Abstract
Background
Recently, dry needling has emerged as a popular treatment for muscular pain and impairments. While there are numerous studies detailing the benefits of dry needling for pain, few studies exist examining the effects on soft tissue mobility.
Purpose
The purpose of this study was to determine if the addition of hamstring dry needling to a standard stretching program results in greater improvements in hamstring flexibility compared to sham dry needling and stretching in subjects with atraumatic knee pain. Additionally, squat range of motion, knee pain, and the Lower Extremity Functional Scale were compared between the two groups.
Study Design
Double blinded randomized controlled trial.
Methods
Thirty-nine subjects were randomized to receive either dry needling (n = 20) or sham (n = 19) dry needling in addition to hamstring stretching, to all detected hamstring trigger points on two visits. All dependent variables were measured at baseline, immediately post intervention, and 1, 3, and 7 days after the initial treatment. Each subject also performed hamstring stretching three times daily for one week.
Results
Significant improvements in hamstring range of motion and all other dependent variables were observed across time regardless of treatment group. However, the lack of significant time by group interactions indicated the improvements were not different between dry needling and sham dry needling groups.
Conclusions
The results of the current randomized controlled trial suggest that two sessions of dry needling did not improve hamstring range of motion or other knee pain-related impairments more than sham dry needling in a young active population with atraumatic knee pain.
Level of Evidence
Therapy, Level 2
Keywords: Flexibility, lower extremity, trigger point
INTRODUCTION
Flexibility and mobility have long been an integral part of many rehabilitation and fitness training programs for patients with non-traumatic knee pain. Muscle tightness, as it contributes to hip and knee range of motion, can limit the execution of large joint, multi-segmental movements such as squatting, lunging, and deadlifting. A decreased ability to perform these movements could potentially lead to decreased physical performance as well as increased risk of injury.1-3 Kibler4 suggests that where there is a deficiency in a proximal segment of the kinetic chain, changed workloads may be required in the more distal segments in order to preserve the same movement outcome at the most distal segment. If this is the case, patients presenting with overuse or overload injuries of the limbs may also experience dysfunction in more proximal segments.
When compared to healthy controls, patients with non-traumatic knee pain have demonstrated significantly less flexibility of lower extremity soft tissues, including the hamstrings.5,6,7 Hamstring mobility, as it contributes to hip and knee range of motion, is important for the proper execution of functional movement patterns such as squatting, deadlifting, lunging, etc commonly required in athletic and training environments.
Hamstring stretching has been used for many years as a common intervention among physical therapists, athletic trainers, and fitness/coaching professionals to improve mobility at the hip and knee as well as to decrease muscle soreness.8 Numerous studies have examined the duration of a single stretch as well as the period of time required to effect significant improvement in hamstring flexibility utilizing a variety of muscle stretching techniques such as active, passive, and assisted stretching. Little consensus exists in the literature about the optimal period of time needed to show improvements with some studies suggesting as little as 4 weeks and other studies suggesting as many as 12 weeks to effect optimal change.9-11 The immediate effects of an acute bout of stretching on knee range of motion have been observed to only last 3-6 minutes.12,13
One potential cause of restricted range of motion related to local muscle dysfunction is the myofascial trigger point (TP).14 TPs are described as localized hyperirritable areas associated with hypersensitive palpable taut bands located in muscle tissue, and are suggested to contribute to joint range of motion restrictions as well as adversely affect muscle activation.15-19 TPs are further described in the literature as being either active or latent.20 Active TPs can be responsible for local pain as well as referred pain or paresthesia21 and may contribute to spontaneous pain at rest.20 Latent TPs are focal areas of tenderness and tightness in muscle tissue. Unless stimulated by direct contact, muscle activation, or stretching, latent TPs are not responsible for local or referred pain. These TPs may lead to altered muscle activation patterns resulting in limited range of motion or weakness of the muscles involved.14,20,21 TPs may also develop secondary to an excessive release of acetylcholine from motor endplates which has been associated with increased motor endplate noise and resulting muscle fiber knots.16
Dry needling (DN) has emerged as a popular intervention to address muscular pain and dysfunction. While multiple theories exist regarding the physiological mechanisms elicited by DN, the functional effects remain largely anecdotal.22-25 These effects appear to be most pronounced when a local twitch response is elicited.23 A local twitch response is an involuntary spinal cord reflex contraction of muscle fibers following needling of the involved fibers.26,27 While numerous studies exist detailing the benefits of dry needling for pain, 18,24,28,29 few studies exist examining the effects on soft tissue flexibility.10,13,27
The primary purpose of this study was to determine if the addition of DN to a standard stretching program results in greater improvements in hamstring flexibility versus stretching alone in patients with atraumatic knee pain over the course of one week. The secondary purpose was to compare changes in knee flexion range of motion while squatting; patient reported changes in knee pain with provocative movements, and self-reported disability. It was hypothesized that subjects who received DN and stretching would have greater improvements in hamstring flexibility, pain, and knee range of motion during a squat compared to subjects who only performed stretching.
METHODS
Participants
Subjects presenting with a chief complaint of atraumatic knee pain were recruited from a direct access physical therapy clinic. Using G Power 3.1.230 we determined a sample size of 36 prior to commencement of the trial. This sample size provided 80% power to detect a change of 10 ° in range of motion on the active straight leg raise (ASLR) and active knee extension (AKE) with an alpha level of 0.05. Previous authors who have studied hamstring injuries and cervicalgia demonstrated 10-12 ° changes after one week of treatment in subjects that responded favorably to a DN intervention.15,29 The study protocol was approved by the institutional review board of Keller Army Community Hospital (West Point, NY) and registered with ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT02498704). All participants signed an informed consent form prior to inclusion in the study. Participants’ rights were protected through the duration of the study.
