Abstract
This study considered effects of timing and duration of iron deficiency (ID) on frontal EEG asymmetry in infancy. In healthy term Chinese infants, EEG was recorded at 9 months in three experimental conditions: baseline, peek-a-boo, and stranger approach. Eighty infants provided data for all conditions. Prenatal ID was defined as low cord ferritin or high ZPP/H. Postnatal ID was defined as ≥ two abnormal iron measures at 9 months. Study groups were pre- and postnatal ID, prenatal ID only, postnatal ID only, and not ID. GLM repeated measure analysis showed a main effect for iron group. The pre- and postnatal ID group had negative asymmetry scores, reflecting right frontal EEG asymmetry (mean ±SE: −.18 ±.07) versus prenatal ID only (.00 ±.04), postnatal ID only (.03 ±.04), and not ID (.02 ±.04). Thus, ID at both birth and 9 months was associated with right frontal EEG asymmetry, a neural correlate of behavioral withdrawal and negative emotions.
Keywords: iron deficiency, emotion, EEG, pre- and postnatal
INTRODUCTION
Iron deficiency (ID) is a global public health problem, affecting primarily women and young children in both developed and developing countries. In early life, there are two peak times of risk for ID: late fetal or early neonatal life (referred to as prenatal ID) and the late infancy/toddler period (6–24 months, referred to as postnatal ID). Although direct estimates of the worldwide prevalence of iron deficiency are problematic (Stoltzfus, 2001), anemia, which is a late manifestation of iron deficiency, affects an estimated 48% of young children (World Health Organization, 2008).
Previous studies have found poorer cognitive, motor, and social–emotional development in ID infants (Lozoff et al., 2006). Our focus here is on the social–emotional domain. Infants and toddlers with iron deficiency anemia are reported to exhibit more shyness, less engagement, decreased positive affect, more difficulty in being soothed when upset, and less optimal interaction with their mothers (Armony-Sivan, Kaplan-Estrin, Jacobson, & Lozoff, 2010; Lozoff et al., 2008, 2010). ID in infancy is also associated with poorer long-term social–emotional outcomes in preschool age (Chang et al., 2011), school age (Lozoff, Castillo, Clark, Smith, & Sturza, 2014), adolescence (Corapci, Calatroni, Kaciroti, Jimenez, & Lozoff, 2010), and young adulthood (Lozoff et al., 2013). Iron status at birth was unknown in these studies. We identified only one study that focused on prenatal ID and social–emotional functioning. Wachs, Pollitt, Cueto, Jacoby, and Creed-Kanashiro (2005) found that lower levels of cord blood hemoglobin and serum ferritin were related to higher levels of negative emotionality and lower levels of alertness and soothability in the newborn period.
Iron plays a critical role in brain morphology, neurochemistry and bioenergetics (Beard, 2003; Georgieff, 2011). ID effects on neurotransmitters are especially relevant for social–emotional outcomes (Lozoff, 2011). Animal models show relations between ID and alterations in metabolism and function of several neurotransmitters, including, dopamine, norepinephrine, and serotonin, and related behaviors (Beard et al., 2006; Kim & Wessling-Resnick, 2014; Unger et al., 2007, 2012; Youdim & Yehuda, 2000). The specific nature of the effects appears to be dependent on the timing of ID and treatment (Unger et al., 2007, 2012). However, identifying central nervous system correlates of ID in human infants is challenging.
