Abstract
BACKGROUND & AIMS
Type 2 diabetes and nonalcoholic steatohepatitis (NASH) are associated with insulin resistance and disordered cholesterol homeostasis. We investigated the basis for hepatic cholesterol accumulation with insulin resistance and its relevance to the pathogenesis of NASH.
METHODS
Alms1 mutant (foz/foz) and wild-type NOD.B10 mice were fed high-fat diets that contained varying percentages of cholesterol; hepatic lipid pools and pathways of cholesterol turnover were determined. Hepatocytes were exposed to insulin concentrations that circulate in diabetic foz/foz mice.
RESULTS
Hepatic cholesterol accumulation was attributed to up-regulation of low-density lipoprotein receptor via activation of sterol regulatory element binding protein 2 (SREBP-2), reduced biotransformation to bile acids, and suppression of canalicular pathways for cholesterol and bile acid excretion in bile. Exposing primary hepatocytes to concentrations of insulin that circulate in diabetic Alms1 mice replicated the increases in SREBP-2 and low-density lipoprotein receptor and suppression of bile salt export pump. Removing cholesterol from diet prevented hepatic accumulation of free cholesterol and NASH; increasing dietary cholesterol levels exacerbated hepatic accumulation of free cholesterol, hepatocyte injury or apoptosis, macrophage recruitment, and liver fibrosis.
CONCLUSIONS
In obese, diabetic mice, hyperinsulinemia alters nuclear transcriptional regulators of cholesterol homeostasis, leading to hepatic accumulation of free cholesterol; the resulting cytotoxicity mediates transition of steatosis to NASH.
Comment
Obesity in the United States and other developed countries is increasing at an alarming rate.1,2 Among the myriad health complications associated with obesity (including diabetes and cardiovascular risk) is non-alcoholic fatty liver disease (NAFLD). NAFLD is a spectrum of liver diseases ranging from simple steatosis, to active inflammation (nonalcoholic steatohepatitis [NASH]), to advanced fibrosis and cirrhosis,3 to hepatocellular carcinoma.4 Risk factors for primary NAFLD (i.e., not secondary to other proximate causes) are analogous to those of the metabolic syndrome (e.g., obesity, type II diabetes, and dyslipidemia).5 The prevalence of simple steatosis in individuals at risk for NAFLD can be very high; for example, the prevalence in the severely obese (body mass index >35) has been reported to be 90%.6 In contrast, the prevalence of NASH is much lower in this population (~40%).6 These factors emphasize that the risk for developing the more severe stages of NAFLD (i.e., NASH and beyond) is not based solely on primary risk factors, but is rather modified by other mitigating genetic or environmental factors. It has been established that physiological/biochemical changes to the liver that are pathologically inert can enhance the hepatotoxic response caused by a second agent; this “two-hit” paradigm has been best exemplified in NAFLD and other fatty liver diseases.7,8
One of the second “hits” in NAFLD appears to be diet composition; specifically a diet richer in saturated fats and cholesterol (a “Western” diet) appears to increase the risk of developing NASH.9 Based on these observations, several studies have investigated the mechanisms by which fat and fat type differentially mediate liver injury and potentially the transition from NAFLD to NASH. The current prevailing hypothesis is that free fatty acid–mediated lipotoxicity is the culprit in NAFLD/NASH progression; however, the clinical evidence is far from conclusive at this time. The main purpose of the study by van Rooyen et al.10 is to test the principle that cholesterol, which is also elevated in NASH livers,11 could also be the hepatotoxic lipid. In short, this purpose was served very well in their work.
The authors employed a mouse strain that contains a spontaneous mutation in the Alms1 gene (foz/foz mice). The phenotype of this mutant strain is analogous to those found in patients suffering from Alström syndrome in humans (e.g., obesity, insulin resistance, dyslipidemia, liver injury),12 which is accelerated by feeding of a high-fat diet (HFD).13 The pathology in these mice is quite impressive and includes robust steatohepatitis with fibrosis as early as 12 weeks of HFD feeding.14 These changes correlated with an increase in both hepatic cholesterol ester (CE) (>50-fold) and free cholesterol (FC) (≈4-fold). Given that cholesterol was only 0.2% of the diet, these data suggest that foz/foz mice somehow accumulate cholesterol. The remainder of the article is dedicated to determining the potential mechanisms.
