Abstract
Purpose of Review
To review and outline the key research contribution to BAV aortopathy over the past 18 months.
Recent Findings
Investigators have further defined the current gaps in knowledge and the scope of the clinical problem of BAV aortopathy. Support for aggressive resection strategies is waning as evidence mounts to suggest that BAV is not similar to genetic connective tissue disorders with respect to aortic risks. The role of cusp fusion patterns and valve-mediated hemodynamics on disease progression is a major area of discovery Molecular and cellular mechanisms remain elusive and contradictory.
Summary
BAV aortopathy is a major public health problem that remains poorly understood. New insights on valve-mediated hemodynamics using novel imaging modalities may lead to more individualized resection strategies and improved clinical guidelines.
Keywords: bicuspid aortic valve, aortopathy, hemodynamics, precision medicine
Introduction
Bicuspid aortic valve is the most common presentation of congenital heart disease (1–2% of the general population) and is frequently associated with pathology of the aorta. Dilatation of any or all segments of the proximal aorta, known as bicuspid aortopathy, is present in ~50% of individuals with congenital BAV(1) and ascending aortic aneurysms occur at a frequency of 1 in 100 BAV patients per year. Importantly, bicuspid aortopathy is often progressive and its presence increases the risk of catastrophic clinical events such as aortic rupture and dissection, both associated with high mortality and morbidity.
Over the past 18 months, substantial research activity has focused on bicuspid aortopathy. For excellent general contemporary overviews of BAV aortopathy written by experts in the field, the reader is referred to publications by Michelena and co-workers(2) in addition to Verma with Siu(1). Recent original publications can be defined into specific themes. First, a series of publications have been focused on clinical guidelines and the development of more personalized and precise approaches to treat bicuspid aortopathy. Second, imaging modalities have been leveraged to gain further insights into risk prediction, clinical outcomes, and disease progression. Third, novel contributions have been published that further the debate on the underlying influences of hemodynamic and genetic factors on BAV aortopathy. Fourth, basic science studies have explored cellular and molecular targets and pathways in the pathophysiology of BAV aortopathy. The purpose of this review is to highlight selected high impact publications from the last 18 months with additional perspectives and commentary.
Clinical Guidelines and the Evolving Approaches to Bicuspid Aortopathy
BAV aortopathy is a major public health problem that remains poorly understood. It is concerning that the number of aortic surgeries in this population is increasing but there is limited scientific evidence for the timing and extent of these prophylactic interventions. The burden of surgery for BAV patients in the USA exceeds 1 billion dollars per year and the frequency of aortic interventions has doubled over the past decade(3). Measures of aortic geometry such as maximal aortic diameter and growth rate are most often used to guide patient management and trigger prophylactic surgical repair of the aorta(4). Based on such contemporary resection guidelines, prophylactic replacement of the ascending aorta is performed in approximately 25% of BAV patients (within 25-years from time of diagnosis)(5). More recently it has been postulated that physician bias and historical local practice within institutions often dictate decision-making regarding surgical interventions for patients with BAV(5–10).
To better understand these issues, surgeons’ perspectives and approaches to bicuspid aortopathy were recently evaluated. A large survey of 100 cardiac surgeons revealed that operative approaches and management of BAV aortopathy are highly variable and not always consistent with current guidelines(10). Perspectives and attitudes on the etiology (inherited aortopathy versus acquired from hemodynamic stress) rather than validated scientific and clinical evidence appeared to influence the surgical treatment of BAV aortopathy. In support of these findings, Hardikar and Marwick published an excellent overview and analysis of the evolution of the clinical guidelines for BAV aortopathy(11). The analysis highlights that from 1998 to 2014 ten different international guidelines have been proposed for the surgical management of BAV aortopathy (Figure 1). Recommended thresholds for intervention began at a conservative cutoff level of 5.5cm in 1998(12), reached an aggressive approach of 4.0 to 4.5cm in 2010(13), and returned to a conservative 5.5cm cutoff level in 2014(14, 15). During this time, no conclusive or objective proof was published to support either an aggressive or conservative strategy for prophylactic aortic resection in BAV aortopathy(11). Based on these wavering guidelines and the increasing number of prophylactic aortic resections in BAV patients it is possible that thousands of unnecessary resections are being performed. It is reassuring to know that in expert surgical programs, as studied by Rinewalt and colleagues, the additional risk of concomitant aortic resection in BAV patients at the time of valve surgery is minimal(16).
Figure 1. Recommendations for aortic surgery in patients with BAV aortopathy.
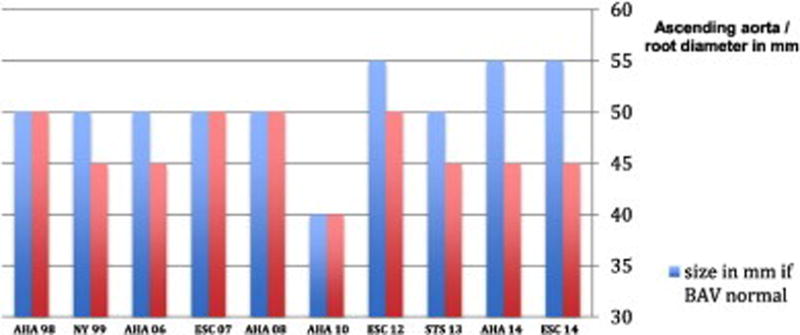
From 1998 to 2014, 10 different international guidelines have focused on the aortopathy related to bicuspid aortic valves. Different criteria are used if the patient is undergoing surgery for aortic valve disease (red) or there are no surgical indications for the aortic valve (blue). With permission (pending) from reference #11.