Asymmetric hemispheric activity is considered as a marker of social–emotional functioning in adulthood (Davidson, 1998) and infancy (Fox, 1991, 1994). Studies show that frontal EEG asymmetry relates to infant temperament and emotional reactivity (Fox, 1991; Harmon-Jones, Gable, & Peterson, 2010). The frontal EEG asymmetry score is based on the difference in alpha power between left and right hemisphere. Alpha power indicates differences in brain activity but in an inverse matter, that is, less alpha power indicates greater brain activity (Davidson, 1998; Vuga, Fox, Cohn, Kovacs, & George, 2008). In general, left frontal EEG asymmetry is associated with joy or anger, and right frontal EEG asymmetry is associated with sadness (Fox & Davidson, 1988). More specifically, greater right frontal EEG asymmetry in infancy is associated with infant withdrawal and inhibited behaviors (Hane, Fox, Henderson, & Marshall, 2008), maternal separation (Davidson & Fox, 1989), maternal depression (Jones, Field, & Almeida, 2009), and high cortisol levels (Buss et al., 2003). We identified only one published study of right frontal EEG asymmetry in ID humans and it involved adults; lower serum ferritin levels were associated with greater right frontal EEG asymmetry (Tucker, Sandstead, Penland, Dawson, & Milne, 1984).
The purpose of the present study was to assess the impact of the timing and duration of ID in infancy (pre- and/or postnatal) on frontal EEG asymmetry at 9 months. As ID in infancy is associated with less positive affect and more behaviors resembling behavioral inhibition, we hypothesized that ID in infancy would be associated with relatively greater right frontal EEG asymmetry but had no basis for predictions about differential effects of the timing and duration of ID. We were also interested in assessing EEG patterns in situations designed to elicit positive or negative affective responses. We predicted that ID-related differences in right frontal EEG asymmetry would be magnified in a stranger approach situation, which is often a negative social–emotional experience for infants at this age.
METHODS
Participants
The EEG assessment was one component of a larger study on the neurodevelopmental effects of ID in early life. Study enrollment occurred between December 2008 and November 2011 in Fuyang County, a rural area in Zhejiang Province, China. Pregnant women with uncomplicated singleton pregnancies were invited to participate when they were randomly screened at a routine visit at 36–37 weeks gestation. After birth, the following inclusion criteria were confirmed: term birth (gestational age 37–42 weeks), birth weight >2,500 g, 5-min APGAR ≥7, no acute or chronic health problems, mother’s age over 18 years, no alcohol or drug use during pregnancy, no perinatal complications or congenital malformations, and no multiple or prolonged hospitalizations (>5 days). Parents provided signed informed consent. The study was approved by the Institutional Review Boards of the University of Michigan and the Children’s Hospital of Zhejiang University.
Procedures
Infant iron status was based on cord blood at birth and venous blood at 9 months. Serum ferritin was assayed by electro-chemiluminescent immunoassay (Cobas 6000-601, Roche Diagnostics Corp., Basel, Switzerland), mean corpuscular volume (MCV) and red cell distribution width (RDW) by autoanalyzer (Sysmex SE-9000 Auto Hematology Analyzer, Kobe, Japan), and zinc protoporphyrin/heme ratio (ZPP/H) by ZPP Hematofluorometer (Model 206D, AVIV Associates, Lakewood, NJ).
There is no consensus on how to define ID in the neonate. Therefore, we considered either low cord ferritin or high ZPP/H as indicative of prenatal ID. We used serum ferritin <75 μg/L as the cutoff for fetal–neonatal ID, as in some previous studies (Amin, Orlando, & Wang, 2013; Armony-Sivan, Eidelman, Lanir, Sredni, & Yehuda, 2004; Tamura et al., 2002). For prenatal ZPP/H, we used a cutoff of ZPP/H >118 μmol/mol, corresponding to the 90th percentile in the US studies (McLimore et al., 2013).
Postnatal ID was defined as two or more abnormal iron measures, a widely used standard in the field (Cook & Finch, 1979). The following age-appropriate cutoffs were used for four measures: MCV <74 fl (Centers for Disease Control, 2001), RDW >14.5% (Centers for Disease Control and Prevention, 1998), serum ferritin <12.0 (Saarinen & Siimes, 1978), and ZPP/H >69 μmol/mol heme (Soldin, Miller, & Soldin, 2003). These cutoffs are based on normative samples in healthy term infants from iron-supplemented populations or studies that excluded infants with evidence of ID. The rationale in these studies is that reference values for iron measures should be based on infants who have access to adequate iron, as data from populations where ID is common will shift the distributions and likely underestimate ID prevalence and fail to identify infants who could benefit from intervention.