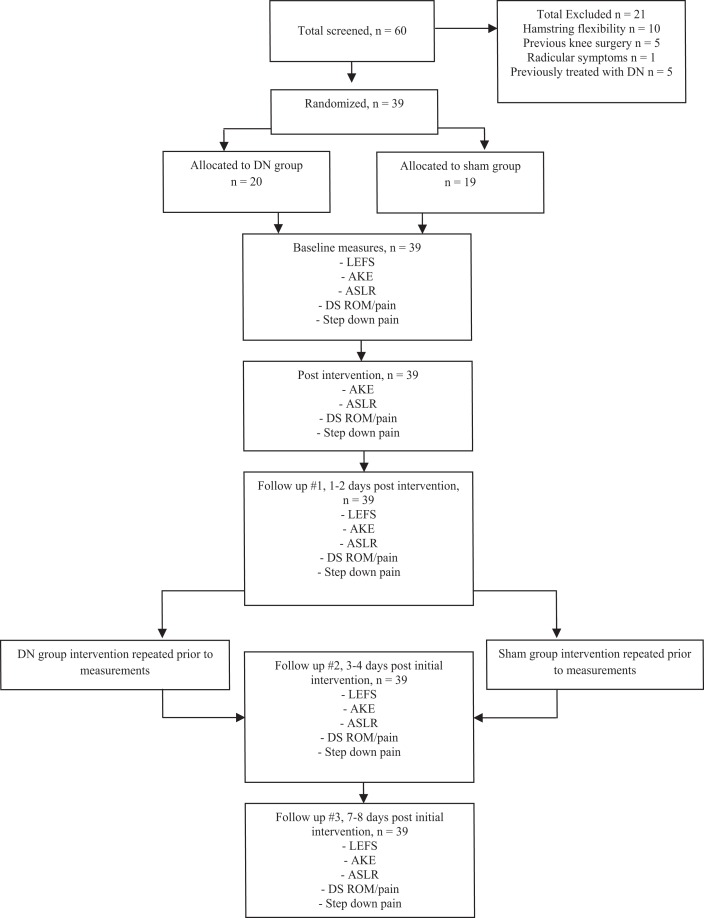
To be included in the study subjects had to present with a chief complaint of atraumatic knee pain with duration of symptoms greater than two weeks. Subjects also had to demonstrate a deficit of at least 20 ° of knee extension on the AKE test. Hamstring tightness was operationally defined as a greater than 20 ° loss of knee extension during the AKE test as measured with the subject supine and the femur held at 90 ° of hip flexion.9 Additional study inclusion and exclusion criteria are presented in Table 1. A screening physical examination of each knee was performed consisting of a Lachman's test, Posterior Drawer Test, Valgus and Varus stress a 0 ° and 30 °, Bounce Home Test, McMurray's Test, and Thessaly Test in order to rule out ligamentous deficiency and/or meniscus tears. Subject flow diagram is presented in Figure 1.
Table 1.
Inclusion and exclusion criteria
| Males and females | History of herniated lumbar disc/radiculopathy |
| Age 18-40 DoD beneficiaries | Prior surgery in the hip, knee, or back |
| Lack of ≥ 20 degrees of supine active knee extension | Self-reported pregnancy |
| Atraumatic knee pain ≥ 2 weeks | History of blood borne pathogens/infections disease/active infection |
| Metal allergy | |
| Positive instability tests indicative of ligamentous tear | |
| Joint line tenderness or positive meniscal tests | |
| Participants not fluent in English | |
| Previous history of DN | |
| Bleeding disorders or current use of anticoagulant medications |
Figure 1.
Subject recruitment/retention flow diagram. DN, dry needling; LEFS, lower extremity functional scale; AKE, active knee extension; DS, deep squat; ASLR, active straight leg raise; ROM, range of motion
INTERVENTIONS
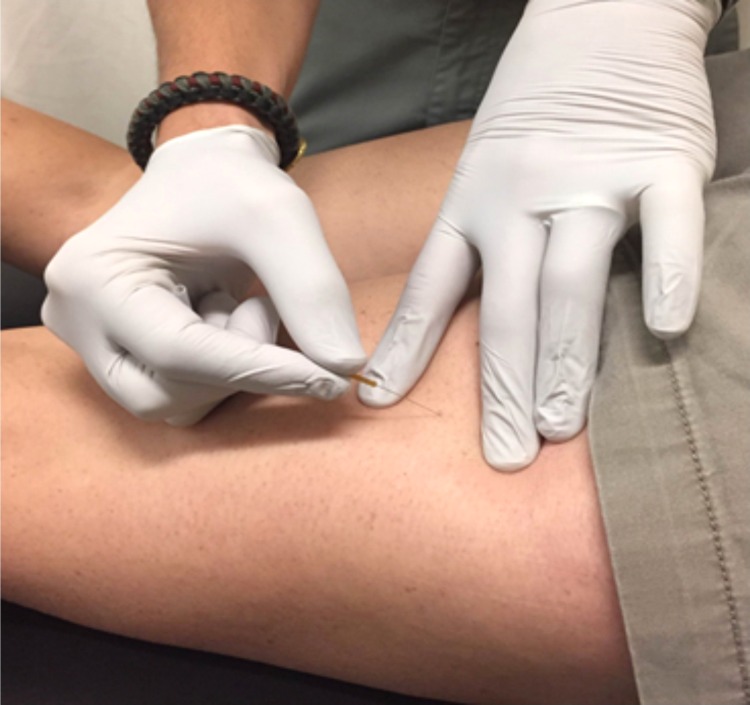
The randomization schedule was computer-generated, with assignments placed in opaque, sequentially numbered envelopes by an investigator not involved with recruitment or data collection. Treatment allocation was revealed to the investigator performing the intervention after collection of baseline measurements. Subjects and investigators taking all measurements were blinded to the intervention. Subjects were randomly assigned to one of two groups: a DN group and a sham DN group. After completion of all initial measurements, each subject received the assigned treatment. Manual palpation of the bilateral biceps femoris, semitendinosus, and semimembranosus was performed to detect the presence of TPs in the DN group. A provider with greater than three years of DN experience performed dry needling to all detected TPs with the subject in the prone position to allow access to the posterior thigh as well as to maintain blinding of the subject. Subjects included in this study complained of anterior knee, not hamstring-region, pain; all TPs identified were latent TPs. Upon identification of a TP a solid monofilament needle (Seirin Corp., Shizuoka, Japan) was inserted into the skin directed towards the target TP (Figure 2). The needle was then repeatedly “pistoned” (inserted and withdrawn rapidly from each TP) without being fully withdrawn from the skin with the goal of eliciting a local twitch response.23,31 Treatment was repeated to produce several local twitch responses and continued until all identified areas of dysfunction had been addressed.
