Abstract
Trade-offs in the allocation of finite-energy resources among immunological defences and other physiological processes are believed to influence infection risk and disease severity in food-limited wildlife populations. However, this prediction has received little experimental investigation. Here we test the hypothesis that food limitation impairs the ability of wild field voles (Microtus agrestis) to mount an immune response against parasite infections. We conducted a replicated experiment on vole populations maintained in large outdoor enclosures during boreal winter, using food supplementation and anthelmintic treatment of intestinal nematodes. Innate immune responses against intestinal parasite infections were compared between food-supplemented and non-supplemented voles. Voles with high food availability mounted stronger immune responses against intestinal nematode infections than food-limited voles. No food effects were seen in immune responses to intracellular coccidian parasites, possibly owing to their ability to avoid activation of innate immune pathways. Our findings demonstrate that food availability constrains vole immune responses against nematode infections, and support the concept that spatio-temporal heterogeneity in food availability creates variation in infectious disease susceptibility.
Keywords: eco-immunology, field experiment, ivermectin, parasite, vole
1. Introduction
Immune responses are nutritionally costly [1,2]. Owing to trade-offs in the allocation of finite-energy resources between immune defences and other physiological processes (e.g. homeostasis and reproduction), food-limited individuals are believed to be more susceptible to infections and suffer greater disease severity when compared with individuals with ample food resources [2,3]. However, the relationship between food resources an infection risk has received little experimental investigation in natural systems (but see [4–7]) and immune defences—as the key mechanism mediating this potential relationship—even less.
In boreal Europe, vole populations display high-amplitude cyclic density fluctuations with peaks in abundance every 3–5 years [8]. Winter food resources limit high-density vole populations [9] and potentially initiate the cyclic abundance ‘crash’. Coinciding winter reductions in immune investment, identified through lymphocyte counts of field voles in the UK [10], suggest that infection risk is elevated during this time and interacts with food limitation to exacerbate vole abundance declines. This hypothesis is supported by the identification of high-intensity infections in declining vole populations [11].
We conducted a replicated field experiment with food supplementation and anthelmintic treatment of intestinal nematodes in high-density field vole populations maintained in large outdoor enclosures during boreal winter. Previously, we reported the effects of these factors on vole population limitation [6]. Food availability limited population growth, and voles in food-supplemented populations were consistently in better physiological condition than non-supplemented voles. Ivermectin treatment reduced nematode prevalence, most pronouncedly in combination with food supplementation. Here we employed new data on innate immune indices to address a fundamental question regarding the relationship between food resources and host immunity: do food-supplemented voles mount a stronger immune response to intestinal parasite infections than food-limited voles?
2. Material and methods
(a). Experimental design
Thirty-two (25 × 20 m) adjoining field enclosures were randomized among four treatment groups: (i) food supplementation and anthelmintic treatment (F+A+), (ii) food supplementation alone (F+A−), (iii) anthelmintic treatment alone (F−A+), and (iv) control (F−A−) (eight replicates per treatment group) [6]. However, for the current analysis the experiment should be considered single factor (food supplementation). A detailed description and justification of the research methods is presented in the electronic supplementary material. Food supplementation, in the form of rodent breeder pellets, was provided ad libitum.
(b). Longitudinal monitoring
Eighteen wild-caught field voles (7 males and 11 females) were released into each enclosure at the beginning of November 2011. Trapping occasions were conducted at four- to six-week intervals until April 2012, when the experiment concluded (six trapping occasions total).
Captured voles were uniquely marked, and blood and faecal samples were collected from the first 10 voles (greater than 20 g) encountered in each enclosure per trapping occasion. Oral ivermectin medication or control (linseed oil) was administered concurrent to sampling [6].
(c). Intestinal parasites
Eggs and oocysts of intestinal parasites were isolated from faecal samples using salt flotations, a common non-invasive technique for assessing intestinal parasitism [6,12]. Two groups of parasites were highly prevalent and used in the subsequent statistical analyses—heligmosomid nematode eggs (prevalence range 15–38%) and Eimeria coccidian oocysts (21–26%). Infection intensity was determined by counting the number of eggs and oocysts in a slide transect, and standardized by faecal sample mass. Negative results may be indicative of a true absence of intestinal parasites, parasitism by only male nematodes or a temporary cessation in shedding. Based on prevalences during the experiment, shedding is unlikely to cease during winter. Furthermore, the randomized experimental design precludes the possibility that temporary cessation of shedding might systematically bias results towards any particular group. For these reasons we did not verify negative flotation results through intestinal dissections.
