Abstract
With millions of years of natural evolution, organisms have achieved sophisticated structures, patterns or textures with complex, spontaneous multifunctionality. Among all the fascinating characteristics observed in biosystems, self-cleaning ability is regarded as one of the most interesting topics in biomimicry because of its potential applications in various fields such as aerospace, energy conversion and biomedical and environmental protection. Recently, in-depth studies have been carried out on various compelling biostructures including lotus leaves, shark skins, butterfly wings and gecko feet. To understand and mimic their self-cleaning mechanisms in artificial structures, in this article, recent progress in self-cleaning techniques is discussed and summarized. Based on the underlying self-cleaning mechanisms, the methods are classified into two categories: self-cleaning with water and without water. The review gives a succinct account of the detailed mechanisms and biomimetic processes applied to create artificial self-cleaning materials and surfaces, and provides some examples of cutting-edge applications such as anti-reflection, water repellence, self-healing, anti-fogging and micro-manipulators. The prospectives and directions of future development are also briefly proposed.
Keywords: self-cleaning, biomimetic, nanostructure, gecko, lotus effect, adhesion
1. Introduction
Regular cleaning involving sanitizing materials and solutions is necessary to maintain freshness on the routine surfaces that we encounter in our day-to-day life. In addition to the economic burden, extensive cleaning potentially introduces hazardous substances to the environment and ecosystem. By contrast, various surfaces in Nature exhibit a high intrinsic ability to clean themselves without any external aid. This phenomenon, due to its unique mechanism and high adaptability, has attracted tremendous research curiosity in past decades [1–10]. The concept of self-cleaning was initially unveiled based on the superhydrophobic nature of certain plant leaves. Among them, the most well-known example is the lotus leaf, which could make water droplets roll off the leaf surface quickly to achieve surface cleaning. Lotus leaves exhibit a contact angle > 150° and a small sliding angle < 2°. The high surface tension of water will assemble the droplets into spheres that drive the droplets to roll off the surface together with embedded dirt from the surface. On the contrary, on superhydrophilic surfaces such as Tillandsia usneoides and sphagnum moss [11], the dirt components on surfaces can be detached using only water. Here, the extremely small or even zero contact angle at the interface between the surface and the water droplets makes the dirt components movable along with the water flow on the surface. Hence, both hydrophobic and hydrophilic surfaces can be efficiently cleaned with the aid of water [12–23]. In general, removing surface-coated/adhered contaminants is difficult because it is hard to dissolve them without the help of high surface energy, solubility and mobility of water. However, intelligent, intricate architectures developed in Nature present remarkable examples of self-cleaning even without the aid of water. For example, geckos can keep their feet clean through the unique, complex structure of their foot skin. Pitcher plants have highly wetting surfaces, which can cause water droplets to spread rapidly across the surfaces. Wetting can enhance the slipperiness and increase the capture rate of small insects as they slip off from its rims [24,25]. Such self-evolved structures give inspiration to researchers around the world to develop artificial materials with self-cleaning capabilities by mimicking natural systems. Although several reviews exist about self-cleaning [15,26–28], rapid progress in this direction has led to the invention of novel structures with enhanced properties, which closely match nano/microstructures with their natural counterparts. Hence, this mini-review focuses on recent developments regarding different approaches for fabrication of biomimetic self-cleaning surfaces with a particular emphasis on the underlying mechanisms. A brief overview of some important practical applications is also presented.
2. Self-cleaning with water
Water, the most abundant and freely available liquid system with optimal density as well as polarity, is being used as an essential medium to remove different types of contaminations on surfaces. In Nature, many ingenious designs exist which combine surface properties and water energetics to scour material surfaces. Lotus leaves, one of the earliest discovered and investigated self-cleaning biosurfaces [29,30], uses a specific water–surface interaction to clean the surface of leaves. Young and co-workers [31–34] proposed wetting models which can be used to explain the self-cleaning mechanism of lotus leaves. Young's equation for the contact angle is given as
| 2.1 |
where 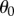 represents the contact angle of the liquid on the surfaces, and
represents the contact angle of the liquid on the surfaces, and 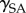 and
and 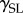 are the surface energies of the solid against air and liquid, respectively, and
are the surface energies of the solid against air and liquid, respectively, and 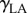 is the surface energy of liquid against air.
is the surface energy of liquid against air.
Young's equation can successfully predict the contact angle of a water droplet on a flat surface with a homogeneous interface. If the surface is rough and the actual surface area is larger than its flat projected area, the contact angle can be given under Wenzel's equation
| 2.2 |
where 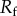 represents the ratio of the actual surface area to its flat projected area. As for a rough surface
represents the ratio of the actual surface area to its flat projected area. As for a rough surface 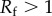 , which means a hydrophobic surface becomes more hydrophobic for rough surfaces and a hydrophilic surface becomes more hydrophilic for rough surfaces. Similarly, for the heterogeneous surfaces composed of two fractions, the Cassie equation can be derived
, which means a hydrophobic surface becomes more hydrophobic for rough surfaces and a hydrophilic surface becomes more hydrophilic for rough surfaces. Similarly, for the heterogeneous surfaces composed of two fractions, the Cassie equation can be derived
| 2.3 |
where f1 and f2 are the fractional area with contact angle θ1 and θ2, respectively.
For a composite interface consisting of a solid–liquid fraction (f1 = fSL and θ1 = θ0) and liquid–air fraction (f2 = fLA and cos θ21 = −1), the Cassie–Baxter equation can be derived
| 2.4 |
Based on these proposed models, it is possible for a surface to achieve self-cleaning ability by controlling surface microstructures to promote free, spontaneous movement of a liquid droplet on the surface, allowing it to extract contaminants from the surfaces. Therefore, for a surface to achieve self-cleaning, the primary goal will be to ensure that droplets can flow or roll off smoothly from the attached surface without any resistance. Natural or biosurfaces principally maximize or minimize the contact angles of the droplets to facilitate free liquid droplet movement. This free movement is achieved by modulating the surface energy of the three phases including liquid, air and solid (on the surface).
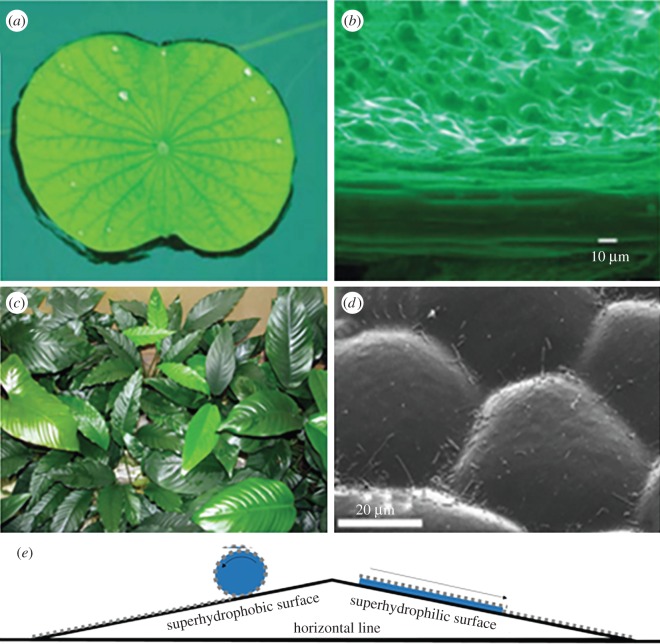
When the contact angle of the droplets is approaching 180°, it is very easy for the droplets to roll off from the surface (e.g. lotus leaves). Zhang et al. [35] studied this effect and found that at this maximized contact angle droplets freely move across the leaf's surface and remove foreign bodies (dirt components) by dissolving them in the liquid along the direction of motion of the droplets (figure 1a,b). However, several other natural systems apply the opposite approach, e.g. pitcher plants have highly wettable surfaces, which can minimize contact angles to form a water film. The wetting surface is so slippery that prey slide off it [25]. When the contact angle approaches zero (e.g. the smooth surface structure of Anubias barteri in figure 1c or Heliamphora nutans), water will flow more freely on it. In the presence of water, the good solubility and high surface energy of water favourably wash the contaminated area, taking out the dissolved contaminants. Moreover, the minuscule contact angle of water droplets will serve as a sharp knife, which can scrape the contaminants off the surface, separating the dirt that is already stabilized via intramolecular forces, resulting in the simple removal of the contaminants. The scanning electron microscopy (SEM) pictures of the lotus leaf surface mentioned above and A. barteri leaf surface studied by Koch & Barthlott [2] are shown in figure 1b and 1d, respectively. The higher surface roughness of lotus leaves compared with A. barteri leaves is evident from the SEM images due to the protuberances on lotus leaves being much smaller and denser.
Figure 1.
