Abstract
Objectives
To test the safety of the CDK4/6 inhibitor palbociclib with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma(HNSCC).
Materials and Methods
A phase I trial using 3+3 design was performed to determine the dose limiting toxicity(DLT) and maximum tolerated dose(MTD) of palbociclib with standard dose weekly cetuximab. Palbociclib was administered orally days 1-21 every 28 days:dose level 1(100 mg/d) and 2(125 mg/d;approved monotherapy dose). Pharmacokinetic assessments were performed on cycle 2, day 15. Cyclin D1,p16INK4a,and Rb protein expression were measured on pre-treatment tumor. Tumor response was assessed using RECIST1.1.
Results
Nine patients(five p16INK4a negative;four positive) were enrolled across dose levels 1(n=3) and 2(n=6) and none experienced a DLT. A MTD of palbociclib was not reached. Myelosuppression was the most common adverse event. Six of nine patients had cetuximab-resistant and 4/9 had platin-resistant disease. Disease control(DC) occurred in 89%,including partial response(PR) in two(22%) and stable disease in six(67%) patients. PRs occurred in p16INK4a negative HNSCC. Five patients(56%) had measurable decreases in tumor target lesions. In cetuximab-resistant HNSCC, best tumor response was PR in 1 and DC in 5 and median TTP was 112 days(range:28-168). In platin-resistant HNSCC, best tumor response:PR in 1, DC in 3 and median TTP was 112 days(range:28-112). The Cmax and AUC0-24h appeared comparable in patients receiving 125vs100 mg dose of palbociclib.
Conclusion
This trial, the first to evaluate a CDK4/6 inhibitor in HNSCC, determined that palbociclib 125 mg/day on days 1-21 every 28 days with cetuximab was safe. Tumor responses were observed, even in cetuximab- or platin-resistant disease.
Keywords: Phase I, Palbociclib, Cetuximab, Head and Neck Cancer, Squamous Cell Carcinoma
INTRODUCTION
Activation of the epidermal growth factor receptor (EGFR) is the most common event in head and neck squamous cell carcinoma (HNSCC), resulting in cellular proliferation, angiogenesis, and radiation resistance[1]. The importance of EGFR signaling was demonstrated by trials that showed improvement in overall survival (OS) when the EGFR inhibitor cetuximab was added to radiation or chemotherapy[2,3]. However, the clinical benefit of cetuximab monotherapy is surprisingly modest, with a time to progression (TTP) of 70 days in platin-resistant recurrent/metastatic (RM) disease[4].
HNSCC is a heterogeneous disease[5,6]. Based on unique gene expression signatures, at least four subgroups have been defined; each with distinct signaling pathways[5-12]. Despite this heterogeneity, aberrant cell cycle regulation is a ubiquitous event. The mechanism underlying unrestrained proliferation varies depending on the tumor’s transcriptionally-active human papillomavirus (HPV) status. In HPV-related HNSCC, E7 viral protein drives unrestrained proliferation by promoting Rb degradation[13]. In HPV-unrelated HNSCC, Rb inactivation occurs through hyperactivation of the Rb inhibitory complex CDK4/cyclin D. CCND1 (encoding cyclin D1, the regulatory subunit of the complex) is amplified and/or the CDK4/6 inhibitor p16INK4a is inactivated in nearly all of these cancers[14-16]. Alterations of CCND1 and p16INK4a are rare in HPV-related HNSCC. As a result, p16INK4a is overexpressed in HPV-related HNSCC and underexpressed in HPV-unrelated HNSCC.
The genetics of HPV-unrelated HNSCC influences the clinical course. CCND1 amplification and p16 INK4a inactivation are poor prognostic factors in HNSCC[15,17], in part because cyclin D1 overexpression adversely affects tumor response to EGFR inhibitors and cisplatin. In HNSCC cell lines, cyclin D1 amplification and/or overexpression correlated with resistance to these drugs[18-20]. Studies in HNSCC reveal that cyclin D1 overexpression is predictive of resistance to cisplatin[21].
The essential roles of CDK4/6 and cyclin D1 in driving cell cycle progression from G1 into S phase motivated intense investigation into blocking this regulatory complex[22-24]. In pre-clinical models, CDK4/6 inhibition inhibits both Rb hyperphosphorylation and cell cycle progression[25], and the efficacy of inhibition in some models correlated with increased cyclin D1 and decreased p16INK4a expression[24]. Palbociclib is the first approved selective inhibitor of the CDK4/6 kinases. Palbociclib exerts potent anti-proliferative effects on Rb-positive cell lines and human breast and colon xenografts[23]. Palbociclib results in decreased Rb phosphorylation and Ki-67 expression in Rb-positive models but has no activity in Rb-negative tumor xenografts[23]. As such, phase I trials restricted the evaluation of palbociclib to patients with Rb-positive cancers[24,25]. These studies determined that the dose-limiting toxicity (DLT) of palbociclib was neutropenia and the maximum tolerated dose (MTD) was 125 mg once daily, administered for 21 days of each 28 day cycle[26,27]. The efficacy of palbociclib was demonstrated in estrogen receptor positive breast cancer[28] and in mantle cell lymphoma[29], tumors in which cyclin D contributes to their pathogenesis.
