Abstract
The restricted, exclusively terrestrial distribution of modern Onychophora contrasts strikingly with the rich diversity of onychophoran-like fossils preserved in marine Cambrian Lagerstätten. The transition from these early forebears to the modern onychophoran body plan is poorly constrained, in part owing to the absence of fossils preserving details of the soft anatomy. Here, we report muscle tissue in a new early Cambrian (Stage 3) lobopodian, Tritonychus phanerosarkus gen. et sp. nov., preserved in the Orsten fashion by three-dimensional replication in phosphate. This first report of Palaeozoic onychophoran musculature establishes peripheral musculature as a characteristic of the ancestral panarthropod, but documents an unexpected muscular configuration. Phylogenetic analysis reconstructs T. phanerosarkus as one of a few members of the main onychophoran lineage—which was as rare and as cryptic in the Cambrian period as it is today.
Keywords: lobopodians, muscle, evolution, taphonomy, phylogenetics, Cambrian Stage 3
1. Introduction
The Panarthropod phyla—Euarthropoda, Tardigrada and Onychophora—evolved from a paraphyletic grade of unsegmented leg-bearing worms, the lobopodians [1]. These organisms illuminate otherwise intractable details of early panarthropod evolution [2,3], even if the full significance of their fossils is difficult to evaluate.
Lobopodians from the onychophoran total group have a deep ancestry. First represented by occurrences of their dorsal armature in the Tommotian (Cambrian Stage 2) shelly fossil record [4,5], they rise to a more obvious prominence in the Chengjiang biota (Cambrian Stage 3), where their carbonaceous compression fossils display a rich array of morphologies [6]. Fine exterior structure, however, is only seen in three-dimensional microfossils of Orsten-type deposits [7]. Orstenotubulus [8], the single lobopodian preserved in this fashion, dates to the Guzhangian (latest mid-Cambrian), substantially after the burst of lobopodian disparity documented by the early Cambrian (Stage 3) Chengjiang biota [9].
This array of onychophoran-like lobopodians may straddle the onychophoran stem lineage [9], or may predominantly belong to an extinct sister group [3]. Some features of the onychophoran body plan are already evident in these Cambrian taxa [1], though other distinctive characteristics, such as slime papillae, necessarily arose after onychophorans colonized the land. New Cambrian fossils, and better resolution of their relationships, are key to elucidating the evolutionary trajectory that led to the specialized anatomy of modern onychophorans. We here present a new phosphatized lobopodian from early Cambrian Orsten-type deposits that provides a unique perspective on the cuticle and musculature of early onychophoran-like lobopodians.
2. Material and methods
The specimen was recovered by 5% acetic acid digestion of carbonate nodules from black shales, and is deposited at the Key Laboratory for Palaeobiology, Yunnan University, Kunming, China (YKLP). Phylogenetic analysis was conducted in TNT [10], using the methods of Smith & Ortega Hernández [1], on a revised matrix of 49 taxa and 115 unordered characters, integrating data from recent lobopodian analyses [1,3,9,11] (data and scripts available at Dryad [12]). Parsimony analysis employed implied weights, with 99 values of Goloboff's concavity constant [13] picked from a lognormal distribution (range: 1.061–259.4; R function qlnorm ((1 : 99)/100, meanlog = log(4), sdlog = log(6)) + 1), and equal weights, with a consensus tree generated from all most parsimonious topologies [14]. Extended implied weighting [15] does not affect the consensus tree.
3. Results
(a). Systematic palaeontology
Superphylum Ecdysozoa, Aguinaldo et al. [16].
Stem-group Onychophora, Grube [17].
Genus Tritonychus Zhang et Smith, gen. nov.
LSID. urn:lsid:zoobank.org:act:959A47D4-3323-47CB-ADB2-B6A8F5B945A0.
Etymology. In reference to the third (τριτος, tritos) claw (ονυχος, onychos), a unique feature among lobopodians.
Diagnosis. Lobopodous appendages paired, 10 times longer than wide, four times narrower than trunk, each ending with three anteriorly directed claws. Trunk and appendages ornamented with bifurcating circumferential wrinkles and bearing dermal papillae. Two discrete layers of longitudinal fibres peripheral to body cavity.
Type species.
Tritonychus phanerosarkus Zhang et Smith gen. et sp. nov.
LSID. urn:lsid:zoobank.org:act:1715A32E-C258-4EC2-A44D-EC1A1E3DC23E.
Etymology. φανερος, phaneros, well-displayed, σαρκος, sarkos, muscle, flesh.