The wavering guidelines may reflect shifting perspectives in the etiology of bicuspid aortopathy. Aggressive approaches to aortic resection (early and wide resection) were based on the assumption that BAV aortopathy is inherited and the entire aorta is fragile and at continued risk of dilatation and rupture. More conservative approaches to resection consider that not all patients are at high risk of aortic complications and that hemodynamic and other risk factors may be at play. Over the past 18 months, original research publications and expert perspective contributions have shown a growing appreciation for the individual variability in the progression of the disease and the possible influence of hemodynamic determinants of the disease(17–29). Experts in this area have assembled into working groups, such as the International BAV Consortium (BAVCon), to define specific gaps in knowledge and develop collaborative research platforms to address these key issues(9, 30). Della Corte and members of the BAVCon provide an excellent overview of the key most critical knowledge gaps and research perspectives that relate to surgical treatment of BAV aortopathy(9). Many experts agree that there is a critical need to develop individualized risk assessments beyond size and growth criteria to offer more precise and individualized strategies for surgical resection of the aorta in BAV patients.
Over the past 18 months a number of key studies have provided further insights that may influence clinical strategies and patient management. Itagaki and co-workers examined the natural history of aortopathy after aortic valve replacement (AVR) in a retrospective comparison of over 13,000 patients(31). The study very clearly showed that the risk of aortic complications in long-term follow-up for BAV patients was much closer to control patients than those with Marfan syndrome. The authors of the study appropriately suggest that BAV patients should not be treated with aggressive approaches like those with Marfan syndrome and perhaps other inherited aortopathies. Recent clinical data from other groups also support the findings of Itagaki and co-workers(32, 33). Similarly, Sundt reviewed the strength of clinical evidence for aortic risks and provides a compelling and critical analysis that supports a more conservative approach to the BAV aorta(7). In large grouped series of BAV patients the risk of aortic events does not appear to be high, although selected patients may be at substantial risk.
The pressing challenge facing clinicians today is a lack of prognostic models to guide the timing and extent of surgical repair. BAV patients are offered aortic resection primarily based on maximal aortic dimension and the rate of aortic expansion; however, it is well recognized that measures of aortic size alone are insufficient to guide treatment strategies(6–8, 34–36). Substantial efforts are thus being made to improve risk prediction for aortic complications, with a focus on precision medicine(37–41). The literature suggests a precision approach, which attempts to account for individual variability in genes, environment, and lifestyle, is required to fully assess risk for BAV aortopathy. Based on recent data for BAV, this would likely include recognition of each individual patient’s underlying genetics and valve-specific hemodynamics based on the pattern of cusp fusion and function (degree of aortic stenosis or regurgitation). In support, Della Corte and his team explored the use of current aortopathy classifications with valve fusion patterns in the determination of risk for aortic growth(42). The authors determined that valve fusion patterns could influence the risk of aortic disease progression. Such studies will aid in the development and validation of factors beyond simple aortic size metrics that may be incorporated into individual resection decisions. Sievers and co-investigators have gone a step further by proposing and implementing an individualized, multifactorial approach to aortic resection in BAV patients, focusing on the BAV phenotype and degree of aortic stenosis (AS) and aortic regurgitation (AR)(43). This study is particularly interesting as it shows the possibilities of using more than simple aortic size criteria for aortic resection decisions can be feasible with excellent outcomes. Further validation of the factors used to influence the decisions to intervene remains to be studied but the approach follows the principles of precision medicine and could serve as a platform for further individualized resection strategies.
Emerging Role for Novel Imaging of Aortic Hemodynamic Biomarkers
Many experts in the area of BAV aortopathy agree that the discovery and implementation of novel aortic risk markers will be critical for the development of consistent guidelines for optimal management of BAV patients with aortic dilatation(2, 29). Aortic hemodynamics are emerging as an important biomarker toward this goal. Past research on the hemodynamic hypothesis for BAV-related aortopathy has primarily focused on on the degree of AS or AR(5, 44, 45). These traditional hemodynamic metrics alone do not reflect the burden on the aortic wall due to the malformed valve. It is increasingly recognized that nontraditional parameters of valve-related changes in aortic hemodynamics (i.e. unrelated to AS or AR) may act as possible mediators of BAV aortopathy(2, 17, 18, 24, 28, 46–48). These findings suggest that the development of BAV aortopathy is not primarily driven by a genetic predisposition (as in Marfan’s syndrome). Supporting this hypothesis are recent studies that have shown that altered aortic flow and valve morphology in BAV patients are associated with the expression of the aortopathy phenotype(49, 50).
