Abstract
Breast cancer metastasis to the bone continues to be a major health problem, with approximately 80% of advanced breast cancer patients expected to develop bone metastasis. Although the problem of bone metastasis persists, current treatment options for metastatic cancer patients are limited. In this study, we investigated the preventive role of the active vitamin D metabolite, 1α,25-dihydroxyvitamin D (1,25(OH)2D), against the metastatic potential of breast cancer cells using a novel three dimensional model (rMET) recapitulating multiple steps of the bone metastatic process. Treatment of MCF10CA1a and MDA-MB-231 cells inhibited metastasis in the rMET model by 70% (±5.7%) and 21% (±6%), respectively. In addition, 1,25(OH)2D treatment decreased invasiveness (20±11% of vehicle) and decreased the capability of MCF10CA1a cells to survive in the reconstructed bone environment after successful invasion through the basement membrane (69±5% of vehicle). An essential step in metastasis is epithelial-mesenchymal transition (EMT). Treatment of MCF10CA1a cells with 1,25(OH)2D increased gene (2.04±0.28 fold increase) and protein (1.87±0.20 fold increase) expression of E-cadherin. Additionally, 1,25(OH)2D treatment decreased N-cadherin gene expression (42±8% decrease), a marker for EMT. Collectively, the present study suggests that 1,25(OH)2D inhibits breast cancer cell metastatic capability as well as inhibits EMT, an essential step in the metastatic process.
Keywords: Metastasis; 1α,25-Dihydroxyvitamin D; Breast Cancer; Prevention; Bone
1. Introduction
Breast cancer continues to be one of the most commonly diagnosed cancers in American women, with 1 in 8 women expected to develop invasive breast cancer during her lifetime [1]. Although treatment of localized breast cancer has been successful with 5 year survival reaching nearly 99%, patient survival drastically decreases to 26% once the tumor has metastasized to distant secondary sites [2]. In fact, metastasis accounts for 90% of all cancer-related deaths [3]. Therefore, it is important to investigate potential strategies which may prevent metastatic breast cancer and thereby prolong survival of breast cancer patients.
Metastasis involves multiple steps including initial cellular changes in the source tissue, cell movement through the basement membrane, escaping into the blood, surviving in the blood, as well as movement into, implantation and growth in the distal site [4]. Emerging evidence also suggests that this metastatic process may occur early in the development of a tumor [5], further emphasizing the importance of discovering safe preventive compounds against cancer metastasis that can be administered early in cancer progression.
An important step in metastasis is epithelial-mesenchymal transition (EMT), a process where epithelial cells become mesenchymal-like by losing their epithelial characteristics [6]. This mesenchymal-like profile increases cancer cell invasiveness and is characterized by a decrease in E-cadherin expression, an epithelial cell-cell adhesion protein, and increase in N-cadherin expression. Therefore, preventing or reversing EMT in breast cancer cells may be an effective strategy for preventing metastasis.
A growing body of evidence suggests that vitamin D may prevent the development of breast cancer [7, 8]. Vitamin D is derived from the diet or synthesized in the skin upon adequate sunlight exposure. Its circulatory form, 25-hydroxyvitamin D (25(OH)D), is a result of hydroxylation of vitamin D in the liver. A recently published meta-analysis demonstrated that serum levels of 25(OH)D dose-dependently correlated with decreased risk of death among breast cancer patients [9]. However, despite an evident relationship between breast cancer mortality and vitamin D, the mechanisms behind this nutrient’s protective effect are still unknown.
Several cell and animal studies suggest that vitamin D may inhibit cancer cell metastasis. Rossdeutscher et al. demonstrated that administration of 25(OH)D to MMTV-PyMT mice significantly decreased the size and number of breast cancer metastases to the lungs [10]. The circulating 25(OH)D is metabolized to its bioactive form, 1α,25-dihydroxyvitamin D (1,25(OH)2D), in the kidney. Multiple studies have now demonstrated that 1,25(OH)2D and its synthetic analogues inhibit metastasis of breast cancer cells both in vitro and in vivo [11–13]. Therefore, vitamin D may be an effective nutrient in preventing breast cancer metastasis.
The most common site of breast cancer metastasis is the bone [14]. However, there is limited availability of validated models designed to study breast to bone metastasis. To address this, a novel in vitro three dimensional reconstructed metastasis model (rMET) was developed in our laboratory (JK) which recapitulates the major steps of breast to bone metastasis including invasion, anchorage independence, and successful implantation into the extracellular matrix (ECM) of the distant secondary site [15]. Through tissue specific matrix compartments that recapitulate the primary tumor site (breast) and the distant metastatic site (bone), the rMET model allows for dissection of the metastatic process in laboratory controlled conditions. Cell subpopulations which successfully migrate to the bone compartment have consistently resulted in selective bone metastasis when injected intracardiacally into nude mice, confirming the validity of the model [15]. Therefore, the rMET model serves as an excellent tool in elucidating the effect of 1,25(OH)2D on essential steps involved in breast to bone metastasis.
