Abstract
Life-history theory predicts that nutrition influences lifespan owing to trade-offs between allocating resources to reproduction, growth and repair. Despite occasional reports that early diet has strong effects on lifespan, it is unclear whether this prediction is generally supported by empirical studies. We conducted a meta-analysis across experimental studies manipulating pre- or post-natal diet and measuring longevity. We found no overall effect of early diet on lifespan. We used meta-regression, considering moderator variables based on experimental and life-history traits, to test predictions regarding the strength and direction of effects that could lead to positive or negative effects. Pre-natal diet manipulations reduced lifespan, but there were no effects of later diet, manipulation type, development mode, or sex. The results are consistent with the prediction that early diet restriction disrupts growth and results in increased somatic damage, which incurs lifespan costs. Our findings raise a cautionary note, however, for placing too strong an emphasis on early diet effects on lifespan and highlight limitations of measuring these effects under laboratory conditions.
Keywords: early development, nutrition, caloric restriction, lifespan, meta-analysis
1. Introduction
Conditions in early development can influence a suite of life-history traits later in life, including the pace of ageing and total lifespan [1–3]. King penguin chicks that experience rapid catch-up growth have shorter telomeres [4], for example, and red deer born under harsh environmental conditions show faster senescence [5]. An important feature of early development is the amount and type of food received, which has immediate effects on growth and can influence later traits. Several studies have manipulated nutrition in early life—providing diets to pregnant mothers or to young before maturity—and measured offspring survival. These studies have traditionally been conducted on laboratory rodents [6], although there are an increasing number of manipulations on a range of species [7,8]. Despite occasional reports of strong effects [9,10], which have raised concerns in the health sciences [11], it is not yet known how general these effects are across biological systems.
Life-history theory provides a framework for understanding how and when early-life diet should influence lifespan. Individuals face trade-offs when allocating resources among traits that enhance growth and reproduction, versus those, such as somatic repair, that increase longevity [12]. Individuals who experience resource limitation in early life may invest in earlier reproduction, incur higher levels of damage and pay a cost of reduced lifespan [13]. Alternatively, those individuals with low resources during development may experience slower growth, delayed reproduction and live a longer life [14].
Whether restricted diet in early life per se, rather than nutritional limitation across development, extends or reduces lifespan depends on several factors. Reducing total energy content might extend lifespan through increasing allocation towards somatic repair [15]; whereas limiting key nutrients for healthy development, such as protein, might impose damage during development and reduce lifespan [16]. The diet experienced beyond early development is likely important. A switch from low to high nutrition can result in catch-up growth, which accrues costs later in life [17]. By contrast, being maintained on a low-nutrition diet could enhance lifespan-extending effects if individuals allocate more to repair [9]. There may also be sex differences in how individuals respond to dietary challenges [7,10]. Increased allocation to growth and reproduction may reduce lifespan to a greater extent for the sex experiencing stronger selection for condition-dependent traits or incurring higher energetic costs to reproduction. Continuous developers might have higher plasticity when conditions improve, compared with organisms with metamorphosis, where adult size is established by larval diet.
Here, we conducted a meta-analysis, selecting studies in which diet was manipulated during early development—at any period from early embryonic stages until age of first reproduction—and later longevity was recorded. We used meta-regression [18] to test hypotheses regarding the causes of heterogeneity across studies (table 1).
Table 1.
Rationale for predictor variables in meta-regression.