The possible combinations of pre- and postnatal ID constituted the four study groups: pre- and postnatal ID, prenatal ID only, postnatal ID only, and not ID at either time.
Electroencephalogram (EEG) Recording
EEG assessments were conducted at the Children’s Hospital of Zhejiang University, Hangzhou, China. Infants were seated on their mothers’ laps in front of a video monitor. Continuous EEG recordings were obtained in three experimental conditions of 2 min each. The experimental conditions were chosen based on previous studies of EEG activity in toddlers and infants in response to neutral, positive, and negative stimuli (Dawson, Klinger, Panagiotides, Hill, & Spieker, 1992; Jones, Field, Fox, Davalos, & Gómez, 2001). For the first condition (baseline), the tester blew soap bubbles to keep the infant’s attention and minimize movement artifact. For the second condition (peek-a-boo), the tester played peek-a-boo with the infant. Although designed to elicit positive affect, testers reported that some infants did not react positively to this condition, perhaps because the game was not played by a familiar person. The third condition (stranger approach) was designed to elicit negative emotions. A stranger entered the room and looked at the infant with a neutral facial expression throughout. The stranger first stood by the door for 40 s, then about half the distance to the infant for 40 s, and finally directly in front of the infant for 40 s.
EEG data were recorded using 128-electrode HydroCel Sensor Net (Electrical Geodesics, Inc., Eugene, OR). The infant’s head was measured and marked with a washable wax pencil in order to ensure accurate placement of the net, which was then placed over the scalp. Scalp impedances were measured prior to recording and kept below 50 kΩ during testing. The EEG signals were amplified, filtered (bandpass .1–100.0 Hz), and sampled (500 Hz). Recording in every electrode was vertex-referenced. With continuous EEG recording, we used an internal marker to indicate the beginning of each experimental condition.
The EEG data were processed offline using Net Station 4.3 (Net Station, Eugene, OR). EEG data were filtered (1–47 Hz band-pass) and segmented by the internal markers for the three experimental conditions. EEG data in each condition were multiple segmented into 120 segments of 1 s. Segmented data were inspected visually for ocular and motion artifact. The two electrodes above the eyes and the two remaining electrodes on the outside canthii of the eyes were used to identify eye blinks and eye movement. Data from individual electrodes were rejected if there was artifact resulting from poor contact or movement or if signal amplitudes exceeded 150 μV. The entire segment was excluded if an eye blink, eye movement, or other significant artifact occurred, or if more than 18 electrodes were rejected. Of the remaining segments, individual electrodes containing artifacts were replaced using spherical spline interpolation, and data were re-referenced to the average reference. At least 15 artifact-free EEG segments were required in each condition for an infant to be included in the analyses described below.
Data Reduction
EEG data were exported to Matlab 10 (2012). Segmented EEG data in each condition were merged. The data were transformed using p-welch Hanning window of 1 s with 50% overlapping. Analysis was focused on alpha frequency band at 6–9 Hz, which is typically used in studies of brain activity of infants in the first 2 years of life (Davidson & Fox, 1982). The alpha power values were averaged into an alpha power score. This score was natural log-transformed to normalize the distribution. Frontal EEG asymmetry score was calculated for each infant by subtracting these normalized values in F3 electrode, located over the frontal left hemisphere, from the mean values in F4 electrode, located over the frontal right hemisphere [LnF4 (Right) minus LnF3 (Left)]. Negative scores indicate greater relative EEG activation (lower power values) in the right hemisphere (right frontal EEG asymmetry), whereas positive scores indicate greater relative EEG activation (lower power values) in the left hemisphere (left frontal EEG asymmetry).
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics for Windows (IBM Corp., 2012) and SAS software version 9.4 (SAS Institute, Inc., Cary, NC). The four iron groups were compared on background characteristics and iron measures using ANOVA for continuous variables and chi-square for categorical ones. To evaluate potential covariates, Pearson’s correlations were used to assess the relation of frontal EEG asymmetry score in the three experimental conditions to background characteristics (Table 1). Potentially confounding variables were considered in the initial models if they were even weakly related to frontal EEG asymmetry score in even one condition (p <.10). Covariates that remained at p <.10 in the final models were retained.