Natural plants and their surface morphology. (a) Lotus leaf. (Reproduced from [35] with permission of The Royal Society of Chemistry.) (b) Scanning electron microscopy (SEM) picture of lotus leaves. (Reproduced from [35] with permission of The Royal Society of Chemistry.) (c) Anubias leaves. (d) SEM picture of Anubias leaves. (Reproduced from [2] with permission of The Royal Society.) (e) Demonstration of self-cleaning on a superhydrophobic surface and a superhydrophilic surface. For the superhydrophobic case, the particles on the surface can be removed by the rolling off of water droplets due to the high contact angle (usually greater than 150°) between the surface and the droplet. For the superhydrophilic surfaces, the particles on the surface can be easily separated by the small contact angle between the droplet and the surface and then taken away due to the good contact between the surface and the droplet.
In short, surfaces that apply the two opposite strategies for maximizing and minimizing the contact angle of droplets to promote free droplet fall-off are termed superhydrophobic and superhydrophilic surfaces, respectively. Figure 1e illustrates the applied mechanism for each surface's self-cleaning process. Although the strategy typically requires a large amount of water to clean the surface, water-assisted self-cleaning is still attracting a high volume of attention from scholars, as successful adaptation and development of the strategies could lead to a considerable reduction in efforts/costs in cleanliness and maintenance.
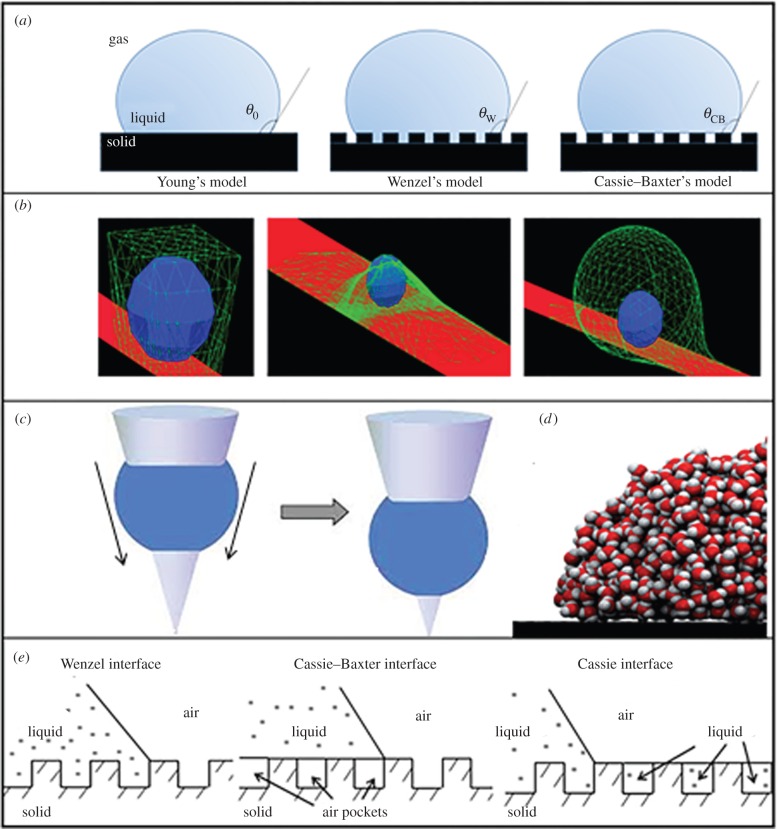
Inspired by the self-cleaning surfaces found in Nature, many technological studies employing a variety of materials and surface modification technologies have been conducted to fabricate artificial superhydrophobic and superhydrophilic surfaces with self-cleaning ability. Although significant progress has been achieved, the level of efficacy attained in Nature is yet to be fully replicated. Figure 2 illustrates the different models proposed to understand the surface-wetting process of diverse surfaces. Figure 2a demonstrates a very traditional wetting model reported by Young, Wenzel and Cassie–Baxter. This model can be used to estimate the contact angle of rough surfaces where air pockets exist, providing a theoretical approach for the design of functional surfaces with special wettability. However, the effect of gravity is not considered in the model, which leads to an imprecise estimation of contact angles of droplets on surfaces compared with the ones simulated with gravity considered, resulting in high variability [40]. Figure 2b shows a droplet profile study by Muller et al. [36] based on surface evolvers. This surface evolver is a simulation tool that can determine the profile of the droplet with minimal surface area, namely minimal surface energy, which indicates that attaining low surface energy is a fundamental property of self-cleaning surfaces [36]. Michielsen et al. [37] considered surfaces to be composed of cones, a very common shape in Nature, and computed the wetting behaviour of such surfaces (figure 2c). This study implied that barrel-shaped droplets could move spontaneously to any location along the cone axis where the defined systems, including the liquid, solid and gas phases, achieve their lowest Gibbs free energy. For a constant cone angle, as the contact angle between the liquid and the cone increases, the drop will move towards the apex of the cone. Likewise, for a constant contact angle, as the cone angle increases, the drop moves toward the apex. According to this theory, it is possible to make a droplet move towards the apex and then roll away from the surface to clean the surface by controlling the geometry and surface energy of the cone. Shih et al. [38] simulated the wetting behaviour of various surfaces with small droplets, such as the tiny droplets in fog that have a complicated intramolecular interaction (figure 2d). This unique approach is used to understand the formation of contact angles by calculating the molecular interaction at the liquid–solid interface. This study indicated that the quantum effect or atomic-level interactions between liquid and solid phases could be quantitatively revealed. However, this study is limited only to small droplets due to the limited capability of the computer processing regarding the volume of the droplets. In all the above modelling approaches, parameters including the Gibbs free energy, surface evolver, and dynamic thermal modelling are widely used. All these models are useful to understand the intricate parameters and factors that control the self-cleaning behaviour of the surfaces (figure 2e).
Figure 2.
Modelling of surface wetting. (a) The progress of wetting models for a droplet on a flat surface from Young's model (flat surface) to the Cassie–Baxter model (with surface roughness). (b) Surface evolver of droplets, which is a calculation of the profile of the droplet with the minimal surface area, namely the minimal surface energy. (Reproduced from [36] with permission of The Royal Society of Chemistry.) (c) Droplet profile on cones, which is a calculation of the droplet profile. (Reprinted with permission from Michielsen S, Zhang J, Du J, Lee HJ. 2011 Gibbs free energy of liquid drops on conical fibres. Langmuir 27, 11 867–11 872. Copyright © 2011 American Chemical Society.) (d) Dynamic thermal simulation of droplets on a flat surface based on the chemistry interactions (this approach has requirement on droplet size, which is limited by the computational capability). (Reproduced from [38] with permission of Nature Publishing Group.) (e) Schematics of configurations described by the Wenzel equation (equation (2.2) for the homogeneous interface, Cassie equation equation (2.3) for the homogeneous interface and the Cassie–Baxter interface for the composite interface with air pockets equation (2.4)). (Reprinted with permission from Bhushan B, Jung YC. 2011 Natural and biomimetic artificial surfaces for superhydrophobicity, selfcleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 56, 1–108. Copyright © 2011 American Chemical Society.)
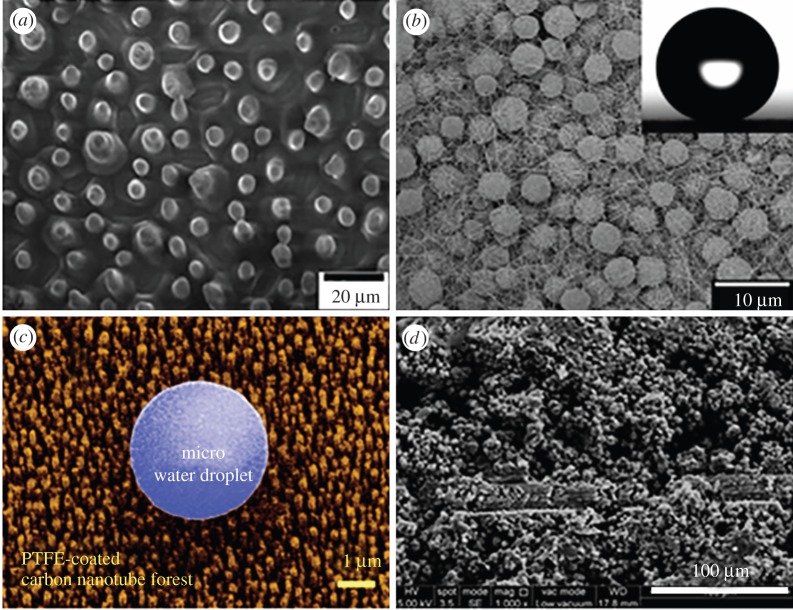
Experimentally, surface modification [41], which only requires limited material consumption, proves to be the most effective and widely used method to create a surface with unique wetting properties. Diverse approaches, including the creation of the three-dimensional gradient porous interconnected network, hydrogel-coated mesh, fluid-infused porous films, reversible capillary-stabilized liquid-filled pores, assembled colloidal photonic crystals, micro/nano-hierarchical structures and proximally immobilized ions, have been employed to build surfaces with self-cleaning properties [41–50]. Figure 3 summarizes some of the compelling strategies reported recently. Liu et al. [51] used the advanced soft-lithographic imprinting technique to fabricate biomimetic and self-cleaning surfaces (figure 3a). The fabricated surface with imprinted protuberances closely mimicked the structure of lotus leaves. The presence of the surface protuberances drastically increases the contact angle of water droplets sitting on this surface due to the trapped air between the droplet and the rough surface, which shares the same scenario as the lotus leaves. Figure 3b illustrates a similar substrate constructed by Wei et al. [52] through electro-brush plating and blackening processes. Figure 3c,d provides an example of a surface coated with aluminium oxide nanoparticles fabricated by Alexander et al. [53] and a self-cleaning surface covered by carbon nanotubes (CNTs) studied by Lau et al. [54]. All the samples in figure 3 are hydrophobic surface-based self-cleaning materials. Compared with the hydrophobic surface self-cleaning materials, the hydrophilic surface does not have much variety in fabrication approach due to the high surface tension requirements. Materials including TiO2 [15,55,56], SiO2 [57], ZnO [58], VO2 [59], Ag [60] and polydopamine-encapsulated octadecylamine [61] have been explored as a novel approach to make superhydrophilic self-cleaning surfaces. Generally, hydrophilic surfaces are more challenging to fabricate because the easily modified surface roughness always increases the hydrophobicity instead of hydrophilicity based on the most fundamental Young's equation on contact angles.