To date, there are limited studies investigating the impact of vitamin D on bone metastasis. The goal of the current study was to investigate the impact of 1,25(OH)2D on EMT and bone metastatic capability of breast cancer cells using the rMET model. The results presented in this study contribute to the understanding of 1,25(OH)2D mediated inhibition of breast cancer metastasis and serve as foundation for further inquiries into the mechanisms explaining this nutrient’s protective effect.
2. Materials and methods
2.1 Chemicals and reagents
The bioactive vitamin D metabolite, 1,25(OH)2D, was purchased from Biomol (Plymouth Meeting, PA). Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) media, horse serum, fetal bovine serum, trypsin and penicillin/streptomycin were obtained from Life Technologies, Gibco-BRL (Rockville, MD). RPMI-1640 was obtained from Sigma (St. Louis, MO).
2.2 Cell culture
MCF10CA1a human breast epithelial cells were a gift from Dr. Julia Kirshner, Purdue University. MCF10CA1a cells were cultured in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, 1:1) supplemented with 5% horse serum, 100 units/ml penicillin, and 0.1 mg/mL streptomycin in a humidified environment at 37°C with 5% CO2 (regular growth conditions). MDA-MB-231 cells were cultured in DMEM/F12, 1:1 with 10% fetal bovine serum, 100 units/ml penicillin, and 0.1 mg/mL streptomycin in a humidified environment at 37°C with 5% CO2. For experiments conducted using the rMET model, cells plated in the transwell were cultured in RPMI-1640 media supplemented with 1% horse serum and 1% penicillin/streptomycin. The two cell lines were chosen due to their variable metastatic capability and stage of progression. The MCF10CA1a cell line was derived from serial passage of MCF10A-Harvey-ras transfected cells in nude mice. It forms primary tumors as well as spontaneous metastasis [16]. In contrast, MDA-MB-231 cells were derived from the metastatic site through pleural effusion and are considered a highly aggressive cell line. When compared in vivo, MDA-MB-231 cells show higher capability for distant metastasis than MCF10CA1a cells [17].
2.3 rMET model
The rMET model was executed as described previously [15]. Briefly, cells were pretreated in 2D culture for 48 hours with either vehicle (100% ethanol <0.1% of final volume) or 1,25(OH)2D (10 nM). After 48 hours, cells were plated into the Matrigel (BD Biosciences Bedford, MA) of the transwell insert of the rMET model at a concentration of 25,000 cells/well. Cells were treated with vehicle (<0.1% of final volume) or 1,25(OH)2D (10nM) for a total of 9 days, including pretreatment, with media changed every 24 hours.
The bone compartment of the rMET model consists of ECM proteins mimicking the endosteum and bone marrow matrix. Reconstructed endosteum in the rMET model consists of collagen I and fibronectin. Layered on top of the reconstructed endosteum is a reconstructed bone marrow matrix consisting of matrigel (rich in collagen IV and laminin), fibronectin and collagen I. In addition, the bone compartment is submerged in human fetal bone marrow mesenchymal stem cell conditioned media to further replicate the environment found in the bone. Cells which successfully metastasize in the rMET model have to degrade the ECM of the primary tumor site (transwell), migrate through the 8µm pores of the transwell membrane, survive through circulation in the bone compartment media, and finally attach to the endosteum/bone marrow matrix complex.
2.4 MTT cell proliferation assay
Cell viability was determined using an MTT assay according to the manufacturer's recommendations (Sigma St. Louis, MO) with slight modifications to accommodate the rMET model. Briefly, after 9 days of treatment the transwell insert was placed in a new 24 well containing 1× MTT in serum free media. Additional serum free MTT media was added into the transwell insert. After two hours of incubation, MTT formazan product was dissolved from the bottom of 8um membrane (invasive cell viability) and the inside of the transwell insert (primary cell population viability) with DMSO. All results were expressed as the relative absorbance (570nm) compared to that in the vehicle treated cells.
2.5 Viability/Cytotoxicity assay
The LIVE/DEAD Viability/Cytotoxicity kit was purchased from Life Technologies (Carlbad, CA). Cell viability was quantified in the bone compartment of the rMET model after 9 days of treatment according to the manufacturer’s instructions.
For experiments investigating the adaptive potential of 1,25(OH)2D treated cells in the bone environment, MCF10CA1a cells were pretreated with vehicle or 1,25(OH)2D (10nM) for five days in regular growth conditions and then re-plated directly into the bone compartment or regular growth conditions (control) at a density of 31,000 cells/well in the absence of 1,25(OH)2D.Cell viability was determined 24 hours after plating according to the manufacturer’s instructions.
2.6 Cell invasion assay
The basement membrane cell invasion assay was purchased from Cell Biolabs, Inc. (San Diego, CA). MCF10CA1a cells were pretreated with vehicle or 10 nM 1,25(OH)2D for 4 days after which they were re-plated at equal densities (750,000 cells/well) into the transwell inserts of the invasion assay. Invasion was quantified according to the manufacturer’s instructions after 48 hours for a total treatment time of 6 days.
