Abstract
Background
As a serious clinical problem, severe burn injury disturbs the immune system, resulting in progressive suppression of immune response. TLRs are associated with immune system activation, but the effect of TLRs levels on circulating cDCs of severe burn injury patients has not been fully assessed.
Material/Methods
Ten patients with total body surface area (TBSA) burned >90% admitted to our institution were enrolled in this study. We analyzed TLR2, TLR4, and TLR9 expression on the circulating cDCs by using multicolor flow cytometric analysis in patients at 14 days to 28 days after burn injury according to mortality, and We also assessed Demographics, clinical outcomes, organ function, and inflammatory and acute-phase responses.
Results
No difference in TBSA, sex, age, or number of operations before the first 14 days after injury were observed between surviving and non-surviving burn patients. The levels of TLR2, TLR4, and TLR9 in circulating cDCs were significantly and consistently elevated in all patients compared to age-matched healthy volunteers, and survivors exhibited higher TLR2 and TLR4 values than non-survivors. Of the survivors, TLR2 and TLR4 levels were higher at 28 days than at 14 days after injury, while the difference in TLR9 levels was not significant. TLR2 levels of non-survivors at 28 days after injury decreased, and the TLR4 and TLR9 levels showed no significant difference.
Conclusions
TLRs levels in circulating cDCs are highly activated in severe burn injury patients up to 28 days after injury. The low expression of TLR2 in cDCs may be useful as a potential marker predicting the poor prognosis of severe burn patients.
MeSH Keywords: Burns, Dendritic Cells, HLA-DR Antigens, Systemic Inflammatory Response Syndrome, Toll-Like Receptors
Background
Severe burn injury is a serious clinical problem worldwide. Due to significant advances in therapeutic strategies, severe burn mortality in the early stage has improved in recent years [1,2]. However, the main contributor to adverse outcomes of severely burned patients is secondary sepsis caused by immune dysfunction in the sub-acute phase following the initial burn injury [3]. The trauma of severe burn injury induces a distinct systemic inflammatory response, and a state of subsequent immunosuppression [4]. In patients with severe burns, 75% of deaths are related to secondary sepsis. Intensive research is needed to focus on immune function of severely burned patients.
Pattern recognition receptors (PRRs) are the central components of the innate immune system that recognizes invading pathogens and initiates the immune response [5]. Of these PRRs, Toll-like receptors (TLRs) are the most studied. To date, 10 functional TLRs have been identified and a broad range of microbial products in many TLRs has been verified [6]. For example, TLR4 interacts with lipopolysaccharide (LPS) from gram-negative bacteria [7] and TLR2 interacts with peptidoglycan, lipoteichoic acid from gram-positive bacteria, lipoproteins from mycobacteria, and others [8]. TLR9 controls bacterial and viral infections by recognizing un-methylated CpG DNA motifs [9]. Because burn injury patients are susceptible to infection from a variety of pathogens, including gram-positive and gram-negative bacteria, as well as viruses, in the present study we focussed on 3 TLRs: TLR2, TLR4, and TLR9.
Toll-like receptors (TLRs) have been implicated in immunoregulation in a variety of disease states, including burn injury [10]. In peripheral blood, dendritic cells (DCs) are potent antigen-presenting immune cells that use TLRs to recognize pathogen-associated molecular patterns. Dendritic cells interface between the innate and adaptive immune systems. However, there has been little research on TLRs expression on monocytes and DCs, which can reflect the innate and adaptive immune responses.
The present prospective study assessed TLRs expression on DCs during the sub-acute phase (14~28 days) in burn patients (TBSA burned >90%) and their association with outcome. Our investigation found that TLR2 expression may be useful as a marker predicting poor prognosis of severe burn injury patients and provide new insight into the complex immunological response accompanied with hyporesponsiveness of innate and adaptive immune systems in severe burn patients, lasting for 28 days after the initial burn injury.
