reticulata (Pisces: Poeciliidae). Anim. Behav. 28, 1195–1201. ( 10.1016/S0003-3472(80)80108-4) [DOI] [Google Scholar]
- 45.Guevara-Fiore P, Stapley J, Watt P. 2010. Mating effort and female receptivity: how do male guppies decide when to invest in sex? Behav. Ecol. Sociobiol. 64, 1665–1672. ( 10.1007/s00265-010-0980-6) [DOI] [Google Scholar]
- 46.Crow RT, Liley NR. 1979. A sexual pheromone in the guppy, Poecilia reticulata (Peters). Can. J. Zool. 57, 184–188. ( 10.1139/z79-016) [DOI] [Google Scholar]
- 47.Long KD, Houde AE. 1989. Orange spots as a visual cue for female mate choice in the guppy (Poecilia reticulata). Ethology 82, 316–324. ( 10.1111/j.1439-0310.1989.tb00511.x) [DOI] [Google Scholar]
- 48.Cole G, Endler J. doi: 10.5061/dryad.cd1sf. 2016 Data from: Courtship decisions are influenced by light environment and female receptivity. Dryad Digital Repository. . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data for this study can be found in the Dryad data repository doi:10.5061/dryad.cd1sf [48].