Figure 3.
Biomimetic self-cleaning surfaces. (a) Fabricated through soft-lithographic imprinting. (Reprinted with permission from [51]. Copyright © 2006 WILEY-VCH Verlag GmbH & Co. KGaA Weinheim.) (b) Fabricated through an electro-brush plating and blackening process. (Reprinted with permission from [52]. Copyright © 2015, Royal Society of Chemistry.) (c) Fabricated through aluminium oxide nanoparticles. (Reprinted with permission from Alexander S, Eastoe J, Lord AM, Guittard F, Barron AR. 2015 Branched hydrocarbon low surface energy materials for superhydrophobic nanoparticle derived surfaces. ACS. Appl. Mater. Interfaces 8, 660–666. Copyright © 2016 American Chemical Society.) (d) Fabricated through carbon nanotubes. (Reprinted with permission from Lau KK, Bico J, Teo KB, Chhowalla M, Amaratunga GA, Milne WI, McKinley GH, Gleason KK. 2003 Superhydrophobic carbon nanotube forests. Nano Lett. 3, 1701–1705. Copyright © 2003 American Chemical Society.) All these approaches increased the contact angles of droplets on them through an increase in the surface roughness based on the Cassie–Baxter model shown in figure 1a. (Online version in colour.)
While water-assisted self-cleaning is an efficient pathway for surface cleaning, under some special conditions where water is not readily accessible, such strategies can fail. For example, in outer space and in cold areas where the temperature is below 0°C, surfaces cannot access abundant amounts of liquid water or the free movement of water will be hindered. Such conditions may limit the usages of water-assisted self-cleaning surfaces. Thus, there is an urgent need to develop advanced technology for surface decontamination in such unique extreme environments.
3. Self-cleaning without water
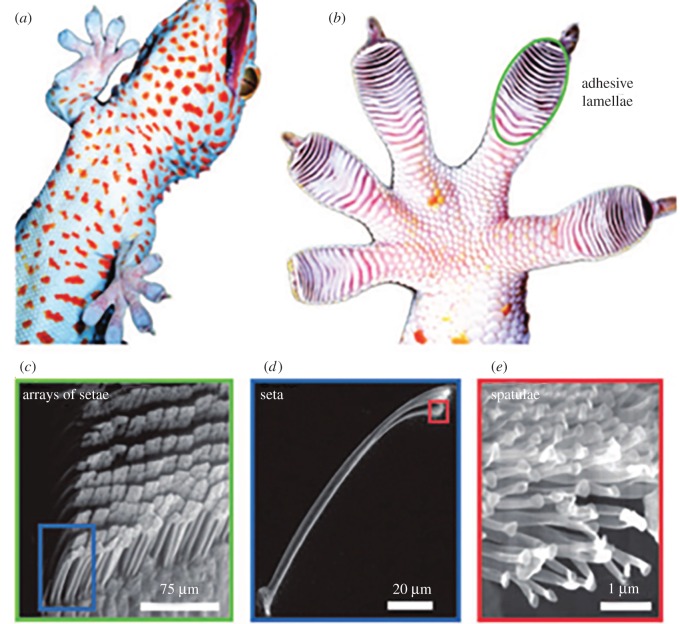
Geckos have the world's most efficient and reversible adhesion system [62–68]. Although their feet are very sticky, geckos have an extraordinary ability to prevent them from fouling while running on dusty ceilings and in corners. Autumn et al. [70] explained the underlying principle behind the gecko's strong adhesion. A gecko's toe pads have a complex hierarchical structure composed of millions of small hairs called ‘setae’. Each seta further branches into hundreds of even smaller hairs, each of which ends in a flattened spatula (figure 4a–e). The van der Waals (vdW) interaction between the millions of spatulae and the surface after coming into intimate contact is sufficient for the gecko to adhere to the surface [70]. This highly evolved mechanism allows the gecko to adhere to surfaces irrespective of whether they are hydrophobic or hydrophilic, dry or wet, rough or smooth. In 2005, Hansen & Autumn [69] discovered that, in addition to this high adhesion ability, the gecko toe pads have a unique self-cleaning capability. Unlike the classic ‘lotus effect’, the gecko's self-cleaning ability does not demand the assistance of water. This ability is regarded as a ‘dry self-cleaning’ property because geckos can successfully dislodge most attached contaminants simply by contacting with external surfaces during their movement from one place to another [71,72]. Another interesting example is tree frogs. Although their strong adhesion is aided by the secretion of mucus, the shear movements and ‘flushing’ play an important role in shedding particles/contaminants and keeping their feet from fouling [73]. Mimicking these hierarchical fibrillary or thin soft film adhesive structures, one can create the next generation of sticky, yet self-cleaning tapes, climbing robots, smart surfaces, etc. that can work efficiently under various temperature, humidity and pressure conditions [74].
Figure 4.
Structure hierarchy of the gecko adhesive system. (a) Macrostructure: ventral view of a tokay gecko climbing a vertical glass wall. (b) Mesostructure: ventral view of the foot. The visible overlapping pads are the adhesion lamellae (scansors). (c) Microstructure: a proximal portion of a single lamella, with a visible array of individual setae. (d and e) Nanostructure: the branched structure of a single seta at upper right, terminating in hundreds of spatula tips. (Reprinted with permission from [69]. Copyright © 2005 National Academy of Sciences.)
Various strategies have been formulated to synthesize gecko feet-like surfaces from different materials including silicones [75], CNTs [76–79], poly(methyl methacrylate) [80], polyurethane acrylate [81], polydimethylsiloxane (PDMS) [82] and more [83–86]. The successful fabrication of gecko-inspired functional materials requires the combination of the physical, chemical and biological principles. The development of such novel functional systems has been extensively discussed in recent review articles [27,39]. Although biomimetic dry self-cleaning surfaces have been well studied, the fabrication of artificial dry self-cleaning surfaces is still at a very preliminary stage. Lee & Fearing [87] reported the first gecko-inspired, dry self-cleaning adhesive using a high-aspect-ratio fibrillar polypropylene (PP) array. The fabricated fibrillar adhesive showed a recovery of around 33% of the shear adhesion on clean samples after 30 simulated steps on a dry, clean and rigid surface (figure 5a–b). Subsequently, Gillies et al. [72] fabricated a dry self-cleaning fibrillar structure made of hard and soft polypropylene. They discovered that hard fibres could recover 96–115% of shear adhesion after fouling with small and large particles (some of them performed better than before). However, soft fibres showed only 55% recovery upon fouling with large particles, as particles prefer to embed readily within the soft polymers (figure 5c–d) and prevent the further self-cleaning of soft polymers. Further, Mengüç et al. [9] designed vertically aligned elastomer microfibres and tested the self-cleaning properties of the biomimetic material after contamination. Followed by a load–drag–unload dry contacting self-cleaning process, they discovered large particles rolling during the drag process. If the particles are smaller than the adjacent fibre distances, they may be embedded in between those fibres. If the particles are far below the size of the fibres, they may stay on the surfaces of those fibres and thus be hard to remove.
