Abstract
Costs of reproduction are expected to be ubiquitous in wild animal populations and understanding the drivers of variation in these costs is an important aspect of life-history evolution theory. We use a 43 year dataset from a wild population of red deer to examine the relative importance of two factors that influence the costs of reproduction to mothers, and to test whether these costs vary with changing ecological conditions. Like previous studies, our analyses indicate fitness costs of lactation: mothers whose calves survived the summer subsequently showed lower survival and fecundity than those whose calves died soon after birth, accounting for 5% and 14% of the variation in mothers' survival and fecundity, respectively. The production of a male calf depressed maternal survival and fecundity more than production of a female, but accounted for less than 1% of the variation in either fitness component. There was no evidence for any change in the effect of calf survival or sex with increasing population density.
Keywords: cost of reproduction, Cervus elaphus, sex allocation, wild ungulate population
1. Introduction
An understanding of the costs of reproduction is fundamental to life-history evolution theory [1]. The energetic costs of raising offspring increase as they progress through the period of parental investment, generating fitness costs for the parents [2]. These costs can vary with the characteristics of both the parents (such as their age [3] or social dominance [4]) and the offspring (such as their size or sex [5]). For example, in sexually size-dimorphic species, offspring of the larger sex commonly require more resources, and producing and rearing them can depress mothers' subsequent survival or breeding success, with implications for sex ratio evolution [4,6,7]. Reproductive costs may be ecologically or physiologically mediated [8] and may also vary with environmental conditions [9,10] though we know little about the relative magnitude of these effects.
Long-term, individual-based studies provide an excellent opportunity to explore the costs of reproduction in wild animals [3,9,10]. Here, we extend earlier work on red deer on the Isle of Rum which has shown that reproduction generates substantial costs to mothers' subsequent survival and fecundity, which vary with both the longevity of the offspring and its sex [4,5,11]. We add an additional 26 years' data and use novel statistical methods to quantify the relative magnitude of costs to maternal survival and fecundity. In addition, we investigate whether the costs of rearing offspring and the relative costs of producing sons and daughters changed with population density, which increased over the study period [12].
2. Material and methods
The unmanaged population of red deer in the North Block of the Isle of Rum, Scotland, has been studied since 1971, with survival and reproductive history of individuals known from regular censuses [4,11]. Females that conceive during the autumn rut give birth to a single calf in May–June, and approximately 10% of calves die in their first two weeks of life. Winter mortality affecting all ages occurs January–March. We used data on all females (aged 3–18 years) that gave birth to a calf of known sex from 1971–2013 inclusive [13].
We examined the effects of calf survival and calf sex in a given year on the subsequent survival and fecundity of the mother. Maternal survival was assessed as survival to 1 May the following year (n = 2888 observations of 636 females), and fecundity by whether she gave birth to a calf the following year, conditional on her survival (n = 2600 observations of 602 females). Calf sex and calf survival to 1 October of the year they were born were included in the models as 2-level fixed factors. Maternal age (linear and quadratic), population size (over the subsequent winter; see the electronic supplementary material) and calf birth date (days since 1 May) were included as fixed covariates. Year was fitted as a random multi-level factor in both models, and maternal identity was fitted as a random term in the fecundity model (see the electronic supplementary material). We tested for differential costs of male and female calves depending on whether they survived to 1 October by including an interaction between calf sex and calf survival in each model, and for changes in costs across varying population densities by including an interaction between either variable and population size.
Maternal survival and fecundity were modelled as binary traits in Bayesian generalized linear mixed models (GLMMs) with the R package MCMCglmm [14], using the categorical family and logit link function. Continuous predictor variables were mean centred prior to inclusion in models. Parameter estimates are presented as the posterior mode with 95% credible intervals of 2000 samples with minimal autocorrelation (iterations: 1.1 × 106; burn-in: 1 × 105; thinning interval: 500). Marginal R2 indicates the percentage of variance explained by the fixed effect component of a model, and can be estimated for GLMMs as the variance of the fixed effects divided by the total variance (×100), calculated on the link scale [15]. We used the change in marginal R2 (ΔR2) when each fixed effect was dropped from the model in turn to estimate the variance in maternal survival and fecundity explained by each of the fixed effects.