Although stimuli for BAV aortopathy are likely multifactorial, results from recent studies provide strong evidence that this stimulus, known as wall shear stress (WSS), may change local matrix homeostasis and, in turn, ascending aortic structure(51–57). Indeed, WSS is known to impact cell function and has been implicated in the development of aortopathy(58–60). Proof-of-concept data was recently obtained using a novel ex vivo tissue model. Atkins and co-workers modeled regional WSS from a TAV as compared to a BAV on the ascending aorta in an ex vivo procine tissue model(48). The impact of BAV-mediated WSS was determined on aortic wall remodeling. The investigators found cellular, molecular (increased MMP-2 activity), and structural changes that are characertistic of human BAV aortopathy. As highlighted by the investgators, the study indictates that altered WSS from a BAV can focally mediate aortic medial degradation. These unique experimental findings provide compelling support for an important role of hemodynamics in mediating BAV aortopathy.
Recent advances in MRI have permitted unobstructed in-vivo assessment of time-resolved 3D blood velocity, using a volumetric technique referred to as 4D flow MRI. 4D flow MRI provides the unique ability to quantify complex 3D blood flow patterns in-vivo and has facilitated new insights and discovery with respect to complex cardiovascular hemodynamics(50, 61–65). Multi-dimensional 4D flow MRI data (3 spatial dimensions describing 3D velocity over time) enables aortic blood flow visualization, quantification of regional flow and velocity,(66–69), and WSS quantification(51, 52, 54, 56, 57, 70). Recent MRI studies provide strong evidence that valve-mediated local flow dynamics(61) and regional differences in wall shear stress (WSS)(50) are associated with changes in regional aortic wall histology and proteolytic events(71), which are known to drive adverse aortic remodeling. Early studies employed non-invasive MRI techniques (2D PC-MRI) to demonstrate BAV-mediated changes in flow and WSS(70). Subsequent 4D flow MRI studies have conclusively documented that aortic WSS is increased in BAV subjects independent of stenosis severity when compared to age- and aortic size-matched controls(61) (Figure 2). Moreover, we have shown that regional variation of WSS within the aorta is dependent on aortic valve fusion phenotype(50, 61) and is associated with aortic diameter(72). A recent study with 30 BAV patients and 30 age-appropriate trileaflet aortic valve (TAV) controls by our group provided evidence that altered aortic hemodynamics may be a pathophysiologic mechanism by which right and left-coronary leaflet (RL-BAV) or right and non-coronary leaflet valve (RN-BAV) fusion influences the expression of aortopathy(50). Similar to the findings of Atkins and Sucosky in the porcine model, we recently discovered that aortic hemodynamic alterations are related to medial wall degeneration(71). In a recent study that included both in-vivo 4D flow MRI and aortic tissue resection in 20 BAV patients, we found that elastin content and structure was severely disrupted in regions of high WSS with a shift in the expression of specific MMPs and TGF-beta (Figure 3). Girdauskas and colleagues found a similar correlation between systolic transvalvular flow patterns and proximal aortic wall changes in the setting of bicuspid aortic valve stenosis(47). With more extensive investigation it is conceivable that quantitative metrics of valve-mediated hemodynamics could be used to guide more precise and individualized surgical resection strategies beyond contemporary empirical size thresholds.
Figure 2. 4D flow MRI of BAV-mediated hemodynamics.
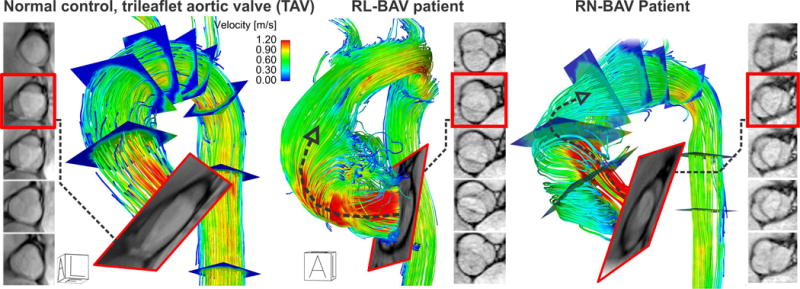
Images show control and BAV patient with a right-left (RL) and right-noncoronary (RN) fusion pattern. Note that the RL-BAV resulted in a marked eccentric aortic outflow jet (but not higher velocity, arrow) impinging on the aortic wall compared to TAV. We have found that the BAV phenotype (RL vs right-noncoronary [RN]) strongly impacts aortic outflow and thus aortic regions exposed to elevated WSS(50, 61).
Figure 3. Correlation of WSS with tissue histopathology.