Figure 2.
Hamstring dry needling. Insertion of monofilament needle into lateral hamstring muscle belly towards suspected trigger point.
Sham DN was implemented using a standard plastic tube as utilized in regular DN, however, instead of a monofilament needle each tube contained a small disinfected finishing nail. Subjects in the sham DN group were positioned identically to subjects in the DN group. Sham DN was performed at three points over the lateral hamstrings and three points over the medial hamstrings without the intention of locating any TPs. Similar technique to the DN was used to include a pistoning motion but the skin was not punctured at any time in this group. The same investigator measured each subject at every time point. The assigned treatment was repeated one additional time in the identical manner as detailed above three days later.
Post-intervention, all subjects were given a standing hamstring stretch to perform one repetition held for 30 seconds, repeated three times daily.32 These parameters have been shown to be effective at improving hamstring flexibility. The stretched was performed with the involved leg elevated on a chair or stool. While standing tall, maintaining neutral spine posture, and keeping the involved knee in full extension, subjects were instructed to lean forward hinging at the hips until moderate stretch discomfort in the hamstrings was felt. Subjects were instructed by demonstration and were provided with a handout of stretching instructions to perform at home. Subjects were also provided with an exercise log to record home exercise compliance.
OUTCOMES
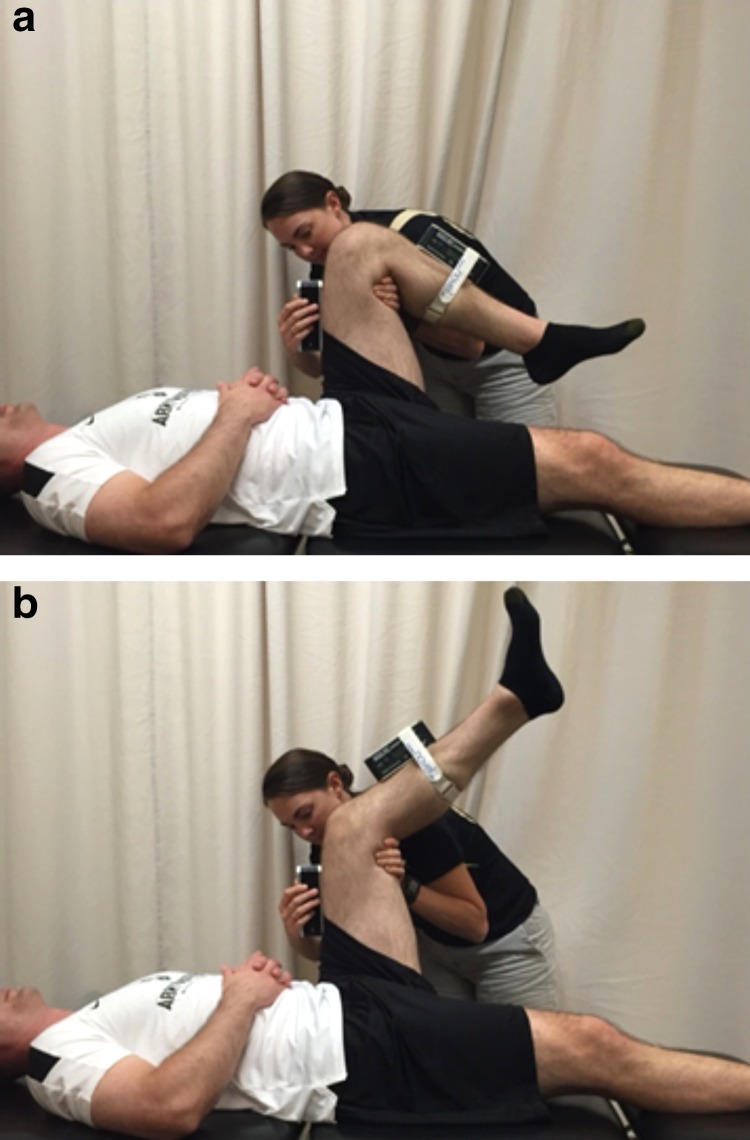
The primary outcome measure was hamstring flexibility (as measured by the AKE and ASLR). Two investigators (one to maintain proper test position, the second to record the measurement) were utilized for the AKE. (Figure 3) The involved extremity was held in 90 ° hip and knee flexion with the contralateral posterior knee in contact with the table. Saunders digital inclinometers (Chaska, Minnesota) were used for measurements. Straps were placed on the distal leg at mid-calf to secure an inclinometer in line with the tibial tuberosity. A second inclinometer was held in place at mid-thigh in order to assure that the subject maintained 90 ° of hip flexion. The subject was directed to actively extend the knee as far as tolerated without loss of the test position. The distal inclinometer was used to measure knee angle. The test was performed twice and the average of the two trials was recorded. The AKE test is a valid and reliable measure of hamstring flexibility.33-35
Figure 3.
(a.) Active knee extension test. Vertical position of the thigh is maintained with one goniometer. (b.) Active knee extension test. Active knee extension angle is measured with a second goniometer.