Because the genetics of HNSCC suggest a crucial role for CDK4/cyclin D in this disease, we performed a phase I trial to determine the DLT and MTD of palbociclib combined with standard weekly doses of cetuximab in patients with RM HNSCC. We built upon the cetuximab platform because palbociclib targets a pathway associated with resistance to EGFR inhibitors[18]. We report the results of the trial along with pharmacokinetic (PK) and biomarker studies and efficacy assessments of this novel combination of targeted agents.
MATERIALS AND METHODS
Patient Inclusion and Exclusion Criteria
Eligibility required RM HNSCC, defined as distant metastases or unresectable, previously irradiated local/regional recurrence. Prior cetuximab or platin for RM disease was allowed. Prior cytotoxic chemotherapy for RM disease was not required because some patients are poor candidates for or decline such therapy. Patients were ≥ 18 years, had Eastern Cooperative Oncology Group performance status of ≤ 2, adequate marrow/organ function (absolute neutrophil count ≥ 1500/mm3, platelet count ≥ 100,000/mm3, creatinine and bilirubin < 1.5x upper limits of normal [ULN], and aspartate transaminase, alanine transaminase, and alkaline phosphatase < 2.5x ULN), and QTc < 480 msec. Patient selection based on p16INK4a, cyclinD1, or Rb status was not performed because this was a phase I trial. Exclusion criteria included inability to swallow and concurrent use of CYP3A4 inhibitors/inducers.
The protocol was approved by the Institutional Review Board. All patients provided signed consent to participate (NCI-2014-01079).
Study Design
Palbociclib was administered orally with food on days 1-21 of each 28 day cycle. Palbociclib doses were level 1 (100 mg/d), level 2 (125 mg/d; the approved monotherapy dose), and level -1 (75 mg/d, if de-escalation needed). Intra-patient dose escalation was not permitted. Cetuximab 400 mg/m2 was given intravenously on cycle 1 day 1, then 250 mg/m2 weekly.
A 3+3 design was employed. Three patients were enrolled per dose level, expanded to 6 if 1 of 3 developed a DLT. If 0 of 3 patients within a dose level developed a DLT, the dose was escalated; if ≥ 2 of 6 within a dose level developed a DLT, the dose below was considered the MTD. DLT and MTD were assessed during cycle one. If a MTD was not established at the maximum dose level, the latter was the recommended phase II dose of palbociclib. Six patients were enrolled at the recommended phase II palbociclib dose.
Hematologic DLT was defined as: grade 4 neutropenia, grade 4 infection with grade 3/4 neutropenia or grade 4 thrombocytopenia with bleeding. Non-hematologic DLT was defined as palbociclib-related grade 3/4 toxicity except: sub-optimally treated nausea/vomiting/diarrhea or grade 3 metabolic abnormalities.
Criteria to initiate subsequent cycles included an absolute neutrophil count (ANC) ≥1 000/mcL, platelets ≥ 50 000/mcL, and non-hematologic toxicities ≤ grade 1. If not met, palbociclib was delayed one week; however, cetuximab was continued. After a 2 week delay, palbociclib was discontinued.
Dose Modifications
Adverse events (AE) were monitored weekly during cycle 1, and then monthly. AEs were graded using NCI-CTCAE version 4.0.
Palbociclib dose was adjusted for selected AEs. A dose decrease by 25 mg/d was recommended for: grade 4 neutropenia/thrombocytopenia, grade 3 neutropenia with infection/fever, grade ≥ 3 non-hematologic toxicity, or treatment delay > 1 week due to persisting AE if recovery occurs within 2 weeks. Patients who required more than two dose reductions were treated with cetuximab alone. The lowest dose permitted was 75 mg/d. Doses omitted for AEs were not replaced within the same cycle.
Tumor Response Assessments
Tumor response assessments were performed every two cycles with neck/chest CT scans using RECIST criteria1.1. Treatment was continued until: disease progression, death, severe AE, or patient withdrawal.
Best overall tumor response was recorded from the start of treatment until disease progression. Disease control was defined as complete response (CR), partial response (PR), stable disease. Efficacy was measured by overall tumor response rate (CR+PR) and time-to-progression (TTP) measured from start of initiation of treatment until progression. OS was defined as the time from start of treatment to death.
Biomarker Assessment Performed on Archived Tumor Tissue
Archival tumor tissue obtained at diagnosis (n= 4) or recurrence (n= 5) were used to assess p16INK4a, cyclin D1, and p-Rb expression in tumor, determined by immunohistochemistry (IHC)[15,30,31]. Anti-cyclin D1 antibody (clone SP4-R) and anti-p16 antibody (clone E6H4) were obtained from Ventana Inc. The Rb antibody (clone G3-254) was purchased from Becton-Dickinson Biosciences. Tumors with no staining were scored as p16INK4a negative and tumors with strong and diffuse p16INK4a staining were scored as positive since HPV-related oropharyngeal (OP) SCC are nearly always strongly/diffusely positive whereas HPV-unrelated HNSCC are nearly always negative. However, focal staining for p16 was scored as negative. Optimal methodology and validated thresholds for a positive or negative result for cyclin D1 and Rb expression as related to palbociclib effect have not been defined. Therefore, any staining was defined as positive and no staining as negative.
