Abstract
During the Mesozoic (242–66 million years ago), terrestrial regions underwent a massive shift in their size, position and connectivity. At the beginning of the era, the land masses were joined into a single supercontinent called Pangaea. However, by the end of the Mesozoic, terrestrial regions had become highly fragmented, both owing to the drifting apart of the continental plates and the extremely high sea levels that flooded and divided many regions. How terrestrial biodiversity was affected by this fragmentation and large-scale flooding of the Earth's landmasses is uncertain. Based on a model using the species–area relationship (SAR), terrestrial vertebrate biodiversity would be expected to nearly double through the Mesozoic owing to continental fragmentation, despite a decrease of 24% in total terrestrial area. Previous studies of Mesozoic vertebrates have generally found increases in terrestrial diversity towards the end of the era, although these increases are often attributed to intrinsic or climatic factors. Instead, continental fragmentation over this time may largely explain any observed increase in terrestrial biodiversity. This study demonstrates the importance that non-intrinsic effects can have on the taxonomic success of a group, and the importance of geography to understanding past biodiversity.
Keywords: Dinosauria, Gondwana, island biogeography, Laurasia, plate tectonics, species-area relationship
1. Introduction
The observation that species diversity increases along with area was noted over 150 years ago [1], and since then has been extensively quantified across almost all major taxonomic groups and regions [2–6], and even into the geological past [7]. It is so ubiquitous that the species-area relationship (SAR) is often called one of the few ‘laws’ of ecology [5,6,8].
Because of the strength and ubiquity of the SAR, it has served as a useful tool for ecologists to estimate diversity into the future, particularly species declines owing to habitat loss [9] or the potential anthropogenic homogenization of faunas (i.e. ‘New Pangaea’ in Rosenzweig [10])[8,11]. For example, Brown [11] calculated that, if the Earth's ecosystems were to be completely homogenized through human influence, biodiversity loss among taxonomic groups could range from 35% to 70%. Similarly, Collins and co-workers [8] predicted an overall loss of 44.5% of global diversity (although see Rosenzweig [10]). While future biodiversity may severely decline owing to habitat loss and a breakdown of geographical barriers, it is unclear how similar changes in land area and terrestrial connectivity in the geologic past may have affected Mesozoic terrestrial vertebrate (MTV) biodiversity.
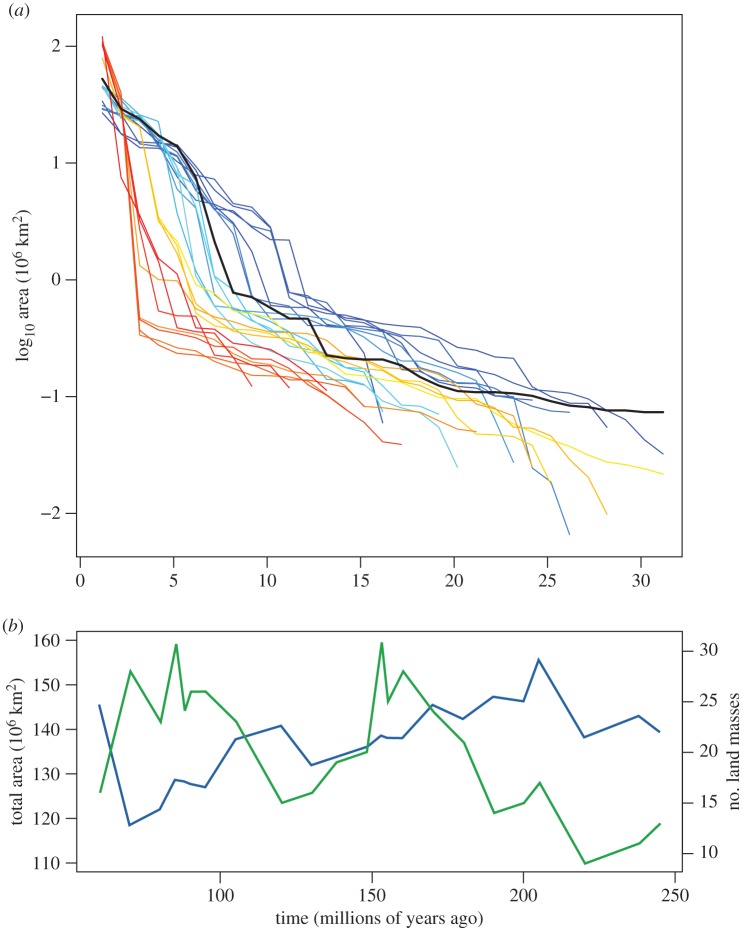
Terrestrial area is largely determined by two long-term processes—plate tectonics and eustatic sea level—interacting to produce the shape and arrangement of coastlines. During the Mesozoic, terrestrial regions went from being their most connected at the start of the era, to their most highly fragmented near the end of the era (figure 1a). At the beginning of the Triassic, the continental plates were almost entirely united into the single supercontinent Pangaea. However, by the Late Cretaceous, these plates had largely moved to their modern day locations [12,13]. At the same time, eustatic sea level rose through this interval, driven in part by these same tectonic processes [14]. Global sea levels peaked up to 250 m above modern during the Late Cretaceous [15] (although other studies have suggested a maximal rise of approximately 150 m [16]), leading to extensive flooding of low-lying regions and a substantial decrease in terrestrial area [13,17] (figure 1b). This rise in sea level further fragmented terrestrial regions through the flooding of continental interiors in Africa, Eurasia and North America, splitting these continental plates into multiple landmasses [17]. While the largest land mass at the beginning of the Triassic was nearly 123 million km2, or 88% of the total terrestrial area, the largest land mass was only 27 million km2 near the end of the Cretaceous, or about 22% of the total terrestrial area (figure 1a). Although the maximum size of a contiguous terrestrial region decreased substantially through this time, it meant that there were a much larger number of moderately sized terrestrial areas.
Figure 1.
Changes in terrestrial area and configuration through time. (a) Rank–area plot of individual terrestrial areas through the Mesozoic. Each time period is represented by a unique colour, with red the oldest (Triassic) through to blue as youngest (Cretaceous). The distribution of present day terrestrial areas is represented by the solid black line. (b) Total terrestrial area (blue) and number of isolated landmasses (green) through time.
The principles of island biogeography [4] could lead to two different expectations for how biodiversity would change in relation to changes in palaeogeography. The isolation of continents should lead to higher numbers of endemic taxa, yet the 24% decrease in total terrestrial area should lead to lower MTV biodiversity. In order to try and understand how MTV biodiversity may have responded to changing palaeogeography, I modelled terrestrial area through the Mesozoic and applied the SAR to predict biodiversity through time.
2. Material and methods
To create a dataset of terrestrial regions during the Mesozoic, I digitized available palaeocoastline maps [13]. For a further discussion of map sources, please consult the electronic supplementary material. Each contiguous land area was digitized individually; this resulted in some cases where several modern continents were joined together into a single polygon, and other cases where modern continents were subdivided into multiple polygons owing to the presence of epeiric seaways. The resulting polygons were analysed using the R Statistical Program [18] (the complete R code, including the full set of polygons, can be downloaded from Dryad [19]). Polygon area was calculated using the areaPolygon function in the geosphere package [20], which is able to calculate true area of an irregular polygon on the surface of an ellipsoid. Expected number of species for each region could then be estimated using the SAR, with total biodiversity for each time period the sum of all estimates.
For this analysis, the interprovincial land mammal SAR formula from Wright [9] was used to calculate the predicted number of species for each individual land mass (see the electronic supplementary material, S1, for a further discussion of SAR equations and alternate estimates of interprovincial SAR equations). For simplicity, this equation was used for the primary analyses in this study. Although ecological roles and taxonomic abundance of MTV groups were different than living animals, terrestrial mammals were used as a proxy for all groups as they represent one of the most abundant and well-studied terrestrial vertebrates. As the SAR varies between groups, it is important to remember that the overall trend of biodiversity through time is of more relevance and probably more accurate than absolute values of standing diversity.