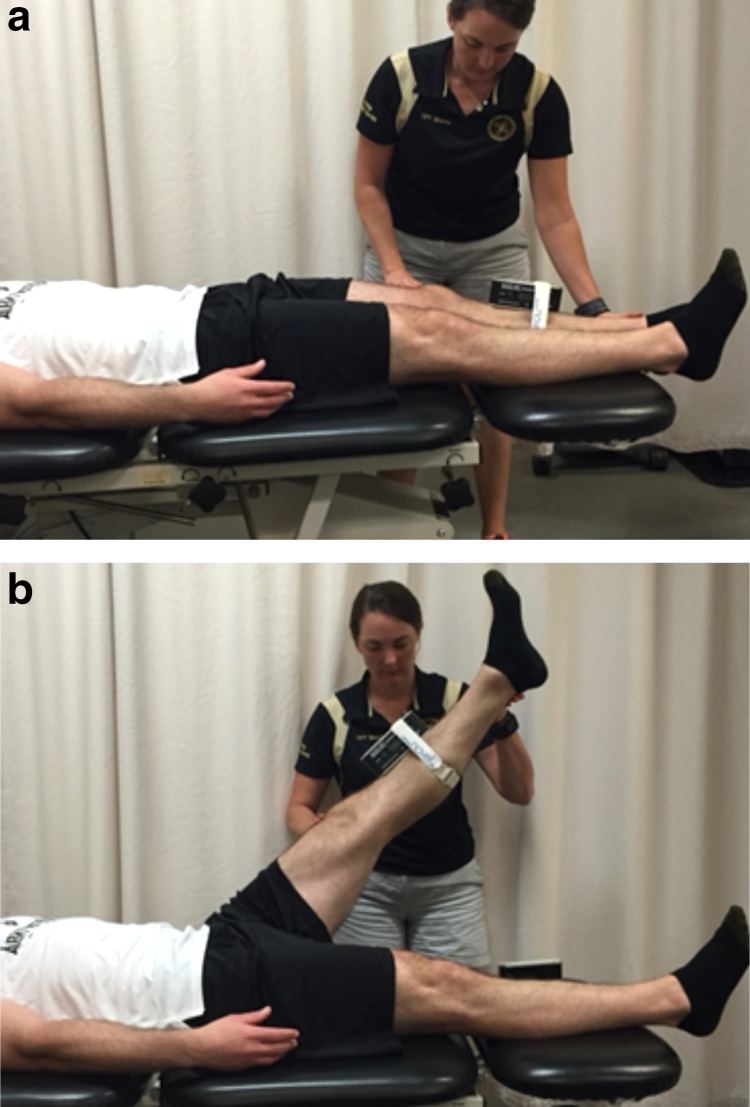
The ASLR was also performed with the subject supine. (Figure 4) The subject was instructed to actively raise the leg by flexing at the hip while maintaining full knee extension and ankle dorsiflexion. The contralateral limb had to remain in contact with the table to prevent contralateral hip flexion. A digital inclinometer was placed on the anterior lower leg in-line with the tibial tubercle. The test was performed twice and the average of the two trials was recorded. There is strong correlation between the AKE and ASLR tests.33 While the ASLR is not a true measure of hamstring flexibility, this test may demonstrate changes in hip and/or nerve mobility influenced by improvements in hamstring flexibility.33
Figure 4.
(a.) Active straight leg raise. Subject actively raises leg while maintaining full knee extension and keeping contralateral posterior knee in contact with the table. (b.) Active straight leg raise. Hip flexion angle measured with goniometer on tibia.
Secondary outcome measures included pain reported during basic functional tasks and self-reported function on the Lower Extremity Functional Scale (LEFS). Following hamstring measurements, subjects performed a deep squat. Subjects started with feet shoulder width apart, shoulders flexed to 90 °, and elbows fully extended. While maintaining heels in contact with the ground, subjects were instructed to squat as deeply as possible or until an increase in knee pain was experienced. Knee flexion was measured at that point with a standard goniometer. Subjects recorded knee pain during the squat on the Visual Analog Scale (VAS). The VAS is a valid and reliable tool for measuring acute and chronic pain.36-38
Next, subjects performed a single leg step down from a 15 centimeter step. Standing on the involved leg, subjects performed a controlled eccentric step down in their normal manner to the uninvolved leg. Subjects recorded knee pain during the step down on the VAS. An investigator blinded to group assignment performed all measurements.
Self-reported knee pain and function were assessed in all subjects at initial enrollment with the LEFS. The LEFS is a self-report questionnaire assessing initial function, ongoing progress, and outcomes concerning 20 different tasks ranging from activities of daily living to hobbies and exercise. The LEFS is a reliable and valid tool for assessing outcomes in lower extremity injuries with a minimal clinically important difference of nine points.39,40
Repeat measurements of all variables were obtained at four time points: immediately post intervention, and one day, three days, and seven days following the initial intervention. Prior to measurements on the third visit, an additional session of DN/sham intervention was performed in the identical manner described above. At the final visit, each subject was asked to predict his or her group assignment. All measurements performed pre-intervention were repeated post-intervention in both groups by the same investigator who remained blinded to group assignment.
DATA ANALYSIS
Data analysis was performed with statistical analysis software R version 3.0.2 and SPSS version 18 (Chicago, IL). Means, standard deviations, and 95% confidence intervals (CIs) were calculated for each variable. A 2x5 mixed model analysis of variance (ANOVA) with Sidak's post hoc testing was used for each outcome measures with time (pre and post intervention, follow up 1, 2, and 3) as the within-subject factor and group (DN or sham DN) as the between-subject factor. Intraclass correlation coefficients (ICCs), model [3,2] were calculated to ensure intra-rater reliability for AKE and ASLR measurements.
RESULTS
Sixty consecutive patients with atraumatic knee pain were screened for eligibility criteria between January and September 2015. Thirty-nine patients (37 males, 2 females) met the inclusion criteria and agreed to participate. Subjects were randomly assigned to the DN group (n = 20) or a sham DN group (n = 19). Baseline statistics for the DN and sham DN group are found in Table 2. No subjects were lost to follow up after initial enrollment and no adverse events were reported. All participants were analyzed in the groups to which they were assigned. Upon visual inspection of the data there appeared to be a difference in baseline pain with step down between the sham (mean VAS 10.5) and DN (mean VAS 22.84) groups. Because of this potential difference analysis of covariance (ANCOVA) was utilized to assess differences in pain with step down. Otherwise, no statistically significant differences were observed between groups at baseline. Substantial intra-rater reliability for AKE and ASLR was demonstrated for both investigators. ICCs for investigator 1 and 2 were between 0.89 and 0.99 for AKE and ASLR respectively.
Table 2.
