Abstract
Objective:
To investigate whether nasogastric tubes (NGTs) increase poststroke pneumonia (PSP), mortality, or poor outcomes in nil-by-mouth acute stroke patients.
Methods:
This study analyzed prespecified outcomes of PSP at 14 days and mortality and function measured by the modified Rankin Scale at 90 days in 1,217 nil-by-mouth stroke patients at ≤48 hours of symptom onset in a multicenter randomized controlled trial of preventive antibiotics between April 21, 2008, and May 17, 2014. Generalized mixed models adjusted for age, comorbidities, stroke type and severity, and quality of care were used. No patients were lost to follow-up at 14 days, and 36 (3%) were lost at 90 days.
Results:
Patients with NGT (298 of 1,217 [24.4%]) had more severe strokes (median NIH Stroke Scale score 17 vs 14, p = 0.0001) and impaired consciousness (39% vs 28%, p = 0.001). NGT did not increase PSP (43 of 298 [14.4%] vs 80 of 790 [10.1%], adjusted odds ratio [OR] 1.26 [95% confidence interval (CI) 0.78–2.03], p = 0.35) or 14- and 90-day mortality (33 of 298 [11.1%] vs 78 of 790 [9.9%], adjusted OR 1.10 [95% CI 0.67–1.78], p = 0.71; and 79 of 298 [26.5%] vs 152 of 790 [19.2%], adjusted OR 0.95 [95% CI 0.67–1.33], p = 0.75, respectively). Ninety-day modified Rankin Scale score distribution was comparable between groups (adjusted OR 1.14 [95% CI 0.87–1.56], p = 0.08). PSP independently increased 90-day mortality (40 of 123 [32.5%] vs 191 of 965 [19.8%], adjusted OR 1.71 [95% CI 1.11–2.65], p = 0.015) and was not prevented by antibiotics in patients with NGT (adjusted OR 1.1 [95% CI 0.89–1.54], p = 0.16).
Conclusions:
Early NGT does not increase PSP incidence, mortality, or poor functional outcomes and can be used safely in acute stroke patients.
Poststroke pneumonia (PSP) is associated with a third of early deaths and a fifth of poor outcomes in stroke.1,2 Dysphagia is present in 51% to 78% of stroke patients and is often managed with nasogastric tubes (NGTs) for nutrition and hydration.3 Dysphagia increases the risk of PSP from 2% to 8% to 16% to 19%,4,5 which may be increased further to 33% to 70% by NGT placement.6–8 Because supportive pharmacological or antimicrobial interventions do not appear to prevent PSP, it remains a major clinical challenge in nil-by-mouth patients, even on stroke units.9–11
Evidence that NGT placement increases PSP incidence remains equivocal; observational studies suggesting this association are limited by heterogeneity in patients, settings, and diagnostic criteria for PSP.6–8 It is possible that PSP and higher mortality are influenced by stroke severity rather than by NGT placement.12,13 Although preventive antibiotics did not reduce PSP in unselected stroke patients,10,11 they may be effective in patients with NGT who have greater gastric reflux and aspiration caused by NGT-induced pharyngeal and lower esophageal sphincter dysfunction.14
The Prophylactic antibiotics for reducing pneumonia in acute stroke patients with dysphagia study (STROKE-INF) was a multicenter, cluster-randomized controlled trial undertaken in 1,217 stroke patients with dysphagia (nil by mouth) recruited from 37 UK stroke units.11 Data from the STROKE-INF trial have been used to test the a priori hypotheses that in nil-by-mouth stroke patients, NGT placement within 48 hours of acute stroke increases PSP incidence and mortality, PSP predicts higher mortality and poor functional outcome independently of stroke severity and treatment, and preventive antibiotics can reduce PSP and 14-day mortality in patients with NGT.
METHODS
Patients.
Patients were recruited from 37 accredited stroke units in the United Kingdom with the use of methods described previously.11 Patients were eligible for inclusion if they were >18 years of age, had a confirmed diagnosis of new stroke (ischemic or hemorrhagic), had an onset of symptoms of ≤48 hours at recruitment, and were unsafe to swallow because of impaired consciousness or failed bedside swallowing assessment. This assessment consisted of a standardized clinical assessment of level of consciousness, oromotor function, and a water swallowing test performed by dysphagia-trained nursing staff.15 Patients with preexisting dysphagia, pyrexia, or known infection at admission; who had taken antibiotics within the last 7 days; or who had expected survival of <14 days were excluded.