(d). Immunological indices
As an index for immune investment, total immunoglobulin G (IgG) antibody titres were measured from blood samples collected during trapping occasions 1, 3 and 6. Differential white blood cell (WBC) counts (neutrophils, lymphocytes, monocytes, eosinophils and basophils from a total count of 100 leucocytes) were performed microscopically (×50/×100 magnification) from blood smears collected during all trapping occasions. WBC indices cannot distinguish immune investment from redistribution. The ratio of neutrophils to lymphocytes (N : L ratio), indicative of a stress response [13], was also calculated. Owing to very low eosinophil and basophil counts (most often none were present; range 0–4 and 0–3, respectively), these cell types were excluded from the statistical analyses.
(e). Statistical analyses
General linear mixed models were used to evaluate the effects of food supplementation, the prevalence and infection intensity of each parasite, and vole sex on the immune indices. Initial models included all interactions between sources of variation (including both parasite groups), with the prevalence and intensity of each infection analysed separately. Enclosure number, trapping occasion, vole identity and the intercept were included as random factors (and ELISA plate number in the IgG models). The most parsimonious models were selected using stepwise reduction based on Akaike information criteria value, and model fit was verified visually via the residual distribution.
3. Results
Throughout the experiment, 567 vole faecal samples were collected and screened for the two parasite groups from a total of 408 unique individuals. Two faecal samples (separate trapping occasions) were collected from 73 voles, three samples from 32 voles, four samples from six voles, and five samples from one vole. Single samples were collected from 296 voles.
Eimeria oocysts were present in 140 samples, and nematode eggs were present in 109 samples. Twenty-eight samples were simultaneously infected by both parasite groups. Infections were acquired and lost throughout the experiment. Voles that transitioned from nematode infected to non-infected had most often received anthelmintic treatment (77%), while voles that became infected during the experiment most often had not (73%). Only four voles were nematode infected during all encountered time points; one received anthelmintic treatment alone, and none received both food supplementation and anthelmintic treatment.
Total IgG was greater in nematode-infected voles that received food supplementation than infected voles without food supplementation and non-infected voles that received food supplementation (FFood×Nematode 1,145 = 6.3, p = 0.013; figure 1). No difference was detected between food-supplemented and non-supplemented voles in the absence of infection, nor were any effects of eimerian infection on total IgG seen (FEimeria 1,207 = 0.0, p = 0.95). The fixed-effects results of all final models are available in the electronic supplementary material, table S2.
Figure 1.
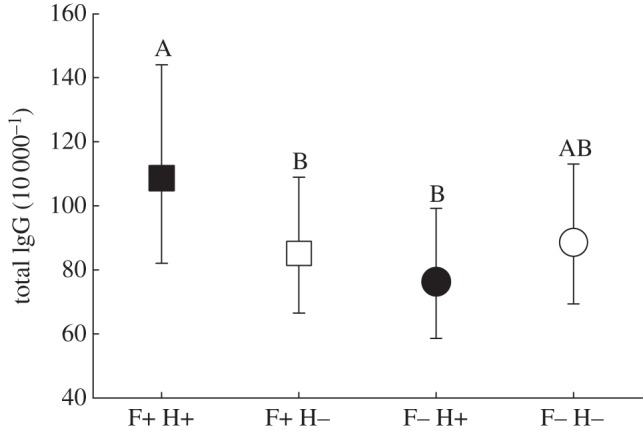
Estimated marginal means of field vole total IgG antibody titres (95% confidence interval) in response to food supplementation (F+) and helminth infection (H+). Treatments sharing letters were not statistically different.
Neutrophil counts were higher in nematode-infected voles that received food supplementation than infected voles without food supplementation and non-infected voles that received food supplementation (FFood×Nematode 1,502 = 9.0, p = 0.003). In this model, the three-way interaction between infection with eimerians, nematodes and vole sex was also significant (FNematode×Eimeria×Sex 1,529 = 7.6, p = 0.006). Neutrophil counts were higher in non-infected males than females (p = 0.027) and in males with double infections than females with double infections (p = 0.022). Nematode-infected voles that received food supplementation had lower lymphocyte counts than non-supplemented voles and non-infected voles which received food supplementation (FFood×Nematode 1,497 = 8.3, p = 0.004). The N : L-ratio was thus highest in nematode-infected voles which received food supplementation and did not vary between non-infected voles regardless of food availability (FFood×Nematode 1,502 = 10.2, p = 0.002; figure 2). Again, three-way interactions between infection with eimerians, nematodes and vole sex were identified in lymphocyte count (FNematode×Eimeria×Sex 1,529 = 7.9, p = 0.005) and N : L-ratio (FNematode×Eimeria×Sex 1,530 = 7.5, p = 0.006) wing to the same pairwise sex differences. None of the explanatory variables influenced monocyte counts (electronic supplementary material, table S2).
