Abstract
Over a decade ago, the discovery of transgenerational immunity in invertebrates shifted existing paradigms on the lack of sophistication of their immune system. Nonetheless, the prevalence of this trait and the ecological factors driving its evolution in invertebrates remain poorly understood. Here, we develop a theoretical host–parasite model and predict that long lifespan and low dispersal should promote the evolution of transgenerational immunity. We also predict that in species that produce both philopatric and dispersing individuals, it may pay to have a plastic allocation strategy with a higher transgenerational immunity investment in philopatric offspring because they are more likely to encounter locally adapted pathogens. We review all experimental studies published to date, comprising 21 invertebrate species in nine different orders, and we show that, as expected, longevity and dispersal correlate with the transfer of immunity to offspring. The validity of our prediction regarding the plasticity of investment in transgenerational immunity remains to be tested in invertebrates, but also in vertebrate species. We discuss the implications of our work for the study of the evolution of immunity, and we suggest further avenues of research to expand our knowledge of the impact of transgenerational immune protection in host–parasite interactions.
Keywords: transgenerational immune effect, local adaptation, dispersal, longevity, Drosophila, eusocial insects
1. Introduction
The immunity of invertebrates was, for a long time, widely assumed to lack the most sophisticated component of the vertebrate immune system: its ability to mount an acquired response where memory effectors produced during an infection protect the individual (within-generational protection) or its offspring (transgenerational protection) against subsequent infections. Yet, recent research has shown that invertebrates have spectacularly plastic immune effectors that can generate true novelty and functional immune responses following exposure to pathogens [1,2]. Experimental evidence of the existence of within-generational immune priming in invertebrates has grown considerably in the last decade [3,4]. It has been documented in a range of invertebrate species, including Decapoda [5], Branchiopoda [6], Lepidoptera [7], Coleoptera [8], Diptera [9], and Hymenoptera [10]. Interestingly, in some cases, immune priming has been shown to persist not only throughout the lifespan of the animal [11,12], but also across generations [13–15]. Transgenerational immunity has been thus far reported in a dozen invertebrate species [13,14,16–24]. Although the mechanisms underlying this transgenerational immune protection remain unclear, this work suggests that this form of parental care may be induced by the transfer of pathogen-derived antimicrobial peptides or mRNA-encoding immune effectors [20,25,26].
Transgenerational immune protection potentially confers a large fitness advantage to offspring [13]. This form of parental protection, however, does not seem to be widespread amongst invertebrates. Indeed, several studies have failed to detect any transgenerational transfer of immunity [27–30], and others have even found a negative impact of maternal infection on offspring resistance to pathogen infections [31]. This raises the question of what are the conditions that favour the evolution of transgenerational immunity in invertebrates.
In this study, we investigate whether the presence or absence of transgenerational immune protection in invertebrates is explained by factors related to the biology and ecology of the species. For this purpose, we first modify the theoretical approach developed by Garnier et al. [32] for a single host population, by considering two invertebrate host populations connected by migration. Each host population is exposed to a different pathogen and migrating hosts have varying degrees of cross-immunity to the resident parasite. We study the impact of host dispersal, host lifespan, immunity costs, force of infection, and parasite virulence on the evolution of transgenerational immunity. We then confront the predictions issued from these models to currently available data. For this purpose, we review all experiments published to date on transgenerational immunity or transgenerational protection in invertebrates, focusing in particular on two traits for which information is readily available at the species level: average dispersal and lifespan. To the best of our knowledge, our study is the first attempt to confront theoretical predictions with empirical patterns of transgenerational immunity in invertebrate species.
2. Material and methods
(a). Theoretical analysis
The evolution of maternal transfer of immunity has been studied elsewhere in a single host population [32,33]. Here, we expand these previous models, and we study the evolution of maternal transfer of immunity in invertebrates in a habitat with two populations connected by migration. Each population is assumed to be exposed to a different pathogen, and the pathogen is not allowed to migrate between populations, which maximizes the heterogeneity of the environment. In population i (where i = 1 or 2) susceptible individuals, Si, are exposed to a constant rate of infection hi which yields infected individuals, Ii. All individuals die naturally, with rate μ, and infected individuals suffer additional parasite-related mortality (i.e. virulence), with rate α. All individuals can produce offspring that can move to a different patch, with probability of dispersal η. We assume that infected individuals can transmit transient immunity to their offspring against the parasite they are infected with. We assume that the investment in immunity transfer may be modulated by the dispersal phenotype of the offspring. The probability of immunity transfer is θP and θD for philopatric and dispersed individuals, respectively. We also consider a scenario where immunity transfer, θ, is not allowed to vary between philopatric and dispersed offspring. The ability to transfer immunity is further assumed to be associated with a fecundity cost cθ. We keep track of the origin of the maternally protected individuals using the notation Mij for the density of maternally protected individuals produced in population i and currently in population j (where i and j = 1 or 2). Hence, Mij is immune to parasites from population i but only partially immune to pathogens from population j. The amount of cross-immunity is governed by the parameter χ, and the force of infection on Mij is (1 − χ)hj, with 0 ≤ χ ≤ 1. Maternal protection is assumed to be transitory and it wanes at rate δM in all populations. We use this model to study the effect of various ecological scenarios on the evolutionary stable investment in transgenerational immunity (see the electronic supplementary material for mathematical details).