Procedures.
All patients received guideline-recommended care for nil-by-mouth patients,3 but the placement of an NGT for fluids or feeding was a local decision. In addition, 615 patients in 18 intervention clusters received preventive amoxicillin or co-amoxiclav with clarithromycin for 7 days, initiated within 48 hours of symptom onset. In accordance with the antibiotic stewardship policies of individual hospitals and UK National Health Service regulations, physicians followed local guidelines for the investigation and treatment of suspected PSP based on local experience of pathogens and antibiotic susceptibility. Patients on preventive antibiotics were given second-line antibiotics as per local antibiotic protocols if PSP was diagnosed. Patient characteristics and stroke severity assessed with the NIH Stroke Scale (NIHSS) with a score ranging from 0 (no deficit) to 42 (severe deficit) were recorded on enrollment. Patients were monitored daily, and respiratory rate, temperature, chest symptoms and signs, white cell counts, and C-reactive protein were recorded at baseline and at 2, 4, 7, 10, and 14 days by research staff not involved in patient care.
Outcome measures.
The primary outcome was PSP in the first 14 days. The diagnosis of PSP was based on the blinded application of predefined criteria derived from the Centers for Disease Control and Prevention criteria for pneumonia16 that interrogated 8 clinical/laboratory observations at the 6 recorded time points in the whole patient group for temperature ≥37.5°C on 2 consecutive measurements or a single measurement of ≥38.0°C; respiratory rate of ≥20 breaths per minute, cough and breathlessness, purulent sputum; and white cell count >11.0 × 109/L, chest infiltrates on X-ray, positive sputum culture/microbiology, or positive blood culture.
Details on the calculation have been provided previously.11 The number of cases of physician-diagnosed PSP in the first 14 days was also recorded. Secondary clinical endpoints included mortality at 14 and 90 days and functional status at 90 days, assessed by trial office researchers masked to baseline characteristics, NGT status, and antibiotic treatment. Functional outcome at 90 days was defined by the modified Rankin Scale (mRS) score, which ranges from 0 (no symptoms) to 6 (death). The length of hospital stay was also recorded. No patients were lost to follow-up at 14 days; follow-up data on mRS score at 90 days were missing in 36 patients (3%).
Analysis.
The analysis was done on an intention-to-treat basis with all 1,217 randomized patients included. Analysis was hypothesis driven, assuming the data structure of a prospective cohort study. Baseline characteristics and outcomes were summarized by treatment group and compared by use of simple between-group comparisons of proportions, means, or medians as appropriate for the data. The relationships between NGT and algorithm-defined or physician-diagnosed PSP were assessed with the same analysis strategy used by Kalra et al.11 A generalized mixed model with PSP as outcome was used, and a fixed contrast for effect of NGT was included to determine the mean difference between the NGT and the non-NGT groups. Other patient-level covariates included age, sex, baseline NIHSS score, premorbid mRS score, stroke type, previous strokes, thrombolysis, chronic lung disease, and smoking. Variations in center performance were adjusted for by the use of patient throughput and quality ranking of the center17 as additional covariates. For the other outcomes in the analysis, i.e., death at 14 days, death at 90 days, binary mRS score, and time to hospital discharge and death, we adjusted for PSP diagnosis because this may confound the influence of NGT on outcome. Separate models were run for algorithm and physician PSP diagnoses because it is unclear whether it is appropriate to adjust for both types of diagnoses in the same model. Adjusted predictive probability of outcomes in patients with and without NGT was calculated as average marginal effects. The distribution of mRS scores between patients with and without NGT was compared using ordinal regression and checked by fitting a marginal model. To account for missing outcome data, models were fitted with the use of multiple imputation by chained equations under the assumption of missingness at random. Twenty-five imputations were generated for each outcome model. Sensitivity analysis assuming different outcomes was performed to assess the effect of algorithm PSP diagnosis not being possible in 129 patients (10%). Analyses were performed with R version 3.0 and Stata software (release 14.1, 2016; StataCorp, College Station, TX).