Material and Methods
Patients and healthy volunteers
We enrolled 10 burn patients older than 18 years, admitted to the ICU of Changhai Hospital, Second Military Medical University (Shanghai, China) within 24 h after injury, with burns covering >90% TBSA from January 2014 to June 2015. All the patients were injured in workplace fires or explosions. Patients were excluded from the study if they were infected with human immunodeficiency virus (HIV), had any neoplastic disease, had received immunosuppressive medications, or if they were participating in any other study.
All of the participants provided written informed consent. The study protocol was approved by the Second Military Medical University’s Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
Samples
For flow cytometry and cytokine measurements, peripheral venous blood samples were drawn in the morning of day 14 and day 28 after admission. The control group included 8 healthy volunteers (sex-matched and age-matched). Blood samples were obtained with the consent of the patient or a legally authorized representative.
Diagnosis of sepsis
According to the American Burn Association Criteria for Sepsis [11], secondary sepsis should be considered when 3 or more of the following criteria are met: 1) Temperature: >39°C or <36.5°C; 2) Progressive tachycardia: >110 beats/min; 3) Progressive tachypnea: a. >25 breaths/min not ventilated; b. Ventilation >12 L/min; 4) Thrombocytopenia (not applied until 3 days after initial resuscitation): <100 000/μl; 5) Hyperglycemia (in the absence of preexisting diabetes mellitus): a. Untreated plasma glucose >200 mg/dl or equivalent mM/l; b. >7 U of insulin/hr IV drip; c. Significant resistance to insulin (>25% increase in insulin requirement over 24 h); 6) Inability to continue enteral feedings >24 h: a. abdominal distension; b. High gastric residuals (residuals 2 times feeding rate); c. Uncontrollable diarrhea (>2500 ml/day).
Flow cytometry
Surface staining was performed for 15 min using standard methods. Briefly, 100–200 ul whole blood was mixed with 0.5–1 ul mouse anti-human monoclonal antibodies for 15 min in the dark at room temperature. Next, erythrocytes were hemolyzed using a Becton Dickinson fluorescence activated cell sorter (BD FACS) lysing solution for 10 min. The leukocytes were washed twice in phosphate-buffered saline (PBS), and finally resuspended in CellFIX solution. Antibodies used were anti-CD11C (eBioscience), anti-HLA-DR(eBioscience), anti-TLR4 (eBioscience), anti-TLR2 (eBioscience), and anti-TLR9 (eBioscience). At least 50 000 cells per sample were counted. DCs were characterized on the basis of the CD11c expression. Flow cytometric analysis (50 000 events/sample) was performed on a FACS Calibur Flow Cytometer (BD Biosciences) and further analysis was performed using FlowJo software (Three Star).
Statistical analysis
Patients’ clinical characteristics are reported as median values and interquartile ranges. Data are expressed as mean ±SE. Comparisons between groups were made using the nonparametric Mann-Whitney U test for continuous variables and the chi-square test was used for categorical data (GraphPad Prism 5.01, GraphPad Software, San Diego, CA). A P value of <0.05 was considered to be statistically significant for all analyses.
Results
Demographics of the patients
Ten patients were enrolled in our study from January 2014 to June 2015 (7 females, 3 males; median age 38.5 years, range 22–53 years). Total body surface area (TBSA) burned was at least 90% in all patients. Inhalation injury occurred in all patients. Prophylactic tracheostomy was applied within 12 h after injury in all patients. Apart from tracheostomy, these patients received standard care, and initial resuscitation was carried out according to the Baxter’s Parkland Hospital formula [12]. Excision and skin grafting were started in the first week (median day 4, range day 3 to day 5). Sepsis was diagnosed in 5 patients between day 16 and day 24 after injury (incidence 50%), and they died between day 28 and day 33. The demographics and characteristics of patients are presented in Table 1.
Table 1.