Holotype. YKLP 12335 (figure 1), the only known specimen.
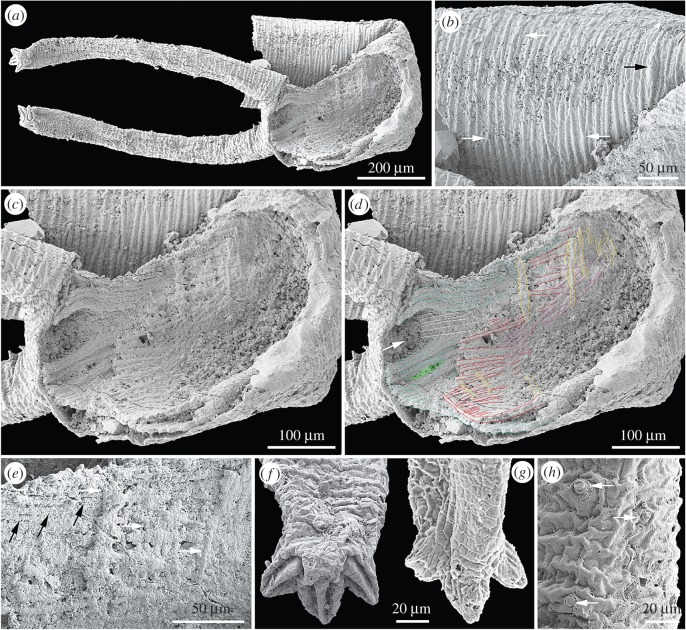
Figure 1.
The Cambrian Stage 3 lobopodian Tritonychus phanerosarkus gen. et sp. nov. from China (YKLP 12335). (a) Overall morphology. (b) Surface ornament; circumferential wrinkles irregularly bifurcate and merge (white arrows) and bear papillae (black arrow). (c) Close up of musculature in panel (a). (d) Three layers of three-dimensionally preserved muscle fibres (myofibrils). Colours denote structures interpreted as: white, ventral longitudinal muscles; cyan, outer layer of longitudinal muscles, parting to leave gap (arrow); crimson/mauve, interwoven layer of oblique muscles; yellow, inner layer of circular muscles; green, point of leg levator insertion. (e) Details of linear fibres subparallel (black arrows) and perpendicular (white arrows) to the body axis. (f) Left appendage tip, bearing impressions of three claws. (g) Right appendage, showing the reverse side of claws. (h) Papillae of different sizes (arrows) on the surface of right appendage.
Occurrence. Yu'anshan Formation (Eoredlichia–Wutingaspis Biozone, approximately late Atdabanian = Cambrian Series 2, Stage 3), Xiaotan section, Yongshan, Yunnan Province.
Diagnosis. As genus.
Description. The specimen is a millimetre-long section of lobopodian trunk that is folded at its midpoint and bears a pair of lobopods on its ventral surface near the presumed posterior margin (figure 1a). It is incomplete at each end, and lacks most of its dorsal surface.
The trunk is ornamented with circumferential wrinkles, spaced at 10 µm, which bifurcate and merge in an irregular fashion (figure 1b). Irregularly positioned conical projections, 5 µm in diameter and 7 µm in height and situated on a round cuticular base (figure 1b,h), presumably correspond to the dermal papillae of Orstenotubulus and extant Onychophora [8]. Moving distally along each appendage, the cuticle wrinkles give way to a reticulate pattern of polygonally arranged ridges that conceivably correspond to cell boundaries, and the papillae are less frequently expressed (figure 1f–h).
The trunk is lined with three layers of fibrous tissue, each around 10 µm thick, which we interpret as muscles. The outer layer (figure 1c–e, cyan) comprises 5–10 µm wide longitudinal fibres; it parts between the appendages to leave a 60 µm wide gap, through which a separate bundle of longitudinal fibres (white in figure 1d) passes. The fibres part again to the right of this point (green in figure 1d), perhaps reflecting the insertion of leg levator musculature. A second layer of interwoven oblique fibres (crimson and mauve in figure 1d) sits within the first, and within that layer lie further fibres oriented perpendicular to the body axis (yellow in figure 1d), presumably representing decayed remnants of an originally extensive layer of circular musculature.
Each of the two appendages is 800 µm long and a uniform 80 µm in diameter, with a circular cross section that is distorted in places by flattening. Each bears the impressions of three terminal claws, separated by 45°; the raised central bosses of these impressions denote an originally hollow claw (figure 1f,g). No distinct foot is present. Assuming the claws to be directed forwards (as in other lobopodians), the legs occupy the posterior limit of the fragment. The ventrolateral location of the appendages suggests that they served a conventional locomotory role, contrasting with the intriguing lateral position of appendages of Orstenotubulus [8].