Standard protocol approvals, registrations, and patient consents.
Written informed consent/assent was provided by patients or the next of kin. The study was registered with International Standard Registered Clinical/Social Study Number registry (37118456) and the European Union Drug Regulating Authorities Clinical Trials Registry (2007-004298-24). Ethics approval was granted by the UK National Research Ethics Committee (08/H0803/1).
RESULTS
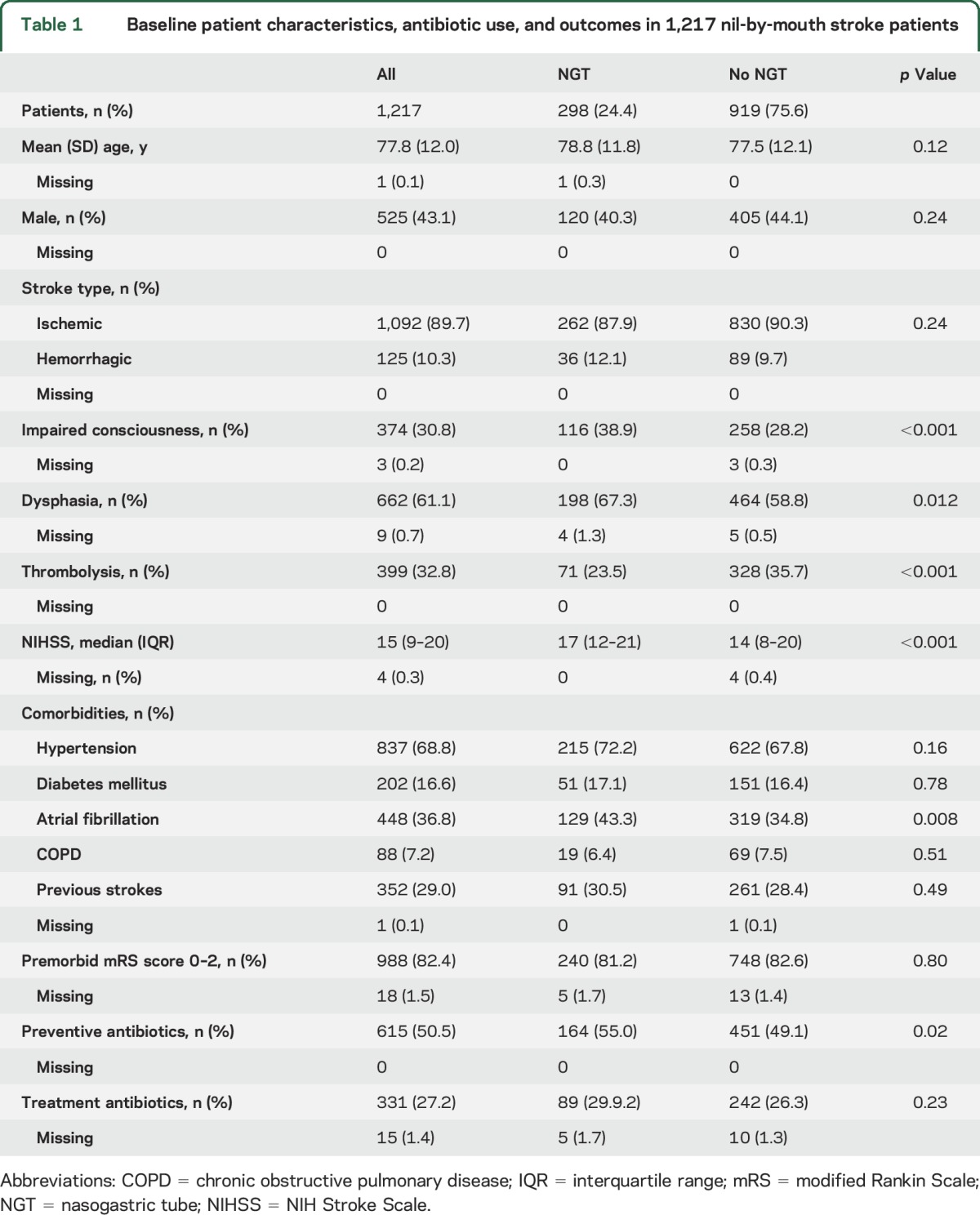
Of the 1,217 stroke patients included in the study, 298 patients (24.4%) had an NGT inserted within 48 hours of onset. There were no significant differences in age, sex, or stroke type between patients with and those without NGT insertion, but patients with NGT had more severe strokes (median NIHSS score 17 vs 14, p = 0.0001) with impaired consciousness, dysphasia, and less frequent use of thrombolysis (table 1). Atrial fibrillation was more common in patients with NGT, but there were no differences in prestroke functional status or the use of antibiotics for either the prevention or treatment of PSP between groups. Antibiotic use was high in both groups, with a third of patients receiving treatment antibiotics (table 1).