Pharmacokinetic Analysis for Palbociclib
Blood samples were collected to obtain PK variables for palbociclib on cycle 2 day 15, when steady state levels were expected [26]. Whole blood samples (2 ml) were collected into an EDTA tube before dosing and at 1, 2, 4, and 8 hours after dosing. Plasma samples were used to determine the concentrations of palbociclib using a validated proprietory assay based on liquid chromatography-tandem mass spectrometry with a dynamic range from 1 to 250 ng/mL (AB Sciex API4000™).
PK parameters (area under the plasma concentration-time curve from 0 to 24 hours after dosing, AUC0-24h using the drug concentration obtained before dosing as a steady-state concentration; maximum plasma concentration, Cmax; time at which Cmax occurred, Tmax) were obtained using non-compartmental analyses (WinNonLin version 5.0.1, Pharsight, Mountain View, CA).
Objectives and Statistical Analyses
The primary objective was to determine the MTD of palbociclib combined with standard doses of cetuximab. Secondary objectives were to assess the AEs of palbociclib with cetuximab and to assess for an efficacy signal by reporting tumor response and disease control rates, and TTP. We explored the relationship between platin- or cetuximab-resistance, expression of p16INK4a, cyclin D1, and Rb status, and efficacy endpoints. Cetuximab-resistant or platin-resistant disease was defined as RM HNSCC that progressed (RECIST criteria1.1) on either agent.
RESULTS
Patient and Tumor Characteristics
Nine patients enrolled on this trial. Most patients had a smoking history, distant metastases, and received ≥ 1 prior therapies for RM disease (Table 1). Six of the nine patients had cetuximab-resistant disease and four of the nine had platin-resistant disease.
Table 1.
Patient, Tumor, and Prior Treatment Characteristics
| Characteristic | Palbociclib + Cetuximab (n=9) |
|---|---|
| Age (years) Mean | 60 |
| Range | 37-78 |
| Sex | |
| M | 7 |
| F | 2 |
| ECOG PSa | |
| 0 | 5 |
| 1 | 4 |
| Smoking History | |
| Yes | 6 |
| No | 3 |
| Primary Site | |
| Oropharynx | 4 |
| Oral Cavity | 3 |
| Hypopharynx | 1 |
| Nasopharynx | 1 |
| p16 | |
| Negative | 5 |
| Positiveb | 4 |
| Site of Recurrence | |
| Local/Regional | 1 |
| Distant | 7 |
| Both | 1 |
| Prior Therapy for RM Disease | |
| Platin | 4 |
| Cetuximab | 6 |
| None | 3 |
| # Lines of Prior Therapy for RM HNSCC | |
| ≥ 2 | 3 |
| 1 | 3 |
| 0 | 3 |
Eastern Cooperative Oncology Group Performance Status
3 OPSCC and 1 Oral Cavity.
DLT, MTD, and Adverse Events
Three patients enrolled on dose level 1 (100 mg/day) of palbociclib and six patients enrolled on dose level 2 (125 mg/day), none experienced a DLT. An MTD of palbociclib was not reached.
Table 2 shows the most frequent AEs that occurred in each dose level of palbociclib. Myelosuppression was the most common AE. Other potential AEs were infrequent and mild. The AEs attributed to palbociclib were different than those attributed to cetuximab, and the non-overlapping AEs of the combination were well tolerated.
Table 2.
Selected Adverse Events Across All Cyclesa
| Adverse Event | Palbociclib 100 mg (n= 3 pts) | Palbociclib 125 mg (n=6 pts) | ||
|---|---|---|---|---|
| Grades 1-2 | Grade 3 | Grades 1-2 | Grade 3 | |
| Hematologic: | ||||
| Neutropenia | 1 | 1 | 2 | 2 |
| Anemia | 2 | 1 | 6 | 0 |
| Thrombocytopenia | 1 | 1 | 3 | 0 |
| Non-Hematologic: | ||||
| Fatigue | 2 | 0 | 3 | 0 |
| Nausea | 0 | 0 | 2 | 0 |
| Vomiting | 0 | 0 | 1 | 0 |
| Diarrhea | 1 | 0 | 1 | 0 |
| Infusion Reaction | 0 | 0 | 1 | 0 |
| Acneiform Rash | 2 | 0 | 5 | 0 |
| Hypomagnesemia | 1 | 1 | 2 | 0 |
Grade 4 AEs did not occur
Dose Delivery of Palbociclib and Cetuximab
The median delivered dose of palbociclib per patient during the first two cycles (administered dose divided by scheduled dose) was 41/42 doses (98%, range 74-100%) in dose level 1 and 38/42 doses (91%, range 50-100%) in dose level 2. Doses were not given due to early progression(1 patient), AE(2), non-compliance(3) and pharmacy delay(1). The median delivered dose of cetuximab per patient during the first two cycles, was 8/8 doses (100%) in both dose levels 1 (range: 88-100%) and 2 (range: 50-100%). Doses were not given due to early progression (1 patient) and AE(2). Intra-patient dose reductions of palbociclib and cetuximab did not occur.