Table 1.
Correlations Between Background Characteristics and Frontal EEG Asymmetry Score in the Three Experimental Conditions*
| Frontal EEG Asymmetry Score
| |||
|---|---|---|---|
| Condition (n =80)a | Baseline | Peek-A-Boo | Stranger Approach |
| Birth weight | .26b | .27b | .25b |
| Gestational age | .14 | .18 | .17 |
| Age at 9-month visit | −.17 | −.08 | −.08 |
| Weight-for-age | .04 | .14 | .12 |
| Maternal age | −.03 | −.17 | .11 |
| HOME score | .03 | .08 | .08 |
| Maternal depression | .22 | .18 | .25b |
Values are Pearson rs for ordinal and continuous measures.
N varies slightly due to occasional missing data for some measures.
p <.05.
General linear mixed model (GLM) statistical analysis was used to compare frontal EEG asymmetry scores by iron group (pre- and postnatal ID, prenatal ID only, postnatal ID only, and not ID) and condition (baseline, peek-a-boo, and stranger approach). Unadjusted means and standard deviations are presented in the text; adjusted means and standard errors are shown in a figure. To further examine hemispheric differences, a mixed model statistical analysis of alpha power score was conducted for group within condition and hemisphere (left, right).
The final sample size (n =80) was adequate to detect only large effect sizes (η2 =.14) among four groups with 80% power and type I error alpha =.05. As the power to detect smaller effect sizes for main effects or interactions was lower and the risk of type II error was of concern, we also report the effect size η2 to indicate the magnitude of association (low =.01, medium =.06, large =.14), regardless of statistical significance.
RESULTS
Of 353 infants assessed at 9 months in the larger study, 238 had some EEG data. After processing and rejecting artifacts, 80 infants had usable data for all three conditions. These infants did not differ from the other 158 in background characteristics, except that, on average, they were 5 days older at testing. Boys and girls were represented approximately equally (52% girls). Many infants were born by C-section (75%). APGAR score at 5 min was 10 for all but four infants, whose scores were 8–9. Gestational age averaged 39.6 weeks (SD =1.0, range: 37–42) and birth weight, 3.47 kg (SD =.41, range: 2.50–4.50). Individual information on cord clamping was not available, but the usual routine in the local hospital was to clamp the cord within 60 s of delivery. The infants remained generally healthy between birth and 9 months. Only five were hospitalized, all briefly for a typical infancy problem (e.g., jaundice, respiratory infection). Data on intercurrent illnesses, available for half the infants, indicated the usual colds and occasional diarrhea (data available on request). The mean chronological age at testing was 9.5 months (mean =285 days, SD =10.7, range: 268–313). Mean age of mothers was 27.0 years (SD =3.7, range: 21–39). The average education of parents was above middle school and below high-school graduation. Maternal mood, evaluated by the Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987), showed relatively low scores (mean =7.5, SD =4.0). None of the mothers reported smoking or drug use. Infant iron status was distributed as follows: pre- and postnatal ID (n =9), prenatal ID only (n =21), postnatal ID only (n =20), and not ID at either time (n =30). The distribution was similar in the 158 infants who did not have usable data in all three conditions.
Background Characteristics and Iron Status
There were no statistically significant group differences in background characteristics (Table 2), except that the prenatal ID only and not ID groups were tested somewhat earlier than the pre- and postnatal ID and postnatal ID only groups. By definition, the groups differed in iron status (Table 3). Cord ferritin was lower and ZPP/H was higher in pre- and postnatal ID and prenatal ID only groups compared to postnatal ID only and not ID groups. At 9 months of age, several iron measures showed poorer iron status (lower hemoglobin and MCV and higher RDW) in pre- and postnatal ID and postnatal ID only groups compared to prenatal ID only and not ID groups.
Table 2.