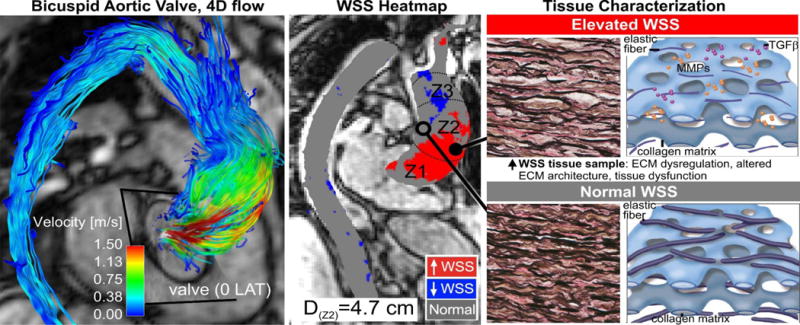
Eccentric transvalvular BAV flow (left) exposes aortic wall regions exposed to elevated WSS (middle, red region) which exhibit abnormal tissue metrics of aortopathy (right)(71).
Cellular and Molecular Mechanisms of BAV Aortopathy
The critical molecular and cellular pathways mediating BAV aortopathy remain elusive. Research over the past 18 months in this domain has provided more new questions rather than specific answers. The critical role of MMP-2 as a key molecular mediator was supported by a recent meta-analysis. Wang and colleagues further showed MMP-2 as a circulating biomarker of aortic dilatation in BAV patients(73). Phillippi with Gleason and Vorp at University of Pittsburgh further characterized the medial matrix remodeling of the BAV aorta and found unique patterns as compared to TAV patients(74). Grewal and co-workers compared the histopathology of BAV, TAV, and Marfan aortic tissue and found both similarities and differences between all three groups with respect to parameters of matrix remodeling and vascular smooth muscle markers(75). The complexity of the histopathology remains high and it is not clear what molecular pathways are unique to the BAV aorta. The complexity is further confounded by the findings of Heng and colleagues. In this recent study, tissue pathology was compared between TAV and BAV patients at matched aortic diameters(76). At odds with conventional wisdom, more severe histologic abnormalities were found in trileaflet aortic valve aorta as compared with BAV, especially when stratified by diameter. The authors suggest that the data do not support a more aggressive approach to surgical intervention for dilatation associated with BAV and the lack of correlation between aortic diameter and histologic abnormality in the setting of BAV highlights the inadequacy of diameter alone as a criterion for aortic resection. Further insights may be provided by biomechanical functional testing of tissue samples in addition to histopathology. Forsell and co-workers recently documented increased collagen-related stiffness in the aortic tissue of BAV patients as compared to TAV controls(77). Aortic stiffness is associated with progressive aortic dilatation and aneurysm formation which is characteristic of BAV aortopathy(34). Indeed, a recent study of abdominal aortic aneurysms found that segmental stiffening of the aorta preceded aneurysm growth and introduced the concept that stiffening may act as an early mechanism triggering elastin breakdown and aneurysm growth(78). Nonetheless, the evidence regarding cellular and molecular mechanisms for BAV aortopathy remain complex and contradictory, with a need for larger cohort, well-controlled studies.
Conclusions
Important contributions to research and perspectives on BAV aortopathy have been made in the past 18 months. Investigators have focused on defining the current gaps in knowledge and the scope of the clinical problem of BAV aortopathy. The role of cusp fusion patterns and valve-mediated hemodynamics on disease progression is a major area of discovery and new insights using novel imaging modalities may lead to more individualized resection strategies and improved resection guidelines. Support for aggressive resection strategies is waning as evidence mounts to suggest that BAV is not similar to Marfan syndrome with respect to aortic risks. Molecular and cellular mechanisms remain elusive and contradictory and deserve continued investigation.
Key Points.
Substantial research efforts over the past 18 months have focused on defining individual risk factors for disease progression
The risks associated with BAV aortopathy may be less than previously assumed warranting more conservative and selective approaches to prophylactic aortic resection
New imaging modalities have been leveraged to obtain novel data that supports valve-mediated hemodynamics as a critical mediator of disease progression
There remain substantial gaps in knowledge with respect to BAV aortopathy, particularly with respect to pathophysiology and molecular mechanisms of disease progression
Expert perspectives highlight a critical need to develop individualized risk assessments beyond size and growth criteria to offer more precise and individualized strategies for surgical resection of the aorta in BAV patients
Acknowledgments
None.
Financial Support and Sponsorship
This work was supported by Melman Bicuspid Aortic Valve Program, Bluhm Cardiovascular Institute, Northwestern University (PWMF), Heart and Stroke Foundation of Canada (PWMF), and National Institutes of Health (NIH) grant K25HL119608 (AJB).
Footnotes
Conflicts of Interest
None.