4. Discussion
The preservation of muscular tissue is in some respects surprising, as body wall musculature is the first feature to decay when onychophorans are rotted in isotonic saline solution [18]. The absence of both labile (gonads, gut) and recalcitrant (claws) tissues in T. phanerosarkus indicates that the sequence of decay in salt water is a poor guide to the sequence of preservation in this fossil material. Here, early phosphatization initiated at the cuticle (evinced by the decreasing quality of preservation away from the body wall) clearly led to enhanced preservation of peripheral tissue (cf. [19]).
More generally, muscle tissue is atypical in phosphatized (‘Orsten-type’) microfossils (reference [20] provides a rare example), and though it may be concealed by overlying tissue layers in some cases [21], in most—including palaeoscolecid cuticles that occur alongside T. phanerosarkus—its absence is genuine. Muscle preservation is no less unusual in Burgess Shale-type settings [22]. The exception is Sirius Passet, where early diagenetic phosphatization records the evolution of muscle anatomy in stem-group Euarthropoda—documenting a conceived transition from peripheral musculature deep in the stem group (Kerygmachela), via peripheral + skeletal muscle (in Pambdelurion), to the skeletal muscle arrangement of crown group euarthropods (and tardigrades) [2]. The presence of peripheral musculature in T. phanerosarkus confirms that peripheral musculature was also ancestral within onychophorans, and thus for panarthropods as a whole.
Nevertheless, the derivation of three-layered musculature from the presumably ancestral twin layers observed in priapulids [23] is not straightforward. In priapulids and extant onychophorans, the outermost muscles are circular and the innermost longitudinal, with onychophorans incorporating an intermediate layer of interwoven oblique muscles [24,25]. Tritonychus phanerosarkus exhibits equivalent layers—though their order is reversed, leaving the homology of each layer with those in other panarthropods unclear, and revealing an unexpected diversity of muscle arrangement in early panarthropods.
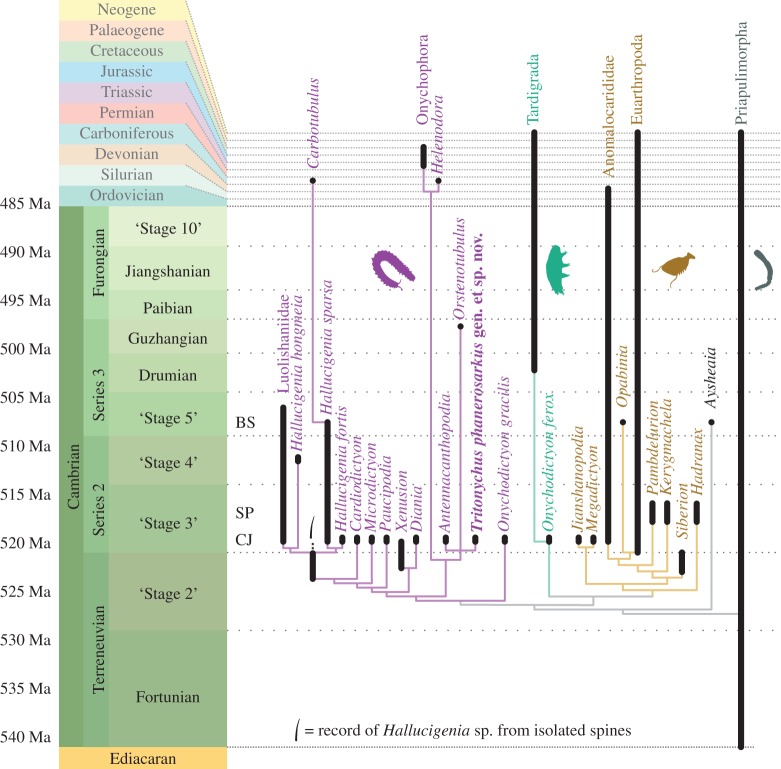
A position within Onychophora is nonetheless robustly supported by phylogenetic analysis, which consistently places the new lobopodian in a clade comprising Orstenotubulus, Antennacanthopodia, Helenodora and crown group Onychophora (electronic supplementary material, summarized in figure 2). This ‘onychophoran-like’ clade is sister to all other Cambrian stem-group onychophorans, with the exception of Onychodictyon gracilis. It reflects cuticular similarities between T. phanerosarkus, Orstenotubulus [8] and extant onychophorans: bifurcating circumferential wrinkles, spinose projections mounted on with circular bases, and (in places) hexagonal patterning.