| predictor | rationale |
|---|---|
| manipulation type (diet quality or quantity) | If dietary restriction extends lifespan, expect positive effect for reduced quantity but not quality of food. By contrast, if certain nutrients have carry-over effects for individual quality, expect quality effect to be stronger. |
| post-treatment diet (control or restricted) | If dietary restriction extends lifespan, expect stronger positive effect if adults are on restricted diet too. If there is a cost of dietary mismatch, expect stronger negative effects when juveniles are on a restricted diet then adults are on a high-food diet. |
| sex | If restriction reduces lifespan owing to allocation trade-offs between growth and reproduction, predict stronger effect in males due to condition-dependent sexual selection; or in females if they experience high costs of reproduction. |
| manipulation stage (pre- or post-natal) | Predict stronger effect of pre-natal diet due to disruption of sensitive stages in development; alternatively predict weaker effect if mothers buffer offspring from nutritional stress. |
| vertebrate versus invertebrate | Expect positive or negative effect sizes to be greater in invertebrates because of indeterminate growth, hence less plasticity in response to early diet. |
| evidence for catch-up growth (yes, no or unknown) | Expect weaker effect if individuals compensate for effect of manipulation through catch-up growth. Alternatively, if catch-up growth incurs costs, expect stronger effect under catch-up growth. |
2. Material and methods
We conducted a comprehensive literature search on Google Scholar and scopus for studies linking early-life diet with longevity, based on keywords (‘ageing’, ‘compensatory growth’, ‘catch-up growth’, damage, development, ‘developmental programming’, ‘early life’, growth, lifespan, longevity, maternal, ‘maternal diet’, oxidative, senescence, stress, survival, telomere) and surveying papers cited by or in several key reviews. We only included studies that conducted a dietary manipulation on pregnant females or offspring before the age of sexual maturity. For studies that provided survival curves, we extracted the log hazards ratio, ln(HR), based on differences in percentage of experimental and control individuals alive at 75%, 50% and 25% of control group survival. However, not all studies report survival curves and we, therefore, repeated our analysis using mean longevity. Where data were provided separately for groups of individuals, for example, by sex, we calculated multiple effect sizes. In total, our search yielded 50 effect sizes of ln(HR) from 18 studies, and 77 effect sizes of mean longevity from 21 studies across 14 species (electronic supplementary material, table S1).
We used meta-regression to investigate whether the effect of early diet on longevity was mediated by manipulation type, post-treatment diet, sex, stage of manipulation, vertebrate versus invertebrate and whether catch-up growth was observed (table 1). We conducted Bayesian mixed-effects meta-analysis using the library MCMCglmm [19] in the statistical environment R (v. 2.15 [20]). We first fitted an intercept-only model to examine an overall effect of early diet on longevity. As ln(HR) provides a measure of risk of death, a negative effect indicates that diet manipulation extends lifespan. We then fitted a model including all moderators and examined their 95% higher posterior densities (HPDs, or credible intervals). Any moderators whose HPD did not overlap zero were considered statistically significant. We tested for publication bias by inspecting funnel plots and conducting Egger's regression [21]. We calculated marginal and conditional R2 to establish the total variance explained by fixed effects or both fixed and random terms in each model, respectively [22]. We included study as a random term. Further details are provided in the electronic supplementary material.
3. Results
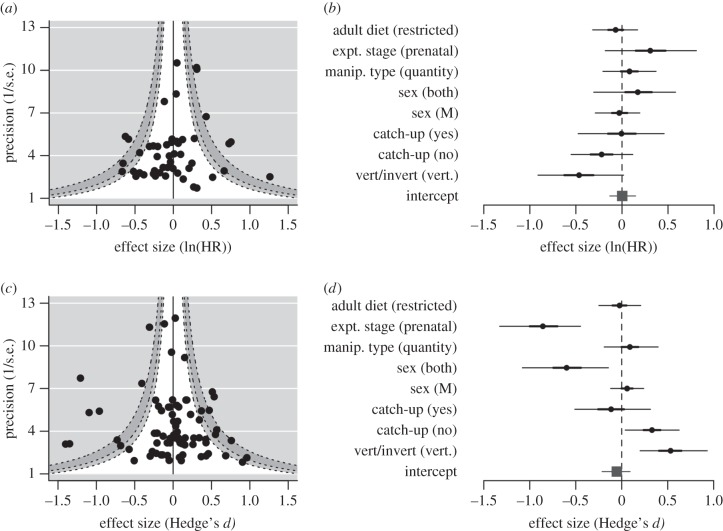
We found no overall effect of early diet on subsequent risk of death (HPD: −0.150, 0.125, figure 1a). The lack of effect may be due to heterogeneity among studies, for example, by combining studies where reducing calories increased lifespan and others where reducing specific nutrients reduced lifespan. Heterogeneity in the data was moderate (I2 = 57.7%, electronic supplementary material, table S2). In our meta-regression to examine differences between studies, we found no significant effects of moderators on risk of death (figure 1b). The marginal R2 was only 0.04 (electronic supplementary material, table S2).
Figure 1.
Funnel plot (a,c) of effect sizes against power, with counter-shaded confidence intervals (90%, 95% and 99% CI) and forest plot (b,d) of HPD intervals (posterior mean and 95% CI) in the meta-analysis on ln(HR) and mean longevity.