References
- 1.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920–1929. doi: 10.1056/NEJMra1207059. [DOI] [PubMed] [Google Scholar]
- 2*.Michelena HI, Corte AD, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M. Bicuspid aortic valve aortopathy in adults: Incidence, etiology, and clinical significance. Int J Cardiol. 2015;201:400–407. doi: 10.1016/j.ijcard.2015.08.106. An excellent contemporary review by an international group of leading experts in the field of both clinical and scientific BAV research. [DOI] [PubMed] [Google Scholar]
- 3.Opotowsky AR, Perlstein T, Landzberg MJ, et al. A shifting approach to management of the thoracic aorta in bicuspid aortic valve. J Thorac Cardiovasc Surg. 2013;146(2):339–346. doi: 10.1016/j.jtcvs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 aha/acc guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129(23):e521–643. doi: 10.1161/CIR.0000000000000031. [DOI] [PubMed] [Google Scholar]
- 5.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. Jama. 2011;306(10):1104–1112. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 6.Wasfy JH, Armstrong K, Milford CE, Sundt TM. Bicuspid aortic disease and decision making under uncertainty - the limitations of clinical guidelines. Int J Cardiol. 2015;181:169–171. doi: 10.1016/j.ijcard.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Sundt TM. Aortic replacement in the setting of bicuspid aortic valve: How big? How much? J Thorac Cardiovasc Surg. 2015;149(2 Suppl):S6–9. doi: 10.1016/j.jtcvs.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 8.Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: Identifying knowledge gaps and rising to the challenge from the international bicuspid aortic valve consortium (bavcon) Circulation. 2014;129(25):2691–2704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Della Corte A, Body SC, Booher AM, et al. Surgical treatment of bicuspid aortic valve disease: Knowledge gaps and research perspectives. J Thorac Cardiovasc Surg. 2014;147(6):1749–1757. 1757 e1741. doi: 10.1016/j.jtcvs.2014.01.021. This is a highly insightful and impactful contribution from international experts that defines the gaps of knowledge and possibilities for future research and discovery for surgical treatment of BAV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma S, Yanagawa B, Kalra S, et al. Knowledge, attitudes, and practice patterns in surgical management of bicuspid aortopathy: A survey of 100 cardiac surgeons. J Thorac Cardiovasc Surg. 2013;146(5):1033–1040 e1034. doi: 10.1016/j.jtcvs.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 11**.Hardikar AA, Marwick TH. The natural history of guidelines: The case of aortopathy related to bicuspid aortic valves. Int J Cardiol. 2015;199:150–153. doi: 10.1016/j.ijcard.2015.06.059. A unique and useful analysis of the evolution of clinical guidelines with detailed and informative outline of the scope of the health problem of BAV aortopathy. [DOI] [PubMed] [Google Scholar]
- 12.Tricoci P, Allen JM, Kramer JM, Califf RM, Smith SC., Jr Scientific evidence underlying the acc/aha clinical practice guidelines. Jama. 2009;301(8):831–841. doi: 10.1001/jama.2009.205. [DOI] [PubMed] [Google Scholar]
- 13.Warnes CA, Williams RG, Bashore TM, et al. Acc/aha 2008 guidelines for the management of adults with congenital heart disease: A report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease) Circulation. 2008;118(23):e714–833. doi: 10.1161/CIRCULATIONAHA.108.190690. [DOI] [PubMed] [Google Scholar]
- 14.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95(6 Suppl):S1–66. doi: 10.1016/j.athoracsur.2013.01.083. [DOI] [PubMed] [Google Scholar]
- 15.Erbel R, Aboyans V, Boileau C, et al. 2014 esc guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the european society of cardiology (esc) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 16.Rinewalt D, McCarthy PM, Malaisrie SC, et al. Effect of aortic aneurysm replacement on outcomes after bicuspid aortic valve surgery: Validation of contemporary guidelines. J Thorac Cardiovasc Surg. 2014;148(5):2060–2069. doi: 10.1016/j.jtcvs.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Uretsky S, Gillam LD. Nature versus nurture in bicuspid aortic valve aortopathy: More evidence that altered hemodynamics may play a role. Circulation. 2014;129(6):622–624. doi: 10.1161/CIRCULATIONAHA.113.007282. [DOI] [PubMed] [Google Scholar]
- 18.Spinale FG, Bolger AF. Fate versus flow: Wall shear stress in the aortopathy associated with bicuspid aortic valves. J Am Coll Cardiol. 2015;66(8):901–904. doi: 10.1016/j.jacc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Sievers HH, Stierle U, Hachmann RM, Charitos EI. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezv087. [DOI] [PubMed] [Google Scholar]
- 20.Michelena HI. The bicuspid aortic valve aortopathy mystery continues: Are we that mediocre? Trends in cardiovascular medicine. 2015;25(5):452–455. doi: 10.1016/j.tcm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Alonso-Montes C, Florez JP, et al. Bicuspid aortic valve syndrome: A heterogeneous and still unknown condition. Int J Cardiol. 2014;177(3):1105. doi: 10.1016/j.ijcard.2014.09.132. [DOI] [PubMed] [Google Scholar]