(b). Empirical data: transgenerational effect scores
To test our theoretical predictions, we carried out an extensive literature review that included all the papers on transgenerational immune priming or transgenerational offspring protection in invertebrates published to date (summarized in electronic supplementary material, table S1). This consisted of 35 published articles comprising a total of 21 invertebrate species. We identified two different protocols for measuring transgenerational immune priming. Some studies investigate the impact of either parental infection or immune stimulation on offspring immunity (we henceforth call this TEI, for transgenerational effect on immunity). These studies quantify and compare immune priming by measuring different immune parameters (melanization, phenoloxidase (PO) production, antibacterial peptide production, haemocyte number, and immune transcripts) in offspring issued from immune-stimulated and naive parents. For simplicity, we scored these studies as either 1 (offspring of infected parents have an increased production of at least one of the immune effectors) or 0 (offspring of infected parents have similar or lower production of a given immune effector). When different studies have been carried out on the same species, the overall TEI score for the species was obtained by averaging across studies. Second, we identified another set of studies where both parents and offspring are exposed to live pathogens. These studies record immune priming by quantifying the outcome of an infection (parasite prevalence, parasite intensity, or survival) in offspring issued from infected and uninfected parents (TER for transgenerational effect on resistance). As above, these studies were scored as either 1 (offspring from infected parents have lower parasite prevalence, lower intensity, or higher survival than offspring from naive parents) or 0 (when the opposite, or when no effect of parental infection is observed), and the average score for the species was obtained by averaging across studies. Finally, for each species, we obtained an overall measure of investment in offspring protection (OTP for overall transgenerational protection) which was scored as 1 when either TEI or TER (or both) were 1, and 0 otherwise.
For each species, we focused on two ecological parameters for which there is available information in the literature: lifespan and dispersal. We define dispersal, as the average distance travelled by adults, in most cases estimated using mark and recapture methods in the field, and lifespan as the average longevity of a species estimated under standard laboratory conditions. Although both parameters are known to vary widely according to environmental and experimental conditions (e.g. nutrition, temperature), these studies provide ballpark estimates of the dispersal (0–6 600 m) and longevity (24–700 days) ranges across species. In three species, no data regarding dispersal were available in the literature and therefore, this analysis was performed on a subset of 18 species.
(c). Statistical analysis
All statistical analyses were performed, using the software R (v. 3.1.0, http://www.cran.r-project.org/). In order to compare TEI, TER, and OTP, we first carried out a Fisher's exact test, using longevity and dispersal as categorical variables. Species were classified as having a short (less than 60 days) or long (greater than or equal to 60 days) lifespan, and those with a short (less than 500 m) and long (greater than or equal to 500 m) dispersal range. We controlled the robustness of our analyses by using several different cut-off points for defining short and long lifespan and dispersal range (nine points for longevity and eight for dispersal; electronic supplementary material, figure S1). Fisher's exact test, however, obviates the fact that species are phylogenetically related and are therefore not statistically independent units. In order to account for this phylogenetic signal, we performed a second analysis, using a linear regression for binary phylogenetic data (binary Phylogenetic Generalized Linear Mixed Models (PGLMM), packages ‘ape’, [34]). Phylogenetic information (electronic supplementary material, figure S2) for the 21 species was obtained from the Interactive Tree of Life (http://itol.embl.de). The branch lengths were obtained from the Timescale of Life (http://timetree.org) and from Niklas Wahlberg (2015, personal communication) for Lepidoptera species.
3. Results
(a). Theory
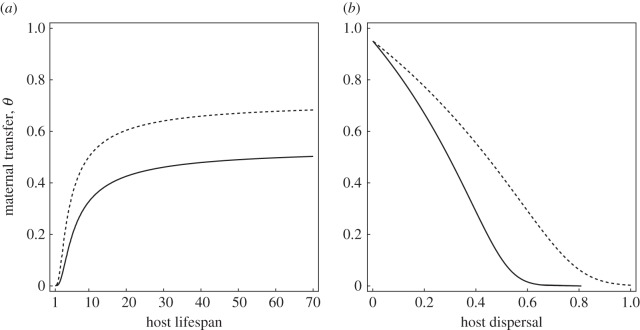
We explored the effect of the different parameters of the model on the evolution of the maternal transfer of immunity. As expected, we show that increasing the force of infection h or decreasing the cost cθ associated with the transfer of immunity always selects for higher values of θ. As pointed out by Garnier et al. [32], pathogen virulence has a non-monotonic effect on the evolution of θ. Both avirulent and very virulent pathogens select for low levels of maternal transfer of immunity. Indeed, when virulence becomes very high, it is not worth investing in a resistance mechanism that will never be expressed as infected individuals have very little opportunity to reproduce before they die from the infection. High levels of investment in θ are only selected when pathogens induce an intermediate reduction in longevity. We also observed the effect of longevity discussed in Garnier et al. [32]. Short-lived species do not invest in transgenerational immunity, because the survival benefit associated with immunity is cancelled out by the intrinsic mortality rate, μ (figure 1a).