2.7 RNA isolation and analysis
RNA isolation and analysis were conducted as described previously [18]. Briefly, RNA was isolated with TriReagent (Molecular Research Center, Cincinnati, OH) following the manufacturer's instructions. Reverse transcription of total RNA was performed using MMLV reverse transcriptase (Promega, Madison, WI). Real-time quantitative PCR was performed using the Brilliant II SYBR Green QPCR Master Mix (Agilent, Santa Clara, CA). The mRNA expression was normalized to 18S expression and results were expressed as arbitrary units. The primers used are shown:
-
E-Cadherin
Forward: 5’- GAAGAGAGACTGGGTTATTC - 3’
Reverse: 5’- GTCAGAGAAGAGAGTGTATG - 3’
-
N-Cadherin
Forward: 5’- CCACCTTAAAATCTGCAGGC – 3’
Reverse: 5’- GTGCATGAAGGACAGCCTCT – 3’
2.8 Immunofluorescent detection of protein expression
For immunofluorescent imaging, MCF10CA1a cells were extracted from the transwell of the rMET model after 9 days of treatment using Cell Recovery Solution (BD Biosciences Bedford, MA). Cells were then fixed with 10% neutral buffered formalin (VWR, West Chester, PA) for 15 minutes at room temperature. Once fixed, samples were blocked using 1% BSA/PBS for 24 hours at 4°C. E-cadherin rabbit mAb (#3195) was purchased from Cell Signaling (Danvers, MA) and the Alexa-488 Fluor® secondary antibody (A11008) was purchased from Life Technologies (Rockville, MD). Samples were additionally stained with DAPI (Life Technologies Rockville, MD) for nucleus visualization. Imaging was done using a Nikon A1R_MP confocal microscope. Since the MCF10CA1a cells grow in three dimensional spheroid structures in the rMET model, multiple images were taken 1µm apart across the width of the whole three dimensional structure. Image stacks were then combined to quantify total E-cadherin intensity. E-cadherin signal was quantified using FIJI software (http://fiji.sc/Fiji) and then normalized to the number of nuclei in the image.
2.9 Statistical Analysis
Values are presented as mean ± SEM. Results are expressed compared to the vehicle within the same cell line, by Student's t-tests, with P<0.05 considered statistically significant.
3. Results
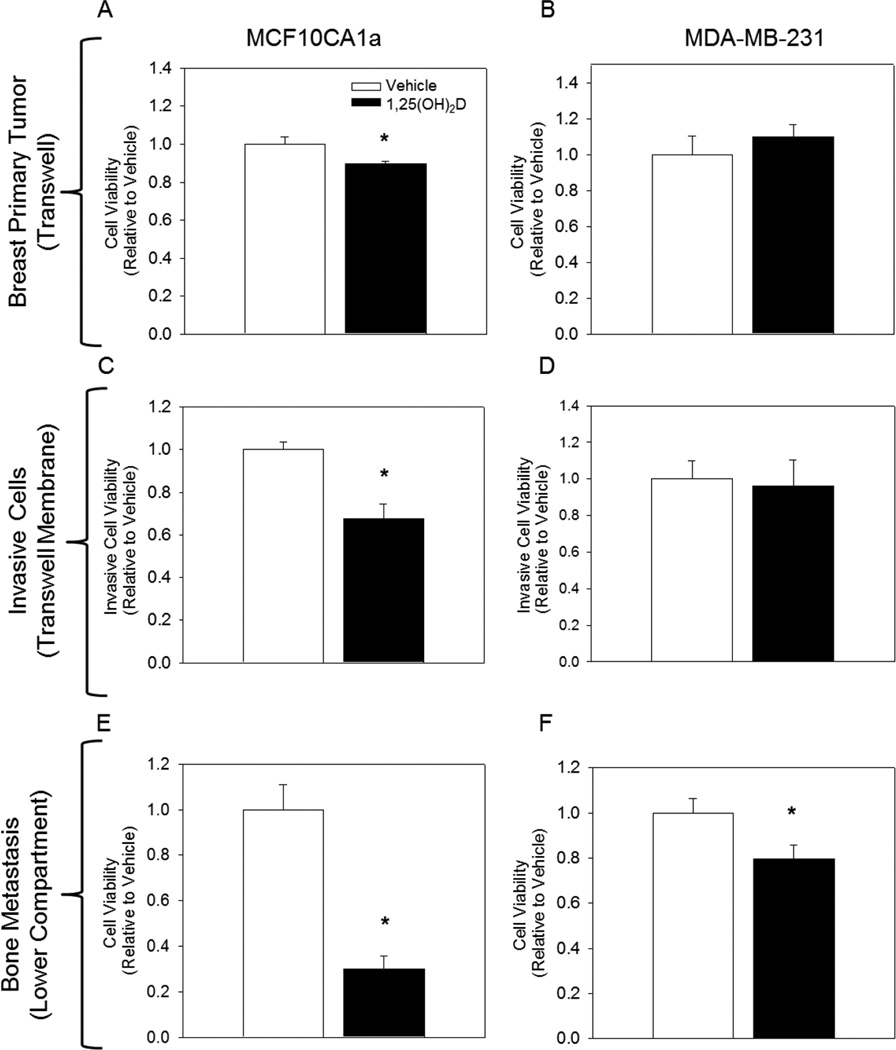
To evaluate the impact of 1,25(OH)2D on the bone metastatic potential of breast cancer cells, MCF10CA1a and MDA-MB-231 cells were treated with vehicle or 1,25(OH)2D (10nM) for a total of 9 days. At day 9, viability of cells that successfully migrated and colonized the ECM of the lower bone compartment was quantified. Treatment of cells with 1,25(OH)2D (10 nM) inhibited breast to bone metastasis of MCF10CA1a and MDA-MB-231 cells in the rMET model by 70% and 21%, respectively (Figure 1). Interestingly, in the transwell insert where cells were originally plated and exclusively treated, treatment of MDA-MB-231 cells with 1,25(OH)2D had no effect on cell viability, while treatment of MCF10CA1a cells only modestly reduced cell viability by 10% (Figure 1). Together, these results suggest that 1,25(OH)2D mediated inhibition of bone metastatic capability cannot be explained by the anti-proliferative effect of the nutrient on the cell population in the upper well.
