Abstract
This study aimed to examine thermoregulatory responses in birds facing two commonly experienced stressors, cold and fasting. Logging devices allowing long-term and precise access to internal body temperature were placed within the gizzards of ducklings acclimated to cold (CA) (5°C) or thermoneutrality (TN) (25°C). The animals were then examined under three equal 4-day periods: ad libitum feeding, fasting and re-feeding. Through the analysis of daily as well as short-term, or ultradian, variations of body temperature, we showed that while ducklings at TN show only a modest decline in daily thermoregulatory parameters when fasted, they exhibit reduced surface temperatures from key sites of vascular heat exchange during fasting. The CA birds, on the other hand, significantly reduced their short-term variations of body temperature while increasing long-term variability when fasting. This phenomenon would allow the CA birds to reduce the energetic cost of body temperature maintenance under fasting. By analysing ultradian regulation of body temperature, we describe a means by which an endotherm appears to lower thermoregulatory costs in response to the combined stressors of cold and fasting.
Keywords: homeothermy, rhythm, body temperature variability, fasting, nocturnal hypothermia
1. Introduction
Organisms show remarkable capacities to adapt to variable environments, notably by optimizing energy balance. Energy conservation allows for the allocation of energy to functions other than survival, such as growth or reproduction, especially when energy is in short supply. Two prominent mechanisms in re-organizing energy balance are (i) the use of internal energy storage from lipids and carbohydrates and (ii) the reduction of energy expenditure via hypometabolism. In endothermic vertebrates in which metabolism is high due in part to thermogenesis, several energy conservation strategies have evolved, such as torpor (metabolic suppression with a daily return to normothermia) or hibernation (profound metabolic suppression and reduced body temperatures over several days) [1–3]. These mechanisms reap large benefits, in part, because of the effects of low body temperature (Tb) on metabolic rate. However, many endotherms do not exhibit such large variations of Tb (i.e. homeotherms) and keep Tb high even under unfavourable periods [4], implying that abandoning homeothermy may have additional, non-energetic costs [5].
Body temperature is determined by the balance between heat production and heat dissipation mechanisms. A well-recognized metabolic adaptation to fasting is the reduction in heat production [6,7], accompanied by reductions in Tb, usually through progressive declines during the inactive period [8–14], which have been argued to result from a regulated thermoregulatory response [8,9,15,16]. Fluctuations in Tb can result from changes in set point [17,18], changes in activity [19,20] or changes in thermal conductance [21]. In birds, regional heterothermy during fasting may provide an explanation for why core Tb does not vary much with fasting in some species, despite a large metabolic suppression [21]. One implication of this is that variability in Tb may itself be a source of energetic savings, a concept that has not been formally tested in the studies mentioned above. Previous research has implicated alterations in vigilance states (i.e. sleep/wake cycles) during prolonged food deprivation in pigeons and geese [15,22], which could be a proximate neurophysiological mechanism leading to the daily changes in Tb observed during fasting.
Body temperature, like many physiological parameters, shows considerable ultradian variation [23]. Although these fluctuations may simply appear to be noise, they have important physiological signatures (e.g. insulin and growth hormone show pulsatile secretion in phase with Tb fluctuations [24,25], fluctuations in core Tb of quail are associated with changes in fuel use [26], alterations in hippocampal activation precedes thermogenic fluctuations [19,20]). As altered vigilance behaviours (i.e. changes in patterns of sleep–wake states) appear essential for the induction and maintenance of fasting-induced nocturnal anapyrexia [11], ultradian Tb rhythms should also be reduced in fasting. In laboratory rodents, ultradian Tb rhythms are themselves circadian in nature and are elevated with cold exposure but decreased in energy-limiting conditions like hypoxia [27]. In wild mole voles, circadian and ultradian Tb rhythms disappear during cold acclimation (CA) [28]. Clearly then, a reduced prevalence of ultradian rhythms should occur with prolonged cold or fasting.
This study aims to investigate the relationship between the diurnal and ultradian regulation of Tb of birds experiencing common environmental stresses. Stressors at early life stages in birds can have long-lasting effects on energetics, growth and thermoregulation [29], thus we submitted ducklings to the energetic constraints of cold and food restriction. We examined thermally acclimated (cold versus thermoneutral, CA versus TN) animals in order to compare animals of differing thermogenic capacity; CA in ducklings upregulates non-shivering heat production and slightly raises basal metabolic rate (BMR) measured within the TN zone [30,31]. Our overall hypothesis was that prolonged energy deficits are countered by adjustments geared at reducing energy requirements. A number of predictions arise from this hypothesis. First, fasting will induce a regulated hypothermia (i.e. anapyrexia). Second, regional heterothermy will be adopted during fasting to decrease thermal conductance and allow for relatively stable homeothermy. Third, as cold exposure will be associated with a greater energy deficit, it will demand enhanced thermoregulatory adjustments during fasting. One mechanism mediating these costs is through changes in Tb variability; therefore, adjustments in ultradian and diurnal regulation of Tb should be geared towards optimizing energy savings based on energy deficit.