Figure 1.
Evolutionary stable investment in maternal transfer of immunity θ (when θP = θD) with or without cross-immunity: χ = 0.5 (dashed line) and χ = 0 (full line) against (a) the longevity of the host, (b) the dispersal of the host. Default parameter values (see the electronic supplementary material for more details on the model): r0 = 1.5, cθ = 0.1, k = 1.1, η = 0.3, K = 20, h = 1.1, α = 3, δM = 1, μ = 0.02.
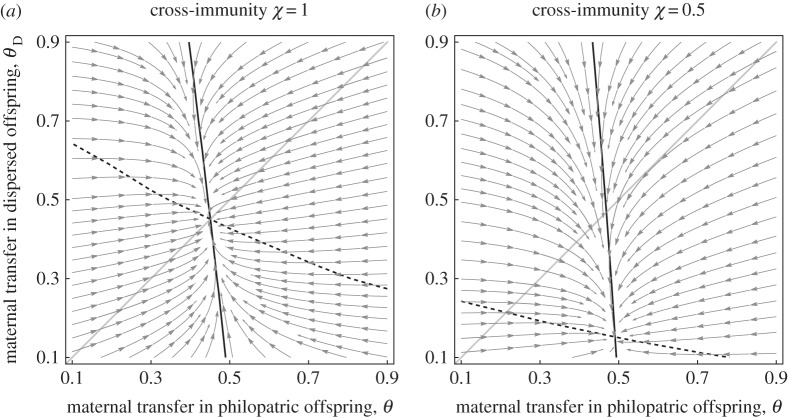
In addition, our model allowed us to explore the effect of dispersal and cross-immunity on the evolutionary outcome. When dispersal is high and cross-immunity is low, maternal investment is unlikely to protect the offspring because they are likely to be exposed to a different pathogen. Consequently, higher investment in maternal transfer is only expected to evolve in philopatric species or in species with high levels of cross--immunity (figure 1b). In the case where mothers have the ability to produce both philopatric and dispersing offspring and cross-immunity is imperfect, maternal investment is predicted to be higher in the philopatric progeny (i.e. θP > θD, figure 2). Indeed, such plastic investment in transgenerational immunity is adaptive, because philopatric offspring are more likely to be exposed to the same pathogens.
Figure 2.
Evolution of maternal transfer of immunity towards philopatric θP or dispersed offspring θD (a) with perfect cross-immunity χ = 1 or (b) with imperfect cross-immunity. The full line is the evolutionary stable value of θP against θD and the dashed line is the evolutionary stable value of θD against θP. The intersection between these two lines indicates the coevolutionary stable strategy of θD and θP. The arrows indicate the direction of evolution on both these traits. Default parameter values (see electronic supplementary material for more details on the model): r0 = 2, cθ = 0.2, k = 1.5, η = 0.25, K = 20, h = 1, α = 3, δM = 1, μ = 0.02.
(b). Empirical data
We focused our attention only on two key life-history traits of the host for which sufficient information is available in the literature: lifespan and dispersal. We investigated the impact of these two parameters in each of the transgenerational immunity scores identified above.
As expected, long-lived species and species with short dispersal ranges have significantly higher TER scores (respectively, Fisher exact test, p = 0.039, figure 3a, p = 0.017, figure 3b). Neither longevity (Fisher exact test, longevity: p = 0.318) nor dispersal range (p = 0.444) has a significant effect on the TEI scores (figure 3a,b). Interestingly, however, both dispersal and lifespan have a significant impact on the overall parental investment in offspring protection as quantified by the OTP score (figure 3a,b). Species with long lifespan and short dispersal ranges have significantly higher OTP scores than their short-lived and highly dispersing counterparts (Fisher exact test, lifespan: p = 0.002, dispersal: p = 0.047). The effect of lifespan on the OTP score is largely robust with respect to the cut-off point between long- and short-lived species (electronic supplementary material, figure S1a). Dispersal, however, is highly sensitive to the cut-off point chosen, and significance is lost in all but the 500 cut-off point (electronic supplementary material, figure S1b).
Figure 3.