Tumor Response Assessment, TTP, and OS
Best tumor response observed with palbociclib and cetuximab was PR in 2 patients (22%), stable disease in 6 (67%), and progressive disease in 1 (11%). The disease control rate was 88%. Figure 1 shows representative CT images of partial responses of target lesions to palbociclib and cetuximab in patient #4 and #8. Patient #4 had cetuximab- and platin-resistant disease and patient #8 had no prior therapy for RM disease.
Figure 1.
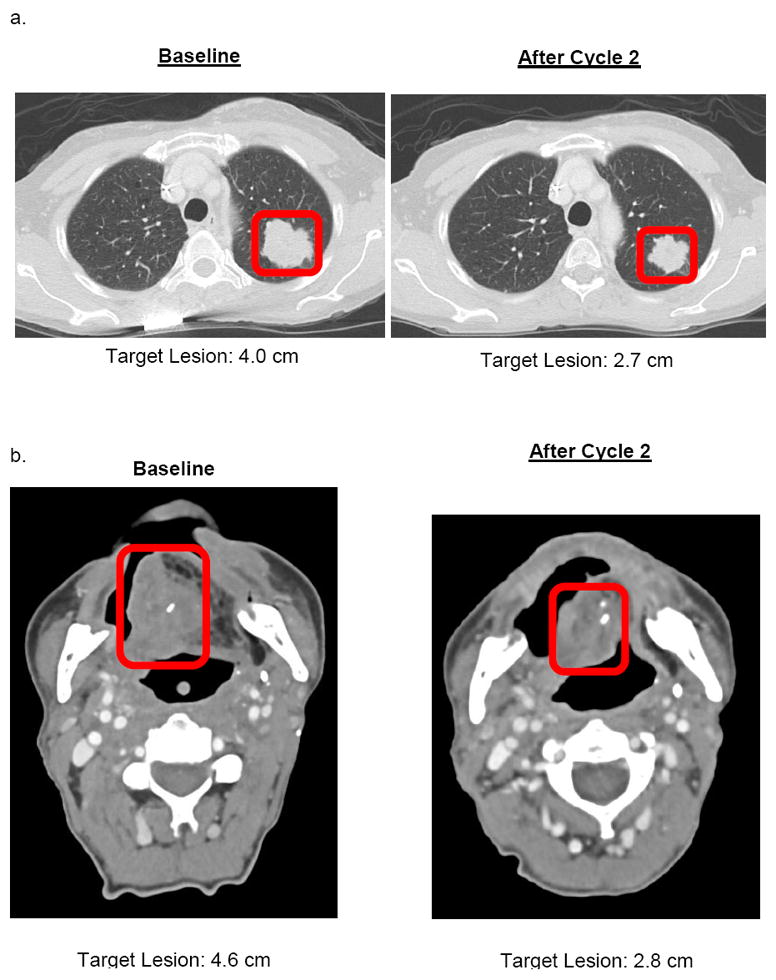
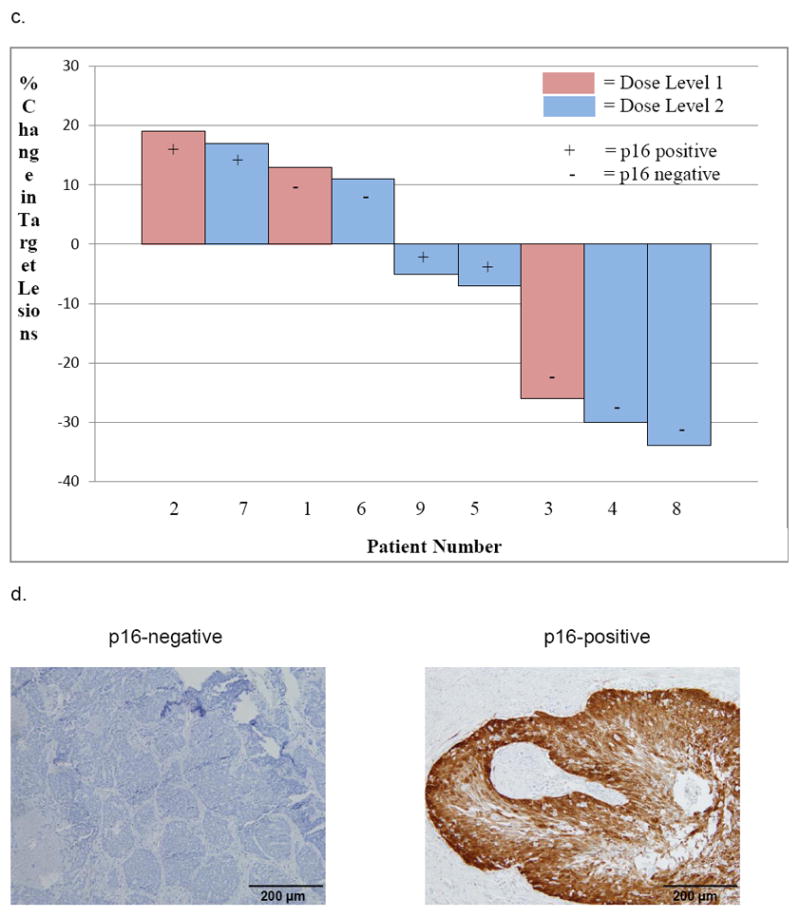
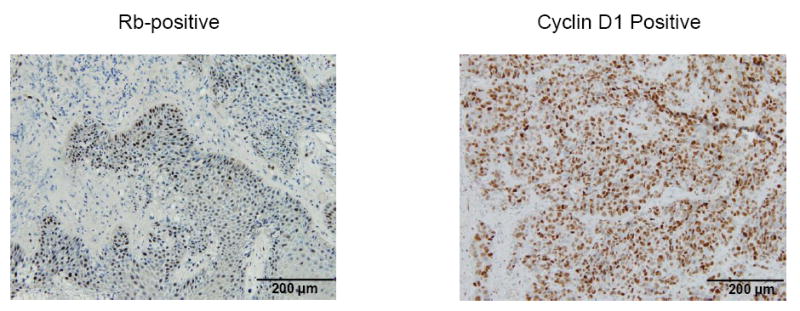
CT images of partial tumor response after two cycles of palbociclib and cetuximab in A) patient 4(Dose Level 2) with p16INK4a negative Hypopharynx SCC, B) patient 8(Dose Level 2) with p16INK4a negative oral cavity SCC, C) Waterfall plot of tumor response(% change in target lesions) achieved after cycle two, and D) Immunohistochemical staining images of tumor sections(nuclei counterstained with hematoxylin).
Overall, 5 of 9 patients (56%) had measureable decreases in target lesions following two cycles of palbociclib and cetuximab (Figure 2). In the 6 of 9 patients with cetuximab-resistant HNSCC, the best tumor response was PR in 1, disease control in 5, and the median TTP was 112 days (range: 28-168). In the 4 of 9 patients with platin-resistant HNSCC, the best tumor response was PR in 1, disease control in 3, and the median TTP was 112 days (range: 28-112).