3. Results
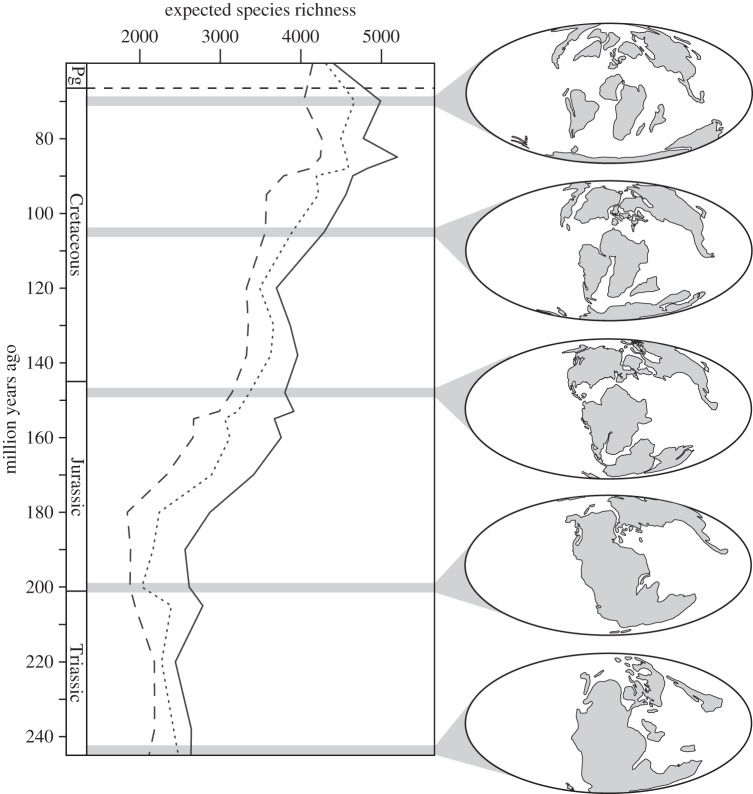
Using this model, MTV diversity would be expected to increase substantially from the earliest Triassic, peak in the Santonian or Campanian, and decline slightly through the Maastrichtian (figure 2). Although alternate interprovincial SAR equations provide different estimates, this overall trend is consistent for a wide range of equations (electronic supplementary material, figure S2). Moving back in time, the rock record becomes less complete, and there is the potential that evidence of smaller terrestrial areas is less likely to be preserved. To test for a potential overestimation due to a higher number of preserved, small, insular regions in more recent time periods, the model was run two additional times, ignoring areas smaller than 250 000 km2 and 500 000 km2, respectively. Removing the smaller land masses decreased estimated diversity for each time period; however, the overall trend of increasing diversity through the Mesozoic was still robust. The large rise in expected biodiversity through time is because of the increasing evenness in land mass size (figure 1a), rather than the absolute number of land masses or the total available terrestrial area (figure 1b).
Figure 2.
Predicted biodiversity for all areas (solid line), areas greater than 250 000 km2 (dotted line) and areas greater than 500 000 km2 (dashed line). Palaeogeographic maps shown for select time periods. Pg, Palaeogene.
As this model was explicitly designed to only investigate the effect of geography on biodiversity, climatic factors were intentionally left out. In particular, all terrestrial regions, even those near the poles, were included in the calculations of expected species richness. Although modern polar regions are relatively species poor, there is ample evidence of diverse terrestrial communities near both poles throughout much of the Mesozoic [21]. Also, there was a distinct warming trend through the Mesozoic, peaking in the Late Cretaceous, with the increase in temperatures generally greater towards the poles, leading to a reduced equator-to-pole thermal gradient [22]. Therefore, integrating climate into the model would probably lead to an even larger predicted increase in biodiversity through the Mesozoic, as polar regions would be able to sustain ever higher numbers of species as high latitude climates became warmer and more equable.
4. Discussion
There has been a recent surge in interest in the shape of terrestrial biodiversity through the Mesozoic [23–28]. However, very few studies have investigated the impact of geographical arrangement on biodiversity estimates. Even among studies that incorporate terrestrial area, these studies almost always test for an effect due to total area [25,26] or geographical spread of localities [28], rather than the arrangement and sizes of individual landmasses. As such, geographically produced effects, particularly owing to the isolation of the continents, may be prematurely ruled out.