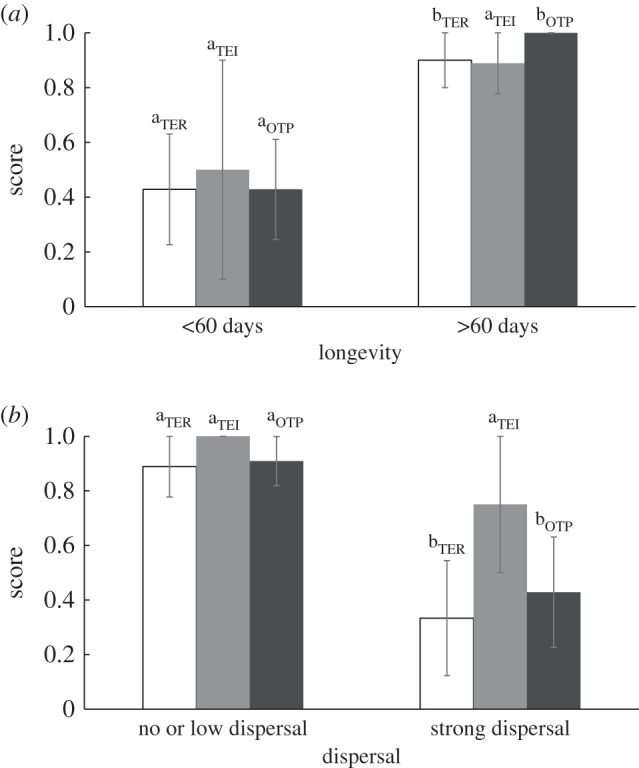
Scores for transgenerational effect on resistance (TER, white bars), transgenerational effect on immunity (TEI, grey bars), and overall transgenerational protection (OTP, black bars) according to species longevity (a) and dispersal (b). Statistical analyses were performed separately for each group (TER, TEI, and OTP). Levels not connected by same letter are significantly different. Error bars represent ± standard error (s.e.).
To verify whether results hold when correcting for phylogenetic correlations, the analyses were repeated using linear regression for binary phylogenetic data. In accordance with the results of the Fisher's exact rest, lifespan has a significant effect on the OTP score (cut-off point: 60 days, Zscore = 2.031, p = 0.042); dispersal, however, loses its significance at the 500 m cut-off point (Zscore = −0.617, p = 0.537).
4. Discussion
Previous work has shown how investment in immunity, and in classic (within-generational) immune memory in particular, should be maximized in species with high or intermediate lifespan [4,35–38]. Simply put, short-lived hosts are unlikely to encounter the same pathogen twice and should therefore not invest in memory. Recently, Garnier et al. [32] and Metcalf & Jones [33] showed that these predictions could also be extended to the evolution of the maternal transfer of immunity in a single host population. Here, we consider a scenario with two host populations connected by migration. In addition, we assume that infected hosts cannot recover from the infection (as is the case in most invertebrates) but may be able to transfer some immunity to its offspring. Our results agree with previous studies in showing that the marginal gain in fitness obtained from transgenerational immunity is higher in long-lived species. Our prediction is supported by empirical data confirming the existence of an association between transgenerational immunity and longevity in invertebrates: immune-challenged, long-lived species have a higher probability of actively protecting their offspring against a subsequent infection than their short-lived counterparts.
The amount of host dispersal is expected to affect the evolution of host–parasite interactions and in particular to shape patterns of parasite local adaptation [39–42]. Because parasites are often found to be adapted to their sympatric hosts [40], host migration may reduce the cost of parasitism and could affect the evolution of immunity [43–45]. For instance, Kurtz et al. [46] showed that after being placed into a new environment the grasshopper (Chorthippus biguttulus) reduces the expression of a non-specific immune trait (i.e. phagocytosis activity), possibly owing to a lower exposure to locally adapted parasites. In this study, we focused on the evolution of immune transfer under the assumption that parasites are locally adapted, and we show that philopatry can promote the evolution of transgenerational immunity because it increases the predictability of the offspring environment. In other words, maternal transfer of protection should be favoured when mothers and offspring share the same environment and are thus likely to be exposed to similar parasites. This prediction, however, could not be satisfactorily confirmed using currently available data. Dispersal is only a marginally significant predictor of maternal transfer of immunity at one of the cut-off points (500 m), and the significance is lost when the phylogeny is taken into account in the analysis.