Figure 2.
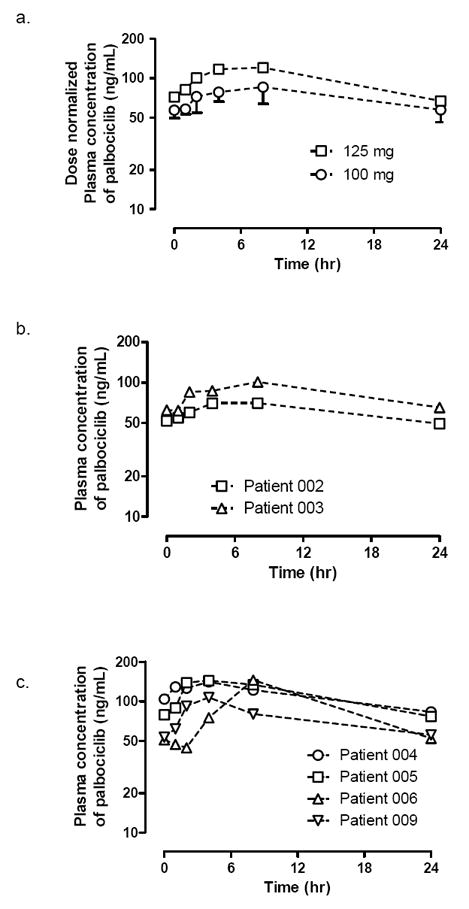
A. Time profiles for dose-normalized plasma concentrations of palbociclib in patients receiving daily oral palbociclib dose of 100 mg(○) or 125 mg(□) on day 15 of cycle 2. B & C. Individual plasma palbociclib concentrations vs time profiles of in patients receiving daily oral palbociclib dose of 100 mg(B) and 125 mg(C) on day 15 of cycle 2.
Overall, the median TTP was 112 days (range: 28-224). All patients came off study due to progressive disease. The median OS was 361 days (range: 124-588+) and four patients remain alive.
Biomarker Studies
Table 3 shows the results of p16INK4a, cyclin D (nuclear) and Rb IHC staining on the patients’ pre-treatment tumor sections. Nine tumors were evaluable for p16INK4a and eight for cyclin D and Rb expression. p16INK4a expression was either strongly and diffusely positive (n=4; 3 were OPSCC) or completely negative (n=5). The two PRs occurred in p16INK4a negative HNSCC. However, three of the four patients with p16INK4a positive tumors had stable disease with palbociclib and cetuximab although two were cetuximab/platin-resistant. Cyclin D expression was positive in seven and Rb expression was positive in eight tumors. Thus, statistical analysis of correlations of cyclin D and Rb expression to tumor response and TTP could not be performed.