2. Material and methods
(a). Animals
A total of 12 ducklings (male Muscovy ducks, Cairina moschata, pedigree R31, Institut National de la Recherche Agronomique, Paris, France) were sourced from a commercial stockbreeder (Eclosion Grimaud-la-Corbière, Roussay, France). Six were assigned randomly to each of the two acclimation temperature groups. The ages of the ducklings at the beginning of the trial was five weeks and had been housed at their acclimation temperatures since 10 days of age: TN ducklings were housed at 25°C and CA ducklings were housed at 5°C following similar published protocols [30]. Ducklings varied in mass throughout the experiments, although at the outset of the measurement period the 12 ducklings chosen for the measurements were size-matched such that both acclimation groups had an average starting mass of approximately 1 kg. The animals were kept at a constant 8 L : 16 D cycle (lights on at 06.00 h). Ducklings were provided with food and water ad libitum, except during the fasting protocol, when food was completely removed. Their normal diet consisted of a commercial poultry mash (Moulin Guenard, 645000MI, Vonnas, France). The same animals were used throughout the feeding protocols, being identified by leg bands. Animal body mass measurements were recorded daily.
(b). Experimental protocol
Three days prior to the experimental period, ducklings were force fed a temperature data logging device (ANIPILL system®; Bodycap, Caen, France). The device has been designed for use in birds to lodge within the gizzard, providing relatively long-term access to an internal body temperature measurement. Following the ingestion of the data logger, birds were monitored for approximately 2 days before commencing the formal data gathering (data logged at 15 min intervals) to assess the patency of the data loggers and the birds' general behaviour. We subsequently divided the experiment into three equal, 4-day time periods: ad libitum feeding, fasting and subsequent re-feeding for the final 4 days of the experiment. The two acclimation groups remained at their respective acclimation temperatures throughout the experimental period. Behavioural observations of drinking and general activity were obtained periodically across all three conditions (N = 32 time points spanning all days, with a minimum of two observations per day, and up to four during the early days of fasting).
(c). Analysis of body temperature
Daily estimates of Tb parameters were summarized using Cosinor analysis (although see electronic supplementary material for summary spectral analysis) to facilitate specific Tb statistical comparisons [32]. Briefly, an individual bird's Tb time series data (electronic supplementary material, figure S1) were modelled using a multiple linear regression model, wherein the daily, 24-h periodic rise and fall in body temperature could be estimated as a linear combination of the cosine- and sine-transformed time variable expressed as radians with a 24 h period:
where M corresponds to the Mesor, or mean Tb, and A and B to the estimated slope values for the cosine- and sine-transformed time vectors. Amplitude (equivalent to the average diurnal deflection in Tb from the Mesor) was extracted from the A and B regression estimates using the following equation:
Extreme short-term, or ultradian, variation in Tb (dTb) was estimated using a sequential difference approach, wherein the daily average estimate of the absolute values of successive Tb measurements (ADT = absolute difference temperature) was taken for each bird:
where n is the number of measurements per day and i is the ith measurement over time. All Tb parameters above were determined daily, for each bird within each feeding condition.
(d). Incremental cost of homeothermy
The energetic costs inherent to the ‘instantaneous’ rate of Tb change can be assessed (assuming 100% efficiency) by dividing daily average ADT measurement by the sampling interval (dt = 900 s), and incorporating these fluctuations into the heat capacity equation:
where cp is the estimated tissue heat capacity (3.4 kJ kg−1), M is the body mass and 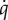 is the estimated power (W = Watts) required to heat the body according to the rate these fluctuations were observed. The 0.5 value in the equation above is included to account for the fact that absolute temperature differences include both the decreases in Tb as well as the increases over time, which would be symmetrical over a 24-h period in a homeothermic animal. These values were normalized to previously measured basal metabolic heat production (5 W kg−1 and 8 W kg−1 for 25°C and 5°C acclimated ducklings; [30,31]).
is the estimated power (W = Watts) required to heat the body according to the rate these fluctuations were observed. The 0.5 value in the equation above is included to account for the fact that absolute temperature differences include both the decreases in Tb as well as the increases over time, which would be symmetrical over a 24-h period in a homeothermic animal. These values were normalized to previously measured basal metabolic heat production (5 W kg−1 and 8 W kg−1 for 25°C and 5°C acclimated ducklings; [30,31]).