Broadly speaking, our ability to test our theoretical predictions concerning dispersal and longevity was limited not only by the difficulties inherent to quantifying these parameters in wild invertebrates, but also by the limited number and phylogenetic breadth of taxa in which transgenerational immune priming has been quantified to date. Transgenerational immunity has thus far been described in a mere dozen invertebrate species, the large majority of which are either aquatic, eusocial, or stored-product species (electronic supplementary material, table S1). This problem is, we suspect, compounded by a publication bias that favours the publication of significant results over non-significant ones. Expanding the range of transgenerational immune protection studies to a large panel of invertebrate taxa with a wide range of life-history traits is an essential first step to understanding the ecological conditions under which this trait evolves. Terrestrial isopod species are good candidates owing to their limited dispersal potential [47] and extended lifespans, which can range between 1 to more than 5 years, depending on the species [48–50]. The confounding effect of phylogeny could be bypassed by working with taxa displaying a range of different life-history traits, such as the bee superfamily of Apoidea that contains both eusocial and solitary bees. Finally, experimental evolution mimicking different ecological scenarios (e.g. high/low dispersal) could provide a powerful tool to test some of these predictions using laboratory-friendly species (e.g. Drosophila, Artemia).
Our theoretical model also generates testable predictions on the evolution of a plastic transfer of immunity in species that can produce both dispersing and non-dispersing morphs. Under the assumption that parasites are locally adapted and that immunity is specific (i.e. that there is low cross-immunity), mothers are expected to invest more in the immune protection of the philopatric, non-dispersing morph, than on the dispersing one. This prediction could be tested in insects producing both apterous and winged (alate) forms, such as aphids [51–53], ants [54,55], and termites [56], or in species that exhibit a sex-biased dispersal, such as gypsy moths [57] and midges [57]. In each of these cases, the philopatric morph or sex is expected to accrue greater benefits from a higher maternal investment in immunity than the dispersing one. Incidentally, this prediction could be validated in vertebrates, such as certain bird and mammal species that exhibit drastic differences in sex-biased dispersal [58]. Finally, our predictions may have implications for when dispersal happens across time rather than across space, as is the case in species that produce dormant stages. Dormancy may favour the evolution of conditional investment in immunity: dormant offspring are often expected to be exposed to maladapted pathogens [59,60] and may require lower investment in immunity than their non-dormant counterparts.
Our review of the experimental literature revealed broad methodological differences between the studies that raise both conceptual and terminological issues regarding what constitutes transgenerational immunity. Two different protocols are used to test for transgenerational immunity and they do not necessarily convey the same information. About half of the studies quantify and compare immune priming by measuring a handful of immune parameters in offspring from immune-stimulated and naive parents (TEI), but do not necessarily verify whether the increased immune effectors result in increased parasite protection. The use of a few (typically one or two) immune assays as a proxy for parasite resistance has come under increased scrutiny, as evidence accumulates that they are not necessarily correlated with each other [61]. In other words, an elevated TEI, does not necessarily imply either that the mother pays any costs for the transfer (immune effectors could diffuse passively into eggs within the ovaries), or indeed that the offspring are better protected as a result (if, for example, immune components are not transmitted in sufficient numbers). Conversely, the other half of the studies, quantify the outcome of an infection (parasite prevalence, parasite intensity, or survival) in offspring issued from infected and uninfected parents (TER) but without delving into whether the underlying mechanisms are immunological or not (for example, through the differential provisioning of offspring with nutritional resources). Our analyses showed that while the results obtained from TER studies are largely consistent with our theoretical predictions, the signal is much less clear for TEI studies. We believe that an integrative view of the transgenerational immune memory requires both approaches [17,18,21,62,63].
In conclusion, there is a growing interest regarding the biology and ecology of transgenerational immune priming in invertebrates [64], not least owing to the key role some of them play as pollinators, vectors of diseases, and agricultural and stored product pests. Transgenerational immune priming is predicted to have not only a strong effect on disease prevalence [65,66], but also on the age structure [65] and population dynamics of invertebrates [66]. Our theoretical model shows that, beyond the effect of host lifespan and host dispersal, several other life-history parameters play a key role in the evolution of transgenerational immunity. Future work needs to expand on currently available data in order to get a wider picture of the transgenerational immune protection and on its impact on the evolutionary ecology of the host–pathogen interactions.
Supplementary Material
Acknowledgements
We thank Niklas Wahlberg for his help with the divergence time of Lepidoptera.
Data accessibility
The complete data can be found in the electronic supplementary material.
Authors' contributions
R.G. and S.G. developed and analysed the theoretical model, R.P. reviewed and analysed empirical data, R.P., S.G., A.R. wrote the paper.
Competing interests
We declare we do not have any competing interest.
Funding
We received no funding for this study.