Baseline and descriptive statistics by group
| Baseline Mean (± SD) DN Group | Baseline Mean (± SD) Sham Group | |
|---|---|---|
| Age (years) | 20.3 (1.08) | 20.16 (2.12) |
| Gender | 20 male | 17 male 2 female |
| Duration of symptoms (weeks) | 17.75 (26.10) | 14.3 (16.36) |
| AKE (degrees)* | 47.72 (9.70) | 49.59 (17.10) |
| ASLR (degrees)* | 52.26 (10.84) | 55.33 (13.35) |
| Deep Squat ROM (degrees)* | 110.35 (24.73) | 108.50 (24.18) |
| Deep Squat pain (VAS)* | 22.40 (22.79) | 24.11 (24.75) |
| Step Down pain (VAS)* | 10.50 (14.27) | 22.84 (22.34) |
| LEFS Score | 65.35 (10.47) | 64.47 (10.78) |
DN = dry needling
AKE = active knee extension
SD = standard deviation
ASLR = active straight leg raise
ROM = range of motion
LEFS = lower extremity functional scale
Measurements taken on the symptomatic limb
The 2-by-5 ANOVA failed to show a significant time by group interaction for AKE (F = 0.83, p = 0.51), ASLR (F = 0.29, p = 0.89), deep squat ROM (F = 0.69, p = 0.60), pain with deep squat (F = 0.58, p = 0.67) and self-reported function (F = 1.73, p = 0.17). The results of the 2-by-5 ANCOVA for pain during a step down also failed to demonstrate a significant difference between groups (F = 2.30, p = 0.47).
A statistically significant main effect for time was observed overall suggesting improvements in AKE (F = 3.94, p < 0.01), ASLR (F = 4.04, p < 0.01), deep squat ROM (F = 10.34, p < 0.001), pain with deep squat (F = 11.44, p < 0.001), pain during a step down (F = 8.78, p < 0.001), and self-reported function (F = 12.79, p < 0.001) across all participants. Post hoc comparisons with Sidak corrections also demonstrated statistically significant improvements from baseline to final follow up for both groups in all variables. Statistically significant improvements in pain and ROM with deep squat were demonstrated for both groups at all time points compared to baseline. Outcome data for primary and secondary outcome measures are presented in Table 3.
Table 3.
Outcome data for LEFS, ROM, and pain by group
| Variable | Baseline mean (± SD) | Post treatment mean (± SD) | FU 1 mean (± SD) | FU 2 mean (± SD) | FU 3 mean (± SD) |
|---|---|---|---|---|---|
| DN group AKE | 47.72 (9.70) | 48.68 (13.34) | 50.34 (11.48) | 49.31 (9.38) | 55.51 (12.66) |
| Sham group AKE | 49.59 (17.10) | 52.98 (9.64) | 52.64 (9.25) | 54.73 (7.41) | 55.20 (7.68) |
| Within-group change score from baseline | |||||
| DN | -- | 0.96 (−6.51, 8.43)‡ | 2.62 (−4.18, 9.42)‡ | 1.59 (−4.52, 7.70)‡ | 7.79 (0.57, 15.01)‡ |
| Sham | -- | 3.39 (−5.74, 12.52)‡ | 3.05 (−6.00, 12.10)‡ | 5.14 (−3.53, 13.81)‡ | 5.61 (−3.11, 14.33)‡ |
| Between-group difference in change score | -- | −2.43 (−10.02, 5.16)‡ | −0.43 (−7.22, 6.36)‡ | −3.55 (−9.05, 1.95)‡ | 2.18 (−4.66, 9.02)‡ |
| DN group ASLR | 52.26 (10.84) | 55.28 (12.43) | 55.60 (7.50) | 57.00 (8.08) | 59.47 (9.41) |
| Sham group ASLR | 55.33 (13.35) | 56.82 (8.38) | 57.71 (7.87) | 59.00 (6.82) | 59.43 (6.88) |
| Within-group change score from baseline | |||||
| DN | -- | 3.02 (−4.45, 10.49)‡ | 3.34 (−2.63, 9.31)‡ | 4.74 (−1.38, 10.86)‡ | 7.21 (0.71, 13.71)‡ |
| Sham | -- | 1.49 (−5.84, 8.82)‡ | 2.38 (−4.83, 9.59)‡ | 3.67 (−3.31, 10.65)‡ | 4.10 (−2.89, 11.09)‡ |
| Between-group difference in change score | -- | 1.53 (−3.43, 6.49)‡ | 0.96 (−4.03, 5.95)‡ | 1.07 (−3.79, 5.93)‡ | 3.11 (−2.26, 8.48)‡ |
| DN group deep squat ROM | 110.35 (24.73) | 114.73 (24.44) | 120.85 (23.86) | 121.78 (23.87) | 123.30 (23.54) |
| Sham group deep squat ROM | 108.50 (24.18) | 115.21 (23.74) | 114.90 (21.85) | 122.03 (17.08) | 119.92 (20.64) |
| Within-group change score from baseline | |||||
| DN | -- | 4.38 (−11.35, 20.11)‡ | 10.50(−5.06, 26.06)‡ | 11.43 (−4.13, 26.99)‡ | 12.95 (−2.51, 28.41)‡ |
| Sham | -- | 6.71 (−9.06, 22.48)‡ | 6.40 (−8.76, 21.56)‡ | 13.53 (−0.24, 27.30)‡ | 11.42 (−3.37, 26.21)‡ |
| Between-group difference in change score | -- | −2.33 (−17.97, 13.31)‡ | 4.10 (−10.77, 18.97)‡ | −2.10 (−15.63,11.43)‡ | 1.53 (−12.87, 15.93)‡ |
| DN group deep squat pain (VAS) | 22.40 (22.79) | 15.48 (20.71) | 10.50 (15.25) | 8.60 (14.82) | 8.95 (16.19) |
| Sham group deep squat pain (VAS) | 24.11 (24.75) | 19.42 (23.14) | 18.21 (20.70) | 13.63 (18.89) | 14.95 (20.96) |
| Within-group change score from baseline | |||||
| DN | -- | −6.92 (−20.86, 7.02)‡ | −11.90 (−24.31, 0.51)‡ | −13.80 (−26.11, −1.49)‡ | −13.45 (−26.10, −0.80)‡ |
| Sham | -- | −4.69 (−20.45, 11.07)‡ | −5.90 (−20.76, 8.96)‡ | −10.48 (−24.97, 4.01)‡ | −9.16 (−24.25, 5.93)‡ |
| Between-group difference in change score | -- | −2.23 (−16.46, 12.00)‡ | −6.00 (−17.75, 5.75)‡ | −3.32 (−14.30, 7.66)‡ | −4.29 (−16.40, 7.82)‡ |
| DN group step down pain (VAS) | 10.50 (14.27) | 6.95 (10.17) | 8.05 (10.10) | 6.00 (10.40) | 6.15 (10.62) |
| Sham group step down pain (VAS) | 22.84 (22.34) | 18.32 (20.79) | 15.00 (18.19) | 10.53 (17.13) | 12.95 (18.76) |
| Within-group change score from baseline | |||||
| DN | -- | −3.55 (−11.48, 4.38)‡ | −2.45 (−10.36, 5.46)‡ | −4.50 (−12.49, 3.49) ‡ | −4.35 (−12.40, 3.70) ‡ |