Demographic data and outcomes of the 10 severe burn patients.
| Patient | age | gender | TBSA burned (%) | TBSA with third-degree burns (%) | Septic shock | Length of stay days | No. of surgical procedure | Survival |
|---|---|---|---|---|---|---|---|---|
| 1 | 38 | F | 90 | 82 | No | 90 | 9 | Yes |
| 2 | 45 | M | 96 | 94 | No | 49 | 10 | Yes |
| 3 | 45 | F | 98 | 90 | No | 73 | 8 | Yes |
| 4 | 32 | F | 90 | 17 | No | 85 | 10 | Yes |
| 5 | 53 | M | 90 | 45 | No | 56 | 6 | Yes |
| 6 | 25 | F | 98 | 81 | Yes | 31 | 6 | No |
| 7 | 22 | M | 92 | 91 | Yes | 28 | 9 | No |
| 8 | 34 | F | 88 | 70 | Yes | 29 | 5 | No |
| 9 | 34 | F | 94 | 82 | Yes | 33 | 6 | No |
| 10 | 39 | F | 98 | 80 | Yes | 32 | 7 | No |
TBAS – total burn surface area.
No differences were observed between survivors and no-survivors in TBSA, sex, age, or number of operations before day 14 (Table 2). Survivors had longer length of stay in the ICU than non-survivors. Patients who survived were transferred to the general ward and were ultimately discharged from the hospital. There was no difference in SOFA scores on day 1 and day 14 after admission. By day 28 after admission, SOFA scores of non-survivors were much higher than scores of survivors (20 vs. 12, p<0.001).
Table 2.
Clinical characteristics of the 10 severe burn patients.
| Survivors | Nosurvivors | |
|---|---|---|
| No. of patients | 5 | 5 |
| Age, yrs | 45 (32–53) | 34 (25–51) |
| Gengder (M/F) | 3/2 | 4/1 |
| TBSA% (III/IV) | 90 (82/10) | 94 (85/8.6) |
| No of surgical procedure in the first 14 days | 6 | 5 |
| Days length of stay, days* | 67 | 30.6 |
| Diagnose of sepsis shock, days | / | 20 (16–24) |
| SOFA scores 1st day | 9 | 10 |
| SOFA scores 14th day | 11 | 14 |
| SOFA scores 28th day*** | 12 | 20 |
Data are presented in median and inter-quartile range.
Significant difference between groups (P<0.05);
SOFA – sequential organ-failure assessment score.
All the patients developed secondary infections: bacteremia (5) or/and pneumonia (n=10). As the result of inhalation injury, pneumonia occurred in every patient. Septic shock was diagnosed in 4 non-surviving patients between day 16 and day 24 after burn injury. The fifth non-surviving patient died due to severe coagulation disorders without any septic complications. The bacterial species involved most often were Pseudomonas aeruginosa, Acinetobacter baumannii, and Klebsiella pneumonia (100%). Soft tissue infections or catheter-related infections were not found in our study.
Expression of TLR2, TLR4, and TLR9 on DCs in severe burn injury patients and healthy volunteers at 28 days after injury
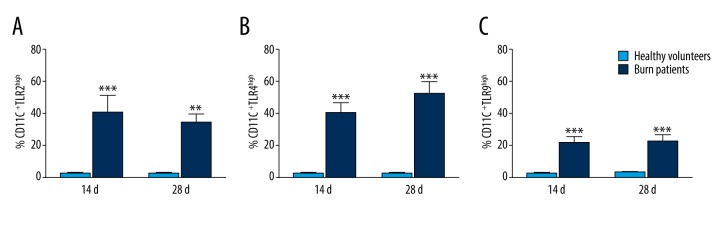
Because our data showed no difference between survivors and non-survivors in clinical parameters or demography (TBSA, sex, age, SOFA scores, and number of operations) during the first 14 days after burn injury, we evaluated TLRs expression levels on DCs at 28 days after injury. The expression of TLR2, TLR4, and TLR9 of DCs were significantly higher in all patients compared to age-matched healthy volunteers (Figure 1A–1C).
Figure 1.
Analysis of the expression of TLR2, TLR4, and TLR9 on DCs of patients at 14 days and 28 days after extreme burn injury. The expression of TLR4 (A), TLR9 (B), and TLR2 (C) on DCs at 14 days and 28 days after major burn injury was higher than in healthy volunteers. TLRs expression was analyzed in histograms and reported as percentages. * P<0.05; ** P<0.01; *** P<0.0001.