Figure 2.
Phylogenetic results. Fossil occurrences marked as thick black lines, ghost ranges as thin lines. Divergence times are unconstrained, except in the case of hallucigeniids, where the record of isolated Hallucigenia-type spines [4] provides a minimum divergence time. BS, Burgess Shale, ca 508 Ma; SP, Sirius Passet, ca 518 Ma; CJ, Chengjiang, ca 520 Ma.
The new fossil extends the record of these features of the modern onychophoran cuticle into the lower Cambrian (Stage 3), along with other characteristics of the onychophoran body plan: the peripheral disposition of multiple muscular layers, the ventrolateral appendage location and conceivably a gonopore—one possible interpretation of the intra-appendicular gap in musculature, suggested by equivalent openings in Orstenotubulus and extant onychophorans [8].
Despite the early evolutionary origin of this suite of onychophoran features, it is striking that the ‘onychophoran-like’ clade is so poorly represented in the fossil record: only six specimens have yet been recovered, four of which are Orsten-type fragments. Whether or not the distinctively onychophoran-like features of the new fossil were also present in other Cambrian lobopodians, the lineage leading to modern Onychophora seems to have been as rare and depauperate during the formative stages of its evolution as it is today.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank H.-Q. Zhang, and M. Tian for sample preparation.
Data accessibility
Phylogenetic data: TreeBASE accession number S18871 [26]. Description of characters and detailed phylogenetic results: Dryad http://dx.doi.org/10.5061/dryad.7r10b [12]. This published work and the nomenclatural acts it contains have been registered in Zoobank: http://zoobank.org/References/D5C6C81C-2EEB-41CD-BE0C-48927554810D.
Authors' contributions
X.G.Z. and M.R.S. conceived the study. X.G.Z., J.Y. and J.B.H. collected the material. M.R.S. performed the phylogenetic analysis. J.Y. took the SEM photos. X.G.Z. and M.R.S. made the figures. M.R.S. drafted the manuscript with input from other authors. All authors have agreed to be held accountable for the content and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This research is supported by the National Natural Science Foundation of China (U1402232 and 41472022 to X.G.Z. and J.Y.), the Department of Science and Technology, Yunnan Province (2015HA045 to X.G.Z.).
References
- 1.Smith MR, Ortega-Hernández J. 2014. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366. ( 10.1038/nature13576) [DOI] [PubMed] [Google Scholar]
- 2.Budd GE. 2001. Tardigrades as ‘stem-group arthropods’: the evidence from the Cambrian fauna. Zool. Anz. 240, 265–279. ( 10.1078/0044-5231-00034) [DOI] [Google Scholar]
- 3.Smith MR, Caron J-B. 2015. Hallucigenia's head and the pharyngeal armature of early ecdysozoans. Nature 523, 75–78. ( 10.1038/nature14573) [DOI] [PubMed] [Google Scholar]
- 4.Caron J-B, Smith MR, Harvey THP. 2013. Beyond the Burgess Shale: Cambrian microfossils track the rise and fall of hallucigeniid lobopodians. Proc. R. Soc. B 280, 20131613 ( 10.1098/rspb.2013.1613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missarzhevsky VV, Mambetov AJ. 1981. Stratigrafiya i fauna pogranichnykh sloev kembriya i dokembriya Malogo Karatau (Stratigraphy and fauna of Cambrian and Precambrian boundary beds of Maly Karatau). Tr. Akad. Nauk. SSSR 326, 1–90. [Google Scholar]
- 6.Liu J-N, Dunlop JA. 2014. Cambrian lobopodians: a review of recent progress in our understanding of their morphology and evolution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 4–15. ( 10.1016/j.palaeo.2013.06.008) [DOI] [Google Scholar]
- 7.Müller KJ. 1985. Exceptional preservation in calcareous nodules. Phil. Trans. R. Soc. Lond. B 311, 67–73. ( 10.1098/rstb.1985.0139) [DOI] [Google Scholar]
- 8.Maas A, Mayer G, Kristensen RM, Waloszek D. 2007. A Cambrian micro-lobopodian and the evolution of arthropod locomotion and reproduction. Chin. Sci. Bull. 52, 3385–3392. ( 10.1007/s11434-007-0515-3) [DOI] [Google Scholar]
