Abstract
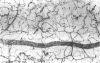
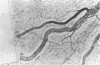
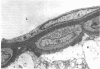
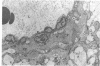
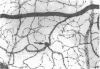
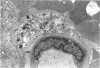
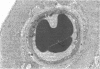
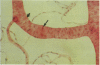
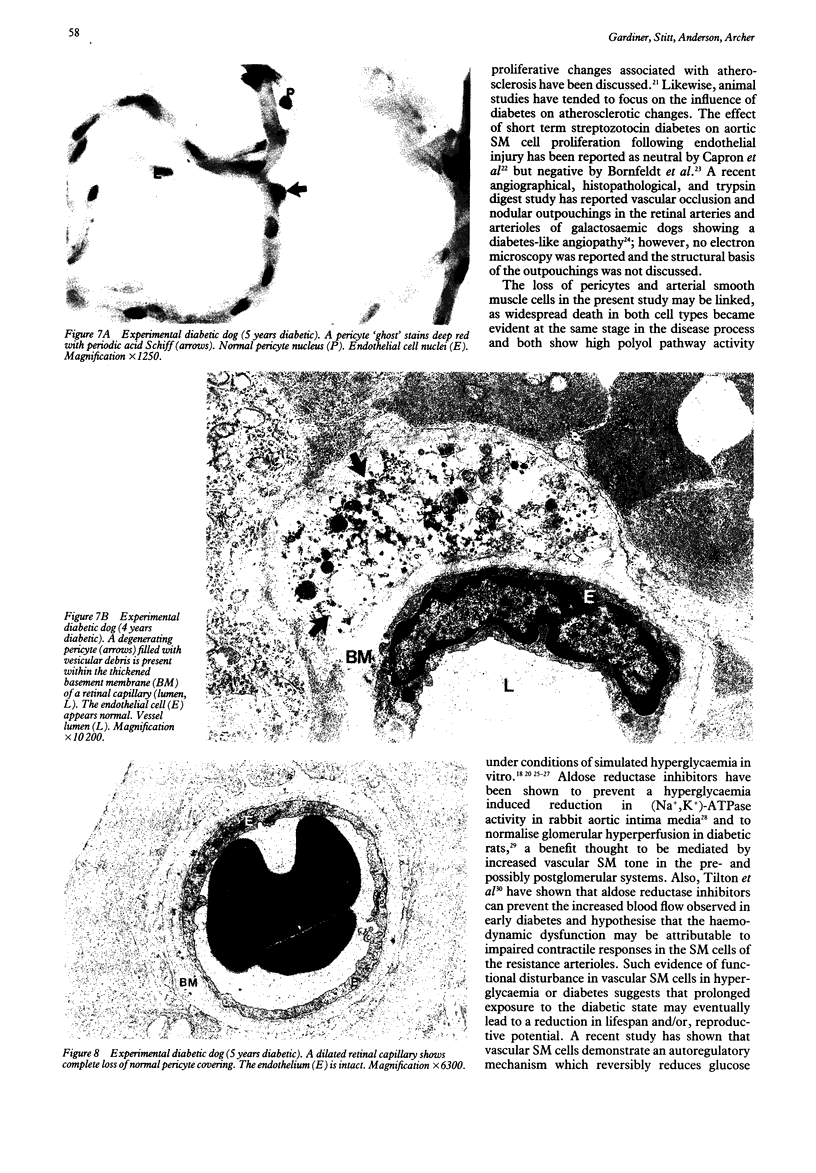
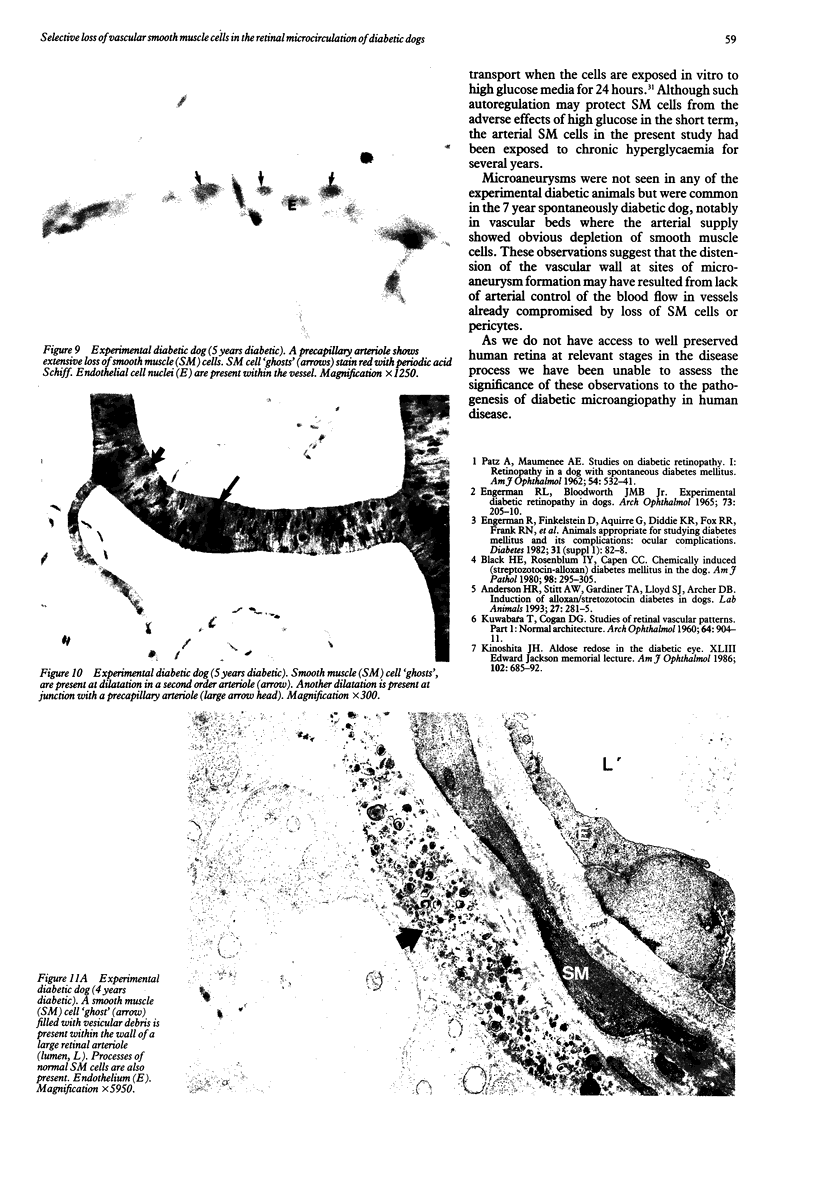
This study was undertaken to further characterise the fine structural changes occurring in the retinal circulation in early diabetes. The eyes of eight alloxan/streptozotocin and three spontaneously diabetic dogs were examined by trypsin digest and electron microscopy after durations of diabetes of between 1 and 7 years. Basement membrane (BM) thickening in the retinal capillaries was the only obvious fine structural change identified during the first 3 years of diabetes and was established within 1 year of induction. Widespread pericyte loss was noted after 4 years of diabetes and was paralleled by loss of smooth muscle (SM) cells, in the retinal arterioles. SM cell loss was most obvious in the smaller arterioles of the central retina. No microaneurysms were noted in the experimental diabetic dogs with up to 5 years' duration of diabetes but were widespread in a spontaneously diabetic animal at 7 years. This study has shown that SM cell loss, a hitherto unrecognised feature of diabetic microangiopathy, accompanies pericyte loss in the retinal circulation of diabetic dogs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi Y., Kador P. F., Kuwabara T., Kinoshita J. H. Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci. 1983 Nov;24(11):1516–1519. [PubMed] [Google Scholar]
- Anderson H. R., Stitt A. W., Gardiner T. A., Lloyd S. J., Archer D. B. Induction of alloxan/streptozotocin diabetes in dogs: a revised experimental technique. Lab Anim. 1993 Jul;27(3):281–285. doi: 10.1258/002367793780745426. [DOI] [PubMed] [Google Scholar]
- Bjerke T., Christensen E. I., Boye N. Tubular handling of neurotensin in the rat kidney as studied by micropuncture and HPLC. Am J Physiol. 1989 Jan;256(1 Pt 2):F100–F106. doi: 10.1152/ajprenal.1989.256.1.F100. [DOI] [PubMed] [Google Scholar]
- Black H. E., Rosenblum I. Y., Capen C. C. Chemically induced (streptozotocin-alloxan) diabetes mellitus in the dog. Biochemical and ultrastructural studies. Am J Pathol. 1980 Feb;98(2):295–310. [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt K. E., Arnqvist H. J., Capron L. In vivo proliferation of rat vascular smooth muscle in relation to diabetes mellitus insulin-like growth factor I and insulin. Diabetologia. 1992 Feb;35(2):104–108. doi: 10.1007/BF00402540. [DOI] [PubMed] [Google Scholar]