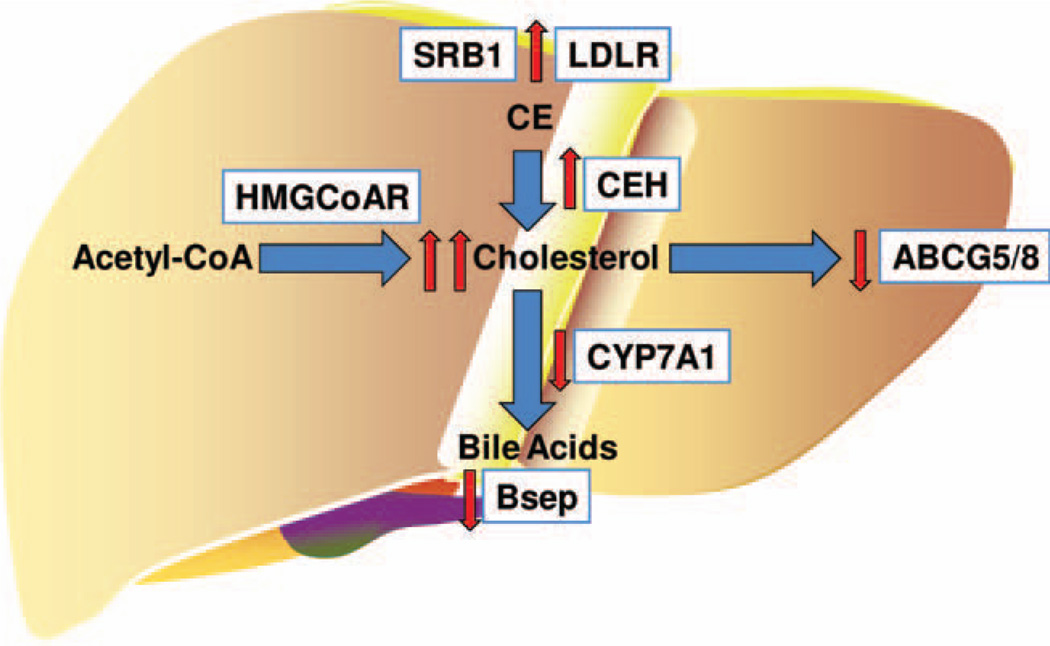
Hepatic free cholesterol can accumulate in the liver via several mechanisms: (1) increased uptake of dietary cholesterol and CEs, (2) increased de novo synthesis, and (3) decreased catabolism via bile acid synthesis and secretion (Fig. 1). HFD feeding in foz/foz mice altered two out of three of these pathways such that hepatic FC accumulation is favored. Specifically, key genes involved in uptake (CD36,14 low-density lipoprotein receptor) and hydrolysis of CE (CE hydrolase) are up-regulated by HFD in foz/foz mice. Furthermore, key genes involved in bile acid synthesis (CYP7A1) and secretion (bile salt export pump), as well as cholesterol secretion (ABCG5/8), were all dramatically down-regulated in the foz/foz strain compared with all other groups. An interesting aspect of this work is that the phenotype in the foz/foz mice fed HFD was so dramatically different than all other groups (wild-type [WT] chow, WT HFD, and foz/foz chow). These results also emphasize the need for researchers to incorporate all groups into a 2 × 2 factorial design so that interactions between the two variables can be studied.
Fig. 1.
Mechanisms of cholesterol homeostasis in the liver. De novo cholesterol synthesis from acetyl coenzyme A is rate-limited by HMG coenzyme A reductase (HMGCoAR). Dietary CEs are taken up by hepatocytes via several receptors, including scavenger receptor B1 (SRB1) and low-density lipoprotein receptor. CEs are hydrolyzed to cholesterol by CE hydrolase (CEH). Excess cholesterol is effluxed from hepatocytes into the bile canaliculi via adenosine triphosphate–binding cassette transporters G5 and G8 (ABCG5/8). Cholesterol is also catabolized to form bile acids via CYP enzymes (e.g., CYP7A1), which are secreted into the bile canaliculi via receptors such as the bile salt export pump (Bsep). The net effect of HFD feeding in foz/foz mice (red arrows) on these pathways favors accumulation of free cholesterol in hepatocytes.