TLRs expression on DCs according to outcome
We also compared TLR2, TLR4, and TLR9 expression between survivors and non-survivors. TLR2 and TLR4 levels were significantly higher in survivors than in non-survivors at enrollment (day 14) and follow-up (day 28) (Figure 2A, 2D). There was no difference in the expression of TLR9 on DCs between non-survivors and survivors (Figure 2B). TLR4 expression on DCs of surviving patients was significantly higher at day 28 compared to that at day 14 (P=0.01; Figure 2B), but expression of TLR4 on DCs of non-surviving patients did not change in these patients between day 14 and day 28 (P=0.11; Figure 2C). Similarly, TLR2 expression on DCs of surviving patients was remarkably higher at day 28 than at day 14 (P=0.001; Figiure 2E), and expression of TLR2 on DCs of non-surviving patients was significantly lower at day 28 than at day 14 (P=0.01; Figure 2F).
Figure 2.
Analysis of the expression of TLR2, TLR4, and TLR9 on DCs of patients at 14 days and 28 days after injury between survivors and non-survivors. The TLR2 and TLR4 levels were significantly higher in survivors than in non-survivors at enrollment (day 14) and follow-up (day 28) (A, D). There was no difference in the expression of TLR9 on DCs between non-survivors and survivors (B). TLR4 expression on DCs of surviving patients was significantly higher at day 28 compared to that at day 14 (P=0.01; B), while expression of TLR4 on DCs of non-survivors did not change in these patients at day 28 compared to day 14 (P=0.11; C). Similarly, TLR2 expression on DCs of surviving patients was remarkably higher at day 28 compared to day 14 (P=0.001; E), and expression of TLR2 on DCs of non-survivors decreased in these patients at day 28 compared to day 14 (P=0.01; F).
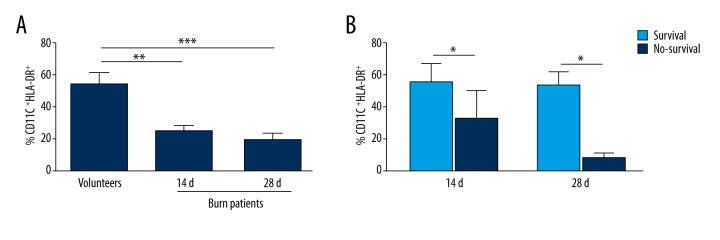
Expression of HLA-DR on DCs in severe burn patients at 14 days and 28 days after injury
Because HLA-DR is a predictor of mortality in septic shock and is a prognostic indicator for susceptibility to secondary infections in burn patients, we measured the level of HLA-DR on DCs during the course of hospitalization after burn injury. We observed low HLA-DR values in all burn patients at 14 days and 28 days after injury (Figure 3A). Non-surviving patients exhibited lower HLA-DR values on DCs than survivors at 14 days after injury, and even lower levels of HLA-DR on DCs were found in non-surviving patients at 28 days after injury (Figure 3B). Results are presented as percentages of DCs expressing HLA-DR.
Figure 3.
Expression of HLA-DR on DCs in severe burn patients at 14 days and 28 days after injury. Low HLA-DR value was found in all burn patients on 14 days and 28 days after injury (A). Non-surviving patients exhibited lower HLA-DR values on DCs than survivors at 14 days after injury, and even lower levels of HLA-DR on DCs were found in non-surviving patients at 28 days after injury (B). Results are presented as percentages of DCs expressing HLA-DR.
Discussion
Our results demonstrate that severe burn injury induced marked increase in TLR2, TLR4, and TLR9 expression on DCs at 14 and 28 days after burn injury covering >90% TBSA. The TLR2 intensity of DCs was associated with mortality. We also found that DCs showed a decreased mHLA-DR expression, confirming that mHLA-DR expression is a reflection of immunosuppression. No relationship was found between TLR4 or TLR9 expression level on DCs and outcome.