3. Results
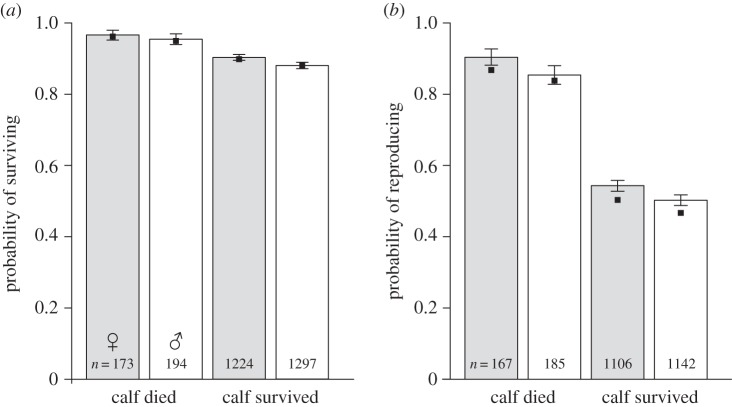
Mothers of calves that survived to 1 October were less likely to survive the next winter (figure 1a; PMCMC < 0.001) or to breed again the following year (figure 1b; PMCMC < 0.001) than mothers whose calves died during the course of the summer; this cost explained a substantial proportion of variation in maternal survival and fecundity (ΔR2 = 5% and 14%, respectively; table 1). Mothers that gave birth to a male calf were also less likely to survive to the following year (figure 1a; PMCMC = 0.023), and less likely to give birth the following spring if they did survive (figure 1b; PMCMC = 0.003). However, calf sex explained less than 1% of the variation both in survival probability and fecundity (table 1). There was no significant interaction between the effects of calf sex and calf survival on maternal survival or fecundity (electronic supplementary material, table S1). In addition, there was no evidence for a significant interaction between population size and the effect of either calf survival or calf sex on either maternal survival or fecundity (electronic supplementary material, table S1). For a summary of other fixed effects, see the electronic supplementary material.
Figure 1.
Effects of calf sex and calf survival on (a) maternal survival and (b) fecundity the year after giving birth. Bars show raw data with standard errors; filled bars represent female calves, and unfilled bars represent males. Black squares show predictions from models incorporating other variables.
Table 1.
Summary of fixed and random effects from generalized linear mixed models (GLMMs) of maternal survival and fecundity the year after giving birth to a calf. (‘Parameter estimate’ gives the mode of the posterior distribution for the coefficient of that variable; parameter estimates are on the link scale for the GLMM (logit link for binomial errors). ΔR2 shows the change in marginal R2 (which is a %) when each fixed effect is dropped from the model in turn.)
| variable | parameter estimate | lower CI | upper CI | PMCMC | ΔR2 | |
|---|---|---|---|---|---|---|
| survival | n = 2888 (636 females) | marginal R2 = 27.64% | ||||
| random effects | year | 0.638 | 0.267 | 1.099 | ||
| fixed effects | age | 0.421 | 0.202 | 0.666 | <0.001 | −16.66 |
| age2 | −0.038 | −0.051 | −0.027 | <0.001 | ||
| population size | −0.014 | −0.025 | −0.003 | 0.004 | −2.79 | |
| calf birth date | −0.021 | −0.030 | −0.014 | <0.001 | −2.21 | |
| calf sex: male | −0.384 | −0.705 | −0.080 | 0.023 | −0.65 | |
| calf survival | −1.844 | −2.582 | −1.198 | <0.001 | −5.03 | |
| fecundity | n = 2600 (602 females) | marginal R2 = 29.04% | ||||
| random effects | year | 0.754 | 0.348 | 1.172 | ||
| maternal ID | 1.963 | 1.381 | 2.612 | |||
| fixed effects | age | 0.901 | 0.697 | 1.088 | <0.001 | −3.55 |
| age2 | −0.054 | −0.065 | −0.043 | <0.001 | ||
| population size | −0.029 | −0.040 | −0.019 | <0.001 | −7.19 | |
| calf birth date | −0.043 | −0.052 | −0.034 | <0.001 | −5.55 | |
| calf sex: male | −0.347 | −0.570 | −0.085 | 0.003 | −0.50 | |
| calf survival | −3.564 | −4.077 | −3.071 | <0.001 | −14.38 | |
4. Discussion
Successful reproduction was costly for red deer females in terms of future survival and fecundity (figure 1 and table 1). Whether or not the calf was alive at the onset of autumn was the greatest determinant of these post-parturition costs, with mothers of calves that survived to 1 October being 6.5% less likely to survive the winter and 36.7% less likely to give birth the following year. This result supports previous findings from this study population and is presumably a consequence of the substantial energetic costs of lactation [11,16]. More than 85% of calves that die in summer die within two weeks of birth, meaning their mothers experience minimal costs of lactation. If calves survive to 1 October, they usually survive the next few months (80% of winter deaths occur in February–April), meaning their mothers bear the full costs of lactation, potentially lactating through the winter months if they fail to conceive again [16].