Table 3.
Immunohistochemical Staining Results for p16INK4a, Cyclin D, and RB in Tumor Sections and Efficacy Endpoints.
| Time to Progression (Days) | Best Response* | Dose Level | Oropharynx Y/N | p16 Status | Cyclin D1 (nuclear) | Rb | Cetuximab - Resistant | Platin- Resistant |
|---|---|---|---|---|---|---|---|---|
| 224 | PRa | 2 | No | Negative | Positive | Positive | N | N |
| 224 | SDb | 1 | Yes | Positive | Positive | Positive | N | N |
| 168 | SD | 1 | Yes | Negative | Positive | Positive | Y | N |
| 168 | SD | 2 | No | Positive | Positive | Positive | N | N |
| 112 | PR | 2 | No | Negative | Positive | Positive | Y | Y |
| 112 | SD | 1 | No | Negative | Positive | Positive | Y | N |
| 112 | SD | 2 | Yes | Positive | Negative | Positive | Y | Y |
| 112 | SD | 2 | No | Negative | Positive | Positive | Y | Y |
| 28 | PDc | 2 | Yes | Positive | Not Doned | Not Doned | Y | Y |
PR=Partial Response
SD=Stable Disease
PD=Progressive Disease
Insufficient tissue
Pharmacokinetics of Palbociclib
The descriptive PK parameters for palbociclib after oral daily dosing of 100 or 125 mg on day 15 of cycle 2 are summarized in Table 4. Six patients were evaluable for PK analysis. Three patients were not evaluable due to failure to collect the pre-dose specimen (1), early progression (1), or missing multiple doses due to a SAE (1). In the plasma samples collected immediately before palbociclib dosing, all patients had detectable pre-dose levels (Ctrough) of palbociclib (Table 4). These Ctrough values were used to obtain AUC0-24h values. When dose-normalized, the Cmax and AUC0-24h values appeared to be comparable between the patients receiving daily oral palbociclib doses of 100 and 125 mg (Table 4).
Table 4.
Pharmacokinetic Parameters Obtained from Plasma Samples of Patients Receiving Daily Oral Dose of Palbociclib on Day 15 of Cycle 2.
| Tmax (h) | Ctrough (ng/mL) | Cmax (ng/mL) | Dose-normalized Cmax (ng/mL) | AUC0-24h (h ng/mL) | Dose-normalized AUC0-24h (h ng/mL) | |
|---|---|---|---|---|---|---|
| 100 mg daily dose cohort (n=2) | ||||||
| Subject 002 | 8 | 49.4 | 70 | 70 | 1,476 | 1,476 |
| Subject 003 | 8 | 65.2 | 101 | 101 | 2,012 | 2,012 |
| Summary | 8 | 56.8 | 84 | 84 | 1,723 | 1,723 |
| 125 mg daily dose cohort (n=4) | ||||||
| Subject 004 | 4 | 83.2 | 141 | 113 | 2,679 | 2,142 |
| Subject 005 | 4 | 76.8 | 144 | 115 | 2,724 | 2,179 |
| Subject 006 | 8 | 52.1 | 145 | 116 | 2,231 | 1,785 |
| Subject 009 | 4 | 55.6 | 107 | 86 | 1,792 | 1,434 |
| Summary | 4 (4-8) | 66 (23) | 133 (14) | 106 (14) | 2,324 (19) | 1,859 (19) |
Data are median (range) for Tmax and geometric mean (coefficient of variation%, CV%) for Cmax, AUC0-24h and dose-normalized AUC0-24h
DISCUSSION
This trial, the first to evaluate a selective CDK 4/6 inhibitor in HNSCC, established the feasibility of combining palbociclib with cetuximab. The palbociclib dose recommended for phase II trials is 125 mg/day on days 1-21 of a 28 day cycle with standard doses of weekly cetuximab. Higher doses were not evaluated because phase I trials of palbociclib monotherapy established the tolerable dose recommended for phase II trials was 125 mg/day[26] and this dose demonstrated anti-tumor activity in patients with HNSCC and other cancer types[28,29].
AEs observed on this trial were those expected for each drug and no overlapping or unexpected AEs were observed. Hematologic AEs were the most common toxicities associated with palbociclib[26] and non-hematologic AEs (rash, hypomagnesemia) were most commonly associated with cetuximab[28].
We assessed the efficacy of palbociclib and cetuximab in RM HNSCC, acknowledging the requirement to validate our results. PRs occurred in two of nine patients (22%) and measureable decreases in target lesions occurred in five (56%). The disease control rate was 89% and median TTP was 112 days (range, 28-224). Six of nine patients had cetuximab-resistant HNSCC: best tumor response was PR in 1 (17%) and disease control in 5 (83%) with median TTP of 112 days (range: 28-168). These data support an independent effect of palbociclib. Four of nine patients had platin-resistant HNSCC: best tumor response was PR in 1 (25%) and disease control in 3 (75%) with median TTP of 112 days (range: 28-112). Although cross-trial comparisons should be interpreted with caution, prior reports with cetuximab monotherapy given for platin-resistant, cetuximab-naïve RM HNSCC showed PR in 13%, disease control in 46%, and the median TTP was 70 days[4,32].