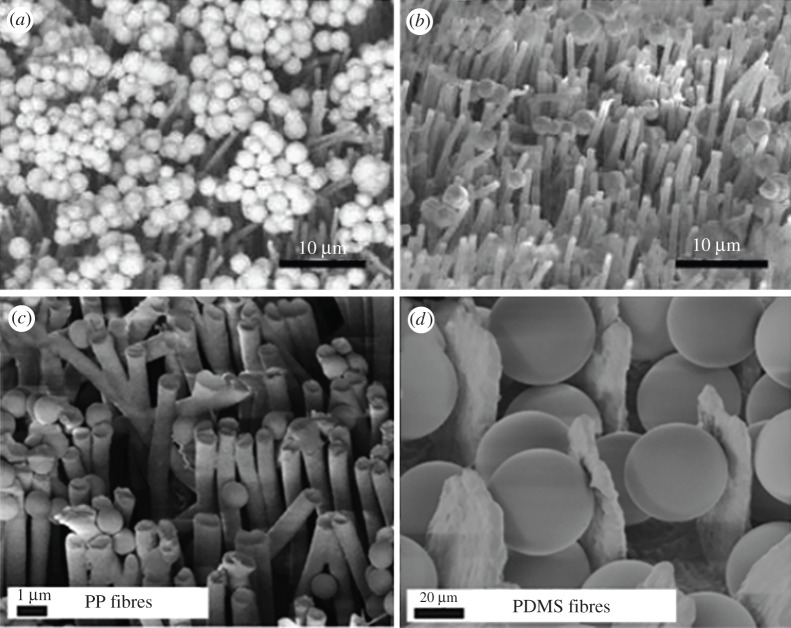
Figure 5.
SEM images of a polypropylene (PP) fibrillary adhesive and conventional pressure-sensitive adhesives. (a) Fibrillar adhesive contaminated by gold microspheres. (Reprinted with permission from Lee J, Fearing RS. 2008 Contact self-cleaning of synthetic gecko adhesive from polymer microfibres. Langmuir 24, 10 587–10 591. Copyright © 2008 American Chemical Society.) (b) Fibrillar adhesive after 30 contacts (simulated steps) on a clean glass substrate. (Reprinted with permission from Lee J, Fearing RS. 2008 Contact self-cleaning of synthetic gecko adhesive from polymer microfibres. Langmuir 24, 10 587–10 591. Copyright © 2008 American Chemical Society.) (c) PP fibres fouled with 3−10 µm glass spheres, where some can be seen deeply embedded (EM) between fibres. (Reprinted with permission from Gillies AG, Puthoff J, Cohen MJ, Autumn K, Fearing RS. 2013 Dry self-cleaning properties of hard and soft fibrillar structures. ACS Appl. Mater. Interfaces 5, 6081–6088. Copyright © 2008 American Chemical Society.) (d) Polydimethylsiloxane (PDMS) fibres contaminated with 40−50 µm glass spheres after 40 recovery steps. Particles can be readily seen embedded between the fibres (EM). (Reprinted with permission from Gillies AG, Puthoff J, Cohen MJ, Autumn K, Fearing RS. 2013 Dry self-cleaning properties of hard and soft fibrillar structures. ACS Appl. Mater. Interfaces 5, 6081–6088. Copyright © 2008 American Chemical Society.)
The fundamental mechanism behind the self-cleaning ability of gecko feet-like structures is also proposed. First, Hansen & Autumn suggested that contact self-cleaning occurs when a particle–substrate adhesive force (Fw−p) overcomes the seta–particle adhesive forces (Fs−p), or simply Fw−p > Fs−p (figure 6a) [69]. However, this model failed to give a complete explanation of why certain particles demonstrate a preference to bind more strongly to the toe pads than to the substrate surfaces. To account for this contradiction, Lee & Fearing [87] proposed that the shaking of the particles during the shearing process was the main reason that seta arrays shed most of the particles (figure 6c). Mengüç et al. [9] believed that the particles rolling under the fibre could enhance the self-cleaning property of a fibrillar adhesion system. Moreover, they suggested that the dragging rate and average load are two critical parameters that affect the performance of the self-cleaning process. Hu et al. [71] reported that geckos clean their feet via a unique digital hyperextension (DH) process (figure 6c). With DH, the gecko can clean 80% of the dust in only four steps of walking while only 40% of the adhesion force can be recovered without DH. During the DH process, the detachment between the gecko seta and substrate becomes a kinetic process. The pull-off velocity at high speed may affect the self-cleaning of gecko seta arrays. Inspired by this, Xu et al. [88] designed experiments to measure a single gecko seta and spatula Fw−p and Fs−p versus different shearing and pull-off velocities. The results revealed that the particle–wall adhesion is velocity dependent, whereas spatula–particle adhesion is velocity independent. This difference leads to the robust self-cleaning capability of gecko feet. During animal locomotion, DH generates high normal pull-off and shear speed before each step. Thus, the gecko can effectively and efficiently dislodge dust from toe pads. Furthermore, this thorough understanding of the self-cleaning mechanism at a single gecko seta level has led to the use of a single gecko seta as a novel powerful micromanipulator tool for various applications. Both single gecko seta and artificially designed seta can quickly pick up, transport and drop off microparticles, helping in precisely assembling complex micro-patterns/structures (figure 6d). The gecko-inspired manipulators [4,89–91] can potentially open up a new window for micromanipulation of particles which could be used in microelectromechanical systems, biomedical devices, etc.
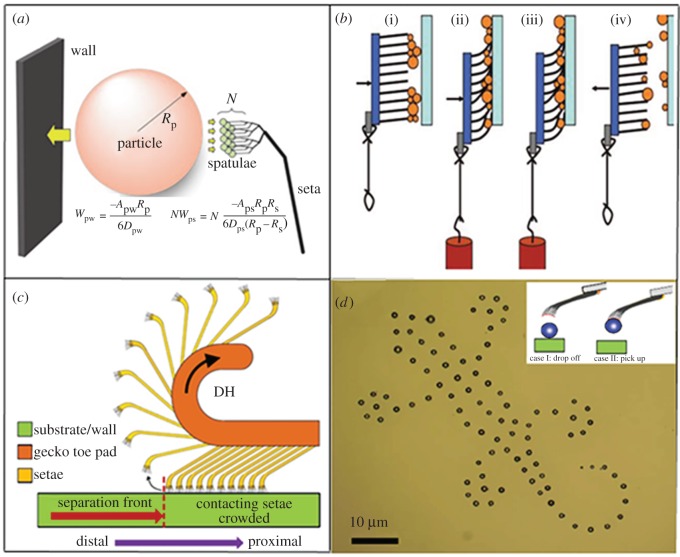
Figure 6.
(a) Model of interactions between N gecko spatulae of radius Rs, a spherical dirt particle of radius Rp and a planar wall. (Reprinted with permission from [69]. Copyright © 2005 National Academy of Sciences.) (b) One cycle of simulated steps, with contact with an initially clean glass slide: (i) applying normal compressive force, (ii) shear load added to the compressive load by a hanging weight, (iii) removing the compressive load (pure shear loading) and (iv) detaching the sample. (Reprinted with permission from Lee J, Fearing RS. 2008 Contact self-cleaning of synthetic gecko adhesive from polymer microfibres. Langmuir 24, 10 587–10 591. Copyright © 2008 American Chemical Society.) (c) Schematics of gecko toe peeling induced by digital hyperextension. (Reproduced from [71] with permission of The Royal Society.) (d) Optical image of a gecko-like log constructed using SiO2 microparticles (size d = 1–25 µm) via precisely assembling them on a glass slide with a biomimetic micromanipulator and using an atomic force microscope. (Reproduced from [88] with permission of Nature Publishing Group.)
4. Multifunctionality of self-cleaning surfaces
A self-cleaning surface usually possesses other functions due to its unique structures and chemistry. The self-cleaning surfaces with multifunctionality may provide additional benefits and properties (figure 7), including anti-icing, anti-fogging, oil–water separation and antimicrobial activity, which brings promising applications in materials engineering, resource reuse, healthcare, as well as safety. For example, Cao et al. [92] fabricated a superhydrophobic surface with anti-icing capability and revealed that the size of the particles exposed on the surface is necessary for the anti-icing property, which gives self-cleaning surfaces new functionality (figure 7a). As the surface is a nanoparticle composite, water on this composite is primarily in contact with air pockets trapped in the rough surface, which leads to superhydrophobicity or self-cleaning. On the other hand, the nanoparticles on the surface directly contact water, which significantly increases the nucleation energy barrier in heterogeneous nucleation, and thus suppress the formation of ice on the surface (i.e. anti-icing property). Park et al. [93] prepared photoanodes with anti-fogging and anti-reflection properties by coating with hydrophilic SiO2 nanoparticles. The SEM and atomic force microscopy figures of the coated surfaces can be seen in figure 7b [93]. Yang et al. [94] fabricated a porous surface made of a PDDA-PFO/SiO2 coating which allows the passage of water but makes oil stand on the top of the surface, making it an anti-oil surface (figure 7c). Self-cleaning surfaces also have significant application for anti-fouling and antimicrobial surfaces, which were initially reported by Ball [96]. Comparison of the antimicrobial activity of a normal surface and a micro-patterned surface, resembling the pattern on shark skin, is given in figure 7d [95]. Meanwhile, a hierarchical structure ranging from the microscale to the nanoscale with a gradient in the surface energy may produce a superhydrophilic slippery surface with continuous, directional water transport on it [97]. Recently, a new mechanism for a slippery surface was discussed by Chen et al. [98] by analysing the peristome surface of Nepenthes alata, which may have practical applications in developing artificial fluid-transport systems. Nowadays, more and more studies focused on slippery surface directions are expected after the discovery of the directional motion of fluid on N. alata surfaces. All those functions make the self-cleaning surface very favourable for different industries, including energy harvesting, civil engineering, medical devices, aerospace and environmental protection.