Producing male calves was more costly than producing female calves (figure 1) [4,5]. Our analysis shows that the effect of calf sex was small in comparison with that of calf survival, explaining less than 1% of the variation in subsequent maternal survival and fecundity (table 1). Previous work suggests that the additional costs of raising sons are greater for subordinate mothers than for dominants so that the relative costs of raising sons may vary in relation to the mother's phenotype [4]. We found no evidence of any interaction between the effects of calf sex and calf survival, suggesting that the relative cost of males was the same regardless of how long they lived. The same was true if we considered only whether the calf survived beyond its first two weeks, so the relative cost of males did not increase even if their mothers experienced the main period of lactation (from birth to three months). One possible interpretation of these results is that the difference in cost of male versus female offspring is generated during gestation (male calves are approx. 5.5% heavier at birth). However, evidence that costs of gestation are small in comparison with those of lactation [11] and that sons suck more than daughters [5] suggest that this is unlikely; the lack of interaction may therefore reflect a lack of statistical power given the small magnitude of the main effect of calf sex.
Our analyses showed strong associations with calf birth date: mothers of early-born calves were more likely to survive and to give birth the following year (explaining 2% and 5% of the variance, respectively; table 1). This effect could be driven by differences in female condition, since mothers in good condition are likely to conceive and give birth earlier, and also have higher future survival and fecundity. Further analysis revealed that the effect of calf birth date was dependent on calf survival, only being significant when the calf survived beyond 1 October (electronic supplementary material, table S2), which suggests that mothers in poor condition (who give birth later) suffer higher costs of successful reproduction, as has also been observed in other ungulates [17].
We found evidence of density-dependence in maternal survival and fecundity (table 1). However, although the effect of calf sex on fecundity was non-significant in the most recent 26 years of data added for this analysis, when population density was high (−0.223 [−0.494− 0.085], PMCMC = 0.137; electronic supplementary material, table S3), there is no indication that the costs associated with calf survival or sex varied with increasing density (electronic supplementary material, table S1). A possible explanation of this effect is that maternal investment in lactation is adjusted to the mother's food intake so that variation in food availability affects the growth and survival of calves rather than the survival and fecundity of their mothers [18].
Several other studies of sexually dimorphic mammals have shown that mothers invest more energy in sons than daughters [19] but relatively few have been able to investigate whether this affects maternal fecundity [9] or survival [20]. Our ability to detect survival costs is unusual for such a long-lived species [18], and may in part be attributable to the large sample sizes available across many years. It may also reflect the relatively harsh conditions on Rum, which are likely to accentuate the reproductive costs of energetic investment [9,10]. We detected reproductive costs despite extensive female heterogeneity, which can frequently mask costs in the highest quality individuals [17,21]. However, it is likely that we are underestimating the total costs of reproduction, because individuals are expected to reduce energetic investment in breeding to minimize fitness costs [18,22].
In summary, we found considerable costs of successful reproduction for female red deer in terms of future survival and fecundity. We found evidence for a significantly higher cost of sons than daughters, although this difference was smaller than the effects of juvenile survival. Despite density-dependence in both aspects of maternal performance, we found no indication that reproductive costs varied with ecological conditions. Our analyses illustrate the value of long-term datasets in affording tests of the generality of life-history patterns across changing environments.
Supplementary Material
Acknowledgements
We are grateful to all those involved in the long-term study of red deer on the Isle of Rum, particularly the field assistants, and to Scottish Natural Heritage for permitting the study. We thank Marco Festa-Bianchet and another anonymous reviewer for constructive feedback on an earlier draft.
Ethics
Ethical approval for the research on the Rum deer has been granted by the appropriate UK Home Office project licenses (currently 70/8818).
Data accessibility
Data are available in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.6rj63 [23].
Authors' contributions
H.F. and L.E.B.K. designed the study; J.M.P., T.H.C.-B. and L.E.B.K. managed data collection; H.F. conducted the analyses and drafted the manuscript; L.E.B.K. and C.A.W. advised on data analysis. All authors contributed substantially to revision of the manuscript, gave final approval and agree to be held accountable for this publication.