References
- 1.Ziauddin J, Schneider DS. 2012. Where does innate immunity stop and adaptive immunity begin? Cell Host Microbe 12, 394–395. ( 10.1016/j.chom.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 2.Armitage SAO, Peuß R, Kurtz J. 2014. Dscam and pancrustacean immune memory – a review of the evidence. Dev. Comp. Immunol. 48, 315–323 ( 10.1016/j.dci.2014.03.004) [DOI] [PubMed] [Google Scholar]
- 3.Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 50, 529–551. ( 10.1146/annurev.ento.50.071803.130420) [DOI] [PubMed] [Google Scholar]
- 4.Best A, Tidbury H, White A, Boots M. 2013. The evolutionary dynamics of within-generation immune priming in invertebrate hosts. J. R. Soc. Interface 10, 20120887 ( 10.1098/rsif.2012.0887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witteveldt J, Cifuentes CC, Vlak JM, van Hulten MCW. 2004. Protection of Penaeus monodon against white spot syndrome virus by oral vaccination. J. Virol. 78, 2057–2061. ( 10.1128/JVI.78.4.2057-2061.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McTaggart SJ, Wilson PJ, Little TJ. 2012. Daphnia magna shows reduced infection upon secondary exposure to a pathogen. Biol. Lett. 8, 972–975. ( 10.1098/rsbl.2012.0581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tidbury HJ, Pedersen AB, Boots M. 2011. Within and transgenerational immune priming in an insect to a DNA virus. Proc. R. Soc. B 278, 871–876. ( 10.1098/rspb.2010.1517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth O, Sadd BM, Schmid-Hempel P, Kurtz J. 2009. Strain-specific priming of resistance in the red flour beetle, Tribolium castaneum. Proc. R. Soc. B 276, 145–151. ( 10.1098/rspb.2008.1157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C. 2010. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329, 1353–1355. ( 10.1126/science.1190689) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadd BM, Schmid-Hempel P. 2006. Insect immunity shows specificity in protection upon secondary pathogen exposure. Curr. Biol. 16, 1206–1210. ( 10.1016/j.cub.2006.04.047) [DOI] [PubMed] [Google Scholar]
- 11.Jacot A, Scheuber H, Kurtz J, Brinkhof MWG. 2005. Juvenile immune system activation induces a costly upregulation of adult immunity in field crickets Gryllus campestris. Proc. R. Soc. B 272, 63–69. ( 10.1098/rspb.2004.2919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas AM, Rudolf VHW. 2010. Challenges of metamorphosis in invertebrate hosts: maintaining parasite resistance across life-history stages. Ecol. Entomol. 35, 200–205. ( 10.1111/j.1365-2311.2009.01169.x) [DOI] [Google Scholar]
- 13.Little TJ, O'Connor B, Colegrave N, Watt K, Read AF. 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13, 489–492. ( 10.1016/S0960-9822(03)00163-5) [DOI] [PubMed] [Google Scholar]
- 14.Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. 2005. Trans-generational immune priming in a social insect. Biol. Lett. 1, 386–388. ( 10.1098/rsbl.2005.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tate AT, Graham AL. 2015. Trans-generational priming of resistance in wild flour beetles reflects the primed phenotypes of laboratory populations and is inhibited by co-infection with a common parasite. Funct. Ecol. 29, 1059–1069. ( 10.1111/1365-2435.12411) [DOI] [Google Scholar]
- 16.Huang CC, Song YL. 1999. Maternal transmission of immunity to white spot syndrome associated virus (WSSV) in shrimp (Penaeus monodon). Dev. Comp. Immunol. 23, 545–552. ( 10.1016/S0145-305X(99)00038-5) [DOI] [PubMed] [Google Scholar]
- 17.Moret Y. 2006. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. B 273, 1399–1405. ( 10.1098/rspb.2006.3465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth O, Joop G, Eggert H, Hilbert J, Daniel J, Schmid-Hempel P, Kurtz J. 2010. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. J. Anim. Ecol. 79, 403–413. ( 10.1111/j.1365-2656.2009.01617.x) [DOI] [PubMed] [Google Scholar]
- 19.Yue F, Zhou Z, Wang L, Ma Z, Wang J, Wang M, Zhang H, Song L. 2013. Maternal transfer of immunity in scallop Chlamys farreri and its trans-generational immune protection to offspring against bacterial challenge. Dev. Comp. Immunol. 41, 569–577. ( 10.1016/j.dci.2013.07.001) [DOI] [PubMed] [Google Scholar]