As a family of cell surface molecules that participate in innate immunity, TLRs have been implicated in immunoregulation in a variety of disease states, including burn injury and sepsis [13]. TLR ligands include the exogenous TLR agonists (pathogen-associated molecular patterns [PAMPS]) displayed in a variety of microorganisms, as well as endogenous TLR agonists (DAMPS), associated with cellular injury and stress responses.
The effect of burn injury on TLR reactivity in the circulating immune cells has been investigated in recent years, and among these immune cells, monocytes are the most studied. According to research on sepsis, TLRs expression on monocytes have little relationship with prognosis or complications [14]. Recent findings from Martin et al. have shown a marked increase in TLR2 and TLR4 expression on circulating γδT cells at 24 h after burn injury, and TLRs were excessively activated on αβT cells at 7 days after burn injury [15]. In the present study, we further measured TLRs kinetics of dendritic cells in severe burn patients in the post-acute stage.
Dendritic cells (DCs) are central mediators in innate and adaptive immune responses, and they play a role in the presentation of pathogens to adaptive immune cells. The effect of burn injury on TLR-mediated DC function remains to be elucidated. The DC family is mainly classified into conventional and plasmacytoid DCs. Conventional DCs (cDCs) efficiently induce antigen-specific T cell responses, whereas plasmacytoid DCs (pDCs) produce high levels of type I interferon (IFN) [16]. Given that DC subsets have unique functional characteristics, in the present study we analyzed the effects of severe burn injury on TLRs expression in the cDCs subpopulation in the circulating blood.
Our data show that levels of TLR2, TLR4, and TLR9 on cDCs at 14–28 days after burn injury were remarkably increased. Patenaude et al. demonstrated that TLR4/MD-2 expression in burn injury patients was down-regulated on splenic CD11c+CD8a+ cDCs [17]. Burn injury-induced impairments in DC immunobiology is a debated topic. The depletion of cDCs following burn injury is clearly established [18], but the ability of cDCs to stimulate Ag-specific T cell proliferation differs among studies. Fujimi et al. reported that numbers and percentages of splenic cDCs and pDCs after burn injury were reduced, while the ability of cDCs to stimulate Ag-specific T cell proliferation was unaffected by burn injury [19]. van den Berg et al. reported that skin DCs have severely reduced T cell stimulatory capacity following burn injury in healthy volunteers [20].
DCs recognize microbes via PRRs, including the TLR family. In our study, we found that levels of TLR2, TLR4, and TLR9 on cDCs at 14–28 days after burn injury was remarkably increased (about 5-fold) compared to healthy volunteers, and TLR2 expression on DCs of non-survivors was downregulated compared to survivors. We postulate that the augmented TLR reactivity of circulating cDCs might contribute to the development of heightened systemic inflammation following severe burn injury. The up-regulation of TLRs in critically ill patients might be related both to the microorganisms involved in inflammation and to the body’s attempt to repair tissue. Because severe burn injury is characterized by extensive tissue damage and tissue necrosis, high-level expression of TLRs may play a role in tissue repair and resolving inflammation in burn injury patients.
Further research with larger numbers of patients is needed. TLRs expression on cDCs should fully be investigated for months or even longer after burn injury, as well as their influence on long-term outcomes in severe burn patients.
Conclusions
The data in the present study suggest that TLRs expression is remarkably upregulated on circulating cDCs at 14–28 days after extremely severe burn injury. In addition, in the presence of severe sepsis, the intensity of this TLR2 expression is related to mortality of patients. Considering the role of DCs in the defense against bacterial infections and sepsis, it appears that DCs are required for maintenance of immune response. High-level expression of TLRs on DCs may play a role in tissue repair and resolution of inflammation in severe burn injury patients.