Figure 2.
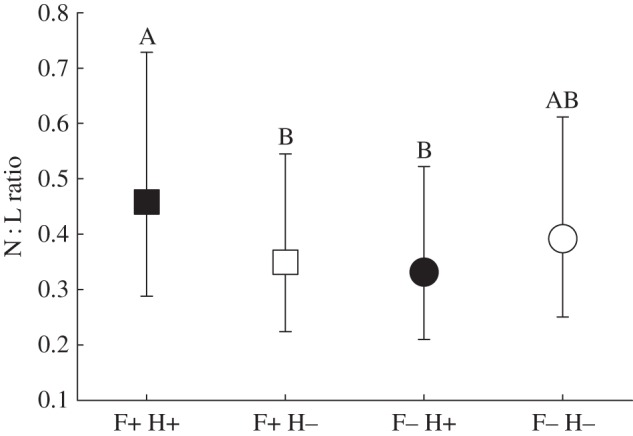
Estimated marginal means of field vole neutrophil : lymphocyte ratio in response to food supplementation (F+) and helminth infection (H+). Treatments sharing letters were not statistically different.
Infection intensity did not influence any of the immune indices. Food significantly affected levels of total IgG, neutrophil and lymphocyte counts and the N : L-ratio in nematode-infected voles. No other significant effects were detected in the infection intensity models (electronic supplementary material, table S2).
4. Discussion
We present a rare investigation of immunity in a wild mammal in relation to the individual and synergistic effects of food availability and intestinal parasite infection. Although a small number of immune parameters were measured, the concordance between total IgG and differential WBC counts is compelling. They demonstrate that food-supplemented voles were able to mount an innate immune response against nematode infections, whereas food-limited voles were not, suggesting that other energetically demanding processes were prioritized in lieu of immunity for food-limited individuals.
Heligmosomid nematodes reside extracellularly in the intestines, and elicit an immunity response characterized by the formation of granulomas rich in neutrophils and elevated levels of non-specific polyclonal IgGs [14,15]. Thus, our findings in helminth-infected, food-supplemented voles are consistent with a typical anti-helminth immune response that was absent in food-limited voles. Eimerian parasites, conversely, complete their life cycle in intestinal epithelial cells [16] to which the immunity response is likely to be more localized and not easily detectable by our non-specific systemic assays.
Here we employed a subset of indices indicative of innate immunity. A large suite of immunological tools exists for laboratory-based host systems, and their continued adaptation for wildlife is important [17]. Wildlife species inherently display high intraspecific variation in physiological indices [17,18], which often necessitates large sample sizes to detect significant effects. This may partly explain why non-food-supplemented voles without nematode infections (F−H−) exhibited IgG and WBC values that were not statistically different from those of food supplemented voles with nematode infections (figure 1; pairwise difference between F+H+ and F−H−: p = 0.12). Notably, F−H− voles did not differ from the other groups either. This counterintuitive finding may also result from a minor immune response elicited in F−H− voles against undetected parasites, or could merely be a random phenomenon.
In summary, our findings demonstrate that food availability constrains vole immune responses against nematode infections, and support the hypothesis that variation in food availability creates spatio-temporal heterogeneity in infectious disease susceptibility. Moreover, we present a functional mechanism through which food limitation and infectious diseases may interact to exacerbate the density crash of wildlife populations in seasonal environments.
Supplementary Material
Acknowledgements
We thank Aitor Barbero Lopez, Chelsea Powell, Elisabet Martínez Sancho, Ilkka Taponen, Jukka Niemimaa, Nuria Blanco and Stephen Ryan for their contributions to fieldwork, Sami Kyröläinen for assistance with the ELISA tests and Dilip Karad for the differential WBC counts. Voitto Haukisalmi provided expertise on parasite identification and Timo Soveri on ivermectin treatments. We thank the anonymous reviewers for constructive comments.
Ethics
The experiment was approved by the Finnish Animal Ethics Council (permit no. ESAVI/1437/04.10.03/2011).
Data accessibility
Data from this experiment is available in Dryad http://dx.doi.org/10.5061/dryad.r59hv [19].
Authors' contributions
K.M.F., T.M., P.S., H.H. and O.H. conceived and designed the experiment, and conducted field monitoring of vole populations. T.M., Ta.S., To.S., P.S., S.M. and O.V. performed the laboratory analyses. K.M.F. conducted the statistical analyses and drafted the manuscript. All authors contributed to manuscript revisions and approved the final version. All authors agree to be held accountable for the content.
Competing interests
We have no competing interests.