A major area of active investigation is the identification of biomarkers of response to palbociclib, which are lacking in clinical trials. The current study does not contain enough patients for analysis of correlative data. We did survey three biomarkers that could influence CDK4 addiction. We stained tumor sections for cyclin D1 and Rb, which has not consistently correlated with palbociclib response in clinical trials. We were unable to perform a correlation of cyclin D1 and Rb expression with tumor response because nearly all tumors expressed these biomarkers.
The biomarker expected to predict lack of tumor response to palbociclib is expression of p16INK4a. Rb is inactivated by the E7 protein in HPV-related disease, which should render CDK4 inhibition ineffective. In our trial, tumor responses (PR) were limited to patients with p16INK4a negative tumors. However, when TTP is considered as another measure of treatment benefit, two of the four patients with the longest TTP had p16INK4a positive HNSCC. Although the prolonged TTP in these patients may be due to cetuximab, the TTP (168 and 224 days) observed was substantially longer than expected (historical median 70 days) for cetuximab monotherapy[4]. Alternatively, palbociclib could have exerted its therapeutic activity in these patients via an off-target effect. Despite its specificity for CDK4/6, palbociclib has been reported to have additional and unexpected targets, such as cell membrane transporters[33]. While the clinical relevance of this finding remains to be validated, the possibility that palbociclib can inhibit tumor growth via a non-CDK dependent mechanism (in addition to CDK4 inhibition) is intriguing. Such a mechanism could explain the inability to find predictive biomarkers amongst canonical G1 cell cycle proteins.
In line with the previous report, palbociclib was absorbed slowly following oral dosing[26]. The dose-normalized Cmax and AUC0-24h values of patients receiving the daily oral palbociclib doses of 100 and 125 mg appeared comparable (Table 4). These results are consistent with the previous report showing dose proportionality in the systemic exposure of palbociclib in the dose range of 25-150 mg[26].
Limitations of this trial exist. The small number of patients does not allow detection of rare or population-specific AEs. Also, alternative doses of palbociclib could be more or equally efficacious. The trial design did not allow us to determine if anti-tumor activity of palbociclib occurs through a direct effect or by reversal of cetuximab resistance. As a phase I trial, we included patients with p16INK4a positive and cetuximab-resistant HNSCC. In patients with p16INK4a negative, cetuximab-naïve HNSCC, palbociclib and cetuximab may be more efficacious. Future trials of palbociclib should include correlative studies that test candidate biomarkers associated with cell cycle regulation and unbiased investigations designed to uncover novel biomarkers of tumor response and resistance.
This phase I trial, the first to evaluate a selective CDK 4/6 inhibitor in HNSCC, found that combining palbociclib with cetuximab is safe. The dose of palbociclib recommended for phase II trials is 125 mg/day orally on days 1-21 of each 28 day cycle, in combination with cetuximab. A signal of efficacy in this dataset led to a confirmatory ongoing international double-blind randomized trial designed to compare the efficacy of palbociclib and cetuximab to cetuximab monotherapy in patients with platin-resistant, cetuximab-naive HPV-unrelated RM HNSCC (NCT02499120).
HIGHLIGHTS.
This is the first trial to evaluate a selective CDK 4/6 inhibitor in HNSCC.
The novel combination of palbociclib and cetuximab was feasible and safe.
The palbociclib dose recommended is 125 mg/day 1-21 q 28 days with cetuximab.
Measureable decreases in target lesions occurred in 56% of patients.
The disease control rate was 89% and median TTP was 112 days (upper range 224).
Acknowledgments
The Siteman Cancer Center is supported in part by NCI Cancer Center Support Grant #P30 CA91842; Dr. Wildes’ research is supported by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH) and Grant Number UL1TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCI, NCATS, or NIH.