Competing interests
The authors declare no competing interests.
Funding
This work was funded by an NERC standard grant (NE/I024925/1) to L.E.B.K. and J.M.P.
References
- 1.Stearns SC. 1992. The evolution of life histories. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Williams GC. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690. ( 10.1086/282461) [DOI] [Google Scholar]
- 3.Descamps S, Boutin S, McAdam AG, Berteaux D, Gaillard J-M. 2009. Survival costs of reproduction vary with age in North American red squirrels. Proc. R. Soc. B 276, 1129–1135. ( 10.1098/rspb.2008.1401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ. 1990. Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343, 261–263. ( 10.1038/343261a0) [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH, Albon SD, Guinness FE. 1981. Parental investment in male and female offspring in polygynous mammals. Nature 289, 487–489. ( 10.1038/289487a0) [DOI] [Google Scholar]
- 6.Stamps JA. 1990. When should avian parents differentially provision sons and daughters? Am. Nat. 135, 671–685. ( 10.1086/285068) [DOI] [Google Scholar]
- 7.Trivers RL, Willard DE. 1973. Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 8.Speakman JR. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398. ( 10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamel S, Côté SD, Festa-Bianchet M. 2010. Maternal characteristics and environment affect the costs of reproduction in female mountain goats. Ecology 91, 2034–2043. ( 10.1890/09-1311.1) [DOI] [PubMed] [Google Scholar]
- 10.Barbraud C, Weimerskirch H. 2005. Environmental conditions and breeding experience affect costs of reproduction in blue petrels. Ecology 86, 682–692. ( 10.1890/04-0075) [DOI] [Google Scholar]
- 11.Clutton-Brock TH, Albon SD, Guinness FE. 1989. Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262. ( 10.1038/337260a0) [DOI] [PubMed] [Google Scholar]
- 12.Stopher KV, Bento AI, Clutton-Brock TH, Pemberton JM, Kruuk LE. 2014. Multiple pathways mediate the effects of climate change on maternal reproductive traits in a red deer population. Ecology 95, 3124–3138. ( 10.1890/13-0967.1) [DOI] [Google Scholar]
- 13.Froy H, Walling CA, Pemberton JM, Clutton-Brock TH, Kruuk LEB. 2016. Data from: relative costs of offspring sex and offspring survival in a polygynous mammal. Dryad Digital Repository ( 10.5061/dryad.6rj63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 15.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 16.Moyes K, Morgan B, Morris A, Morris S, Clutton-Brock T, Coulson T. 2011. Individual differences in reproductive costs examined using multi-state methods. J. Anim. Ecol. 80, 456–465. ( 10.1111/j.1365-2656.2010.01789.x) [DOI] [PubMed] [Google Scholar]
- 17.Hamel S, Côté SD, Gaillard J-M, Festa-Bianchet M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151. ( 10.1111/j.1365-2656.2008.01459.x) [DOI] [PubMed] [Google Scholar]
- 18.Martin JGA, Festa-Bianchet M. 2010. Bighorn ewes transfer the costs of reproduction to their lambs. Am. Nat. 176, 414–423. ( 10.1086/656267) [DOI] [PubMed] [Google Scholar]
- 19.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Hamel S, Gaillard J-M, Yoccoz NG, Loison A, Bonenfant C, Descamps S. 2010. Fitness costs of reproduction depend on life speed: empirical evidence from mammalian populations. Ecol. Lett. 13, 915–935. ( 10.1111/j.1461-0248.2010.01478.x) [DOI] [PubMed] [Google Scholar]
- 21.Weladji RB, Loison A, Gaillard J-M, Holand Ø, Mysterud A, Yoccoz NG, Nieminen M, Stenseth NC. 2008. Heterogeneity in individual quality overrides costs of reproduction in female reindeer. Oecologia 156, 237–247. ( 10.1007/s00442-008-0961-x) [DOI] [PubMed] [Google Scholar]
- 22.Berube CH, Festa-Bianchet M, Jorgenson JT. 1996. Reproductive costs of sons and daughters in Rocky Mountain bighorn sheep. Behav. Ecol. 7, 60–68. ( 10.1093/beheco/7.1.60) [DOI] [Google Scholar]
- 23.Froy H, Walling C, Pemberton J, Clutton-Brock T, Kruuk L. 2016 doi: 10.5061/dryad.6rj63. Data from: Relative costs of offspring sex and offspring survival in a polygynous mammal. Dryad Digital Repository. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.6rj63 [23].