| Sham | - | −4.52 (−18.72, 9.68)‡ | −7.84 (−21.24, 5.56)‡ | −12.31 (−25.41, 0.79)‡ | −9.89 (−23.46, 3.68)‡ |
| Between-group difference in change score | -- | 0.97 (−9.56, 11.50)‡ | 5.39 (−4.09, 14.87)‡ | 7.81 (−1.33, 16.95)‡ | 5.54 (−4.29, 15.37)‡ |
| DN group step down pain (VAS)† | 16.51 (14.27) | 6.95 (10.17) | 8.05 (10.10) | 6.00 (10.40) | 6.15 (10.62) |
| Sham group step down pain (VAS)† | 16.51 (22.34) | 18.32 (20.79) | 15.00 (18.19) | 10.53 (17.13) | 12.95 (18.76) |
| Within-group change score from baseline | |||||
| DN† | -- | −9.56 (−17.49, −1.63)‡ | −8.46 (−16.37, −0.55)‡ | −10.51 (−18.50, −2.52)‡ | −10.36 (−18.41, −2.31)‡ |
| Sham† | -- | 1.81 (−12.39, 16.01)‡ | −1.51 (−14.91, 11.89)‡ | −5.98 (−19.08, 7.12)‡ | −3.56 (−17.13, 10.01)‡ |
| Between-group† difference in change score | -- | −11.37 (−21.90,−0.84)‡ | −6.95 (−16.43, 2.53)‡ | −4.53 (−13.67, 4.61)‡ | −6.80 (−16.63, 3.03)‡ |
| DN group LEFS | 65.35 (10.47) | -- | 67.75 (8.32) | 70.95 (6.14) | 72.30 (5.97) |
| Sham group LEFS | 64.47 (10.78) | -- | 67.11 (9.65) | 66.53 (10.65) | 69.26 (11.37) |
| Within-group change score from baseline | |||||
| DN | -- | -- | 2.40 (−3.65, 8.45)‡ | 5.60 (0.11, 11.09)‡ | 6.95 (1.49, 12.41)‡ |
| Sham | -- | -- | 2.64 (−4.09, 9.37)‡ | 2.06 (−4.99, 9.11)‡ | 4.79 (−2.50, 12.08)‡ |
| Between-group difference in change score | -- | -- | −0.24 (−6.08, 5.60)‡ | 3.54 (−2.06, 9.14)‡ | 2.16 (−3.69, 8.01)‡ |
DN = dry needling
AKE = active knee extension (degrees)
VAS = visual analog scale
SD = standard deviation
ASLR = active straight leg raise (degrees)
LEFS = lower extremity functional scale
ROM = range of motion (degrees)
FU = follow up; 1, 3, and 7 days after initial intervention
mean (95% confidence interval)
calculations based on ANCOVA adjusted mean
DISCUSSION
The outcomes of the current randomized controlled trial suggest that two sessions of hamstring DN with daily stretching for one week did not result in larger improvements in ROM, pain, and self-reported function compared to daily stretching and sham needling in patients with atraumatic knee pain. Participants in both groups demonstrated statistically significant improvements across all measures at the final follow up when compared to baseline. These observations may be a result of hamstring stretching, sham DN, or simply the passage of time, however, which of these influenced the results cannot be known, because a group that received no intervention was not included in this study.
These results are consistent with previous observations of Huguenin et al,41 who reported no significant changes in straight leg raise or hip internal rotation following gluteal DN or sham DN. The current results indicating no changes in ROM for hamstrings are contrary to previous studies demonstrating significant changes in upper extremity and cervical ROM following DN intervention.29,42,43 It is possible that mobility limited by pain rather than muscle dysfunction may demonstrate larger improvements in ROM following DN intervention. In addition, the hamstrings of the included subjects were not directly injured unlike the subjects of other studies who had muscles treated that were directly involved in an injury. This may explain in part the conflicting results between studies.
It is noteworthy that when asked, 85% (17/20) of subjects in the experimental group, correctly identified true DN, whereas 89.5% (17/19) of subjects in the sham group incorrectly identified true DN. For future studies utilizing sham needling, these results indicate this methodology could be repeated as subjects unfamiliar with this treatment are not likely to know the difference between sham and true needling. These results also suggest that improvement via placebo effect or patient expectations with treatment cannot be ruled out as previous studies have demonstrated positive results may be based on positive expectations of the subject.44
There are a number of limitations in this study. First, DN was only performed twice and to only one muscle group. More demonstrable effects of DN may potentially have been observed with increased frequency and longer duration of treatment and/or treatment of multiple muscle groups involved in hip/knee ROM. While observation of the immediate effect of DN on HS flexibility was desired, a one week follow up period may not have been sufficient to detect overall differences in changes between groups. Second, subjects with atraumatic knee pain of varying origins/sources were included in this study. Hip/core weakness, strength imbalance, and impaired neuromuscular control and timing have also been suggested as contributing factors to apparent hamstring inflexibility and anterior knee pain.45-48 These additional contributory factors were not assessed in this population. Additional methods of needling to include treatment of corresponding spinal levels as proposed by Gunn49 were not performed. It is not unreasonable to hypothesize that utilizing various applications of this modality to a more clearly defined diagnostic criterion may yield different results.