- 9.Yang J, Ortega-Hernández J, Gerber S, Butterfield NJ, Hou J-B, Lan T, Zhang X-G. 2015. A superarmored lobopodian from the Cambrian of China and early disparity in the evolution of Onychophora. Proc. Natl Acad. Sci. USA 112, 8678–8683. ( 10.1073/pnas.1505596112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. ( 10.1111/j.1096-0031.2008.00217.x) [DOI] [Google Scholar]
- 11.Yang J, Ortega-Hernández J, Butterfield NJ, Liu Y, Boyan GS, Hou J-B, Lan T, Zhang X-G. 2016. Fuxianhuiid ventral nerve cord and early nervous system evolution in Panarthropoda. Proc. Natl Acad. Sci. USA 113, 2988–2993. ( 10.1073/pnas.1522434113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X-G, Smith MR, Yang J, Hou J-B. 2016. Data from: Onychophoran-like musculature in a phosphatized Cambrian lobopodian. Dryad Digital Repository. ( 10.5061/dryad.7r10b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goloboff PA. 1993. Estimating character weights during tree search. Cladistics 9, 83–91. ( 10.1111/j.1096-0031.1993.tb00209.x) [DOI] [PubMed] [Google Scholar]
- 14.Mirande JM. 2009. Weighted parsimony phylogeny of the family Characidae (Teleostei: Characiformes). Cladistics 25, 574–613. ( 10.1111/j.1096-0031.2009.00262.x) [DOI] [PubMed] [Google Scholar]
- 15.Goloboff PA. 2014. Extended implied weighting. Cladistics 30, 260–272. ( 10.1111/cla.12047) [DOI] [PubMed] [Google Scholar]
- 16.Aguinaldo AM, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA. 1997. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 387, 489–493. ( 10.1038/387489a0) [DOI] [PubMed] [Google Scholar]
- 17.Grube AE. 1853. Über den Bau von Peripatus Edwardsii. In Müller's Archives of anatomy and physiology, pp. 322–360. Berlin, Germany: G. Eichler. [Google Scholar]
- 18.Murdock DJE, Gabbott SE, Mayer G, Purnell MA. 2014. Decay of velvet worms (Onychophora), and bias in the fossil record of lobopodians. BMC Evol. Biol. 14, 222 ( 10.1186/s12862-014-0222-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hof CHJ, Briggs DEG. 1997. Decay and mineralization of mantis shrimps (Stomatopoda: Crustacea)—a key to their fossil record. Palaios 12, 420–438. ( 10.2307/3515381) [DOI] [Google Scholar]
- 20.Andres D. 1989. Phosphatisierte Fossilien aus dem unteren Ordoviz von Südschweden. Berliner Geowissenschaftliche Abhandlungen (A) 106, 9–19. [Google Scholar]
- 21.Eriksson ME, Terfelt F, Elofsson R, Marone F. 2012. Internal soft-tissue anatomy of Cambrian ‘Orsten’ arthropods as revealed by synchrotron X-ray tomographic microscopy. PLoS ONE 7, e42582 ( 10.1371/journal.pone.0042582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterfield NJ. 2006. Hooking some stem-group ‘worms’: fossil lophotrochozoans in the Burgess Shale. BioEssays 28, 1161–1166. ( 10.1002/bies.20507) [DOI] [PubMed] [Google Scholar]
- 23.Carnevali MDC, Ferraguti M. 1979. Structure and ultrastructure of muscles in the priapulid Halicryptus spinulosus: functional and phylogenetic remarks. J. Mar. Biol. Assoc. UK 59, 737–744. ( 10.1017/S0025315400045719) [DOI] [Google Scholar]
- 24.Hoyle G, Williams M. 1980. The musculature of Peripatus and its innervation. Phil. Trans. R. Soc. Lond. B 288, 481–510. ( 10.1098/rstb.1980.0024) [DOI] [Google Scholar]
- 25.Snodgrass RE. 1938. Evolution of the Annelida, Onychophora and Arthropoda. Smithson Misc. Collect. 97, 1–159. [Google Scholar]
- 26.Zhang X-G, Smith MR, Yang J, Hou J-B. 2016. Data from: Onychophoran-like musculature in a phosphatized Cambrian lobopodian. TreeBASE S18871, purl.org/phylo/treebase/phylows/study/TB2:S18871.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Phylogenetic data: TreeBASE accession number S18871 [26]. Description of characters and detailed phylogenetic results: Dryad http://dx.doi.org/10.5061/dryad.7r10b [12]. This published work and the nomenclatural acts it contains have been registered in Zoobank: http://zoobank.org/References/D5C6C81C-2EEB-41CD-BE0C-48927554810D.