- 20.Freitak D, Schmidtberg H, Dickel F, Lochnit G, Vogel H, Vilcinskas A. 2014. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 5, 547–554. ( 10.4161/viru.28367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López JH, Schuehly W, Crailsheim K, Riessberger-Gallé U. 2014. Trans-generational immune priming in honeybees. Proc. R. Soc. B 281, 20140454 ( 10.1098/rspb.2014.0454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamara KB, van Lieshout E, Simmons LW. 2014. The effect of maternal and paternal immune challenge on offspring immunity and reproduction in a cricket. J. Evol. Biol. 27, 1020–1028. ( 10.1111/jeb.12376) [DOI] [PubMed] [Google Scholar]
- 23.Fisher JJ, Hajek AE. 2015. Maternal exposure of a beetle to pathogens protects offspring against fungal disease. PLoS ONE 10, e0125197 ( 10.1371/journal.pone.0125197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norouzitallab P, Biswas P, Baruah K, Bossier P. 2015. Multigenerational immune priming in an invertebrate parthenogenetic Artemia to a pathogenic Vibrio campbellii. Fish Shellfish Immunol. 42, 426–429. ( 10.1016/j.fsi.2014.11.029) [DOI] [PubMed] [Google Scholar]
- 25.Salmela H, Amdam GV, Freitak D. 2015. Transfer of immunity from mother to offspring is mediated via egg-yolk protein vitellogenin. PLoS Pathog. 11, e1005015 ( 10.1371/journal.ppat.1005015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubuffet A, Zanchi C, Boutet G, Moreau J, Teixeira M, Moret Y. 2015. Trans-generational immune priming protects the eggs only against gram-positive bacteria in the mealworm beetle. PLoS Pathog. 11, e1005178 ( 10.1371/journal.ppat.1005178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vorburger C, Gegenschatz SE, Ranieri G, Rodriguez P. 2008. Limited scope for maternal effects in aphid defence against parasitoids. Ecol. Entomol. 33, 189–196. ( 10.1111/j.1365-2311.2007.00949.x) [DOI] [Google Scholar]
- 28.Voordouw MJ, Lambrechts L, Koella J. 2008. No maternal effects after stimulation of the melanization response in the yellow fever mosquito Aedes aegypti. Oikos 117, 1269–1279. ( 10.1111/j.0030-1299.2008.16741.x) [DOI] [Google Scholar]
- 29.Linder JE, Promislow DEL. 2009. Cross-generational fitness effects of infection in Drosophila melanogaster. Fly (Austin) 3, 143–150. ( 10.4161/fly.8051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pigeault R, Vézilier J, Nicot A, Gandon S, Rivero A. 2015. Transgenerational effect of infection in Plasmodium-infected mosquitoes. Biol. Lett. 11, 20141025 ( 10.1098/rsbl.2014.1025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vantaux A, Dabiré KR, Cohuet A, Lefèvre T. 2014. A heavy legacy: offspring of malaria-infected mosquitoes show reduced disease resistance. Malar. J. 13, 442 ( 10.1186/1475-2875-13-442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garnier R, Boulinier T, Gandon S. 2012. Coevolution between maternal transfer of immunity and other resistance strategies against pathogens. Evol. Int. J. Org. Evol. 66, 3067–3078. ( 10.1111/j.1558-5646.2012.01665.x) [DOI] [PubMed] [Google Scholar]
- 33.Metcalf CJE, Jones JH. 2015. The evolutionary dynamics of timing of maternal immunity: evaluating the role of age-specific mortality. J. Evol. Biol. 28, 493–502. ( 10.1111/jeb.12583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ives AR, Garland T. 2010. Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26. ( 10.1093/sysbio/syp074) [DOI] [PubMed] [Google Scholar]
- 35.Boots M, Bowers RG. 2004. The evolution of resistance through costly acquired immunity. Proc. R. Soc. Lond. B 271, 715–723. ( 10.1098/rspb.2003.2655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee KA. 2006. Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 46, 1000–1015. ( 10.1093/icb/icl049) [DOI] [PubMed] [Google Scholar]
- 37.Miller MR, White A, Boots M. 2007. Host life span and the evolution of resistance characteristics. Evol. Int. J. Org. Evol. 61, 2–14. ( 10.1111/j.1558-5646.2007.00001.x) [DOI] [PubMed] [Google Scholar]
- 38.Best A, Hoyle A. 2013. The evolution of costly acquired immune memory. Ecol. Evol. 3, 2223–2232. ( 10.1002/ece3.611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I. 1996. Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc. R. Soc. Lond. B 263, 1003–1009. ( 10.1098/rspb.1996.0148) [DOI] [Google Scholar]
- 40.Kaltz O, Shykoff JA. 1998. Local adaptation in host–parasite systems. Heredity 81, 361–370. ( 10.1046/j.1365-2540.1998.00435.x) [DOI] [Google Scholar]
- 41.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 42.Blanquart F, Kaltz O, Nuismer SL, Gandon S. 2013. A practical guide to measuring local adaptation. Ecol. Lett. 16, 1195–1205. ( 10.1111/ele.12150) [DOI] [PubMed] [Google Scholar]
- 43.Boulinier T, McCoy KD, Sorci G. 2001. Dispersal and parasitism. In Dispersal (eds JC Clobert, E Danchin, AA Dhondt, JD Nichols), pp. 169–179. Oxford, UK: Oxford University Press. [Google Scholar]