- 22.Itagaki S, Chiang Y, Tang GH. Why does the bicuspid aortic valve keep eluding us? Cardiology in review. 2015 doi: 10.1097/CRD.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 23.Girdauskas E, Borger MA. Surgical threshold for bicuspid aortic valve-associated aortopathy: Does the phenotype matter? JACC Cardiovascular imaging. 2014;7(3):318. doi: 10.1016/j.jcmg.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Fedak PW, Verma S. Bicuspid aortopathy and the development of individualized resection strategies. J Thorac Cardiovasc Surg. 2014;148(5):2080–2081. doi: 10.1016/j.jtcvs.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 25*.Della Corte A, Bancone C, Dialetto G, et al. Towards an individualized approach to bicuspid aortopathy: Different valve types have unique determinants of aortic dilatation. Eur J Cardiothorac Surg. 2014;45(4):e118–124. doi: 10.1093/ejcts/ezt601. discussion e124. This study examines possible phenotypes at risk of progression aortic dilatation and identified some selected patients at increased risk. These data could be useful in developing more individualized approaches to treatment in BAV aortopathy. [DOI] [PubMed] [Google Scholar]
- 26.Della Corte A. Trying to overcome the ‘chicken or egg’ impasse in bicuspid aortopathy research. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezv173. [DOI] [PubMed] [Google Scholar]
- 27.Della Corte A. Phenotypic heterogeneity of bicuspid aortopathy: A potential key to decode the prognosis? Heart. 2014;100(2):96–97. doi: 10.1136/heartjnl-2013-305004. [DOI] [PubMed] [Google Scholar]
- 28.Atkins SK, Sucosky P. Etiology of bicuspid aortic valve disease: Focus on hemodynamics. World journal of cardiology. 2014;6(12):1227–1233. doi: 10.4330/wjc.v6.i12.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamo L, Braverman AC. Surgical threshold for bicuspid aortic valve aneurysm: A case for individual decision-making. Heart. 2015;101(17):1361–1367. doi: 10.1136/heartjnl-2014-306601. [DOI] [PubMed] [Google Scholar]
- 30.Prakash SK, Bosse Y, Muehlschlegel JD, et al. A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: Insights from the international bavcon (bicuspid aortic valve consortium) J Am Coll Cardiol. 2014;64(8):832–839. doi: 10.1016/j.jacc.2014.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus marfan syndrome. J Am Coll Cardiol. 2015;65(22):2363–2369. doi: 10.1016/j.jacc.2015.03.575. An important analysis of clinical data that defines the relative risk of BAV aortopathy as compared to those with a genetic connective tissue disease (Marfan). The data have important implications for the development of clinical guidelines that have traditionally treated BAV patients similarly to those with Marfan syndrome. [DOI] [PubMed] [Google Scholar]
- 32.Detaint D, Michelena HI, Nkomo VT, Vahanian A, Jondeau G, Sarano ME. Aortic dilatation patterns and rates in adults with bicuspid aortic valves: A comparative study with marfan syndrome and degenerative aortopathy. Heart. 2014;100(2):126–134. doi: 10.1136/heartjnl-2013-304920. [DOI] [PubMed] [Google Scholar]
- 33.Charitos EI, Stierle U, Petersen M, et al. The fate of the bicuspid valve aortopathy after aortic valve replacement. Eur J Cardiothorac Surg. 2014;45(5):e128–135. doi: 10.1093/ejcts/ezt666. [DOI] [PubMed] [Google Scholar]
- 34.Prakash A, Adlakha H, Rabideau N, et al. Segmental aortic stiffness in children and young adults with connective tissue disorders: Relationships with age, aortic size, rate of dilation, and surgical root replacement. Circulation. 2015;132(7):595–602. doi: 10.1161/CIRCULATIONAHA.114.014934. [DOI] [PubMed] [Google Scholar]
- 35.Della Corte A, De Santo LS, Forte A. Missing link between aortic wall pathology and aortic diameter: Methodological bias or worrisome finding? Eur J Cardiothorac Surg. 2012;42(1):195–196. doi: 10.1093/ejcts/ezs006. author reply 196. [DOI] [PubMed] [Google Scholar]
- 36.Della Corte A. The conundrum of aortic dissection in patients with bicuspid aortic valve: The tissue, the mechanics and the mathematics. Eur J Cardiothorac Surg. 2014 doi: 10.1093/ejcts/ezu418. [DOI] [PubMed] [Google Scholar]
- 37.Della Corte A, Bancone C, Buonocore M, et al. Pattern of ascending aortic dimensions predicts the growth rate of the aorta in patients with bicuspid aortic valve. JACC Cardiovascular imaging. 2013;6(12):1301–1310. doi: 10.1016/j.jcmg.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Della Corte A, Bancone C, Conti CA, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: A new risk marker for aortopathy. J Thorac Cardiovasc Surg. 2012;144(2):360–369. 369 e361. doi: 10.1016/j.jtcvs.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Della Corte A, Bancone C, Quarto C, et al. Predictors of ascending aortic dilatation with bicuspid aortic valve: A wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31(3):397–404. doi: 10.1016/j.ejcts.2006.12.006. discussion 404–395. [DOI] [PubMed] [Google Scholar]
- 40.Della Corte A, Buonocore M, Del Viscovo L. Rationale and methods for quantifying ascending aortic flow eccentricity: Back to the underlying mechanism? Journal of magnetic resonance imaging: JMRI. 2012;36(2):505–506. doi: 10.1002/jmri.23656. author reply 507. [DOI] [PubMed] [Google Scholar]