Fig. I.
1,25(OH)2D inhibits bone metastatic capability of MCF10CA1a and MDA-MB-231 epithelial cells. Cells were treated with vehicle or 1,25(OH)2D (10 nM) for 2 days and an additional 7 days after re-plating into the transwell of the rMET model. (A–B) Relative viability of MCF10CA1a and MDA-MB-2331 cells in the transwell insert of the rMET model. (C–D) Relative viability of cells that successfully invaded through the ECM basement membrane, but have not yet migrated to the bone compartment as measured by an MTT assay. (E–F) Relative viability of cells that successfully metastasized to the bone compartment of the rMET model as assessed by Live/Dead Cytotoxicity Assay. * indicates a significant difference from vehicle within that group (P<0.05 2-tailed), # indicates a significant difference from vehicle within that group (P<0.05 1-tailed).
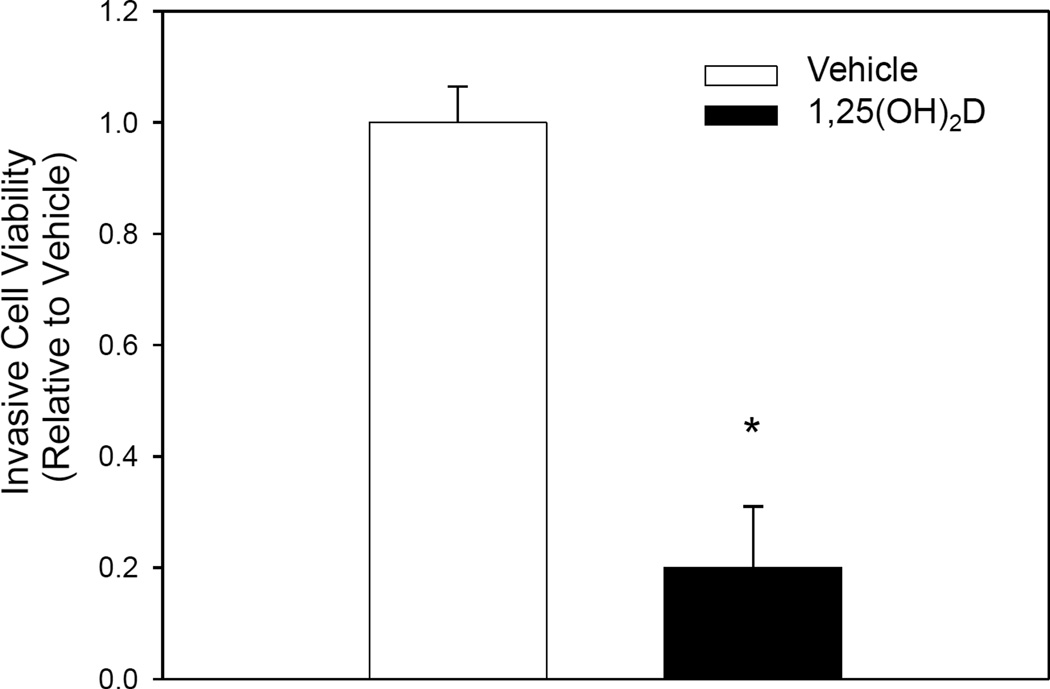
Invasion of cells through the basement membrane is an initial step of metastasis recapitulated in the rMET model. Cells attached to the bottom side of the transwell membrane in the rMET model represent a population of cells that have already invaded through the basement membrane but have not yet detached and entered circulation [15]. To assess the effect of 1,25(OH)2D on the invasive potential of breast cancer cells, we measured viability of cells which successfully migrated to the bottom of this transwell membrane. MCF10CA1a cells treated with 1,25(OH)2D had decreased viability on the transwell membrane relative to vehicle by 33%, indicating decreased invasive potential of breast cancer cells with 1,25(OH)2D treatment in the rMET model (Figure 1). To further confirm this effect, we conducted a basement membrane invasion assay independent of the rMET model. Cells treated with 1,25(OH)2D for six days demonstrated a significant 80% decrease in invasiveness relative to vehicle treated cells (Figure 2). Together, these results suggest that 1,25(OH)2D mediated inhibition of breast to bone metastasis may be in part attributed to decreased invasiveness of 1,25(OH)2D treated MCF10CA1a cells.