Table 1.
Baseline patient characteristics, antibiotic use, and outcomes in 1,217 nil-by-mouth stroke patients
An objective algorithm–based diagnosis of PSP or no PSP could not be established because of missing data in 129 patients (10.6%). PSP, defined by the objective Centers for Disease Control and Prevention–based algorithm and assessed by researchers masked to baseline characteristics and patient condition, was identified in 123 of the remaining 1,088 patients (11.3%) and was more frequent in patients with NGT (14.4% vs 10.1%, p = 0.046) compared with those without NGT (table 2). PSP was diagnosed by physicians in 192 of 1,217 patients (15.7%), with no differences between patients with and those without NGT (18.5% vs 15.3%, p = 0.21). The higher incidence of algorithm-defined PSP in patients with NGT did not remain significant after adjustment for age, stroke type, severity, and chronic lung disease (adjusted odds ratio [OR] 1.26 [95% confidence interval [CI] 0.78–2.03], p = 0.35; table 3). Sensitivity analyses using different outcome assumptions for the 129 of the 1,217 patients (10.6%) in STROKE-INF in whom algorithm diagnosis was not possible did not show results different from the results for 1,088 patients in whom algorithm diagnosis was possible. Preventive antibiotics did not reduce the incidence of PSP in patients with NGT (adjusted OR 1.05 [95% CI 0.73–1.52], p = 0.803), and NGT placement did not influence antibiotic treatment for PSP (OR for interaction 1.33 [95% CI 0.51–3.48], p = 0.56).
Table 2.
Unadjusted comparisons of outcomes between nil-by-mouth stroke patients stratified by nasogastric tube (NGT) use and poststroke pneumonia (PSP) incidence
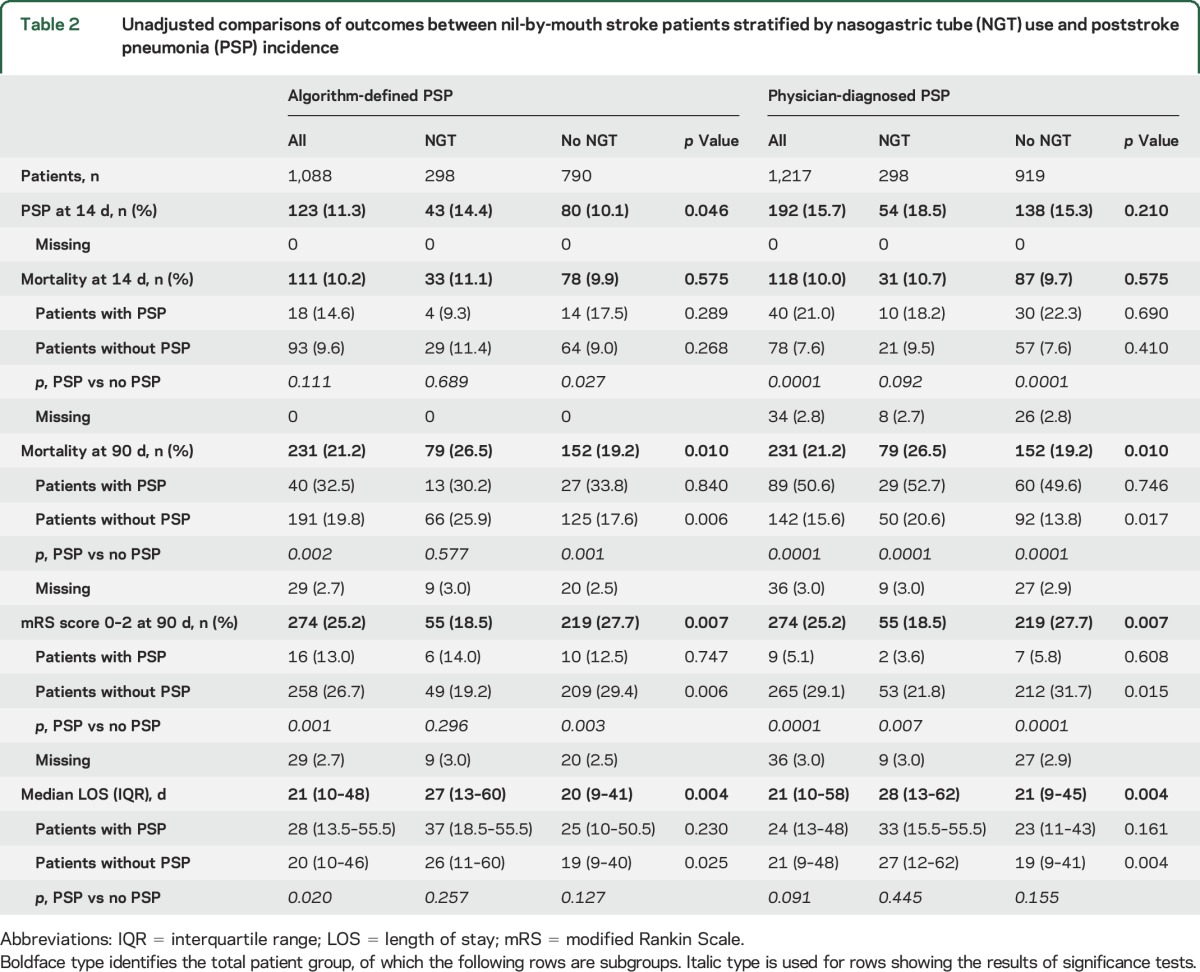
Table 3.
Contribution of nasogastric tube (NGT) insertion and poststroke pneumonia (PSP) to outcomes adjusted for age, stroke severity, comorbidities, and stroke unit care