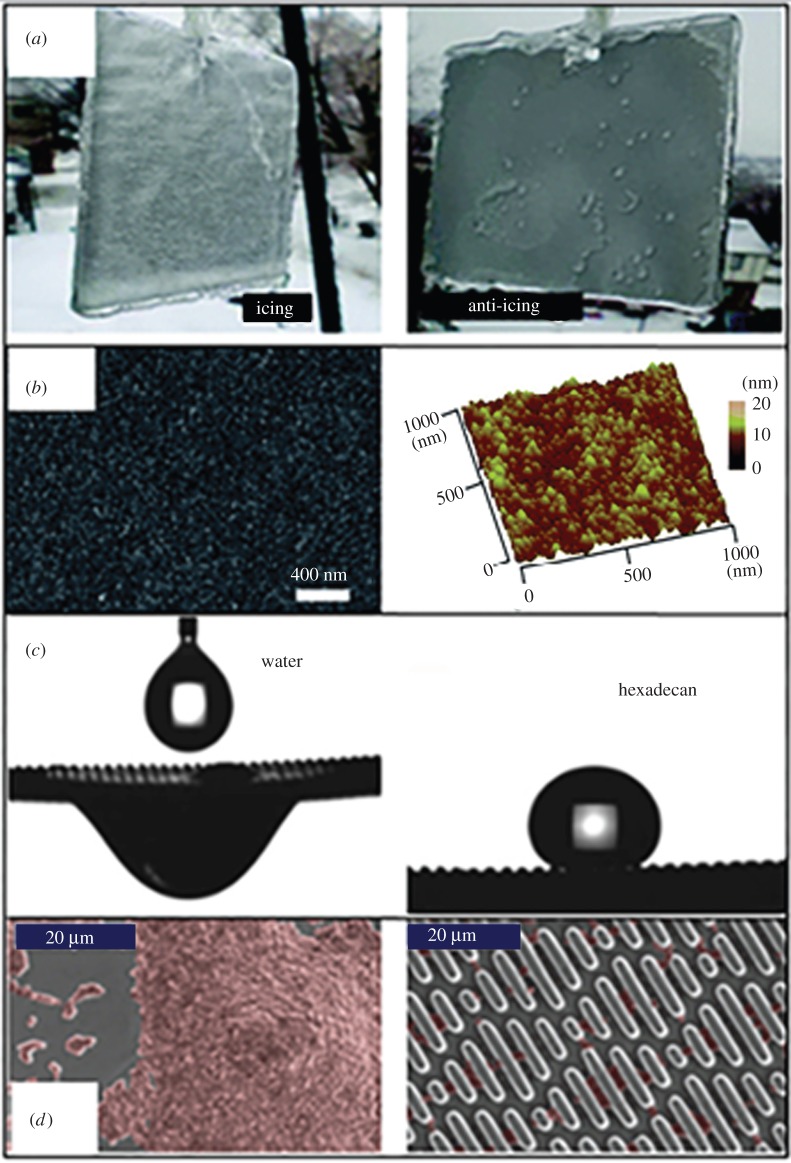
Figure 7.
Multifunctionality of self-cleaning surfaces and their applications. (a) Anti-icing surfaces obtained through increasing both the contact angle of water due to air pockets trapped in the rough surfaces and the heterogeneous nucleation energy barrier by reducing the size of particles on the surface. (Reprinted with permission from Cao L, Jones AK, Sikka VK, Wu J, Gao D. 2009 Anti-icing superhydrophobic coatings. Langmuir 25, 12 444–12 448. Copyright © 2009 American Chemical Society.) (b) Anti-fogging surfaces obtained through creating a superhydrophobic surface which can prevent the condensation of water. (Reprinted with permission from [93]. Copyright © 2014, Royal Society of Chemistry.) (c) Water/oil separation based on the difference between the contact angles of water and oil on the same surfaces. (Reproduced from [94] with permission of The Royal Society.) (d) Antimicrobial surface inspired by shark skin which uses a certain micro-sized pattern to resist bacteria adhesion to the surface. (Reprinted with permission from [95]. Copyright © 2014 Springer International Publishing Switzerland.) (Online version in colour.)
5. Conclusion and outlook
Robust self-cleaning surfaces are highly desired material systems for various applications in society, including solar energy [99], anti-fogging [100], self-healing [101], water–oil separation [102,103], water purification surfaces [104] and smart devices [105–107]. Deriving inspiration from Nature, scientists are working hard to fabricate multifunctional self-cleaning surfaces. In this article, two pathways of self-cleaning, namely the water-assisted and water-free (dry) pathways, are reviewed. A concise overview of the state of the art and an in-depth analysis of the fundamental mechanism of the self-cleaning capability of this fascinating class of materials is presented with a particular focus on recent developments. The latter part of the review also demonstrates the application possibilities of these captivating structures.
The future trend of self-cleaning surfaces is expected to see various other cutting-edge functionalities being incorporated into self-cleaning surfaces including anti-reflection, water repellence, self-healing, anti-fogging, micromanipulation, anti-stickiness, etc. From the engineering aspect, future developments in the fabrication process will make these smart surfaces more cost-effective, flexible, sustainable, durable and reliable. Going forward, the control of these smart surfaces will be attained via the application of various kinds of external stimuli, such as mechanical, thermal, electrical and magnetic fields. At the same time, research will find ways to incorporate additional novel and intriguing properties into self-cleaning structures to increase their utility. Moreover, Nature has evolved many more multifunctional systems which are waiting to be discovered [108,109]. A more fundamental understanding of the mechanisms in natural systems is necessary to mimic and replicate the properties intimately in next-generation, multifunctional self-cleaning surfaces. Hence, a continuous, sustained, rigorous study of different biosurfaces present in Nature and construction of futuristic multifunctional surfaces with unprecedented properties are expected.
Competing interests
We declare we have no competing interests.
Funding
We thank the National Nature Science Foundation of China (no. 51505501) and the National Key Research and Development Plan (no. 2016YFC0303700) for support.