(e). Thermal image acquisition and analysis
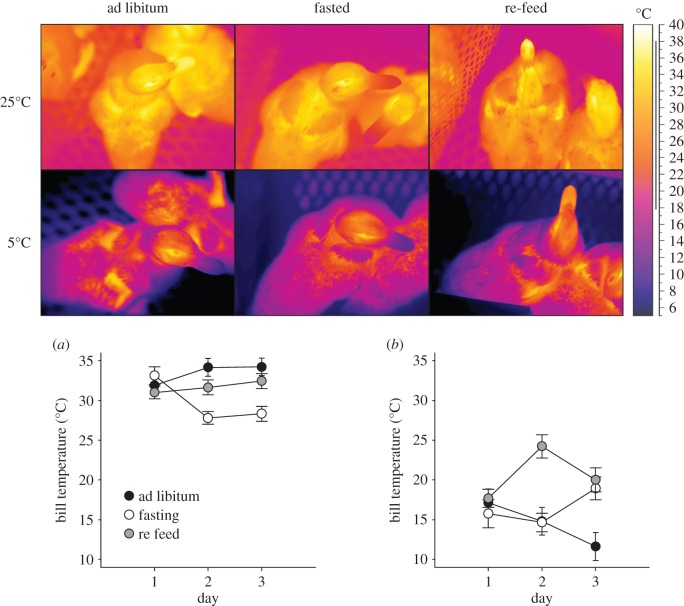
Infrared thermal images were manually captured at various time points throughout the experimental protocol, spanning the active and inactive periods of the ducklings during days 1–3 of each feeding condition. Thermographic images were collected using an IR thermal imaging camera (Model SC660, FLIR Systems, Wilsonville, Oregon). The camera was allowed time to warm up and electronically stabilize, and was calibrated prior to every experiment against an internal thermocouple (NIST standard). Using specialized software (Thermacam Researcher Pro), regions of interest on the body surface were digitally ‘drawn’ on each frame to obtain the average surface bill (Tbill) temperature, which was used to assess how a known vascular thermal window might be recruited to vasodilate or vasoconstrict over time [33,34]. In all cases, emissivity was assumed to be 0.96 [35]. Ducklings were not handled during the measurements, as preliminary observations from an initial pilot study indicated a significant lowering (1.3°C, p = 0.001) of surface temperatures as a result of a brief handling (less than 1 min duration) and replacement into the home cage.
(f). Statistical analyses
For descriptions of Tb and to test hypotheses regarding the influence of acclimation temperature, feeding condition and time, we employed a generalized and/or linear mixed effects modelling (GLMM or LMM) approach, including bird identity as a random effect (intercept) to account for repeated measurements over time. For the drinking behaviour, we used a GLMM (link = logit, where drinking was a factor variable of 1 or 0). For the analysis of Tb amplitude variation (short term versus long term), we allowed a random slope and intercept effect in the model to account for individual bird responses. For each parameter, we constructed a linear model that included all relevant predictor variables and their interactions if appropriate (i.e. condition, day, acclimation temperature, drinking status). We then employed an information-theoretic approach for model selection [36]; we ranked models based on how well they fit the data using AICc [37] by comparing with the model with the lowest AIC score, and choosing the most comprehensive model with ΔAICc < 2; comparisons with the null, intercept model were also included (ΔAICcn = Null AICc − Model AICc) to assess the degree to which the model explained the data. We performed all analyses with R [38], using the MuMIn [39] package for the information-theoretic approach, and nlme [40] or lme4 [41] packages for the GLMMs. Residuals were verified for normality [42] and when visual inspection revealed deviations from homoscedasticity, we refit the model weighted by parameter specific variance estimates [43]. We present model weights (from the informatic approach), ΔAICc values, model coefficients (B) or marginal mean values (±model s.e.) as measures of support. Rather than presenting inaccurate or inflated p-values inherent to the uncertainty in mixed model d.f. estimation, or over-inflating type II error rates, we present bootstrapped confidence limits for model parameters (B) in place of classical significance and multiple comparison testing [41,43].
3. Results
(a). Body mass and behaviour
Ducklings were still growing during the study (BDay = 89 g d−1), so body mass rose during the ad libitum phase, declined during fasting (BFasting×Day = −192 g d−1) and rapidly rose during the re-feeding phase (figure 1a). The TN group exhibited a steeper rate of recovery in body mass compared with the CA group during the re-feeding phase, due to a significant three-way interaction between Day × Condition × Acclimation (b = 32.5 g d−1, 95% CI: 6.48–59.26; ω = 0.408, ΔAICcn = 373, p = 0.0136). Relative mass loss during fasting did differ between the TN and CA ducklings (TN: −61 ± 3 g kg−1 d−1; CA: −73 ± 7 g kg−1 d−1; t7 = 4; p = 0.007). Over the last 3 days of fasting, CA ducklings lost body mass 20% faster than TN birds. The top GLMM describing the odds of drinking included the Condition × Day interaction (ω = 0.292, ΔAICcn = 12), although the strength of this interaction was weak (p = 0.074). There was, however, strong support for feeding condition influencing the odds of drinking; fasted ducklings were less likely to drink than ad libitum ducklings (b = −3.35, or = 0.035, p = 0.0322). Although ducklings were observed to drink on 5–25% of observations, the increased odds of drinking during the re-feeding phase was not different from the ad libitum period (p = 0.74). Drinking was positively associated with Tb (Tb when drinking was 0.25°C higher; p = 0.0032, LMM of Tb versus Drinking Status).