There is some support for continental fragmentation promoting biodiversity from several empirical studies. In Late Cretaceous North America, the Western Interior Seaway bisected the continent from north to south, isolating eastern and western regions from each other, and these two regions developed distinct faunal assemblages during this time [29]. Likewise, Late Cretaceous Europe was fragmented into a large number of isolated islands. Each of these smaller islands, though individually less diverse than larger contemporaneous land masses, nonetheless possessed a high degree of endemism [30], creating an overall higher diversity than a single landmass of equivalent size.
The slight decrease in expected biodiversity during the Maastrichtian shows the large effect terrestrial isolation can have. During the very last 5 million years of the Cretaceous, sea level began to fall, reconnecting previously separate regions, leading to a predicted homogenization of ecosystems and overall decrease in biodiversity. This decrease in expected biodiversity is occurring despite the increase in total terrestrial area (figure 1b). Although a decline in diversity shortly before the extinction has been previously suggested [31], the subject is of some debate [23]. Further work is needed to disentangle regional and global signals of biodiversity, in order to understand how biodiversity was actually responding at this time. In particular, incorporating species occurrence data, and estimates of biodiversity derived from that data, with the geographical model presented here is a logical next step in understanding the influence of geography on Mesozoic terrestrial biodiversity. Most importantly, palaeogeography provides an explicit alternative to climatic or intrinsic drivers of biodiversity, and can provide an important framework for further examining terrestrial biodiversity changes throughout the Mesozoic.
Supplementary Material
Acknowledgements
Pat A. Holroyd, Brian D. Rankin, and Nicolás E. Campione provided constructive criticism that greatly improved the manuscript. I would also like to thank the several reviewers and editors whose comments greatly improved the final version of this paper.
Data accessibility
Full data analysis code, including polygons for all land massess and time periods, is downloadable from Dryad [19] http://dx.doi.org/10.5061/dryad.81tf9.
Competing interests
The author declares that he has no competing interests.
Funding
I received no funding for this research.
References
- 1.Rosenzweig ML. 1995. Species diversity in space and time. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Williams CB. 1943. Area and number of species. Nature 152, 264–267. ( 10.1038/152264a0) [DOI] [Google Scholar]
- 3.Preston FW. 1960. Time and space and the variation of species. Ecology 41, 611–627. ( 10.2307/1931793) [DOI] [Google Scholar]
- 4.MacArthur RH, Wilson EO. 1967. The theory of island biogeography, vol. 1 Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Connor EF, McCoy ED. 1979. The statistics and biology of the species-area relationship. Am. Nat. 113, 791–833. ( 10.1086/283438) [DOI] [Google Scholar]
- 6.Lomolino MV. 2000. Ecology's most general, yet protean pattern: the species-area relationship. J. Biogeogr. 27, 17–26. ( 10.1046/j.1365-2699.2000.00377.x) [DOI] [Google Scholar]
- 7.Barnosky AD, Carrasco MA, Davis EB. 2005. The impact of the species-area relationship on estimates of paleodiversity. PLoS Biol. 3, 1–6. ( 10.1371/journal.pbio.0030266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins MD, Vázquez DP, Sanders NJ. 2002. Species-area curves, homogenization and the loss of global diversity. Evol. Ecol. Res. 4, 457–464. ( 10.1086/324787) [DOI] [Google Scholar]
- 9.Wright DH. 1987. Estimating human effects on global extinction. Int. J. Biometeorol. 31, 293–299. ( 10.1007/BF02188940) [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig ML. 2001. The four questions: what does the introduction of exotic species do to diversity? Evol. Ecol. Res. 3, 361–367. [Google Scholar]
- 11.Brown JH. 1995. Macroecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 12.Irving E. 1983. Fragmentation and assembly of the continents, mid-Carboniferous to present. Geophys. Surv. 5, 299–333. ( 10.1007/BF01453985) [DOI] [Google Scholar]
- 13.Smith AG, Smith DG, Funnell BM. 1994. Atlas of Cenozoic and Mesozoic coastlines. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Poulsen CJ, Gendaszek AS, Jacob RL. 2003. Did the rifting of the Atlantic Ocean cause the Cretaceous thermal maximum? Geology 31, 115–118. () [DOI] [Google Scholar]
- 15.Haq BU, Hardenbol JAN, Vail PR. 1987. Chronology of fluctuating sea levels since the Triassic. Science 235, 1156–1167. ( 10.1126/science.235.4793.1156) [DOI] [PubMed] [Google Scholar]
- 16.Miller KG, Kominz MA, Browning JV, Wright JD. 2005. The Phanerozoic record of global sea-level change. Science 310, 1293–1298. ( 10.1126/science.1116412) [DOI] [PubMed] [Google Scholar]
- 17.Hancock JM, Kauffman EG. 1979. The great transgressions of the Late Cretaceous. J. Geol. Soc. Lond. 136, 175–186. ( 10.1144/gsjgs.136.2.0175) [DOI] [Google Scholar]
- 18.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Development Core Team. See http://www.R-project.org.