Finally, mean duration of symptoms was sixteen weeks (2-104 weeks) and median duration was four weeks. Potential differences may be observed among a population with more chronic symptoms. Finally, detection of the presence of trigger points was not attempted prior to enrollment as part of this study's inclusion criteria as previous studies have failed to establish adequate reliability for detection with physical exam.50 Consequently, subjects without active trigger points in the HS may have been included. If an insufficient number of trigger points are present within the treated musculature, potential effects of DN may not be as demonstrable.
The results of this study are not conclusive with regard to the effect of DN on hamstring flexibility. While not statistically significant, the 95% confidence intervals for the between group difference change score for the AKE (-4.66, 9.02) and ASLR (-2.26, 8.48) include a potentially clinically meaningful change. As a result, research investigating muscle flexibility changes associated with DN may warrant further consideration.
Future research is needed to investigate the characteristics of subgroups of the population (acute versus chronic injury, physical/psychosocial attributes) that respond favorably to this intervention and more clearly identify those more likely to experience most favorable outcomes. In addition, DN research should aim to identify optimal treatment parameters and the effectiveness of DN in various body regions and musculoskeletal conditions.
CONCLUSION
The results of the current randomized controlled trial suggest that two sessions of DN and daily stretching did not result in larger improvements in hamstring ROM, pain, and self-reported function compared to daily stretching and sham DN, over one week, in a young active population with atraumatic knee pain. Although potentially relevant within-group changes were observed, it is unclear whether these changes were a result of treatment or merely the result of passing time. Additional research is needed to more clearly define the effects of DN on tissue flexibility for different body regions as well as to identify subgroups of the population more likely to obtain optimal outcomes following DN intervention.
REFERENCES
- 1.Cook G Burton L Hoogenboom BJ Voight M. Functional movement screening: The use of fundamental movement screening as an assessment of function - Part 1. Int J Sports Phys Ther. 2014;9(3):396-409. [PMC free article] [PubMed] [Google Scholar]
- 2.Kiesel K Plisky PJ Voight ML. Can serious injury in professional football be predicted by a preseason functional movement screen? North Am J Sports Phys Ther. 2007;2(3):147-158. [PMC free article] [PubMed] [Google Scholar]
- 3.Schneiders AG Davidsson A Horman E Sullivan SJ. Functional movement screen normative values in a young, active population. Int J Sports Phys Ther. 2011;6(2):75-82. [PMC free article] [PubMed] [Google Scholar]
- 4.Kibler WB. Shoulder rehabilitation: principles and practice: Med Amp Sci Sports Amp Exerc. 1998;30(Supplement 1):40-50. [DOI] [PubMed] [Google Scholar]
- 5.White LC Dolphin P Dixon J. Hamstring length in patellofemoral pain syndrome. Physiotherapy. 2009;95(1):24-28. [DOI] [PubMed] [Google Scholar]
- 6.Piva SR Goodnite EA Childs JD. Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35(12):793-801. [DOI] [PubMed] [Google Scholar]
- 7.Smith AD Stroud L McQueen C. Flexibility and anterior knee pain in adolescent elite figure skaters. J Pediatr Orthop. 1991;11(1):77-82. [DOI] [PubMed] [Google Scholar]
- 8.Decoster LC Cleland J Altieri C. The effects of hamstring stretching on range of motion: A systematic literature review. J Orthop Sports Phys Ther. 2005;35(6):377-387. [DOI] [PubMed] [Google Scholar]
- 9.Davis DS Ashby PE McCale KL McQuain JA Wine JM. The effectiveness of 3 stretching techniques on hamstring flexibility using consistent stretching parameters. J Strength Cond Res Natl Strength Cond Assoc. 2005;19(1):27-32. [DOI] [PubMed] [Google Scholar]
- 10.Fasen JM O'Connor AM Schwartz SL, et al. A randomized controlled trial of hamstring stretching: comparison of four techniques. J Strength Cond Res Natl Strength Cond Assoc. 2009;23(2):660-667. [DOI] [PubMed] [Google Scholar]
- 11.Sainz de Baranda P Ayala F. Chronic flexibility improvement after 12 week of stretching program utilizing the ACSM recommendations: hamstring flexibility. Int J Sports Med. 2010;31(6):389-396. [DOI] [PubMed] [Google Scholar]
- 12.DePino GM Webright WG Arnold BL. Duration of maintained hamstring flexibility after cessation of an acute static stretching protocol. J Athl Train. 2000;35(1):56-59. [PMC free article] [PubMed] [Google Scholar]
- 13.Spernoga SG Uhl TL Arnold BL Gansneder BM. Duration of maintained hamstring flexibility after a one-time, modified hold-relax stretching protocol. J Athl Train. 2001;36(1):44-48. [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas KR Polus BI Rich PA. Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8(3):160-166. [Google Scholar]
- 15.Dembowski SC Westrick RB Zylstra E Johnson MR. Treatment of hamstring strain in a collegiate pole-vaulter integrating dry needling with an eccentric training program: a resident's case report. Int J Sports Phys Ther. 2013;8(3):328-339. [PMC free article] [PubMed] [Google Scholar]
- 16.Simons DG Travell JG Simons Lois S Travell JG. Travell & Simons’ Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore: Williams & Wilkins; 1999. [Google Scholar]
- 17.Srbely JZ Dickey JP Lee D Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42(5):463-468. [DOI] [PubMed] [Google Scholar]
- 18.Tekin L Akarsu S Durmuş O Çakar E Dinçer Ü Kıralp MZ. The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double-blinded placebo-controlled trial. Clin Rheumatol. 2013;32(3):309-315. [DOI] [PubMed] [Google Scholar]