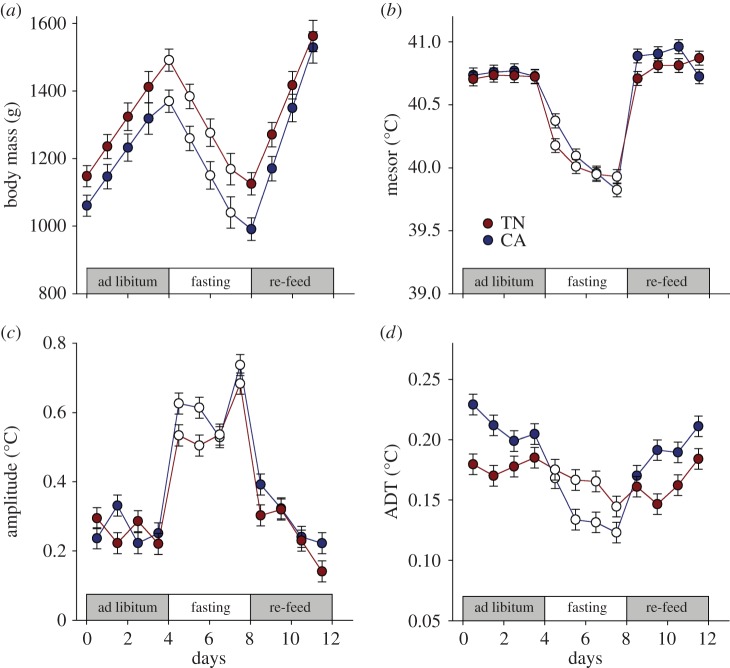
Figure 1.
Body mass (a) effects plots (mean ± model s.e.) in thermoneutral (TN) and cold-acclimated (CA) ducklings during similar durations (4 days) of ad libitum feeding, fasting and re-feeding. (b–d) Body temperature effects plots (marginal model fits ± s.e.) of TN and CA ducklings (N = 6 per temperature group) from the daily Cosinor analysis during the three different feeding conditions. Mesor results (b) reflect the daily average Tb. Amplitude (c) reflects the peak or nadir deviation from the mesor, and ADT (d) reflects the extreme short-term variation across the sampled time interval (15 min). (Online vesion in colour.)
(b). Body temperature analysis
Mesor values were similar for the most part between acclimation temperatures. The top model describing Tb mesor included the Acclimation Temperature × Day and Condition × Day interactions (ω = 0.671, ΔAICcn = 320), while the second highest model included the three-way interaction (ω = 0.224, ΔAICcn = 318). As a consequence, Mesor changed in a manner dependent on Acclimation Temperature, Day and Condition (figure 1b). During the ad libitum stage, Mesor values were similar, and while fasting and re-feeding declined faster in the CA group (BCA×Fasting = −0.091°C/day, p = 0.033 and BCA×Re-feeding = −0.085°C day−1, p = 0.045, respectively), although the TN ducklings did appear to show Mesor stabilizing during fasting. The CA ducklings did exhibit an overshoot in mesor values during the re-feeding phase, by virtue of the overall higher values (BCA = 0.26°C, p = 0.0302).
Amplitude values were similar for the most part between acclimation temperatures (figure 1c). The top ranked model (ω = 0.408) describing Amplitude included the Condition × Day interactions and their main effects, while the second ranked model also included Acclimation Temperature (ω = 0.292). Amplitude was higher during fasting than during ad libitum feeding (B = 0.22, p < 0.00001) and remained higher during re-feeding (B = 0.13, p = 0.001), returning throughout the 4 days of re-feeding to baseline values (B = −0.047°C d−1, p = 0.0006). Although Acclimation Temperature did appear in the higher ranked models, the strength of the effect was negligible (p = 0.19). Instead, throughout fasting at both temperatures, Amplitude rose (B = 0.048°C d−1, p = 0.0010), reaching peak values by the fourth day. Similarly, the return of Amplitude to baseline values followed a much steeper slope than that observed during the initial ad libitum period (B = −0.048°C d−1, p = 0.0014).
Short-term variation in Tb values (ADT) varied between the experimental groups (figure 1d). The top model describing ADT included the Acclimation Temperature × Day and Condition × Day interactions and their main effects (ω = 0.491, ΔAICcn = 103), while the second highest model included all two-way interactions (ω = 0.328, ΔAICcn = 102). As a consequence, the ADT changed in a manner dependent on Acclimation Temperature, Day and Condition (figure 1d). Overall, CA ducklings had higher ADT when fed, but a lower ADT during fasting compared with TN ducklings (Acclimation Temperature × Condition: BFasting = −0.0569, p < 0.0001). The daily rates of change in ADT were generally more negative during fasting (B = −0.0084, p = 0.039) and more positive during re-feeding (B = 0.013, p = 0.0006).
(c). Metabolic cost of absolute difference temperature heat increment
The top model explaining 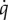 included Mass (included as a covariate to account mass effects), Acclimation Temperature × Condition and their main effects (ω = 1, ΔAICcn = 146). TN ducklings maintained a lower overall
included Mass (included as a covariate to account mass effects), Acclimation Temperature × Condition and their main effects (ω = 1, ΔAICcn = 146). TN ducklings maintained a lower overall 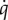 , which also continued to decline during the re-feeding phase (B = −0.046, p = 0.0076), while cold-acclimated ducklings had overall much higher
, which also continued to decline during the re-feeding phase (B = −0.046, p = 0.0076), while cold-acclimated ducklings had overall much higher 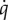 values (B = 0.087, p < 0.0001) that underwent an extremely large decline during fasting (B = −0.12, p < 0.0001), and recovered to baseline levels during re-feeding (figure 2).
values (B = 0.087, p < 0.0001) that underwent an extremely large decline during fasting (B = −0.12, p < 0.0001), and recovered to baseline levels during re-feeding (figure 2).