- 19.Vavrek MJ.2016. Data from: the fragmentation of Pangaea and Mesozoic terrestrial vertebrate biodiversity. Dryad Digital Repository. http://dx.doi.org/doi:10.5061/dryad.81tf9 .
- 20.Hijmans RJ. 2015. Geosphere: spherical trigonometry. R package version 1.4–3. See https://cran.r-project.org/package=geosphere. [Google Scholar]
- 21.Rich TH, Vickers-Rich PA, Gangloff RA. 2002. Polar dinosaurs. Science 295, 979–980. ( 10.1126/science.1068920) [DOI] [PubMed] [Google Scholar]
- 22.Frakes LA, Francis JE, Syktus JI. 1992. Climate modes of the Phanerozoic Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Wang SC, Dodson P. 2006. Estimating the diversity of dinosaurs. Proc. Natl Acad. Sci. USA 103, 13 601–13 605. ( 10.1073/pnas.0606028103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler RJ, Barrett PM, Nowbath S, Upchurch P. 2009. Estimating the effects of sampling biases on pterosaur diversity patterns: implications for hypotheses of bird/pterosaur competitive replacement. Paleobiology 35, 432–446. ( 10.1666/0094-8373-35.3.432) [DOI] [Google Scholar]
- 25.Butler RJ, Brusatte SL, Andres B, Benson RBJ. 2012. How do geological sampling biases affect studies of morphological evolution in deep time? A case study of pterosaur (Reptilia: Archosauria) disparity. Evolution (N. Y.) 66, 147–162. ( 10.1111/j.1558-5646.2011.01415.x) [DOI] [PubMed] [Google Scholar]
- 26.Mannion PD, Benson RBJ, Upchurch P, Butler RJ, Carrano MT, Barrett PM. 2012. A temperate palaeodiversity peak in Mesozoic dinosaurs and evidence for Late Cretaceous geographical partitioning. Glob. Ecol. Biogeogr. 21, 898–908. ( 10.1111/j.1466-8238.2011.00735.x) [DOI] [Google Scholar]
- 27.Nicholson DB, Holroyd PA, Benson RBJ, Barrett PM. 2015. Climate-mediated diversification of turtles in the Cretaceous. Nat. Commun. 6, 7848 ( 10.1038/ncomms8848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson RBJ, Butler RJ, Alroy J, Mannion PD, Carrano MT, Lloyd GT. 2016. Near-stasis in the long-term diversification of Mesozoic tetrapods. PLoS Biol. 14, e1002359 ( 10.1371/journal.pbio.1002359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwimmer DR. 1997. Late Cretaceous dinosaurs in eastern USA: a taphonomic and biogeographic model of occurrences. Dinofest Int. Proceedings, Philadelphia Acad. Nat. Sci., pp. 203–211. [Google Scholar]
- 30.Csiki-Sava Z, Buffetaut E, Ősi A, Pereda-Suberbiola X, Brusatte SL. 2015. Island life in the Cretaceous: faunal composition, biogeography, evolution, and extinction of land-living vertebrates on the Late Cretaceous European archipelago. Zookeys 469, 1–161. ( 10.3897/zookeys.469.8439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan RE, Rigby JK, Van Valen LM, Gabriel D. 1986. Gradual dinosaur extinction and simultaneous ungulate radiation in the Hell Creek Formation. Science 232, 629–633. ( 10.1126/science.232.4750.629) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full data analysis code, including polygons for all land massess and time periods, is downloadable from Dryad [19] http://dx.doi.org/10.5061/dryad.81tf9.