References
- 1.Bhushan B, Jung YC, Koch K. 2009. Self-cleaning efficiency of artificial superhydrophobic surfaces. Langmuir 25, 3240–3248. ( 10.1021/la803860d) [DOI] [PubMed] [Google Scholar]
- 2.Koch K, Barthlott W. 2009. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Phil. Trans. R. Soc. A 367, 1487–1509. ( 10.1098/rsta.2009.0022) [DOI] [PubMed] [Google Scholar]
- 3.Wang B, Liang W, Guo Z, Liu W. 2015. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: a new strategy beyond nature. Chem. Soc. Rev. 44, 336–361. ( 10.1039/C4CS00220B) [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Chary S, Das S, Tamelier J, Pesika NS, Turner KL, Israelachvili JN. 2011. Gecko-inspired dry adhesive for robotic applications. Adv. Funct. Mater. 21, 3010–3018. ( 10.1002/adfm.201100493) [DOI] [Google Scholar]
- 5.Jin K, Cremaldi JC, Erickson JS, Tian Y, Israelachvili JN, Pesika NS. 2014. Adhesives: biomimetic bidirectional switchable adhesive inspired by the gecko. Adv. Funct. Mater. 24, 573 ( 10.1002/adfm.201470028) [DOI] [Google Scholar]
- 6.Zheng Y, Bai H, Huang Z, Tian X, Nie FQ, Zhao Y, Zhai J, Jiang L. 2010. Directional water collection on wetted spider silk. Nature 463, 640–643. ( 10.1038/nature08729) [DOI] [PubMed] [Google Scholar]
- 7.Vukusic P, Sambles JR. 2003. Photonic structures in biology. Nature 424, 852–855. ( 10.1038/nature01941) [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka S, Fujita H, Kinoshita S, Matsuhana B. 2014. Alignment of crystal orientations of the multi-domain photonic crystals in Parides sesostris wing scales. J. R. Soc. Interface 11, 20131029 ( 10.1098/rsif.2013.1029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mengüç Y, Rohrig M, Abusomwan U, Holscher H, Sitti M. 2014. Staying sticky: contact self-cleaning of gecko-inspired adhesives. J. R. Soc. Interface 11, 20131205 ( 10.1098/rsif.2013.1205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson GS, Cribb BW, Watson JA. 2010. How micro/nanoarchitecture facilitates anti-wetting: an elegant hierarchical design on the termite wing. ACS Nano 4, 129–136. ( 10.1021/nn900869b) [DOI] [PubMed] [Google Scholar]
- 11.Koch K, Bhushan B, Barthlott W. 2008. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 4, 1943–1963. ( 10.1039/B804854A) [DOI] [Google Scholar]
- 12.Frankiewicz C, Attinger D. 2016. Texture and wettability of metallic lotus leaves. Nanoscale 8, 3982–3990. ( 10.1039/C5NR04098A) [DOI] [PubMed] [Google Scholar]
- 13.Fürstner R, Barthlott W, Neinhuis C, Walzel P. 2005. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 21, 956–961. ( 10.1021/la0401011) [DOI] [PubMed] [Google Scholar]
- 14.Lim HS, Lee SG, Lee DH, Lee DY, Lee S, Cho K. 2008. Superhydrophobic to superhydrophilic wetting transition with programmable ion-pairing interaction. Adv. Mater. 20, 4438–4441. ( 10.1002/adma.200801069) [DOI] [Google Scholar]
- 15.Liu K, Jiang L. 2012. Bio-inspired self-cleaning surfaces. Annu. Rev. Mater. Res. 42, 231–263. ( 10.1146/annurev-matsci-070511-155046) [DOI] [Google Scholar]
- 16.Ming W, Wu D, van Benthem R, de With G. 2005. Superhydrophobic films from raspberry-like particles. Nano Lett. 5, 2298–2301. ( 10.1021/nl0517363) [DOI] [PubMed] [Google Scholar]
- 17.Nakajima A, Hashimoto K, Watanabe T, Takai K, Yamauchi G, Fujishima A. 2000. Transparent superhydrophobic thin films with self-cleaning properties. Langmuir 16, 7044–7047. ( 10.1021/la000155k) [DOI] [Google Scholar]
- 18.Wang S, Liu H, Liu D, Ma X, Fang X, Jiang L. 2007. Enthalpy-driven three-state switching of a superhydrophilic/superhydrophobic surface. Angew. Chem. Int. Ed. 46, 3915–3917. ( 10.1002/anie.200700439) [DOI] [PubMed] [Google Scholar]
- 19.Xia F, Feng L, Wang S, Sun T, Song W, Jiang W, Jiang L. 2006. Dual-responsive surfaces that switch between superhydrophilicity and superhydrophobicity. Adv. Mater. 18, 432–436. ( 10.1002/adma.200501772) [DOI] [Google Scholar]
- 20.Xu L, Chen W, Mulchandani A, Yan Y. 2005. Reversible conversion of conducting polymer films from superhydrophobic to superhydrophilic. Angew. Chem. Int. Ed. 44, 6009–6012. ( 10.1002/anie.200500868) [DOI] [PubMed] [Google Scholar]
- 21.Yu Y, Zhao Z-H, Zheng Q-S. 2007. Mechanical and superhydrophobic stabilities of two-scale surfacial structure of lotus leaves. Langmuir 23, 8212–8216. ( 10.1021/la7003485) [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zhou Z, Cheng B, DeSimone JM, Samulski ET. 2006. Superhydrophobic behavior of a perfluoropolyether lotus-leaf-like topography. Langmuir 22, 8576–8580. ( 10.1021/la061400o) [DOI] [PubMed] [Google Scholar]
- 23.Zheng S, Li C, Fu Q, Li M, Hu W, Wang Q, Du M, Liu X, Chen Z. 2015. Fabrication of self-cleaning superhydrophobic surface on aluminum alloys with excellent corrosion resistance. Surf. Coat. Tech. 276, 341–348. ( 10.1016/j.surfcoat.2015.07.002) [DOI] [Google Scholar]
- 24.Nishimoto S, Bhushan B. 2013. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 3, 671–690. ( 10.1039/C2RA21260A) [DOI] [Google Scholar]
- 25.Bohn HF, Federle W. 2004. Insect aquaplaning: nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl Acad. Sci. USA 101, 14 138–14 143. ( 10.1073/pnas.0405885101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu K, Yao X, Jiang L. 2010. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 39, 3240–3255. ( 10.1039/B917112F) [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Xia Z, Dai L. 2013. Advanced gecko-foot-mimetic dry adhesives based on carbon nanotubes. Nanoscale 5, 475–486. ( 10.1039/C2NR33027J) [DOI] [PubMed] [Google Scholar]
- 28.Su B, Tian Y, Jiang L. 2016. Bioinspired interfaces with superwettability: from materials to chemistry. J. Am. Chem. Soc. 138, 1727–1748. ( 10.1021/jacs.5b12728) [DOI] [PubMed] [Google Scholar]
- 29.Barthlott W, Neinhuis C. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8. ( 10.1007/s004250050096) [DOI] [Google Scholar]
- 30.Chen S, Wu B-H, Fang J-B, Liu Y-L, Zhang H-H, Fang L-C, Guan L, Li S-H. 2012. Analysis of flavonoids from lotus (Nelumbo nucifera) leaves using high performance liquid chromatography/photodiode array detector tandem electrospray ionization mass spectrometry and an extraction method optimized by orthogonal design. J. Chromatogr. A 1227, 145–153. ( 10.1016/j.chroma.2011.12.098) [DOI] [PubMed] [Google Scholar]
- 31.Whyman G, Bormashenko E, Stein T. 2008. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 450, 355–359. ( 10.1016/j.cplett.2007.11.033) [DOI] [Google Scholar]
- 32.Wenzel RN. 1936. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28, 988–994. ( 10.1021/ie50320a024) [DOI] [Google Scholar]
- 33.Cassie A, Baxter S. 1944. Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551. ( 10.1039/tf9444000546) [DOI] [Google Scholar]
- 34.Bittoun E, Marmur A. 2012. The role of multiscale roughness in the lotus effect: is it essential for super-hydrophobicity? Langmuir 28, 13 933–13 942. ( 10.1021/la3029512) [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Wang J, Zhao Y, Xu L, Gao X, Zheng Y, Jiang L. 2008. How does the leaf margin make the lotus surface dry as the lotus leaf floats on water? Soft Matter 4, 2232–2237. ( 10.1039/B807857B) [DOI] [Google Scholar]
- 36.Muller A, Meyer J, Paumer T, Pompe T. 2014. Cytoskeletal transition in patterned cells correlates with interfacial energy model. Soft Matter 10, 2444–2452. ( 10.1039/C3SM52424H) [DOI] [PubMed] [Google Scholar]
- 37.Michielsen S, Zhang J, Du J, Lee HJ. 2011. Gibbs free energy of liquid drops on conical fibers. Langmuir 27, 11 867–11 872. ( 10.1021/la202952e) [DOI] [PubMed] [Google Scholar]
- 38.Shih C-J, Strano MS, Blankschtein D. 2013. Wetting translucency of graphene. Nat. Mater. 12, 866–869. ( 10.1038/nmat3760) [DOI] [PubMed] [Google Scholar]