There were no differences in early (14-day) mortality between patients with and those without NGT (table 2). Patients with NGT who developed PSP did not have a higher mortality than those who did not develop PSP. In contrast, mortality was significantly higher in patients without NGT with PSP compared with those without PSP (table 2). Neither NGT placement (adjusted OR 1.10 [95% CI 0.67–1.78], p = 0.71) nor algorithm-defined PSP (adjusted OR 1.37 [95% CI 0.77–2.44], p = 0.29) predicted early mortality (table 3). In contrast, physician-diagnosed PSP had an independent association with early mortality (adjusted OR 2.41 [95% CI 1.53–3.80], p < 0.001).
At 90 days, mortality was higher and functional outcomes were poorer in patients with NGT (table 2), but the differences compared with patients without NGT did not remain significant after adjustment for age and other patient characteristics, stroke severity, PSP incidence, and quality of stroke care (table 3). There was also no difference in the distribution of mRS score categories at 90 days between patients with NGT and those without NGT (adjusted OR 1.14 [95% CI 0.87–1.56], p = 0.081; figure). Predicted probability for various outcomes for patients with or without NGT and with or without PSP (table 4) showed no differences in any outcome that could be attributed to NGT placement. Algorithm-defined PSP and physician-diagnosed PSP were independent determinants of 90-day mortality, but only physician-diagnosed PSP was associated with poor functional outcomes (table 3). The median length of hospital stay was significantly longer for patients with NGT (table 2). Patients with NGT had longer time to death (adjusted hazard ratio 3.84 [95% CI 2.76–5.94], p = 0.038) and longer time to discharge (adjusted hazard ratio 2.34 [95% CI 2.05–2.73], p = 0.05), even after adjustment for baseline differences, PSP incidence, and quality of care.