Figure 2.
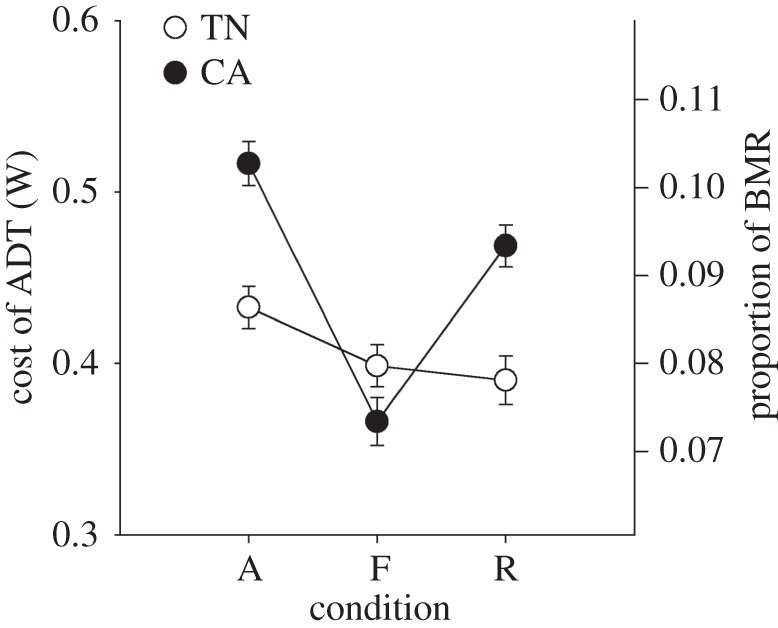
Estimated energetic costs of short-term change in Tb during ad libitum feeding (A), fasting (F) and re-feeding (R) as calculated from the heat capacity equation. The metabolic costs associated with the periodic rises in Tb were typically higher in cold-acclimated ducklings, but declined more profoundly during fasting. The metabolic costs are not insubstantial; when expressed as a portion of basal metabolic rate [30] are between 7 and 11%.
(d). Surface temperature analysis
As drinking was observed approximately 5–25% of the time and was shown to be influenced by Tb measurements, we included Drinking Status in models of surface temperature assessment, especially for Tbill (Drinking Status always ranked in the top most models). We examined Tbill separately for each Acclimation Temperature because of known physical (i.e. non-biological) determinants of surface temperatures [35]. The top model describing Tbill for TN ducklings included Drinking, Condition × Day interactions and their main effects (ω = 0.95, ΔAICcn = 82). In TN ducklings, Tbill declined more rapidly during the fasting period (B = −2.2°C d−1, p = 0.0114) compared with the ad libitum period (figure 3). In CA ducklings, on the other hand, only the re-feeding period was associated with a change in the rate at which Tbill changed (B = 3.8°C day−1, p = 0.0142). Drinking Status had negative influential effects on bill temperatures for both TN and CA ducklings (B = −5.97, and p < 0.001, −7.19°C and p < 0.0001, respectively).
Figure 3.
Bill surface temperature in thermoneutral (TN) (25°C) and cold-acclimated (CA) (5°C) ducklings obtained from thermal images. Representative thermal images are depicted in the top panel. The lower traces show the marginal model fits (±s.e.) for the TN- (a) and CA (b) duckling. Bill temperatures declined during the first 3 days of fasting in TN ducklings, while remaining constant, but lower in the CA ducklings. Bill temperatures during re-feeding were similar to those prior to fasting, although in the CA ducklings fluctuated heavily as shown in the sample thermal image.
4. Discussion
Thermoregulatory changes in ducklings were profoundly related to both energy status and acclimation temperature. Fasting led to progressive declines in body mass, as well as profound changes in thermoregulatory parameters. Mean Tb declined throughout the 4-day fast, while diurnal variability (e.g. Amplitude) rose. The greater day-to-night variability with fasting was likely reflective of adjustments in Tb set point, where nocturnal anapyrexia was progressively related to the duration of fasting. Ultradian Tb variation was generally lower during the fasting period, especially in CA birds. During re-feeding, CA ducklings more rapidly recovered body mass and exhibited a sustained overshoot in Tb compared with TN ducklings, presumably due to an excessive digestion-related thermogenesis from the more rapid reprovisioning and biosynthesis. Although our measurements involved a temperature sensor within the crop, ingestion of cool water did not account for any of the reductions in Tb, suggesting that this approach provides a robust measure of core Tb (see electronic supplementary material).
(a). Temperature changes during fasting
The decline in Tb that occurred with fasting was unaffected by acclimation temperature, although the diurnal amplitude in Tb was higher with CA. Previous work has shown that both low ambient temperature and fasting lead to nocturnal anapyrexia in Japanese quail and that they will act synergistically [8]. The energetic benefits of nocturnal anapyrexia were estimated to be approximately 27% energy savings for birds below the TNZ and approximately 16% energy savings for those within the TNZ. Similarly, in barn owls, Tb declines diurnally in the inactive phase during fasting, associated with total metabolic rate decreases of approximately 40% [9]. Bill surface temperatures during fasting suggest that TN ducklings decrease blood flow to their thermal windows (figure 3), thereby reducing thermal conductance. This effect is not apparent in CA ducklings, presumably because vasoconstriction is already maximal in the cold. Interestingly, Japanese quail at TN temperatures exhibited much lower Tb decline, yet still significant energetic savings during fasting [8], which suggests a change in thermal conductance. Our surface temperature results offer a potential explanation for how fasting-induced decreases in thermal conductance can allow birds to reduce night-time heat production and effect energy savings while still exhibiting relatively low diurnal changes in Tb.