Abbreviations
- TBSA
total body surface area
- TLRs
toll-like receptors
- cDCs
conventional dendritic cells
- SOFA
sequential organ-failure assessment score
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81276025 to Dr. XM Deng; 81471845 to Dr. XM Deng; 81571877 to Dr. JB Li, 81171843 to Dr. JJ Bian, 81401575 to Dr. R Bao) and Key Project on Basic Research of Shanghai (12JC1410700 to Dr. XM Deng)
References
- 1.Brusselaers N, Monstrey S, Vogelaers D, et al. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity, and mortality. Crit Care. 2010;14(5):R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brusselaers N, Monstrey S, Snoeij T, et al. Morbidity and mortality of bloodstream infections in patients with severe burn injury. Am J Crit Care. 2010;19(6):e81–87. doi: 10.4037/ajcc2010341. [DOI] [PubMed] [Google Scholar]
- 3.D’Avignon LC, Hogan BK, Murray CK, et al. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: An autopsy series. Burns. 2010;36(6):773–79. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Yizhi P, Jing C, Zhiqiang Y, et al. Editorial board of guidelines for the treatment of burn infection CMA: Diagnostic criteria and treatment protocol for post-burn sepsis. Crit Care. 2013;17(1):406. doi: 10.1186/cc11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–50. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 7.Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162(7):3749–52. [PubMed] [Google Scholar]
- 8.Song DH, Lee JO. Sensing of microbial molecular patterns by Toll-like receptors. Immunol Rev. 2012;250(1):216–29. doi: 10.1111/j.1600-065X.2012.01167.x. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H. Interactions between bacterial CpG-DNA and TLR9 bridge innate and adaptive immunity. Curr Opin Microbiol. 2002;5(1):62–69. doi: 10.1016/s1369-5274(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 10.Paterson HM, Murphy TJ, Purcell EJ, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171(3):1473–83. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 11.Paratz JD, Lipman J, Boots RJ, et al. A New marker of sepsis post burn injury? Crit Care Med. 2014;42(9):2029–36. doi: 10.1097/CCM.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 12.Haberal M, Sakallioglu Abali AE, Karakayali H. Fluid management in major burn injuries. Indian J Plast Surg. 2010;43(Suppl):S29–36. doi: 10.4103/0970-0358.70715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14(8):546–58. doi: 10.1038/nri3713. [DOI] [PubMed] [Google Scholar]
- 14.Mohri T, Ogura H, Koh T, et al. Enhanced expression of intracellular heme oxygenase-1 in deactivated monocytes from patients with severe systemic inflammatory response syndrome. J Trauma. 2006;61(3):616–23. doi: 10.1097/01.ta.0000238228.67894.d7. discussion 623. [DOI] [PubMed] [Google Scholar]
- 15.Schwacha MG, Zhang Q, Rani M, et al. Burn enhances toll-like receptor induced responses by circulating leukocytes. Int J Clin Exp Med. 2012;5(2):136–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Muralimohan G, Vella AT. A role for IFNgamma in differential superantigen stimulation of conventional versus plasmacytoid DCs. Cell Immunol. 2006;242(1):9–22. doi: 10.1016/j.cellimm.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patenaude J, D’Elia M, Hamelin C, Bernier J. Selective effect of burn injury on splenic CD11c(+) dendritic cells and CD8alpha(+)CD4(−)CD11c(+) dendritic cell subsets. Cell Mol Life Sci. 2010;67(8):1315–29. doi: 10.1007/s00018-009-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Arpa A, Accardo-Palumbo A, Amato G, et al. Decrease of circulating dendritic cells in burn patients. Ann Burns Fire Disasters. 2007;20(4):199–202. [PMC free article] [PubMed] [Google Scholar]
- 19.Fujimi S, Lapchak PH, Zang Y, et al. Murine dendritic cell antigen-presenting cell function is not altered by burn injury. J Leukoc Biol. 2009;85(5):862–70. doi: 10.1189/jlb.0408257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Berg LM, de Jong MA, Witte Ld, et al. Burn injury suppresses human dermal dendritic cell and Langerhans cell function. Cell Immunol. 2011;268(1):29–36. doi: 10.1016/j.cellimm.2011.01.007. [DOI] [PubMed] [Google Scholar]