- 19.Westrick RB Zylstra E Issa T Miller JM Gerber JP. Evaluation and treatment of musculoskeletal chest wall pain in a military athlete. Int J Sports Phys Ther. 2012;7(3):323. [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett R. Myofascial pain syndromes and their evaluation. Best Pract Res Clin Rheumatol. 2007;21(3):427-445. [DOI] [PubMed] [Google Scholar]
- 21.Dommerholt J Huijbregts P. Myofascial Trigger Points: Pathophysiology and Evidence-Informed Diagnosis and Management. Jones & Bartlett Publishers; 2010. [Google Scholar]
- 22.Cotchett MP Landorf KB Munteanu SE Raspovic AM. Consensus for dry needling for plantar heel pain (plantar fasciitis): a modified Delphi study. Acupunct Med J Br Med Acupunct Soc. 2011;29(3):193-202. [DOI] [PubMed] [Google Scholar]
- 23.Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point. The importance of the local twitch response. Am J Phys Med Rehabil Assoc Acad Physiatr. 1994;73(4):256-263. [DOI] [PubMed] [Google Scholar]
- 24.Rainey CE. The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther. 2013;8(2):145. [PMC free article] [PubMed] [Google Scholar]
- 25.Shah JP Danoff JV Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16-23. [DOI] [PubMed] [Google Scholar]
- 26.Hong CZ. Electrophysiological characteristics of localized twitch responses in responsive taut bands of rabbit skeletal muscle fibers. J Musculoskelet Pain. 1994;2(2):17-43. [Google Scholar]
- 27.Hong CZ. Persistence of local twitch response with loss of conduction to and from the spinal cord. Arch Phys Med Rehabil. 1994;75(1):12-16. [PubMed] [Google Scholar]
- 28.Mason JS Tansey KA Westrick RB. Treatment of subacute posterior knee pain in an adolescent ballet dancer utilizing trigger point dry needling: a case report. Int J Sports Phys Ther. 2014;9(1):116. [PMC free article] [PubMed] [Google Scholar]
- 29.Mejuto-Vázquez MJ Salom-Moreno J Ortega-Santiago R Truyols-Domínguez S Fernández-de-las-Peñas C. Short-term changes in neck pain, widespread pressure pain sensitivity, and cervical range of motion after the application of trigger point dry needling in patients with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. February 2014:1-30. doi:10.2519/jospt.2014.5108. [DOI] [PubMed] [Google Scholar]
- 30.Faul F Erdfelder E Lang A-G Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh Y-L Kao M-J Kuan T-S Chen S-M Chen J-T Hong C-Z. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil Assoc Acad Physiatr. 2007;86(5):397-403. [DOI] [PubMed] [Google Scholar]
- 32.Tsai C-T Hsieh L-F Kuan T-S Kao M-J Chou L-W Hong C-Z. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil Assoc Acad Physiatr. 2010;89(2):133-140. [DOI] [PubMed] [Google Scholar]
- 33.Majlesi J Unalan H. Effect of Treatment on Trigger Points. Curr Pain Headache Rep. 2010;14(5):353-360. doi:10.1007/s11916-010-0132-8. [DOI] [PubMed] [Google Scholar]
- 34.Bandy WD Irion JM Briggler M. The Effect of Time and Frequency of Static Stretching on Flexibility of the Hamstring Muscles. Phys Ther. 1997;77(10):1090-1096. [DOI] [PubMed] [Google Scholar]
- 35.Davis DS Quinn RO Whiteman CT Williams JD Young CR. Concurrent validity of four clinical tests used to measure hamstring flexibility. J Strength Cond Res. 2008;22(2):583-588. [DOI] [PubMed] [Google Scholar]
- 36.Gajdosik R Lusin G. Hamstring muscle tightness reliability of an active-knee-extension test. Phys Ther. 1983;63(7):1085-1088. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan M Dejulia J Worrell T. Effect of pelvic position and stretching method on hamstring muscle flexibility. Med Sci Sports Exerc. 1992;24(12):1383-1389. [PubMed] [Google Scholar]
- 38.Bijur PE Silver W Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8(12):1153-1157. [DOI] [PubMed] [Google Scholar]
- 39.Gallagher EJ Liebman M Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633-638. [DOI] [PubMed] [Google Scholar]
- 40.McCormack HM de L. Horne DJ Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007-1019. [DOI] [PubMed] [Google Scholar]
- 41.Binkley JM Stratford PW Lott SA Riddle DL. The lower extremity functional scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79(4):371-383. [PubMed] [Google Scholar]
- 42.Watson CJ Propps M Ratner J Zeigler DL Horton P Smith SS. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136-146. [DOI] [PubMed] [Google Scholar]
- 43.Huguenin L. Effect of dry needling of gluteal muscles on straight leg raise: a randomised, placebo controlled, double blind trial. Br J Sports Med. 2005;39(2):84-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bialosky JE Bishop MD Robinson ME Barabas JA George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: An experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brindle TJ Mattacola C McCrory J. Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003;11(4):244-251. [DOI] [PubMed] [Google Scholar]
- 46.Cook G Burton L Hoogenboom BJ Voight M. Functional movement screening: The use of fundamental movements as an assessment of function-part 2. Int J Sports Phys Ther. 2014;9(4):549-563. [PMC free article] [PubMed] [Google Scholar]
- 47.Bolgla LA Malone TR Umberger BR Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285. [PMC free article] [PubMed] [Google Scholar]
- 48.Sahrmann S. Diagnosis and Treatment of Movement Impairment Syndromes. Elsevier Health Sciences; 2002. [Google Scholar]
- 49.Gunn CC. The Gunn Approach to the Treatment of Chronic Pain: Intramuscular Stimulation for Myofascial Pain of Radiculopathic Origin. Elsevier Churchill Livingstone; 1996. [Google Scholar]
- 50.Lucas N Macaskill P Irwig L Moran R Bogduk N. Reliability of physical examination for diagnosis of myofascial trigger points: a systematic review of the literature. Clin J Pain. 2009;25(1):80-89. [DOI] [PubMed] [Google Scholar]