We did not find an overall effect of early diet on mean longevity (HPD: −0.200, 0.101, figure 1c), and these data had low heterogeneity (I2 = 32.5%, electronic supplementary material, table S2). Several moderators had significant effects (figure 1d), although the marginal R2 was only 0.08 (electronic supplementary material, table S2). Early diet restriction extended longevity to a greater extent in vertebrates than in invertebrates (HPD: 0.219, 0.944), and when there was no catch-up growth (HPD: 0.039, 0.624). Longevity was reduced when dietary restriction occurred before birth (HPD: −1.343, −0.471), and in studies combining both sexes (HPD: −1.086, −0.152).
To understand contrasting results in models analysing ln(HR) and longevity, we repeated our analysis on those studies measuring both. Only the effect of pre- versus post-natal stage on longevity remained significant (HPD: −1.069, −0.024). Publication bias was weak or absent.
4. Discussion
The impact of early-life nutrition has recently come to the forefront of concerns regarding healthy ageing [23]. Life-history theory provides explanations for why early diet restriction should influence lifespan [13,15]. However, we find that experimental studies generally fail to demonstrate these effects. A plausible explanation for the lack of an overall effect is that positive and negative effects cancel out. Indeed, there are evolutionary rationales for expecting opposite patterns across studies. We found little evidence, however, that these factors explain the overall lack of an effect of early diet on mortality risk and longevity. The general conclusion of narrative reviews, that early nutrition affects later-life mortality [6], thus appears to be driven by a small number of key studies (e.g. [8,9]; electronic supplementary material, figure S2).
While it is tempting to draw conclusions about the evolutionary basis for early diet effects on lifespan, studies testing these effects are almost always conducted under laboratory conditions. In the laboratory, causes of mortality typical of natural conditions are absent, and individuals experience predictable food, no predation and a different reproductive regime to that in the wild. Thus, evidence for weak or absent effects in laboratory studies may simply be due to the fact that intrinsic damage may not be sufficiently strong to cause increased mortality risk [24].
Nevertheless, our analysis identified predictors of the effect of early-life diet on mean longevity. We found pre-natal diet manipulations had stronger negative effects compared with post-natal manipulations. Thus, at least in live-bearing species, mothers do not fully buffer their offspring from nutritional stress. This is also consistent with observations that variations in biomarkers of ageing, such as telomere length, primarily accrue early in life [4]. Early diet extends lifespan in vertebrate but not invertebrate species, potentially because juvenile and adult functions are decoupled through metamorphosis. Our results may thus be more easily interpreted in the light of mechanistic theory concerning the link between diet, damage reduction and lifespan [25] than broader life-history explanations.
Our analyses suggest weak general evidence that nutrient reduction early in life influence lifespan. Whatever effects exist, and we have theoretical reasons to believe that they should, may be specific to the study system. This conclusion is similar to a recent extensive meta-analysis on lifespan-enhancing effects of diet restriction [26]. This study found that protein restriction had stronger life-extending effects than caloric restriction, yet replication in our study was not sufficient to make this comparison. Indeed, animals show plasticity in growth and development across their life, such that single effects of diet restriction may be weak and context-dependent. Insofar as laboratory conditions are informative, the overall evidence as it stands does not provide strong support that food restriction during development causes major effects on adult intrinsic mortality or lifespan.
Supplementary Material
Acknowledgements
We are grateful to all authors who contributed data (listed in the electronic supplementary material) and to Shinichi Nakagawa for statistical advice.
Data accessibility
Data and code are available on GitHub [27]: https://github.com/sineadenglish/early-diet-longevity.
Authors' contributions
S.E. and T.U. designed the study. S.E. extracted the data and conducted the analysis. S.E. and T.U. wrote the paper. Both authors approve the final version of the manuscript and agree to be held accountable for the content therein.
Competing interests
We declare we do not have competing interests.
Funding
This research was funded by the EU FP7 programme (agreement 259679, IDEAL). S.E. and T.U. were supported by the Royal Society of London. T.U. received support from the Knut and Alice Wallenberg Foundations.