(b). Short-term Tb fluctuations
When fed, CA ducklings have higher ADT compared with TN ducklings, therefore, the more costly energetic demands of endothermic homeothermy in the cold are mediated through more intense bouts of thermogenesis. During fasting, however, these short-term fluctuations are more dramatically reduced in CA ducklings and inversely related to the degree of diurnal Tb variation (electronic supplementary material, figure S2). The net result is that fasting enhances short-term homeothermy. These ultradian rhythms in Tb are reflective of orchestrated changes in underlying neurophysiological adjustments. Blessing et al. [19,20] argued that short-term fluctuations in Tb are part of a brain-coordinated rest–activity cycle where non-shivering thermogenesis is activated, which episodically warms the brain in a manner associated with prior hippocampal activation rather than thermoregulatory control. Therefore, although these ultradian rhythms in Tb are not necessarily thermoregulatory in nature they would incur energetic costs, and their diminution appears to be a mode of energetic conservation only recruited in the cold. Nevertheless, changes in thermoregulatory control likely accompany fasting. Heller [44] demonstrated that the threshold spinal temperature in pigeons (a proxy for Tb set point) progressively reduces with nights into a fast. Therefore, the decline in short-term Tb fluctuations that accompanies fasting in CA ducklings must also reflect altered regulation of threshold temperatures that activate thermogenesis. It is plausible, therefore, that CA ducklings alter their vigilance states more than TN ducklings [19], leading to longer periods of reduced vigilance states where the Tb set point is reduced. In terms of energetics, we estimate the energy cost associated with the heating portion of ultradian Tb rhythms to be approximately 7–10.5% of BMR. The reduction in ultradian Tb rhythms during fasting is imperceptible in TN ducklings (electronic supplementary material, figure S3), but in CA ducklings leads to a profound reduction in the estimated energetic costs of warming (from 10.5% of BMR down to 7% of BMR), independent of the metabolic savings that are traditionally associated with the large diurnal Tb changes.
5. Conclusion
The results of this study demonstrate the dynamic nature of homeothermy in an avian model of fasting. We describe two novel thermoregulatory efficiencies of fasting. Under TN conditions, blood perfusion to thermal windows is reduced, which would lower thermal conductance and permit the maintenance of Tb at lower energetic costs. We also describe how short-term Tb changes are reduced in CA, fasted birds. To our knowledge, an explicit, proximate cost of homeothermy has not previously been estimated, although thermoregulatory states like fever have been shown to be energetically costly (30% rise for a 1.4°C change in Tb in Pekin ducks [45]). Future research using long-term records of Tb could make use of ultradian rhythms to assess energy-sparing strategies to novel stressors.
Supplementary Material
Ethics
The present investigation was carried out according to the ethical principles of the French (Ministère de l'Agriculture) and European Convention for the Protection of Vertebrate Animals Used for Experimental and Scientific Purposes. The protocol has been approved by the Ethical Committee of Claude Bernard University (authorization number: DR2013-54).
Data accessibility
Data from this study are available at the following repository: http://dx.doi.org/10.5061/dryad.gt5c0 [46].
Authors' contributions
G.J.T., L.T. and D.R. carried out the laboratory work, participated in data analysis; G.J.T., L.T., D.R. and Y.V. participated in the design of the study; G.J.T. carried out the statistical analyses and drafted the manuscript; G.J.T. and L.T. conceived of and designed the study. All authors were involved in the interpretation of the data, edited the manuscript and provided final approval for publication.
Competing interests
We have no competing interests.
Funding
We thank the Université de Lyon for providing financial support for the visiting professorship period that supported this research. The research programme of G.J.T. is supported by the Natural Sciences and Engineering Research Council of Canada (RGPIN-2014-05814).