Figure. Distribution of modified Rankin Scale (mRS) scores at 90 days in nil-by-mouth stroke patients managed with nasogastric tubes (NGTs) and without NGTs during acute admission.
Unadjusted odds ratio (OR) 1.19 (95% confidence interval [CI] 1.01–1.79), p = 0.022. Adjusted OR 1.14 (95% CI 0.87–1.56), p = 0.081 (adjusted for age, sex, baseline NIH Stroke Scale score, premorbid modified Rankin Scale [mRS] score, stroke type, previous strokes, thrombolysis, stroke unit care). mRS 0 = no symptoms; mRS 1 = no disability despite symptoms; mRS 2 = slight disability, no assistance required; mRS 3 = moderate disability, requiring some help; mRS 4 = moderately severe disability, unable to walk or attend to personal needs without assistance; mRS 5 = severe disability, bedridden, incontinent, and requiring constant nursing care; and mRS 6 = dead.
Table 4.
Predicted probability for outcomes for patients with or without nasogastric tubes (NGTs) and with or without diagnosis of poststroke pneumonia (PSP)

DISCUSSION
This study showed that the incidence of PSP, mortality, and poor functional outcomes are not increased by NGT placement within 48 hours of presentation. Although PSP was an independent predictor of 90-day mortality in nil-by-mouth stroke patients, its incidence was not reduced by preventive antibiotics in patients with NGT. NGT use was associated with a 2- to 3-fold prolongation in hospital stay in both those who died and those who were discharged.
PSP incidence in patients with NGT (14.4% algorithm defined, 16.2% physician diagnosis) was considerably lower than the 33% to 70% incidence reported previously, and the risk of developing PSP was the same rather than increased 3- to 4-fold compared with patients without NGT as described in observation studies.5,8 Previous observational studies have included all stroke patients, did not adjust for stroke severity and other baseline characteristics, and compared patients with NGT, who were likely to have swallowing difficulties, impaired consciousness, advanced age, or greater stroke severity, with patients without NGT who lacked these characteristics. In contrast, this study was limited to those nil by mouth and balanced risk factors for PSP between the groups with NGT and without NGT. The low incidence of PSP may also be explained by the routine use of preventive measures such as positioning, regular suction, swallowing techniques, and modified diet in nil-by-mouth patients on specialist stroke units.18 The rate of PSP diagnosis is dependent on the diagnostic criteria used.19 Physicians are influenced by stroke severity and poor expectation of outcome in diagnosing pneumonia20; it is likely that the presence of NGT further increases this clinical bias. This study applied an objective, criteria-based algorithm blindly to the whole dataset to diagnose PSP, which not only minimized the likelihood of false-positive diagnosis but also reduced bias from PSP diagnoses that may have been missed clinically.
PSP incidence rather than NGT placement is associated with higher mortality and poor outcomes at 90 days; the differences between patients with and those without NGT disappear when corrected for age, stroke type, and stroke severity. Fourteen-day mortality was not influenced by PSP incidence in patients with NGT (table 2), confirming that early mortality is more likely to result from neurologic complications such as hemorrhage and cerebral edema rather than infection. The significantly higher 14-day mortality in patients with PSP who did not have NGT is most likely to be explained by end-of-life care decisions in terminal stroke patients. The association between PSP and 90-day outcomes depended on the diagnostic criteria used (table 3). Algorithm-diagnosed PSP had an independent association only with mortality at 90 days. In contrast, physician-diagnosed PSP independently correlated with increased 14- and 90-day mortality and with poor functional outcomes at 90 days. This difference may be attributable to the influence of conservative care/end-of-life decisions in older patients and patients with more severe stroke on physician diagnosis of PSP, even in the absence of confirmatory investigations.21 This is supported by research showing that twice as many patients receive antibiotics as diagnosed with infections.10,11 The effects of such physician decisions on research are unknown, and a structured approach to PSP diagnosis, as used in this study, may be useful for reducing bias in future studies.22