Fig. II.
1,25(OH)2D inhibits the invasive potential of MCF10CA1a cells. MCF10CA1a cells were treated for 4 days and plated onto the basement membrane of the transwell insert for an additional 2 days of treatment. Viability of cells which successfully migrated through the basement membrane relative to vehicle treated cells. * indicates a significant difference from vehicle within that group (P<0.05 2-tailed).
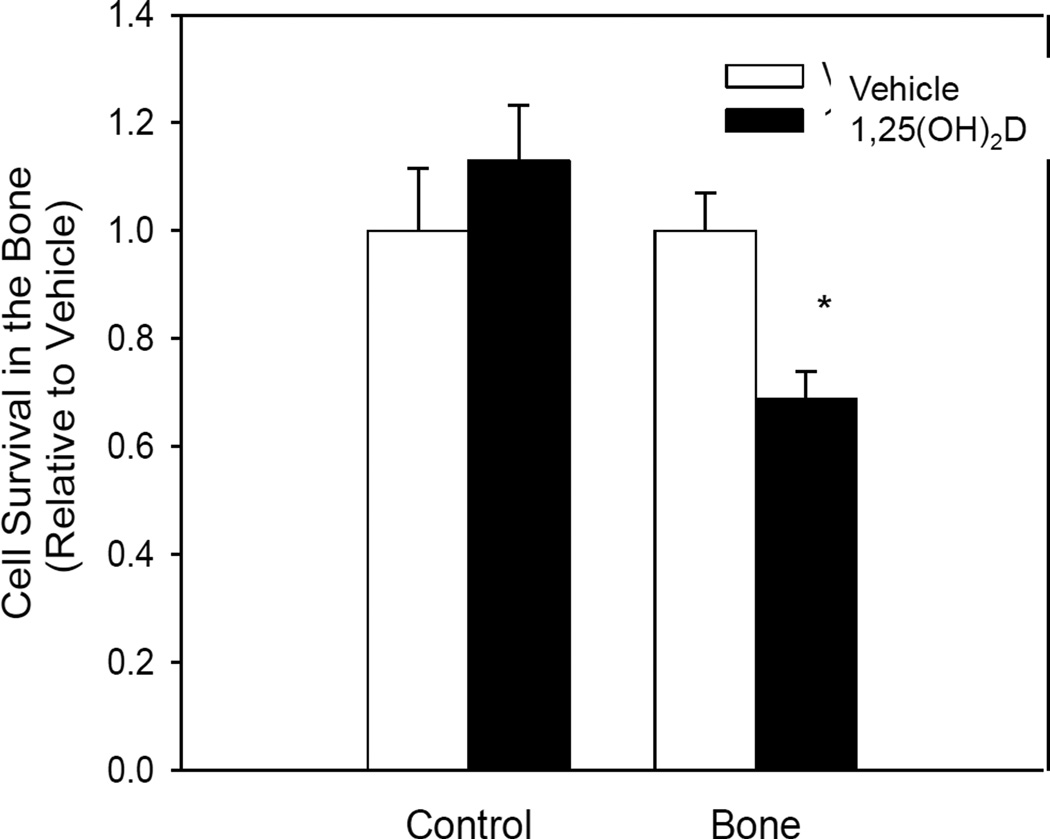
Another essential step in metastasis is successful adaptation to the environment of the distant secondary metastatic site. The bone compartment in the rMET model consists of ECM proteins mimicking the endosteum and bone marrow matrix. In addition, the media in this compartment is derived from human fetal bone marrow mesenchymal cells which release multiple factors into their environment. To evaluate if 1,25(OH)2D inhibits successful adaptation of breast cancer cells to this environment, MCF10CA1a cells were pretreated for five days in normal growth conditions with 1,25(OH)2D (10nM) and plated at equal cell density directly into the bone environment of the rMET model. Pretreatment of cells with 1,25(OH)2D resulted in reduced cell viability in the bone compartment by 31% relative to vehicle 24 hours after plating (Figure 3). On the other hand, cells pretreated with 1,25(OH)2D for the same duration, but then re-plated into regular growth conditions showed no reduction in viability. This result suggests that in addition to inhibition of invasion, 1,25(OH)2D also inhibits breast cancer cells’ ability to adapt to the drastically different environment of the secondary metastatic site.
Fig. III.
1,25(OH)2D inhibits the ability of MCF10CA1a cells to adapt to the bone environment of the rMET Model. Cells were pretreated for 5 days and then plated at equal density directly into regular growth conditions (Control) or the bone compartment of the rMET model (Bone). Viability was assessed using an MTT Assay and is presented relative to vehicle of each group. * indicates a significant difference from vehicle within that group (P<0.05 2-tailed).