Footnotes
Presented in abstract form at the 2015 Annual Meeting of the American Society of Clinical Oncology
Conflict of Interest/Disclosures
Disclosures: Research Support – Pfizer
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harari PM, Allen GW, Bonner JA. Biology of interactions: antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–65. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- 2.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 3.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25(16):2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 5.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 6.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristic of a high risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–8. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 7.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivanchenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactiving mutations in NOTCH1. Science. 2011;333(6046):1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mountzios G, Rampias T, Psyrri A. The mutational spectrum of squamous-cell carcinoma of the head and neck: targetable genetic events and clinical impact. Ann Oncol. 2014;25(10):1889–1900. doi: 10.1093/annonc/mdu143. [DOI] [PubMed] [Google Scholar]
- 11.Seiwert TY, Zuo Z, Keck MK, Khattri A, Pedamallu CS, Stricker T, et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21(3):632–41. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–81. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 13.Allen CT, Lewis JS, Jr, El-Mofty SK, Haughey BH, Nussenbaum B. Human Papillomavirus and oropharynx cancer: Biology, detection and clinical implications. Laryngoscope. 2010;120:1756–72. doi: 10.1002/lary.20936. [DOI] [PubMed] [Google Scholar]
- 14.Smeets SJ, Braakhuis BJM, Abbas S, Snijders PJ, Ylstra B, van de Wiel MA, et al. Genome-wide DNA copy number alterations in head and neck squamous cell carcinomas with or without oncogene-expressing human papillomavirus. Oncogene. 2006;25:2558–64. doi: 10.1038/sj.onc.1209275. [DOI] [PubMed] [Google Scholar]
- 15.Scantlebury JB, Luo J, Thorstad WL, El-Mofty SK, Lewis JS., Jr Cyclin D1 a prognostic marker in oropharyngeal squamous cell carcinoma that is tightly associated with high-risk human papillomavirus status. Human Pathol. 2013;44(8):1672–80. doi: 10.1016/j.humpath.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed AL, Califano J, Cairns P, Westra WH, Jones RM, Koch W, et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3. [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalish LH, Kwong RA, Cole IE, Gallagher RM, Sutherland RL, Musgrove EA. Deregulated Cyclin D1 Expression Is Associated with Decreased Efficacy of the Selective Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Gefitinib in Head and Neck Squamous Cell Carcinoma Cell Lines. Clin Cancer Res. 2004;10:7764–74. doi: 10.1158/1078-0432.CCR-04-0012. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Zhang Z, Zhou X, Qiu W, Chen F, Chen W. Identification of genes associated with cisplatin resistance in human oral squamous cell carcinoma cell line. BMC Cancer. 2006;6:224. doi: 10.1186/1471-2407-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Zhang Z, Yang X, Chen W, Zhang P. Inhibition of cyclin D1 expression by cyclin D1 shRNAs in human oral squamous cell carcinoma cells is associated with increased cisplatin chemosensitivity. Int J Cancer. 2009;124:483–9. doi: 10.1002/ijc.23964. [DOI] [PubMed] [Google Scholar]
- 21.Feng Z, Guo W, Zhang C, Xu Q, Zhang P, Sun J, et al. CCND1 as a Predictive Biomarker of Neoadjuvant Chemotherapy in Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma. PLoS ONE. 2011;6:e26399. doi: 10.1371/journal.pone.0026399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirama T, Koeffler HP. Role of the Cyclin-Dependent Kinase Inhibitors in the Development of Cancer. Blood. 1995;86:841–54. [PubMed] [Google Scholar]
- 23.Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, et al. Specific Inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–37. [PubMed] [Google Scholar]
- 24.Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):1–13. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baughn LB, Di Liberto MD, Wu K, Toogood P, Louie T, Gottschalk R, et al. A Novel Orally Active Small Molecule Potently Induces G1 Arrest in Primary Myeloma Cells and Prevents Tumor Growth by Specific Inhibition of Cyclin-Dependent Kinase 4/6. Cancer Res. 2006;66(15):7661–7. doi: 10.1158/0008-5472.CAN-06-1098. [DOI] [PubMed] [Google Scholar]
- 26.Flaherty KT, LoRusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, et al. Phase I, Does-Escalation Trial of the Oral Cyclin-Dependent Kinase 4/6 Inhibitor PD 0332991, Administered Using a 21-Day Schedule in Patients with Advanced Cancer. Clin Cancer Res. 2012;18:568–76. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1) Brit J Cancer. 2011;104:1862–8. doi: 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of estrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 29.Leonard JP, LaCasce AS, Smith MR, Noy A, Chirieac LR, Rodig SJ, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119:4597–607. doi: 10.1182/blood-2011-10-388298. [DOI] [PubMed] [Google Scholar]
- 30.Reis-Filho JS, Savage K, Lambros MB, James M, Steele D, Jones RL, Dowsett M. Cyclin D1 protein overexpression and CCND1amplification in breast carcinomas: an immunohistochemical and chromogenic in situ hybridization analysis. Mod Pathol. 2006;19:999–1009. doi: 10.1038/modpathol.3800621. [DOI] [PubMed] [Google Scholar]
- 31.Evans MF, Peng Z, Clark KM, Adamson CS, Ma XJ, Wu X, et al. HPV E6/E7 rna in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PLoS ONE. 2014;9(3):e91142. doi: 10.1371/journal.pone.0091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baselga J, Trigo JM, Bourhis J, Tortochaux J, Cortes-Funes H, Hitt R, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23(24):5568–77. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 33.Pabla N, Gibson AA, Buege M, Ong SS, Li L, Hu S, et al. Mitigation of acute kidney injury by cell-cycle inhibitors that suppress both CDK4/6 and OCT2 functions. PNAS. 2015;112(16):5231–6. doi: 10.1073/pnas.1424313112. [DOI] [PMC free article] [PubMed] [Google Scholar]