- 39.Bhushan B, Jung YC. 2011. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 56, 1–108. ( 10.1016/j.pmatsci.2010.04.003) [DOI] [Google Scholar]
- 40.Quéré D. 2008. Wetting and roughness. Annu. Rev. Mater. Res. 38, 71–99. ( 10.1146/annurev.matsci.38.060407.132434) [DOI] [Google Scholar]
- 41.Xu Q, Lv Y, Dong C, Sreeprased TS, Tian A, Zhang H, Tang Y, Yu Z, Li N. 2015. Three-dimensional micro/nanoscale architectures: fabrication and applications. Nanoscale 7, 10 883–10 895. ( 10.1039/C5NR02048D) [DOI] [PubMed] [Google Scholar]
- 42.Ma R, Wang J, Yang Z, Liu M, Zhang J, Jiang L. 2015. Bioinspired gas bubble spontaneous and directional transportation effects in an aqueous medium. Adv. Mater. 27, 2384–2389. ( 10.1002/adma.201405087) [DOI] [PubMed] [Google Scholar]
- 43.Xue Z, Wang S, Lin L, Chen L, Liu M, Feng L, Jiang L. 2011. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 23, 4270–4273. ( 10.1002/adma.201102616) [DOI] [PubMed] [Google Scholar]
- 44.Yao X, Hu Y, Grinthal A, Wong T-S, Mahadevan L, Aizenberg J. 2013. Adaptive fluid-infused porous films with tunable transparency and wettability. Nat. Mater. 12, 529–534. ( 10.1038/nmat3598) [DOI] [PubMed] [Google Scholar]
- 45.Hou X, Hu Y, Grinthal A, Khan M, Aizenberg J. 2015. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature 519, 70–73. ( 10.1038/nature14253) [DOI] [PubMed] [Google Scholar]
- 46.Huang Y, et al. 2012. Colloidal photonic crystals with narrow stopbands assembled from low-adhesive superhydrophobic substrates. J. Am. Chem. Soc. 134, 17 053–17 058. ( 10.1021/ja304751k) [DOI] [PubMed] [Google Scholar]
- 47.Liu M, Wang S, Wei Z, Song Y, Jiang L. 2009. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 21, 665–669. ( 10.1002/adma.200801782) [DOI] [Google Scholar]
- 48.Gao X, Jiang L. 2004. Biophysics: water-repellent legs of water striders. Nature 432, 36 ( 10.1038/432036a) [DOI] [PubMed] [Google Scholar]
- 49.Chu Z, Seeger S. 2014. Superamphiphobic surfaces. Chem. Soc. Rev. 43, 2784–2798. ( 10.1039/C3CS60415B) [DOI] [PubMed] [Google Scholar]
- 50.Ma CD, Wang C, Acevedo-Velez C, Gellman SH, Abbott NL. 2015. Modulation of hydrophobic interactions by proximally immobilized ions. Nature 517, 347–350. ( 10.1038/nature14018) [DOI] [PubMed] [Google Scholar]
- 51.Liu B, He Y, Fan Y, Wang X. 2006. Fabricating super-hydrophobic lotus-leaf-like surfaces through soft-lithographic imprinting. Macromol. Rapid Commun. 27, 1859–1864. ( 10.1002/marc.200600492) [DOI] [Google Scholar]
- 52.Wei Y, Hongtao L, Wei Z. 2015. Preparation of anti-corrosion superhydrophobic coatings by an Fe-based micro/nano composite electro-brush plating and blackening process. RSC Adv. 5, 103 000–103 012. ( 10.1039/C5RA15640H) [DOI] [Google Scholar]
- 53.Alexander S, Eastoe J, Lord AM, Guittard F, Barron AR. 2015. Branched hydrocarbon low surface energy materials for superhydrophobic nanoparticle derived surfaces. ACS. Appl. Mater. Interfaces 8, 660–666. ( 10.1021/acsami.5b09784) [DOI] [PubMed] [Google Scholar]
- 54.Lau KK, Bico J, Teo KB, Chhowalla M, Amaratunga GA, Milne WI, McKinley GH, Gleason KK. 2003. Superhydrophobic carbon nanotube forests. Nano Lett. 3, 1701–1705. ( 10.1021/nl034704t) [DOI] [Google Scholar]
- 55.Guo M-Z, Maury-Ramirez A, Poon CS. 2016. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Prod. 112, 3583–3588. ( 10.1016/j.jclepro.2015.10.079) [DOI] [Google Scholar]
- 56.Bergamonti L, Bondioli F, Alfieri I, Lorenzi A, Mattarozzi M, Predieri G, Lottici PP. 2016. Photocatalytic self-cleaning TiO2 coatings on carbonatic stones. Appl. Phys. A 122, 1–12. ( 10.1007/s00339-015-9560-y) [DOI] [Google Scholar]
- 57.Jesus MAMdL, Neto JTdS, Timò G, Paiva PRP, Dantas MSS, Ferreira AdM. 2015. Superhydrophilic self-cleaning surfaces based on TiO2 and TiO2/SiO2 composite films for photovoltaic module cover glass. Appl. Adhes. Sci. 3, 1–9. ( 10.1186/s40563-015-0034-4) [DOI] [Google Scholar]
- 58.He HY. 2015. Photoinduced superhydrophilicity and high photocatalytic activity of ZnO-reduced graphene oxide nanocomposite films for self-cleaning applications. Mater. Sci. Semicond. Proc. 31, 200–208. ( 10.1016/j.mssp.2014.11.029) [DOI] [Google Scholar]
- 59.Zheng J, Bao S, Jin P. 2015. TiO2(R)/VO2(M)/TiO2(A) multilayer film as smart window: combination of energy-saving, antifogging and self-cleaning functions. Nano Energy 11, 136–145. ( 10.1016/j.nanoen.2014.09.023) [DOI] [Google Scholar]
- 60.Li J-H, Yan B-F, Shao X-S, Wang S-S, Tian H-Y, Zhang Q-Q. 2015. Influence of Ag/TiO2 nanoparticle on the surface hydrophilicity and visible-light response activity of polyvinylidene fluoride membrane. Appl. Surf. Sci. 324, 82–89. ( 10.1016/j.apsusc.2014.10.080) [DOI] [Google Scholar]
- 61.Liu Y, Liu Z, Liu Y, Hu H, Li Y, Yan P, Yu B, Zhou F. 2015. One-step modification of fabrics with bioinspired polydopamine@octadecylamine nanocapsules for robust and healable self-cleaning performance. Small 11, 426–431. ( 10.1002/smll.201402383) [DOI] [PubMed] [Google Scholar]
- 62.Autumn K, Liang YA, Hsieh ST, Zesch W, Chan WP, Kenny TW, Fearing R, Full RJ. 2000. Adhesive force of a single gecko foot-hair. Nature 405, 681–685. ( 10.1109/NEMS.2006.334844) [DOI] [PubMed] [Google Scholar]
- 63.Loskill P, Puthoff J, Wilkinson M, Mecke K, Jacobs K, Autumn K. 2013. Macroscale adhesion of gecko setae reflects nanoscale differences in subsurface composition. J. R. Soc. Interface 10, 20120587 ( 10.1098/rsif.2012.0587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou M, Pesika N, Zeng H, Wan J, Zhang X, Meng Y, Wen S, Tian Y. 2012. Design of gecko-inspired fibrillar surfaces with strong attachment and easy-removal properties: a numerical analysis of peel-zone. J. R. Soc. Interface 9, 2424–2436. ( 10.1098/rsif.2012.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartlett MD, Croll AB, King DR, Paret BM, Irschick DJ, Crosby AJ. 2012. Looking beyond fibrillar features to scale gecko-like adhesion. Adv. Mater. 24, 1078–1083. ( 10.1002/adma.201104191) [DOI] [PubMed] [Google Scholar]
- 66.Prowse MS, Wilkinson M, Puthoff JB, Mayer G, Autumn K. 2011. Effects of humidity on the mechanical properties of gecko setae. Acta Biomater. 7, 733–738. ( 10.1016/j.actbio.2010.09.036) [DOI] [PubMed] [Google Scholar]
- 67.Chen B, Wu PD, Gao H. 2008. Hierarchical modelling of attachment and detachment mechanisms of gecko toe adhesion. Proc. R. Soc. A 464, 1639–1652. ( 10.1098/rspa.2007.0350) [DOI] [Google Scholar]
- 68.Peattie AM, Majidi C, Corder A, Full RJ. 2007. Ancestrally high elastic modulus of gecko setal β-keratin. J. R. Soc. Interface 4, 1071–1076. ( 10.1098/rsif.2007.0226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hansen W, Autumn K. 2005. Evidence for self-cleaning in gecko setae. Proc. Natl Acad. Sci. USA 102, 385–389. ( 10.1073/pnas.0408304102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Autumn K et al. 2002. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 99, 12 252–12 256. ( 10.1073/pnas.192252799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu S, Lopez S, Niewiarowski PH, Xia Z. 2012. Dynamic self-cleaning in gecko setae via digital hyperextension. J. R. Soc. Interface 9, 2781–2790. ( 10.1098/rsif.2012.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gillies AG, Puthoff J, Cohen MJ, Autumn K, Fearing RS. 2013. Dry self-cleaning properties of hard and soft fibrillar structures. ACS Appl. Mater. Interfaces 5, 6081–6088. ( 10.1021/am400839n) [DOI] [PubMed] [Google Scholar]
- 73.Crawford N, Endlein T, Barnes WJP. 2012. Self-cleaning in tree frog toe pads; a mechanism for recovering from contamination without the need for grooming. J. Exp. Biol. 215, 3965–3972. ( 10.1242/jeb.073809) [DOI] [PubMed] [Google Scholar]