Preventive antibiotics did not reduce PSP incidence or early mortality in patients with NGT. Explanations include a lower PSP incidence in patients with NGT than previously reported and the finding that NGT placement, in itself, did not increase the risk of PSP. It is also possible that preventive antibiotics do not add to existing measures to prevent aspiration on specialist stroke units.18 Antibiotic prophylaxis is based on the assumption of an infective pathogenesis for PSP; it has been suggested that aspiration of gastric/esophageal contents may result in pneumonitis of complex bacterial, chemical, and immunologic origins,23 for which novel strategies need to be investigated.24
A limitation of the study was that the data were derived from a randomized controlled trial and a prospective cohort data structure was assumed. The inclusion criteria of the randomized controlled trial may have resulted in a selection bias. Patients with NGT were older; had more severe strokes, altered consciousness levels, and dysphasia; and were less likely to have been thrombolyzed. Swallowing was assessed with the bedside water swallowing test rather than instrumental techniques. Because these factors can influence NGT placement6 and confound outcomes, these factors and the quality of stroke unit care were included as additional explanatory variables in regression models of the prespecified outcomes. Bias from missing data was reduced by the use of multiple imputation assuming missingness at random. Sensitivity analyses with different assumptions of outcomes for patients in whom algorithm PSP diagnosis was not possible did not have a significant effect, suggesting that inclusion of these patients would not have changed outcomes. The diagnostic criteria used for PSP are another source of potential bias. Although the algorithm-based diagnosis minimized detection bias, STROKE-INF allowed the use of local guidelines for investigations of suspected PSP, and not all patients had radiographic or microbiological confirmation of PSP diagnosis.11 However, because this was true for both groups, any bias arising from this source will be balanced. Information was not collected on the duration between stroke and NGT placement, number of tubes used, or total duration of NGT placement. The algorithm diagnosis of PSP was based on a blinded review of prespecified criteria being fulfilled over 14 days after stroke, and the exact time of PSP onset could not be determined. There is a possibility that these factors may have increased or expedited PSP incidence or worsened stroke outcomes. The increased length of stay in patients with NGT who did not develop PSP remains unexplained. It is possible that this was associated with delays in starting nutrition, rehabilitation, or decision making on future care, but these data were not collected.
NGT placement does not increase PSP incidence, early mortality, mortality, and poor functional outcomes at 90 days independently of age, stroke type, and stroke severity in stroke patients unable to swallow. The relationship between PSP, increased mortality, and poor functional outcomes is ambiguous and depends on the criteria used for PSP diagnosis. PSP probably increased 90-day mortality but may have had no effect on functional outcome in survivors. The study suggests that NGT can be used early for hydration and nutritional support in nil-by-mouth stroke patients. It also supports the need for consensus on well-defined diagnostic criteria for PSP for future research.
Supplementary Material
GLOSSARY
- CI
confidence interval
- mRS
modified Rankin Scale
- NGT
nasogastric tube
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PSP
poststroke pneumonia
- STROKE-INF
Prophylactic antibiotics for reducing pneumonia in acute stroke patients with dysphagia study
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
L.K., D.S., and D.M. contributed to generating the hypothesis for post hoc analyses, study design, data collation and interpretation, and drafting of the paper. S.I. contributed to trial coordination, data acquisition and interpretation, and drafting of the paper. J.H. contributed to the analysis plan before collection of data, data analysis and interpretation, and drafting of the report. The corresponding author (L.K.) and the statistician (J.H.) have access to all the data and vouch for the completeness and accuracy of the analysis. L.K. had the final responsibility for the decision to submit for publication.
STUDY FUNDING
The research was funded by the National Institute for Health Research (project No. PB-PG-0906-11103). The funders had no role in the study design; collection, analysis, or interpretation of the data; or writing of the report. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, National Institute for Health Research, or Department of Health.