- 44.Møller AP, Erritzøe J. 2001. Dispersal, vaccination and regression of immune defence organs. Ecol. Lett. 4, 484–490. ( 10.1046/j.1461-0248.2001.00259.x) [DOI] [Google Scholar]
- 45.Møller AP, Martín-Vivaldi M, Soler JJ. 2004. Parasitism, host immune defence and dispersal. J. Evol. Biol. 17, 603–612. ( 10.1111/j.1420-9101.2004.00694.x) [DOI] [PubMed] [Google Scholar]
- 46.Kurtz J, Klappert K, Schneider W, Reinhold K. 2002. Immune defence, dispersal and local adaptation. Evol. Ecol. Res. 4, 431–439. [Google Scholar]
- 47.Beck ML, Price JO. 1981. Genetic variation in the terrestrial isopod, Armadillidium vulgare. J. Hered. 72, 15–18. [DOI] [PubMed] [Google Scholar]
- 48.Miller RH, Cameron GN. 1983. Intraspecific variation of life history parameters in the terrestrial isopod, Armadillidium vulgare. Oecologia 57, 216–226. ( 10.1007/BF00379583) [DOI] [PubMed] [Google Scholar]
- 49.Alikhan AM. 1995. Terrestrial isopod biology. Rotterdam, Netherlands: CRC Press. [Google Scholar]
- 50.Quadros AF, Caubet Y, Araujo PB. 2009. Life history comparison of two terrestrial isopods in relation to habitat specialization. Acta Oecol. 35, 243–249. ( 10.1016/j.actao.2008.10.007) [DOI] [Google Scholar]
- 51.Mackay PA, Lamb RJ. 1996. Dispersal of five aphids (Homoptera: Aphididae) in relation to their impact on Hordeum vulgare. Environ. Entomol. 25, 1032–1044. ( 10.1093/ee/25.5.1032) [DOI] [Google Scholar]
- 52.Weisser WW, Braendle C, Minoretti N. 1999. Predator-induced morphological shift in the pea aphid. Proc. R. Soc. Lond. B 266, 1175–1181. ( 10.1098/rspb.1999.0760) [DOI] [Google Scholar]
- 53.Sloggett JJ, Weisser WW. 2002. Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos 98, 323–333. ( 10.1034/j.1600-0706.2002.980213.x) [DOI] [Google Scholar]
- 54.Heinze J, Tsuji K. 1995. Ant reproductive strategies. Res. Popul. Ecol. 37, 135–149. ( 10.1007/BF02515814) [DOI] [Google Scholar]
- 55.Cremer S, Heinze J. 2003. Stress grows wings: environmental induction of winged dispersal males in Cardiocondyla ants. Curr. Biol. 13, 219–223. ( 10.1016/S0960-9822(03)00012-5) [DOI] [PubMed] [Google Scholar]
- 56.Korb J, Katrantzis S. 2004. Influence of environmental conditions on the expression of the sexual dispersal phenotype in a lower termite: implications for the evolution of workers in termites. Evol. Dev. 6, 342–352. ( 10.1111/j.1525-142X.2004.04042.x) [DOI] [PubMed] [Google Scholar]
- 57.Dingle H. 1996. Polymorphisms and polyphenism. In Migration: the biology of life on the move, pp. 231–247. New York, NY: Oxford University Press. [Google Scholar]
- 58.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 59.Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Van Kerckhoven L, Ebert D, De Meester L. 2007. Host–parasite ‘red queen’ dynamics archived in pond sediment. Nature 450, 870–873. ( 10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- 60.Blanquart F, Gandon S. 2013. Time-shift experiments and patterns of adaptation across time and space. Ecol. Lett. 16, 31–38. ( 10.1111/ele.12007) [DOI] [PubMed] [Google Scholar]
- 61.Adamo SA. 2004. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 68, 1443–1449. ( 10.1016/j.anbehav.2004.05.005) [DOI] [Google Scholar]
- 62.Eggert H, Kurtz J, Diddens-de Buhr MF. 2014. Different effects of paternal trans-generational immune priming on survival and immunity in step and genetic offspring. Proc. R. Soc. B 281, 20142089 ( 10.1098/rspb.2014.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trauer-Kizilelma U, Hilker M. 2015. Insect parents improve the anti-parasitic and anti-bacterial defence of their offspring by priming the expression of immune-relevant genes. Insect Biochem. Mol. Biol. 64, 91–99. ( 10.1016/j.ibmb.2015.08.003) [DOI] [PubMed] [Google Scholar]
- 64.Contreras-Garduño J, Lanz-Mendoza H, Franco B, Nava A, Pedraza-Reyes M, Canales-Lazcano J. 2016. Insect immune priming: ecology and experimental evidences. Ecol. Entomol. 41, 351–366. ( 10.1111/een.12300) [DOI] [Google Scholar]
- 65.Tate AT, Rudolf VHW. 2012. Impact of life stage specific immune priming on invertebrate disease dynamics. Oikos 121, 1083–1092. ( 10.1111/j.1600-0706.2011.19725.x) [DOI] [Google Scholar]
- 66.Tidbury HJ, Best A, Boots M. 2012. The epidemiological consequences of immune priming. Proc. R. Soc. B 279, 20121841 ( 10.1098/rspb.2012.1841) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete data can be found in the electronic supplementary material.