References
- 1.Geiser F. 2004. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239–274. ( 10.1146/annurev.physiol.66.032102.115105) [DOI] [PubMed] [Google Scholar]
- 2.Heldmaier G, Ortmann S, Elvert R. 2004. Natural hypometabolism during hibernation and daily torpor in mammals. Respir. Physiol. Neurobiol. 141, 317–329. ( 10.1016/j.resp.2004.03.014) [DOI] [PubMed] [Google Scholar]
- 3.Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. 2011. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331, 906–909. ( 10.1126/science.1199435) [DOI] [PubMed] [Google Scholar]
- 4.Tattersall GJ, Sinclair BJ, Withers PC, Fields PA, Seebacher F, Cooper CE, Maloney SK. 2012. Coping with thermal challenges: physiological adaptations to environmental temperatures. Compr. Physiol. 2, 2151–2202. ( 10.1002/cphy.c110055) [DOI] [PubMed] [Google Scholar]
- 5.Ivanov KP. 2006. The development of the concepts of homeothermy and thermoregulation. J. Therm. Biol. 31, 24–29. ( 10.1016/j.jtherbio.2005.12.005) [DOI] [Google Scholar]
- 6.McCue MD. 2010. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 156, 1–18. ( 10.1016/j.cbpa.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Hung CC, Randall DJ. 2006. The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol. 68, 223–251. ( 10.1146/annurev.physiol.68.040104.105739) [DOI] [PubMed] [Google Scholar]
- 8.Ben-Hamo M, Pinshow B, McCue MD, McWilliams SR, Bauchinger U. 2010. Fasting triggers hypothermia, and ambient temperature modulates its depth in Japanese quail Coturnix japonica. Comp. Biochem. Physiol. A 156, 84–91. ( 10.1016/j.cbpa.2009.12.020) [DOI] [PubMed] [Google Scholar]
- 9.Thouzeau C, Duchamp C, Handrich Y. 1999. Energy metabolism and body temperature of barn owls fasting in the cold. Physiol. Biochem. Zool. 72, 170–178. ( 10.1086/316659) [DOI] [PubMed] [Google Scholar]
- 10.Prinzinger R, Goppel R, Lorenz A, Kulzer E. 1981. Body temperature and metabolism in the red-backed mousebird (Colius castanotus) during fasting and torpor. Comp. Biochem. Phys. A 69, 689–692. ( 10.1016/0300-9629(81)90157-2) [DOI] [Google Scholar]
- 11.Rashotte ME, Pastukhov IF, Poliakov EL, Henderson RP. 1998. Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columba livia). Am. J. Physiol. 275, R1690–R1702. [DOI] [PubMed] [Google Scholar]
- 12.Aitboulahsen A, Garlich JD, Edens FW. 1989. Effect of fasting and acute heat-stress on body temperature, blood acid–base and electrolyte status in chickens. Comp. Biochem. Phys. A 94, 683–687. ( 10.1016/0300-9629(89)90617-8) [DOI] [PubMed] [Google Scholar]
- 13.Hohtola E, Hissa R, Pyörnilä A, Rintamäko H, Saarela S. 1991. Nocturnal hypothermia in fasting Japanese quail: the effect of ambient temperature. Physiol. Behav. 49, 563–567. ( 10.1016/0031-9384(91)90281-R) [DOI] [PubMed] [Google Scholar]
- 14.Laurila M, Pilto T, Hohtola E. 2005. Testing the flexibility of fasting-induced hypometabolism in birds: effect of photoperiod and repeated food deprivations. J. Therm. Biol. 30, 131–138. ( 10.1016/j.jtherbio.2004.09.002) [DOI] [Google Scholar]
- 15.Graf R, Krishna S, Heller HC. 1989. Regulated nocturnal hypothermia induced in pigeons by food-deprivation. Am. J. Physiol. 256, R733–R738. [DOI] [PubMed] [Google Scholar]
- 16.Reinertsen RE. 1996. Physiological and ecological aspects of hypothermia. In Avian energetics and nutritional ecology, pp. 125–157. New York, NY: Chapman & Hall. [Google Scholar]
- 17.Refinetti R. 1997. Homeostasis and circadian rhythmicity in the control of body temperature. In Annals of the New York Academy of Sciences. Thermoregulation: Tenth Int. Symp. on the Pharmacology of Thermoregulation (ed. Blatteis CM.), pp. 63–70. New York, NY: New York Academy of Science. [DOI] [PubMed] [Google Scholar]
- 18.Refinetti R. 1999. Amplitude of the daily rhythm of body temperature in eleven mammalian species. J. Therm. Biol. 24, 477–481. ( 10.1016/S0306-4565(99)00077-7) [DOI] [Google Scholar]
- 19.Blessing W, Mohammed M, Ootsuka Y. 2012. Heating and eating: brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest–activity cycle in rats. Physiol. Behav. 105, 966–974. ( 10.1016/j.physbeh.2011.11.009) [DOI] [PubMed] [Google Scholar]
- 20.Ootsuka Y, de Menezes RC, Zaretsky DV, Alimoradian A, Hunt J, Stefanidis A, Oldfield BJ, Blessing WW. 2009. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest–activity cycle. Neuroscience 164, 849–861. ( 10.1016/j.neuroscience.2009.08.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hohtola E. 2012. Thermoregulatory adaptations to starvation in birds. In Comparative physiology of fasting, starvation, and food limitation (ed. McCue MD.), pp. 155–170. Berlin, Germany: Springer. [Google Scholar]
- 22.Dewasmes G, Cohenadad F, Koubi H, Lemaho Y. 1984. Sleep changes in long-term fasting geese in relation to lipid and protein metabolism. Am. J. Physiol. 247, R663–R671. [DOI] [PubMed] [Google Scholar]