DISCLOSURE
L. Kalra reports no disclosures relevant to the manuscript. S. Irshad was supported by an NIHR grant for the study. J. Hodsoll is supported by the NIHR Maudsley Biomedical Research Centre. D. Smithard and D. Manawadu report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koennecke W, Belz W, Berfelde D, et al. . Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurology 2011;77:965–972. [DOI] [PubMed] [Google Scholar]
- 3.Intercollegiate Stroke Working Party. National Clinical Guidelines for Stroke. 4th ed. London, UK: Clinical Effectiveness & Evaluation Unit, Royal College of Physicians; 2012. [Google Scholar]
- 4.Martino R, Martin RE, Black S. Dysphagia after stroke and its management. CMAJ 2012;184:1127–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martino R, Foley N, Bhogal S. Dysphagia after stroke: incidence, diagnosis and pulmonary complications. Stroke 2005;36:2756–2763. [DOI] [PubMed] [Google Scholar]
- 6.Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Respiratory infections in acute stroke: nasogastric tubes and immobility are stronger predictors than dysphagia. Dysphagia 2014;29:340–345. [DOI] [PubMed] [Google Scholar]
- 7.Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Dysphagia and factors associated with respiratory infections in the first week post stroke. Neuroepidemiology 2014;43:140–144. [DOI] [PubMed] [Google Scholar]
- 8.Langdon PC, Lee AH, Binns CW. High incidence of respiratory infections in “nil by mouth” tube-fed acute ischemic stroke patients. Neuroepidemiology 2009;32:107–113. [DOI] [PubMed] [Google Scholar]
- 9.Geeganage C, Beavan J, Ellender S, Bath PMW. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev 2012:CD000323. [DOI] [PubMed] [Google Scholar]
- 10.Westendorp WF, Vermeij JD, Zock E, et al. . for the PASS Investigators. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015;385:1519–1526. [DOI] [PubMed] [Google Scholar]
- 11.Kalra L, Irshad S, Hodsoll J, et al. . Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 2015;386:1835–1844. [DOI] [PubMed] [Google Scholar]
- 12.Vargas M, Horcajada JP, Obach V, et al. . Clinical consequences of infection in patients with acute stroke: is it prime time for further antibiotic trials? Stroke 2006;37:461–465. [DOI] [PubMed] [Google Scholar]
- 13.Meisel A. Preventive antibiotic therapy in stroke: PASSed away? Lancet 2015;385:1486–1487. [DOI] [PubMed] [Google Scholar]
- 14.Satou Y, Oguro H, Murakami Y, et al. . Gastroesophageal reflux during enteral feeding in stroke patients: a 24-hour esophageal pH-monitoring study. J Stroke Cerebrovasc Dis 2013;22:185–189. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey DJ, Smithard DG, Kalra L. Early assessments of dysphagia and aspiration risk in acute stroke patients. Stroke 2003;34:1252–1257. [DOI] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 17.Royal College of Physicians. Sentinel Stroke National Audit Programme (SSNAP). Available at: https://www.strokeaudit.org/results/Organisational/National-Organisational.aspx. Accessed May 27, 2015.
- 18.Titsworth WL, Abram J, Fullerton A, et al. . Prospective quality initiative to maximize dysphagia screening reduces hospital-acquired pneumonia prevalence in patients with stroke. Stroke 2013;44:3154–3160. [DOI] [PubMed] [Google Scholar]
- 19.Kishore AK, Vail A, Chamorro A, et al. . How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke 2015;46:1202–1209. [DOI] [PubMed] [Google Scholar]
- 20.Harms H, Hoffmann S, Malzahn U, et al. . Decision-making in the diagnosis and treatment of stroke-associated pneumonia. J Neurol Neurosurg Psychiatry 2012;83:1225–1230. [DOI] [PubMed] [Google Scholar]
- 21.Meisel A, Smith CJ. Prevention of stroke-associated pneumonia: where next? Lancet 2015;386:1802–1804. [DOI] [PubMed] [Google Scholar]
- 22.Smith CJ, Kishore AK, Vail A, et al. . Diagnosis of stroke-associated pneumonia: recommendations from the Pneumonia in Stroke Consensus Group. Stroke 2015;46:2335–2340. [DOI] [PubMed] [Google Scholar]
- 23.Marik PE. Aspiration pneumonitis and pneumonia. N Engl J Med 2001;344:665–671. [DOI] [PubMed] [Google Scholar]
- 24.Warusevitane A, Karunatilake D, Sim J, Lally F, Roffe C. Safety and effect of metoclopramide to prevent pneumonia in patients with stroke fed via nasogastric tubes trial. Stroke 2015;46:454–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