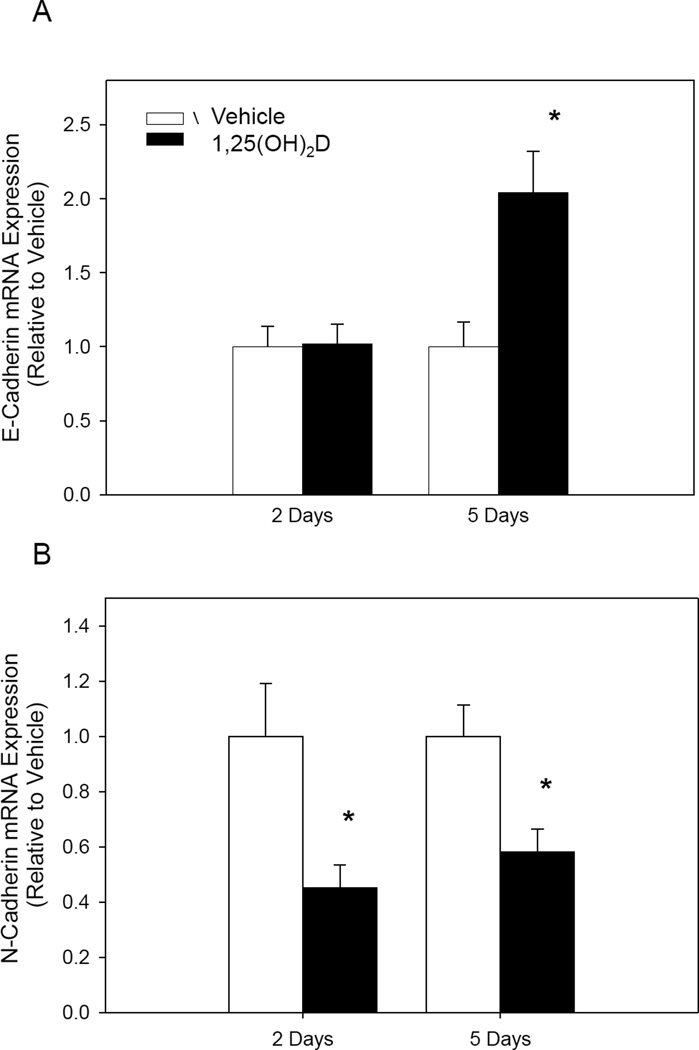
EMT is an important part of the metastatic process. To evaluate the effect of 1,25(OH)2D on EMT, we analyzed expression of major genes involved in EMT. Treatment with 1,25(OH)2D for 5 days resulted in a two fold increase in E-cadherin mRNA expression (Figure 4). N-cadherin mRNA expression, on the other hand, was decreased by 65% and 48% at 48 hours and 5 days of treatment, respectively (Figure 4). Together, these results suggest that 1,25(OH)2D contributes to inhibition of EMT.
Fig. IV.
1,25(OH)2D changes gene expression of EMT markers. MCF10CA1a cells were treated for 48 hours and 5 days. (A) E-Cadherin mRNA expression and (B) N-Cadherin expression was normalized to 18S, a housekeeping gene. * indicates a significant difference from vehicle within that group (P<0.05 2-tailed).
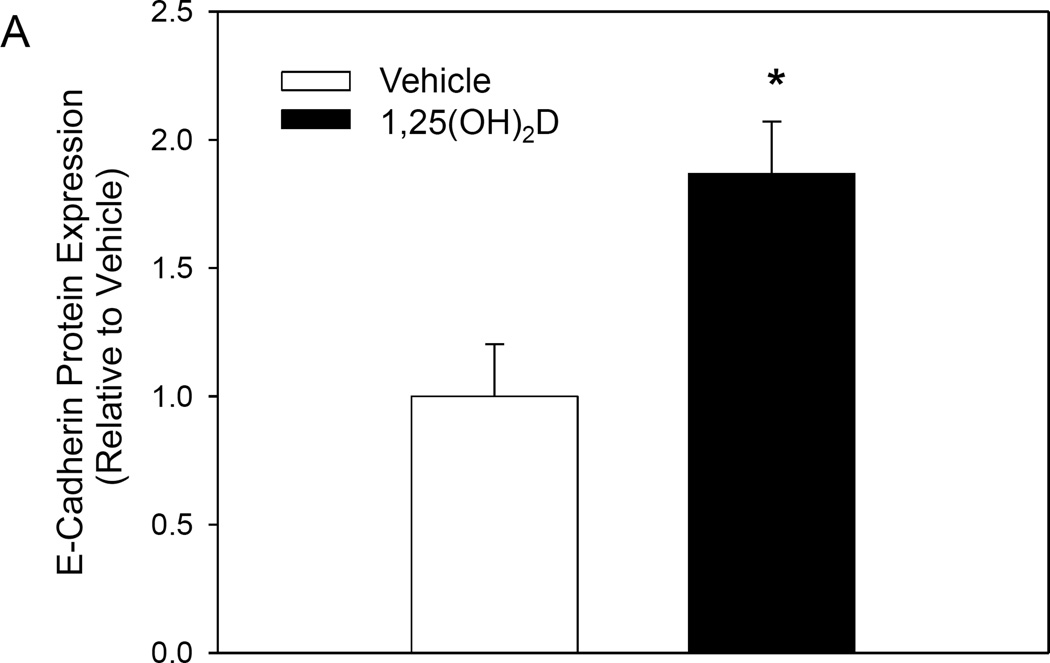
E-cadherin is a major cell-cell adhesion glycoprotein. Loss of E-cadherin expression by the cell is an essential step in the metastatic process [19]. To confirm that the 1,25(OH)2D mediated increase in gene expression corresponds with increased protein expression, we used immunofluorescence to measure E-cadherin protein levels at day 9 of treatment in the rMET model. Consistent with our mRNA data, treatment of MCF10CA1a cells with 1,25(OH)2D increased E-cadherin protein expression by 1.87 fold in the transwell insert of the rMET model (Figure 5). Together these results confirm that 1,25(OH)2D inhibits EMT in breast cancer cells.