Funding
The experiment was funded by grants from the Academy of Finland (grant no. 133495 to O.H., grant no. 132190 to T.M.). K.M.F. is financially supported by the Finnish Cultural Foundation.
References
- 1.Zuk M, Stoehr AM. 2002. Immune defence and host life history. Am. Nat. 160, s9–s22. ( 10.1086/342131) [DOI] [PubMed] [Google Scholar]
- 2.Sheldon BC, Verhulst S. 1996. Ecological immunity: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 3.Beldomenico PM, Begon M. 2010. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 25, 21–27. ( 10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 4.Vandergrift KJ, Raffel TR, Hudson PJ. 2008. Parasites prevent summer breeding in white-footed mice, Peromyscus leucopus. Ecology 89, 2251–2258. ( 10.1890/07-1935.1) [DOI] [PubMed] [Google Scholar]
- 5.Pedersen AB, Greives TJ. 2008. The interaction of parasites and resources causes crashes in a wild mouse population. J. Anim. Ecol. 77, 370–377. ( 10.1098/rsbl.2013.0205) [DOI] [PubMed] [Google Scholar]
- 6.Forbes KM, Stuart P, Mappes T, Henttonen H, Huitu O. 2014. Food resources and intestinal parasites as limiting factors for boreal vole populations during winter. Ecology 95, 3139–3148. ( 10.1890/13-2381.1) [DOI] [Google Scholar]
- 7.Forbes KM, Henttonen H, Hirvelä-Koski V, Kipar A, Mappes T, Stuart P, Huitu O. 2015. Food provisioning alters infection dynamics in populations of a wild rodent. Proc. R. Soc. B 282, 20151939 ( 10.1098/rspb.2015.1939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson L, Henttonen H. 1985. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia 67, 394–402. ( 10.1007/BF00384946) [DOI] [PubMed] [Google Scholar]
- 9.Huitu O, Koivula M, Korpimäki E, Klemola T, Norrdahl K. 2003. Winter food supply limits growth of northern vole populations in the absence of predation. Ecology 84, 2108–2118. ( 10.1890/02-0040) [DOI] [Google Scholar]
- 10.Beldomenico PM, Telfer S, Gerbert S, Lukomski L, Bennett M, Begon M. 2008. The dynamics of health in wild field vole populations: a haematological perspective. J. Anim. Ecol. 77, 984–997. ( 10.1111/j.1365-2656.2008.01413.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soveri T, Henttonen H, Rudbäck E, Schildt R, Tanskanen R, Husu-Kallio J, Haukisalmi V, Sukura A, Laakkonen J. 2000. Disease patterns in field and bank vole populations during a cyclic decline in central Finland. Comp. Immunol. Microbiol. Infect. Dis. 23, 73–89. ( 10.1016/S0147-9571(99)00057-0) [DOI] [PubMed] [Google Scholar]
- 12.Pedersen AB, Fenton A. 2015. The role of antiparasite treatment experiments in assessing the impact of parasites on wildlife. Trends Parasitol. 31, 200–211. ( 10.1016/j.pt.2015.02.004) [DOI] [PubMed] [Google Scholar]
- 13.Davis AK, Maney DL, Maerz JC. 2008. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 22, 760–772. ( 10.1111/j.1365-2435.2008.01467.x) [DOI] [Google Scholar]
- 14.Anthony RM, Rutitzky LI, Urban JF, Stadecker MJ, Gause WC. 2007. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 7, 975–987. ( 10.1038/nri2199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy KD, et al. 2008. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4, 362–373. ( 10.1016/j.chom.2008.08.014) [DOI] [PubMed] [Google Scholar]
- 16.Ovington KS, Alleva LM, Kerr EA. 1995. Cytokines and immunological control of Eimeria spp. Int. J. Parasitol. 25, 1331–1351. ( 10.1016/0020-7519(95)00069-E) [DOI] [PubMed] [Google Scholar]
- 17.Pedersen AB, Babayon SA. 2011. Wild immunology. Mol. Ecol. 20, 872–880. ( 10.1111/j.1365-294X.2010.04938.x) [DOI] [PubMed] [Google Scholar]
- 18.Shutler D. 2011. Sexual selection: when to expect trade-offs. Biol. Lett. 7, 101–104. ( 10.1098/rsbl.2010.0531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes KM, Mappes T, Sironen T, Strandin T, Stuart P, Meri S, Vapalahti O, Henttonen H, Huitu O. 2016. Data from: Food limitation constrains host immune responses to nematode infections. Dryad Digital Repository ( 10.5061/dryad.r59hv). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this experiment is available in Dryad http://dx.doi.org/10.5061/dryad.r59hv [19].