- 41.Ikonomidis JS, Ivey CR, Wheeler JB, et al. Plasma biomarkers for distinguishing etiologic subtypes of thoracic aortic aneurysm disease. The Journal of thoracic and cardiovascular surgery. 2013;145(5):1326–1333. doi: 10.1016/j.jtcvs.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Della Corte A, Bancone C, Dialetto G, et al. The ascending aorta with bicuspid aortic valve: A phenotypic classification with potential prognostic significance. Eur J Cardiothorac Surg. 2014;46(2):240–247. doi: 10.1093/ejcts/ezt621. discussion 247. [DOI] [PubMed] [Google Scholar]
- 43*.Sievers HH, Stierle U, Mohamed SA, et al. Toward individualized management of the ascending aorta in bicuspid aortic valve surgery: The role of valve phenotype in 1362 patients. J Thorac Cardiovasc Surg. 2014;148(5):2072–2080. doi: 10.1016/j.jtcvs.2014.04.007. A clinical surgical series with excellent outcomes in which an individualized approach to management using multiple criteria beyond aortic size alone. [DOI] [PubMed] [Google Scholar]
- 44.Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. Jama. 2008;300(11):1317–1325. doi: 10.1001/jama.300.11.1317. [DOI] [PubMed] [Google Scholar]
- 45.Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2012;42(5):832–837. doi: 10.1093/ejcts/ezs137. discussion 837–838. [DOI] [PubMed] [Google Scholar]
- 46.Sievers HH, Sievers HL. Aortopathy in bicuspid aortic valve disease - genes or hemodynamics? Or scylla and charybdis? Eur J Cardiothorac Surg. 2011;39(6):803–804. doi: 10.1016/j.ejcts.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Girdauskas E, Rouman M, Disha K, et al. Correlation between systolic transvalvular flow and proximal aortic wall changes in bicuspid aortic valve stenosis. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2014;46(2):234–239. doi: 10.1093/ejcts/ezt610. discussion 239. [DOI] [PubMed] [Google Scholar]
- 48*.Atkins SK, Cao K, Rajamannan NM, Sucosky P. Bicuspid aortic valve hemodynamics induces abnormal medial remodeling in the convexity of porcine ascending aortas. Biomechanics and modeling in mechanobiology. 2014;13(6):1209–1225. doi: 10.1007/s10237-014-0567-7. A novel ex vivo porcine tissue model was used to to validate BAV valve-related hemodynamics on aortic tissue remodeling. [DOI] [PubMed] [Google Scholar]
- 49.Kang JW, Song HG, Yang DH, et al. Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: Comprehensive evaluation using mdct and echocardiography. JACC Cardiovascular imaging. 2013;6(2):150–161. doi: 10.1016/j.jcmg.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 50.Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129(6):673–682. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bock J, Frydrychowicz A, Lorenz R, et al. In vivo noninvasive 4d pressure difference mapping in the human aorta: Phantom comparison and application in healthy volunteers and patients. Magn Reson Med. 2011;66(4):1079–1088. doi: 10.1002/mrm.22907. [DOI] [PubMed] [Google Scholar]
- 52.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2d and 3d phase contrast mri: Optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60(5):1218–1231. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 53.Harloff A, Wallis W, Strecker C, et al. Global pulse wave velocity in 87 patients with acute ischemic stroke and aortic atherosclerosis. Flow Velocity Measurement in the Carotid Bifurcation Using 4D Flow-Sensitive MRI and Doppler Ultrasound. 2010 [Google Scholar]
- 54.Harloff A, Nussbaumer A, Bauer S, et al. In vivo assessment of wall shear stress in the atherosclerotic aorta using flow-sensitive 4d mri. Magn Reson Med. 2010;63(6):1529–1536. doi: 10.1002/mrm.22383. [DOI] [PubMed] [Google Scholar]
- 55.Markl M, Bauer S, Bock J, Stalder A, Frydrychowicz A, Harloff A. Wall shear stress in normal & atherosclerotic carotid arteries. Proceedings: 21st Annual International Conference on MR Angiography; Lansing, USA. 2009. p. 68. [Google Scholar]
- 56.van Ooij P, Potters WV, Nederveen AJ, et al. A methodology to detect abnormal relative wall shear stress on the full surface of the thoracic aorta using four-dimensional flow mri. Magn Reson Med. 2015;73(3):1216–1227. doi: 10.1002/mrm.25224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia J, Markl M, Schnell S, et al. Evaluation of aortic stenosis severity using 4d flow jet shear layer detection for the measurement of valve effective orifice area. Magn Reson Imaging. 2014 doi: 10.1016/j.mri.2014.04.017. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.den Reijer PM, Sallee D, 3rd, van der Velden P, et al. Hemodynamic predictors of aortic dilatation in bicuspid aortic valve by velocity-encoded cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:4. doi: 10.1186/1532-429X-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hope MD, Hope TA, Crook SE, et al. 4d flow cmr in assessment of valve-related ascending aortic disease. JACC Cardiovascular imaging. 2011;4(7):781–787. doi: 10.1016/j.jcmg.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: Four-dimensional mr evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255(1):53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 61.Barker AJ, Markl M, Burk J, et al. Bicuspid aortic valve is associated with altered wall shear stress in the ascending aorta. Circ Cardiovasc Imaging. 2012;5(4):457–466. doi: 10.1161/CIRCIMAGING.112.973370. [DOI] [PubMed] [Google Scholar]