Fig. V.
1,25(OH)2D increases expression of E-Cadherin in MCF10CA1a cells. E-Cadherin expression was determined in the transwell insert of the rMET model at day 9 of treatment using immunofluorescent confocal microscopy. (A) Protein expression per cell after vehicle or 1,25(OH)2D treatment. * indicates a significant difference from vehicle (P<0.05 2-tailed). (B) Representative immunofluorescent images of MCF10CA1a cells extracted from the transwell in their typical spheroid growth orientation.
4. Discussion
Bone is the major site of breast cancer metastasis. Development of bone metastasis is associated with both severe morbidity and increased mortality [20, 21]. However, despite the severe consequences of breast metastatic disease, treatment options for metastatic cancer are limited. In this study, we demonstrated that 1,25(OH)2D significantly inhibits breast to bone metastatic capability of MCF10CA1a and MDA-MB-231 cells in a novel three dimensional model recapitulating major steps of the bone metastatic cascade [15]. We confirmed that 1,25(OH)2D inhibits the invasive properties of metastatic breast cancer cells as well as their ability to adapt to the environment of the secondary metastatic site. These results suggest there may be multiple mechanisms by which 1,25(OH)2D contributes to inhibition of metastasis.
EMT is an essential process through which epithelial cells gain migratory and invasive properties [6]. Treatment of MCF10CA1a cells with 1,25(OH)2D resulted in nearly a two-fold increase in E-cadherin protein expression, suggesting inhibition of EMT. The same effect of 1,25(OH)2D on E-cadherin has been demonstrated previously in breast cancer cells in several studies [22–24]. Pendás-Franco et al. (2007) not only demonstrated an increase in E-cadherin expression with 1,25(OH)2D treatment in MDA-MB-453 cells, but also a significant decrease in N-cadherin expression and decreased cell invasiveness, as measured by a Matrigel invasion assay. Therefore, our study confirms previously reported effects of 1,25(OH)2D on EMT and cell invasion in breast cancer cells using a model that better recapitulates metastasis as it occurs in vivo.
An interesting observation in our study was the difference in magnitude of 1,25(OH)2D mediated inhibition of bone metastatic capability between MCF10CA1a and MDA-MB-231 cells. MCF10CA1a cells exhibited approximately a 3.5 greater inhibition of bone metastasis with 1,25(OH)2D treatment compared to MDA-MB-231 cells. In addition, MDA-MB-231 cells showed no inhibition of invasion or decrease in cell number with 1,25(OH)2D treatment in the rMET model. A reason for this may be that MDA-MB-231 cells are a more aggressive cell line and have already lost E-cadherin expression [25, 26]. Additionally, MDA-MB-231 cells have lower vitamin D receptor expression compared to most established breast cancer cell lines [27]. Therefore, the decrease in effect observed between MCF10CA1a and MDA-MB-231 cells is likely due to resistance to 1,25(OH)2D treatment as well as absence of E-cadherin expression in the MDA-MB-231 cell line.
The rMET model’s strengths lie in its more accurate depiction of breast to bone metastasis relative to other available in vitro models. Bone metastasis, however, is an immensely complex process involving cancer cell interaction with both the bone microenvironment as well cells found in the bone, primarily osteoblasts and osteoclasts [28]. The major limitation of the rMET model is that it does not incorporate cell to cell interactions. Therefore, follow up studies are necessary to confirm the inhibitory effect of 1,25(OH)2D on breast to bone metastasis in vivo.
5. Conclusion
Our study is the first to investigate the impact of 1,25(OH)2D on breast to bone metastatic capability. The results of the current experiments not only confirm inhibited metastatic potential of breast cancer cells, but also demonstrate that 1,25(OH)2D inhibits breast cancer cells’ ability to adapt to the drastically different bone environment, an observation not reported previously. Since cells were only treated in the transwell where they were originally plated, the decreased adaptability to the bone environment is likely due to 1,25(OH)2D mediated changes occurring in the primary tumor site, supporting the potential use of vitamin D in preventing metastasis. However, further studies are needed to elucidate the mechanism underlying this effect.
Acknowledgments
This work was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number UL1TR001108, as well as the National Institutes of Health, National Cancer Institute R25CA128770. Additional support was received from the Indiana Elks Charities administered by the Purdue Cancer Center.