The results with cholesterol homeostasis genes correlated with changes in nuclear localization of key regulators of cholesterol and bile acid homeostasis (e.g., sterol regulatory element binding protein-2 and liver receptor homolog-1); however, the pattern of these changes was less clear. For example, although expression of CYP7A1 in foz/foz mice fed HFD was less than half that of WT mice fed chow, nuclear localization of liver receptor homolog-1 was not different between these groups. The authors then showed that insulin in vitro at similar levels found in foz/foz mice (up to 20 ng/mL)13 changed the expression of some of the cholesterol synthesis genes in a pattern similar to what was observed in vivo (e.g., sterol regulatory element binding protein-1, low-density lipoprotein receptor, and bile acid export protein). These in vitro results, although supportive of the concept that the authors promote, have some limitations. First, the authors did not study the effect of these insulin concentrations directly on cholesterol flux in these cells. Second, these results ignore the fact that plasma insulin levels are equally elevated in foz/foz mice fed chow,13 which would imply that they would expect similar expression changes with foz/foz mice on chow diet, which was not observed in their animal models (see Figs. 1–3 in van Rooyen DM et al.10).
The last series of experiments are to test the effect of titrating cholesterol into the standard HFD on liver damage in the foz/foz mice. The results demonstrate that foz/foz mice accumulate CE and FC and develop inflammatory liver damage even on HFD without cholesterol and that these variables increase as the percentage of dietary cholesterol rises. Interestingly, HFD-fed WT mice do not develop significant liver injury until dietary cholesterol levels are high enough to accumulate in the liver. However, it should be noted that there are key differences between WT and foz/foz mice that cannot be explained simply by differential abilities to accumulate cholesterol. For example, at even the highest concentration of cholesterol (2%), the authors observed few fibrotic changes in WT mice, which is in contrast to foz/foz mice that were fibrotic even in the absence of added dietary cholesterol (see Fig. 6 in van Rooyen DM et al.10).
Issues that remain after this study include whether the results observed here are specific to Alström syndrome, or are more generally applicable to fatty liver disease. First, it is unclear if the magnitude of increase in cholesterol observed in this study is relevant to human fatty liver disease. For example, hepatic CE levels do not differ between control, NAFLD and NASH in humans, but were >50-fold higher in foz/foz mice.11 Furthermore, hepatic FC levels are only 20% elevated in NASH versus NAFL, versus a much stronger increase in foz/foz mice.11 Second, the dyslipidemia observed in Alström patients is only one piece of a complex phenotype.12 Given that the mechanisms by which Alms1 mutations cause the phenotypes of Alström disease are incompletely understood at best, it is premature at this time to state that the results with the foz/foz mice are not unique to this mutation, but rather generally applicable to NAFLD. In their defense, the authors are careful to point this out and couch their results accordingly.
In conclusion, this work clearly supports the concept that cholesterol could, in principle, be a hepatotoxic culprit in NAFLD/NASH. Previous work by others has shown that dietary FC loading depletes hepatic mitochondrial glutathione and sensitizes the liver to cell killing via tumor necrosis factor-α or Fas signaling.15 Such a mechanism is in line with the observation in the current work that indices of apoptosis (cytokeratin-18) were dramatically higher in the foz/foz mice fed HFD with cholesterol (see Fig. 6A in van Rooyen DM et al.10). Whether these results translate generally to fatty liver diseases remains to be seen. However, even in the case that these results do not, the finding that foz/foz mice are relatively normal when restricted to a low-fat diet may at least be useful information for the management of the metabolic disorder of Alström; disease.
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Day CP. Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med. 2006;6:19–25. doi: 10.7861/clinmedicine.6-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40:S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 6.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 7.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 10.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, et al. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–1403. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 12.Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, et al. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- 13.Arsov T, Larter CZ, Nolan CJ, Petrovsky N, Goodnow CC, Teoh NC, et al. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006;342:1152–1159. doi: 10.1016/j.bbrc.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Larter CZ, Yeh MM, Van Rooyen DM, Teoh NC, Brooling J, Hou JY, et al. Roles of adipose restriction and metabolic factors in progression of steatosis to steatohepatitis in obese, diabetic mice. J Gastroenterol Hepatol. 2009;24:1658–1668. doi: 10.1111/j.1440-1746.2009.05996.x. [DOI] [PubMed] [Google Scholar]
- 15.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]