- Buzney S. M., Frank R. N., Varma S. D., Tanishima T., Gabbay K. H. Aldose reductase in retinal mural cells. Invest Ophthalmol Vis Sci. 1977 May;16(5):392–396. [PubMed] [Google Scholar]
- Capron L., Jarnet J., Kazandjian S., Housset E. Growth-promoting effects of diabetes and insulin on arteries. An in vivo study of rat aorta. Diabetes. 1986 Sep;35(9):973–978. doi: 10.2337/diab.35.9.973. [DOI] [PubMed] [Google Scholar]
- ENGERMAN R. L., BLOODWORTH J. M., Jr EXPERIMENTAL DIABETIC RETINOPATHY IN DOGS. Arch Ophthalmol. 1965 Feb;73:205–210. doi: 10.1001/archopht.1965.00970030207013. [DOI] [PubMed] [Google Scholar]
- Engerman R., Finkelstein D., Aguirre G., Diddie K. R., Fox R. R., Frank R. N., Varma S. D. Ocular complications. Diabetes. 1982;31(Suppl 1 Pt 2):82–88. doi: 10.2337/diab.31.1.s82. [DOI] [PubMed] [Google Scholar]
- Ganda O. P. Pathogenesis of macrovascular disease in the human diabetic. Diabetes. 1980 Nov;29(11):931–942. doi: 10.2337/diab.29.11.931. [DOI] [PubMed] [Google Scholar]
- Hohman T. C., Nishimura C., Robison W. G., Jr Aldose reductase and polyol in cultured pericytes of human retinal capillaries. Exp Eye Res. 1989 Jan;48(1):55–60. doi: 10.1016/0014-4835(89)90018-3. [DOI] [PubMed] [Google Scholar]
- Jiang Z. Y., Zhou Q. L., Eaton J. W., Koppenol W. H., Hunt J. V., Wolff S. P. Spirohydantoin inhibitors of aldose reductase inhibit iron- and copper-catalysed ascorbate oxidation in vitro. Biochem Pharmacol. 1991 Aug 22;42(6):1273–1278. doi: 10.1016/0006-2952(91)90265-7. [DOI] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960 Dec;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Kador P. F., Akagi Y., Takahashi Y., Ikebe H., Wyman M., Kinoshita J. H. Prevention of retinal vessel changes associated with diabetic retinopathy in galactose-fed dogs by aldose reductase inhibitors. Arch Ophthalmol. 1990 Sep;108(9):1301–1309. doi: 10.1001/archopht.1990.01070110117035. [DOI] [PubMed] [Google Scholar]
- Kaiser N., Sasson S., Feener E. P., Boukobza-Vardi N., Higashi S., Moller D. E., Davidheiser S., Przybylski R. J., King G. L. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993 Jan;42(1):80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- Kern T. S., Engerman R. L. Retinal polyol and myo-inositol in galactosemic dogs given an aldose-reductase inhibitor. Invest Ophthalmol Vis Sci. 1991 Dec;32(13):3175–3177. [PubMed] [Google Scholar]