Abbreviations
- EMT
epithelial-mesenchymal transition
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D
1α,25-dihydroxyvitamin D
- rMET
reconstructed metastasis model
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Cancer of the Breast: SEER Stat Fact Sheets. National Cancer Institute; 2014. [Google Scholar]
- 2.Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J, Cristofanilli M, Dontu G, Bidaut L, Valero V, Hortobagyi GN, Yu D. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69:4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285–1297. doi: 10.2217/fon.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foroni C, Broggini M, Generali D, Damia G. Epithelial-mesenchymal transition and breast cancer: role, molecular mechanisms and clinical impact. Cancer Treat Rev. 2012;38:689–697. doi: 10.1016/j.ctrv.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 9.Mohr SB, Gorham ED, Kim J, Hofflich H, Garland CF. Meta-analysis of vitamin D sufficiency for improving survival of patients with breast cancer. Anticancer Res. 2014;34:1163–1166. [PubMed] [Google Scholar]
- 10.Rossdeutscher L, Li J, Luco AL, Fadhil I, Ochietti B, Camirand A, Huang DC, Reinhardt TA, Muller W, Kremer R. Chemoprevention activity of 25-hydroxyvitamin D in the MMTV-PyMT mouse model of breast cancer. Cancer Prev Res (Phila) 2014 doi: 10.1158/1940-6207.CAPR-14-0110. [DOI] [PubMed] [Google Scholar]
- 11.Chiang KC, Yeh CN, Hsu JT, Jan YY, Chen LW, Kuo SF, Takano M, Kittaka A, Chen TC, Chen WT, Pang JH, Yeh TS, Juang HH. The vitamin D analog, MART-10, represses metastasis potential via downregulation of epithelial-mesenchymal transition in pancreatic cancer cells. Cancer Lett. 2014;354:235–244. doi: 10.1016/j.canlet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 12.So JY, Smolarek AK, Salerno DM, Maehr H, Uskokovic M, Liu F, Suh N. Targeting CD44-STAT3 signaling by Gemini vitamin D analog leads to inhibition of invasion in basal-like breast cancer. PLoS One. 2013;8:e54020. doi: 10.1371/journal.pone.0054020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Abdaimi K, Dion N, Papavasiliou V, Cardinal PE, Binderup L, Goltzman D, Ste-Marie LG, Kremer R. The vitamin D analogue EB 1089 prevents skeletal metastasis and prolongs survival time in nude mice transplanted with human breast cancer cells. Cancer Res. 2000;60:4412–4418. [PubMed] [Google Scholar]
- 14.NCI. Metastatic Cancer Fact Sheet - National Cancer institute. NCI; 2015. [Google Scholar]
- 15.Parikh MR, Minser KE, Rank LM, Glackin CA, Kirshner J. A reconstructed metastasis model to recapitulate the metastatic spread in vitro. Biotechnol J. 2014;9:1129–1139. doi: 10.1002/biot.201400121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santner S, Dawson P, Tait L, Soule H, Mohamed A, Wolman S, Heppner G, Miller F. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Cancer Research Treatment. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 17.Drabsch Y, He S, Zhang L, Snaar-Jagalska BE, ten Dijke P. Transforming growth factor-β signalling controls human breast cancer metastasis in a zebrafish xenograft model. Breast Cancer Res. 2013;15:R106. doi: 10.1186/bcr3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng W, Tayyari F, Gowda GA, Raftery D, McLamore ES, Shi J, Porterfield DM, Donkin SS, Bequette B, Teegarden D. 1,25-Dihydroxyvitamin D regulation of glucose metabolism in Harvey-ras transformed MCF10A human breast epithelial cells. J Steroid Biochem Mol Biol. 2013;138:81–89. doi: 10.1016/j.jsbmb.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 20.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 21.Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011;14:177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 22.Pendás-Franco N, González-Sancho JM, Suárez Y, Aguilera O, Steinmeyer A, Gamallo C, Berciano MT, Lafarga M, Muñoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75:193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Lee D, Sysounthone V, Chandraratna RAS, Christakos S, Korah R, Wieder R. 1,25-dihydroxyvitamin D3 and retonic acid analogues induce differentiation in breast cancer cells with function- and cell-specific additive effects. Breast Cancer Res Treat. 2001;67:157–168. doi: 10.1023/a:1010643323268. [DOI] [PubMed] [Google Scholar]
- 24.Lopes N, Carvalho J, Durães C, Sousa B, Gomes M, Costa JL, Oliveira C, Paredes J, Schmitt F. 1Alpha,25-dihydroxyvitamin D3 induces de novo E-cadherin expression in triple-negative breast cancer cells by CDH1-promoter demethylation. Anticancer Res. 2012;32:249–257. [PubMed] [Google Scholar]
- 25.Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T. E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res. 1996;56:4063–4070. [PubMed] [Google Scholar]
- 26.Yang JY, Zong CS, Xia W, Wei Y, Ali-Seyed M, Li Z, Broglio K, Berry DA, Hung MC. MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol Cell Biol. 2006;26:7269–7282. doi: 10.1128/MCB.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend K, Banwell CM, Guy M, Colston KW, Mansi JL, Stewart PM, Campbell MJ, Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin Cancer Res. 2005;11:3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 28.Theriault RL. Biology of bone metastases. Cancer Control. 2012;19:92–101. doi: 10.1177/107327481201900203. [DOI] [PubMed] [Google Scholar]