- 74.Labonte D, Clemente CJ, Dittrich A, Kuo C-Y, Crosby AJ, Irschick DJ, Federle W. 2016. Extreme positive allometry of animal adhesive pads and the size limits of adhesion-based climbing. Proc. Natl Acad. Sci. USA 113, 1297–1302. ( 10.1073/pnas.1519459113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hu S, Xia Z. 2012. Rational design and nanofabrication of gecko-inspired fibrillar adhesives. Small 8, 2464–2468. ( 10.1002/smll.201200413) [DOI] [PubMed] [Google Scholar]
- 76.Qu L, Dai L, Stone M, Xia Z, Wang ZL. 2008. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 322, 238–242. ( 10.1126/science.1159503) [DOI] [PubMed] [Google Scholar]
- 77.Sethi S, Ge L, Ci L, Ajayan PM, Dhinojwala A. 2008. Gecko-inspired carbon nanotube-based self-cleaning adhesives. Nano Lett. 8, 822–825. ( 10.1021/nl0727765) [DOI] [PubMed] [Google Scholar]
- 78.Xu M, Futaba DN, Yumura M, Hata K. 2012. Alignment control of carbon nanotube forest from random to nearly perfectly aligned by utilizing the crowding effect. ACS Nano 6, 5837–5844. ( 10.1021/nn300142j) [DOI] [PubMed] [Google Scholar]
- 79.Qu L, Dai L. 2007. Gecko-foot-mimetic aligned single-walled carbon nanotube dry adhesives with unique electrical and thermal properties. Adv. Mater. 19, 3844–3849. ( 10.1002/adma.200700023) [DOI] [Google Scholar]
- 80.Sitti M, Fearing RS. 2003. Synthetic gecko foot-hair micro/nano-structures as dry adhesives. J. Adhes. Sci. Technol. 17, 1055–1073. ( 10.1163/156856103322113788) [DOI] [Google Scholar]
- 81.Jeong HE, Lee SH, Kim P, Suh KY. 2006. Stretched polymer nanohairs by nanodrawing. Nano Lett. 6, 1508–1513. ( 10.1021/nl061045m) [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Qu S, Cheng X, Gao X, Guo X. 2016. Fabrication and characterization of gecko-inspired dry adhesion, superhydrophobicity and wet self-cleaning surfaces. J. Bionic Eng. 13, 132–142. ( 10.1016/S1672-6529(14)60167-0) [DOI] [Google Scholar]
- 83.King DR, Bartlett MD, Gilman CA, Irschick DJ, Crosby AJ. 2014. Creating gecko-like adhesives for ‘real world’ surfaces. Adv. Mater. 26, 4345–4351. ( 10.1002/adma.201306259) [DOI] [PubMed] [Google Scholar]
- 84.Ho AYY, Yeo LP, Lam YC, Rodríguez I. 2011. Fabrication and analysis of gecko-inspired hierarchical polymer nanosetae. ACS Nano 5, 1897–1906. ( 10.1021/nn103191q) [DOI] [PubMed] [Google Scholar]
- 85.Tuma J, Peressadko A, Varenberg M, Gorb S. 2007. Biomimetic mushroom-shaped fibrillar adhesive microstructure. J. R. Soc. Interface 4, 271–275. ( 10.1098/rsif.2006.0164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kustandi TS, Samper VD, Yi DK, Ng WS, Neuzil P, Sun W. 2007. Self-assembled nanoparticles based fabrication of gecko foot-hair-inspired polymer nanofibers. Adv. Funct. Mater. 17, 2211–2218. ( 10.1002/adfm.200600564) [DOI] [Google Scholar]
- 87.Lee J, Fearing RS. 2008. Contact self-cleaning of synthetic gecko adhesive from polymer microfibers. Langmuir 24, 10 587–10 591. ( 10.1021/la8021485) [DOI] [PubMed] [Google Scholar]
- 88.Xu Q, et al. 2015. Robust self-cleaning and micromanipulation capabilities of gecko spatulae and their bio-mimics. Nat. Commun. 6, 8949 ( 10.1038/ncomms9949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeong J, Kim J, Song K, Autumn K, Lee J. 2014. Geckoprinting: assembly of microelectronic devices on unconventional surfaces by transfer printing with isolated gecko setal arrays. J. R. Soc. Interface 11, 20140627 ( 10.1098/rsif.2014.0627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gillies AG, Kwak J, Fearing RS. 2013. Controllable particle adhesion with a magnetically actuated synthetic gecko adhesive. Adv. Funct. Mater. 23, 3256–3261. ( 10.1002/adfm.201203122) [DOI] [Google Scholar]
- 91.Mengüç Y, Yang SY, Kim S, Rogers JA, Sitti M. 2012. Gecko-inspired controllable adhesive structures applied to micromanipulation. Adv. Funct. Mater. 22, 1246–1254. ( 10.1002/adfm.201101783) [DOI] [Google Scholar]
- 92.Cao L, Jones AK, Sikka VK, Wu J, Gao D. 2009. Anti-icing superhydrophobic coatings. Langmuir 25, 12 444–12 448. ( 10.1021/la902882b) [DOI] [PubMed] [Google Scholar]
- 93.Park JT, Kim JH, Lee D. 2014. Excellent anti-fogging dye-sensitized solar cells based on superhydrophilic nanoparticle coatings. Nanoscale 6, 7362–7368. ( 10.1039/C4NR00919C) [DOI] [PubMed] [Google Scholar]
- 94.Yang J, Zhang Z, Xu X, Zhu X, Men X, Zhou X. 2012. Superhydrophilic-superoleophobic coatings. J. Mater. Chem. 22, 2834–2837. ( 10.1039/C2JM15987B) [DOI] [Google Scholar]
- 95.Lee M. 2014. Shark skin: taking a bite out of bacteria. In Remarkable natural material surfaces and their engineering potential (ed. Lee M.), pp. 15–27. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 96.Ball P. 1999. Engineering shark skin and other solutions. Nature 400, 507–509. ( 10.1038/22883) [DOI] [Google Scholar]
- 97.Wong T, Kang SH, Tang S, Smythe E, Hatton BD, Grinthal A, Aizenberg J. 2011. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 477, 443–447. ( 10.1038/nature10447) [DOI] [PubMed] [Google Scholar]
- 98.Chen H, Zhang P, Zhang L, Liu H, Jiang Y, Zhang D, Han Z, Jiang L. 2016. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 532, 85–89. ( 10.1038/nature17189) [DOI] [PubMed] [Google Scholar]
- 99.Strauss JA, Soave PA, Ribeiro RS, Horowitz F. 2015. Absorber and self-cleaning surfaces on modified polymer plates for solar harvesting in the humid (sub)tropics. Sol. Energy 122, 579–586. ( 10.1016/j.solener.2015.09.020) [DOI] [Google Scholar]
- 100.Shang Q, Zhou Y. 2016. Fabrication of transparent superhydrophobic porous silica coating for self-cleaning and anti-fogging. Ceram. Int. 42, 8706–8712. ( 10.1016/j.ceramint.2016.02.105) [DOI] [Google Scholar]
- 101.Chen K, Wu Y, Zhou S, Wu L. 2016. Recent development of durable and self-healing surfaces with special wettability. Macromol. Rapid Commun. 37, 463–485. ( 10.1002/marc.201500591) [DOI] [PubMed] [Google Scholar]
- 102.Liu M, Xue Z, Liu H, Jiang L. 2012. Surface wetting in liquid–liquid–solid triphase systems: solid-phase-independent transition at the liquid–liquid interface by Lewis acid–base interactions. Angew. Chem. Int. Ed. 51, 8348–8351. ( 10.1002/anie.201202293) [DOI] [PubMed] [Google Scholar]
- 103.Ge J, et al. 2014. Pumping through porous hydrophobic/oleophilic materials: an alternative technology for oil spill remediation. Angew. Chem. Int. Ed. 53, 3612–3616. ( 10.1002/anie.201310151) [DOI] [PubMed] [Google Scholar]
- 104.Xu Q, Xu H, Chen J, Lv Y, Dong C, Sreeprasad TS. 2015. Graphene and graphene oxide: advanced membranes for gas separation and water purification. Inorg. Chem. Front. 2, 417–424. ( 10.1039/C4QI00230J) [DOI] [Google Scholar]
- 105.Lee SG, Lee DY, Lim HS, Lee DH, Lee S, Cho K. 2010. Switchable transparency and wetting of elastomeric smart windows. Adv. Mater. 22, 5013–5017. ( 10.1002/adma.201002320) [DOI] [PubMed] [Google Scholar]
- 106.Jeong HE, Lee J-K, Kim HN, Moon SH, Suh KY. 2009. A nontransferring dry adhesive with hierarchical polymer nanohairs. Proc. Natl Acad. Sci. USA 106, 5639–5644. ( 10.1073/pnas.0900323106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee H, Lee BP, Messersmith PB. 2007. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 448, 338–341. ( 10.1038/nature05968) [DOI] [PubMed] [Google Scholar]
- 108.Peisker H, Michels J, Gorb SN. 2013. Evidence for a material gradient in the adhesive tarsal setae of the ladybird beetle Coccinella septempunctata. Nat. Commun. 4, 1661 ( 10.1038/ncomms2576) [DOI] [PubMed] [Google Scholar]
- 109.Watson GS, Green DW, Schwarzkopf L, Li X, Cribb BW, Myhra S, Watson JA. 2015. A gecko skin micro/nano structure—a low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 21, 109–122. ( 10.1016/j.actbio.2015.03.007) [DOI] [PubMed] [Google Scholar]