- 62.Markl M, Geiger J, Arnold R, et al. Comprehensive 4-dimensional magnetic resonance flow analysis after successful heart transplantation resolves controversial intraoperative findings and reveals complex hemodynamic alterations. Circulation. 2011;123(11):e381–383. doi: 10.1161/CIRCULATIONAHA.110.979971. [DOI] [PubMed] [Google Scholar]
- 63.Markl M, Wegent F, Zech T, et al. In vivo wall shear stress distribution in the carotid artery: Effect of bifurcation geometry, internal carotid artery stenosis, and recanalization therapy. Circ Cardiovasc Imaging. 2010;3(6):647–655. doi: 10.1161/CIRCIMAGING.110.958504. [DOI] [PubMed] [Google Scholar]
- 64.Harloff A, Simon J, Brendecke S, et al. Complex plaques in the proximal descending aorta: An underestimated embolic source of stroke. Stroke. 2010;41(6):1145–1150. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 65.Harloff A, Strecker C, Dudler P, et al. Retrograde embolism from the descending aorta: Visualization by multidirectional 3d velocity mapping in cryptogenic stroke. Stroke; a journal of cerebral circulation. 2009;40(4):1505–1508. doi: 10.1161/STROKEAHA.108.530030. [DOI] [PubMed] [Google Scholar]
- 66.Markl M, Geiger J, Kilner PJ, et al. Time-resolved three-dimensional magnetic resonance velocity mapping of cardiovascular flow paths in volunteers and patients with fontan circulation. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2011;39:206–212. doi: 10.1016/j.ejcts.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 67.Bock J, Frydrychowicz A, Stalder AF, et al. 4d phase contrast mri at 3 t: Effect of standard and blood-pool contrast agents on snr, pc-mra, and blood flow visualization. Magn Reson Med. 2010;63(2):330–338. doi: 10.1002/mrm.22199. [DOI] [PubMed] [Google Scholar]
- 68.Frydrychowicz A, Arnold R, Harloff A, et al. In vivo 3-dimensional flow connectivity mapping after extracardiac total cavopulmonary connection. Circulation. 2008;118(2):e16–17. doi: 10.1161/CIRCULATIONAHA.107.761304. [DOI] [PubMed] [Google Scholar]
- 69.Markl M, Harloff A, Bley TA, et al. Time-resolved 3d mr velocity mapping at 3t: Improved navigator-gated assessment of vascular anatomy and blood flow. Journal of magnetic resonance imaging: JMRI. 2007;25(4):824–831. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 70.Barker AJ, Lanning C, Shandas R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast mri. Annals of biomedical engineering. 2010;38(3):788–800. doi: 10.1007/s10439-009-9854-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Guzzardi DG, Barker AJ, van Ooij P, et al. Valve-related hemodynamics mediate human bicuspid aortopathy: Insights from wall shear stress mapping. Journal of the American College of Cardiology. 2015;66(8):892–900. doi: 10.1016/j.jacc.2015.06.1310. These data implicate a critical role for valve-related hemodynamics as measured using 4D flow MRI in mediating BAV aortopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bissell MM, Hess AT, Biasiolli L, et al. Aortic dilation in bicuspid aortic valve disease: Flow pattern is a major contributor and differs with valve fusion type. Circ Cardiovasc Imaging. 2013;6(4):499–507. doi: 10.1161/CIRCIMAGING.113.000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Wu B, Dong L, Wang C, Wang X, Shu X. Circulating matrix metalloproteinase patterns in association with aortic dilatation in bicuspid aortic valve patients with isolated severe aortic stenosis. Heart and vessels. 2014 doi: 10.1007/s00380-014-0593-5. [DOI] [PubMed] [Google Scholar]
- 74.Phillippi JA, Green BR, Eskay MA, et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2014;147(3):1056–1064. doi: 10.1016/j.jtcvs.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grewal N, Franken R, Mulder BJ, et al. Histopathology of aortic complications in bicuspid aortic valve versus marfan syndrome: Relevance for therapy? Heart and vessels. 2015 doi: 10.1007/s00380-015-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heng E, Stone JR, Kim JB, Lee H, MacGillivray TE, Sundt TM. Comparative histology of aortic dilatation associated with bileaflet versus trileaflet aortic valves. Ann Thorac Surg. 2015 doi: 10.1016/j.athoracsur.2015.05.105. [DOI] [PubMed] [Google Scholar]
- 77.Forsell C, Bjorck HM, Eriksson P, Franco-Cereceda A, Gasser TC. Biomechanical properties of the thoracic aneurysmal wall: Differences between bicuspid aortic valve and tricuspid aortic valve patients. Ann Thorac Surg. 2014;98(1):65–71. doi: 10.1016/j.athoracsur.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 78.Raaz U, Zollner AM, Schellinger IN, et al. Segmental aortic stiffening contributes to experimental abdominal aortic aneurysm development. Circulation. 2015;131(20):1783–1795. doi: 10.1161/CIRCULATIONAHA.114.012377. [DOI] [PMC free article] [PubMed] [Google Scholar]