- Kinoshita J. H. Aldose reductase in the diabetic eye. XLIII Edward Jackson memorial lecture. Am J Ophthalmol. 1986 Dec 15;102(6):685–692. doi: 10.1016/0002-9394(86)90394-6. [DOI] [PubMed] [Google Scholar]
- McCaleb M. L., McKean M. L., Hohman T. C., Laver N., Robison W. G., Jr Intervention with the aldose reductase inhibitor, tolrestat, in renal and retinal lesions of streptozotocin-diabetic rats. Diabetologia. 1991 Oct;34(10):695–701. doi: 10.1007/BF00401513. [DOI] [PubMed] [Google Scholar]
- Morrison A. D. Effect of inhibition of polyol pathway activity on aortic smooth muscle metabolism. Clin Invest Med. 1990 Jun;13(3):119–122. [PubMed] [Google Scholar]
- PATZ A., MAUMENEE A. E. Studies on diabetic retinopathy. I. Retinopathy in a dog with spontaneous diabetes mellitus. Am J Ophthalmol. 1962 Oct;54:532–541. [PubMed] [Google Scholar]
- Robison W. G., Jr, Kador P. F., Kinoshita J. H. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science. 1983 Sep 16;221(4616):1177–1179. doi: 10.1126/science.6612330. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I. Mechanisms in rabbit aorta for hyperglycaemia-induced alterations in angiotensin II and norepinephrine effects. Diabetologia. 1992 Aug;35(8):725–729. doi: 10.1007/BF00429091. [DOI] [PubMed] [Google Scholar]
- Stevens V. J., Rouzer C. A., Monnier V. M., Cerami A. Diabetic cataract formation: potential role of glycosylation of lens crystallins. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2918–2922. doi: 10.1073/pnas.75.6.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Wyman M., Ferris F., 3rd, Kador P. F. Diabeteslike preproliferative retinal changes in galactose-fed dogs. Arch Ophthalmol. 1992 Sep;110(9):1295–1302. doi: 10.1001/archopht.1992.01080210113037. [DOI] [PubMed] [Google Scholar]
- Tawata M., Ohtaka M., Hosaka Y., Onaya T. Aldose reductase mRNA expression and its activity are induced by glucose in fetal rat aortic smooth muscle (A10) cells. Life Sci. 1992;51(10):719–726. doi: 10.1016/0024-3205(92)90480-d. [DOI] [PubMed] [Google Scholar]
- Tilton R. G., Chang K., Pugliese G., Eades D. M., Province M. A., Sherman W. R., Kilo C., Williamson J. R. Prevention of hemodynamic and vascular albumin filtration changes in diabetic rats by aldose reductase inhibitors. Diabetes. 1989 Oct;38(10):1258–1270. doi: 10.2337/diab.38.10.1258. [DOI] [PubMed] [Google Scholar]
- Wilson D. K., Bohren K. M., Gabbay K. H., Quiocho F. A. An unlikely sugar substrate site in the 1.65 A structure of the human aldose reductase holoenzyme implicated in diabetic complications. Science. 1992 Jul 3;257(5066):81–84. doi: 10.1126/science.1621098. [DOI] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]