References
- 1.Ricklefs RE. 2010. Embryo growth rates in birds and mammals. Funct. Ecol. 24, 588–596. ( 10.1111/j.1365-2435.2009.01684.x) [DOI] [Google Scholar]
- 2.Barnes SK, Ozanne SE. 2011. Pathways linking the early environment to long-term health and lifespan. Prog. Biophys. Mol. Biol. 106, 323–336. ( 10.1016/j.pbiomolbio.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe N, Monaghan P. 2003. Growth versus lifespan: perspectives from evolutionary ecology. Exp. Gerontol. 38, 935–940. ( 10.1016/S0531-5565(03)00159-1) [DOI] [PubMed] [Google Scholar]
- 4.Geiger S, LE Vaillant M, Lebard T, Reichert S, Stier A, Le Maho Y, Criscuolo F. 2012. Catching-up but telomere loss: half-opening the black box of growth and ageing trade-off in wild king penguin chicks. Mol. Ecol. 21, 1500–1510. ( 10.1111/j.1365-294X.2011.05331.x) [DOI] [PubMed] [Google Scholar]
- 5.Nussey DH, Kruuk LEB, Morris A, Clutton-Brock TH. 2007. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 17, R1000–R1001. ( 10.1016/j.cub.2007.10.005) [DOI] [PubMed] [Google Scholar]
- 6.Sayer AA, Cooper C. 2002. Early diet and growth: impact on ageing. Proc. Nutr. Soc. 61, 79–85. ( 10.1079/PNS2001138) [DOI] [PubMed] [Google Scholar]
- 7.Houslay TM, Hunt J, Tinsley MC, Bussière LF. 2015. Sex differences in the effects of juvenile and adult diet on age-dependent reproductive effort. J. Evol. Biol. 28, 1067–1079. ( 10.1111/jeb.12630) [DOI] [PubMed] [Google Scholar]
- 8.Saastamoinen M, Hirai N, van Nouhuys S. 2013. Direct and trans-generational responses to food deprivation during development in the Glanville fritillary butterfly. Oecologia 171, 93–104. ( 10.1007/s00442-012-2412-y) [DOI] [PubMed] [Google Scholar]
- 9.Ozanne SE, Hales CN. 2004. Lifespan: catch-up growth and obesity in male mice. Nature 427, 411–412. ( 10.1038/427411b) [DOI] [PubMed] [Google Scholar]
- 10.Boggs CL, Freeman KD. 2005. Larval food limitation in butterflies: effects on adult resource allocation and fitness. Oecologia 144, 353–361. ( 10.1007/s00442-005-0076-6) [DOI] [PubMed] [Google Scholar]
- 11.Mentis A-FA, Kararizou E. 2010. Does ageing originate in utero? Biogerontology 11, 725–729. ( 10.1007/s10522-010-9293-4) [DOI] [PubMed] [Google Scholar]
- 12.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer Associates Inc. [Google Scholar]
- 13.Kirkwood T. 1977. Evolution of ageing. Nature 270, 301–304. ( 10.1038/270301a0) [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood TBL. 2005. Understanding the odd science of aging. Cell 120, 437–447. ( 10.1016/j.cell.2005.01.027) [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood TBL, Shanley DP. 2005. Food restriction, evolution and ageing. Mech. Ageing Dev. 126, 1011–1016. ( 10.1016/j.mad.2005.03.021) [DOI] [PubMed] [Google Scholar]
- 16.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503. ( 10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalfe NB, Monaghan P. 2001. Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254–260. ( 10.1016/S0169-5347(01)02124-3) [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S, Santos ESA. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 19.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 20.R Development Core Team. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Development Core Team; http://www.R-project.org. [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634. ( 10.1136/bmj.315.7109.629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 23.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73. ( 10.1056/NEJMra0708473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speakman JR, et al. 2015. Oxidative stress and life histories: unresolved issues and current needs. Ecol. Evol. 5, 5745–5757. ( 10.1002/ece3.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavrilov LA, Gavrilova N. 2003. Early-life factors modulating lifespan. In Modulating aging and longevity (ed. Rattan SIS.), pp. 27–50. Dordecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- 26.Nakagawa S, Lagisz M, Hector KL, Spencer HG. 2012. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell 11, 401–409. ( 10.1111/j.1474-9726.2012.00798.x) [DOI] [PubMed] [Google Scholar]
- 27.English S. 2016. Code and data from: Does early-life diet affect longevity? A meta-analysis across experimental studies. Zenodo: https://github.com/sineadenglish/early-diet-longevity. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available on GitHub [27]: https://github.com/sineadenglish/early-diet-longevity.