- 23.Lavie P, Kripke DF. 1981. Ultradian circa 112 hours rhythms: a multioscillatory system. Life Sci. 29, 2445–2450. ( 10.1016/0024-3205(81)90698-6) [DOI] [PubMed] [Google Scholar]
- 24.Ho KY, Veldhuis JD, Johnson ML, Furlanetto R, Evans WS, Alberti KG, Thorner MO. 1988. Fasting enhances growth hormone secretion and amplifies the complex rhythms of growth hormone secretion in man. J. Clin. Invest. 81, 968–975. ( 10.1172/JCI113450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefcourt AM, Huntington JB, Akers RM, Wood DL, Bitman J. 1999. Circadian and ultradian rhythms of body temperature and peripheral concentrations of insulin and nitrogen in lactating dairy cows. Domest. Anim. Endocrinol. 16, 41–55. ( 10.1016/S0739-7240(98)00047-2) [DOI] [PubMed] [Google Scholar]
- 26.McCue MD, Amaya JA, Yang AS, Erhardt EB, Wolf BO, Hanson DT. 2013. Targeted C-13 enrichment of lipid and protein pools in the body reveals circadian changes in oxidative fuel mixture during prolonged fasting: a case study using Japanese quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 166, 546–554. ( 10.1016/j.cbpa.2013.08.009) [DOI] [PubMed] [Google Scholar]
- 27.Cadena V, Tattersall GJ. 2014. Body temperature regulation during acclimation to cold and hypoxia in rats. J. Therm. Biol. 46, 56–64. ( 10.1016/j.jtherbio.2014.10.007) [DOI] [PubMed] [Google Scholar]
- 28.Petrovski DV, Novikov EA, Burns JT, Moshkin MP. 2010. Wintertime loss of ultradian and circadian rhythms of body temperature in the subterranean euthermic mole vole, Ellobius talpinus. Chronobiol. Int. 27, 879–887. ( 10.3109/07420521003786792) [DOI] [PubMed] [Google Scholar]
- 29.Burness G, Huard JR, Malcolm E, Tattersall GJ. 2013. Post-hatch heat warms adult beaks: irreversible physiological plasticity in Japanese quail. Proc. R. Soc. B 280, 20131436 ( 10.1098/Rspb.2013.1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teulier L, Rouanet JL, Letexier D, Romestaing C, Belouze M, Rey B, Duchamp C, Roussel D. 2010. Cold-acclimation-induced non-shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J. Exp. Biol. 213, 2476–2482. ( 10.1242/jeb.043489) [DOI] [PubMed] [Google Scholar]
- 31.Teulier L, Rouanet JL, Rey B, Roussel D. 2014. Ontogeny of non-shivering thermogenesis in Muscovy ducklings (Cairina moschata). Comp. Biochem. Physiol. AMol. Integr. Physiol. 175, 82–89. ( 10.1016/j.cbpa.2014.05.012) [DOI] [PubMed] [Google Scholar]
- 32.Cornelissen G. 2014. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 11, 16 ( 10.1186/1742-4682-11-16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tattersall GJ, Andrade DV, Abe AS. 2009. Heat exchange from the toucan bill reveals a controllable vascular thermal radiator. Science 325, 468–470. ( 10.1126/science.1175553) [DOI] [PubMed] [Google Scholar]
- 34.Scott GR, Cadena V, Tattersall GJ, Milsom WK. 2008. Body temperature depression and peripheral heat loss accompany the metabolic and ventilatory responses to hypoxia in low and high altitude birds. J. Exp. Biol. 211, 1326–1335. ( 10.1242/Jeb.015958) [DOI] [PubMed] [Google Scholar]
- 35.Tattersall G. In press Infrared thermography: a non-invasive window into thermal physiology. Comp. Biochem. Physiol. A ( 10.1016/j.cbpa.2016.02.022) [DOI] [PubMed] [Google Scholar]
- 36.Burnham KP, Anderson DR, Burnham KP. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 37.Akaike H. 1973. Information theory as an extension of the maximum likelihood principle. In Second Int. Symp. on Information Theory (eds Petrov BN, Csaki F), pp. 267–281. Budapest, Hungary: Akademiai Kiado. [Google Scholar]
- 38.R Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Bartoń K. 2016. MuMIn: multi-model inference. R package version 1.15.6.
- 40.Knowles J, Frederick C. 2015. merTools: tools for analyzing mixed effect regression models. R package version 0.1.0.
- 41.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 42.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Thousand Oaks, CA: Sage. [Google Scholar]
- 43.Zuur AF. 2009. Mixed effects models and extensions in ecology with R. In Statistics for biology and health, p. 1 online resource New York, NY: Springer. [Google Scholar]
- 44.Heller HC. 1988. Sleep and hypometabolism. Can. J. Zool. 66, 61–69. ( 10.1139/z88-008) [DOI] [Google Scholar]
- 45.Marais M, Maloney SK, Gray DA. 2011. The metabolic cost of fever in Pekin ducks. J. Therm. Biol. 36, 116–120. ( 10.1016/j.jtherbio.2010.12.004) [DOI] [Google Scholar]
- 46.Tattersall GJ, Roussel D, Voituron Y, Teulier L. 2016. Data from: Novel energy-saving strategies to multiple stressors in birds: the ultradian regulation of body temperature. Dryad Digital Repository. ( 10.5061/dryad.gt5c0) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available at the following repository: http://dx.doi.org/10.5061/dryad.gt5c0 [46].