Background Characteristics by Iron Status Group*
| (n)a | Pre- and Postnatal ID (9) | Prenatal ID Only (21) | Postnatal ID Only (20) | Not ID (30) | p-Values |
|---|---|---|---|---|---|
| Infant and pregnancy | |||||
| Gender, % male (n) | 56 (5) | 42 (9) | 55 (11) | 43 (13) | .70 |
| C-section, % yes (n) | 78 (7) | 86 (18) | 75 (15) | 63 (19) | .21 |
| Birth weight (g) | 3,522 ±386 | 3,477 ±466 | 3,487 ±445 | 3,458 ±375 | .98 |
| Gestational age (weeks) | 39.5 ±.9 | 39.2 ±1.0 | 39.8 ±1.1 | 39.7 ±1.0 | .61 |
| Age at 9-month visit (days) | 288.3 ±10.4 | 281.0 ±8.7 | 292.7 ±13.0 | 282.9 ±8.2 | .00 |
| Weight-for-age, z-score | .84 ±1.01 | .71 ±.86 | .82 ±.87 | .64 ±1.17 | .91 |
| Family | |||||
| Maternal age, years | 25.6 ±3.1 | 27.9 ±3.9 | 26.7 ±4.4 | 27.3 ±3.3 | .43 |
| Educationb | 4.1 ±1.2 | 3.7 ±1.2 | 3.9 ±.9 | 3.8 ±.9 | .84 |
| Number people/household | 5.5 ±1.5 | 4.9 ±1.0 | 5.1 ±1.3 | 5.0 ±1.1 | .67 |
| HOME scorec | 34.3 ±4.1 | 35.2 ±3.7 | 36.1 ±3.0 | 35.4 ±3.7 | .64 |
| Maternal depressiond | 6.9 ±4.0 | 7.2 ±3.5 | 6.9 ±3.3 | 8.0 ±4.1 | .72 |
ID, iron deficiency.
Values are expressed as means ±SD or % (n) for categorical variables, and p-values are based on ANOVA for continuous variables and chi-square analyses for categorical variables.
N varies slightly due to occasional missing data for some measures.
Values represent parents education (mean) on an ordinal scale (1 =below elementary school, 4 =high-school graduate, 8 =postgraduate studies).
HOME, Home Observation for Measurement of the Environment.
Score on the Edinburgh Postnatal Depression Scale.
Table 3.
Maternal and Infant Iron Status by Study Group*
| (n)a | Pre- and Postnatal ID (9) | Prenatal ID Only (21) | Postnatal ID Only (20) | Not ID (30) | p-Values |
|---|---|---|---|---|---|
| Maternal hemoglobinb, g/L | 110.4 ±9.4 | 117.9 ±15.0 | 113.1 ±9.9 | 112.0 ±9.5 | .24 |
| Cord blood | |||||
| Hemoglobin, g/L | 138.0 ±17.9 | 145.0 ±19.0 | 143.10 ±31.6 | 147.3 ±17.2 | .72 |
| Ferritin, μg/L | 84.5 ±27.7c | 73.5 ±79.8c | 202.4 ±78.0d | 177.8 ±58.6d | .00 |
| sTfR, nmol/L | 27.7 ±16.2 | 28.4 ±16.7 | 27.7 ±11.0 | 26.5 ±16.5 | .97 |
| ZPP/H, μmol/mol | 126.2 ±20.8c | 132.2 ±33.6c | 91.0 ±16.3d | 94.5 ±17.1d | .00 |
| 9 months | |||||
| Hemoglobin, g/L | 100.7 ±6.1c | 113.0 ±5.1d | 104.7 ±4.5c | 114.6 ±7.4d | .00 |
| MCV, fl | 72.1 ±3.4c | 80.2 ±1.9d | 70.9 ±7.6c | 79.5 ±2.6d | .00 |
| RDW, % | 15.0 ±1.9c | 12.5 ±0.8d | 15.2 ±2.0c | 12.8 ±0.8d | .00 |
| Ferritin, μg/L | 28.1 ±27.6 | 46.3 ±27.6 | 41.4 ±42.2 | 45.3 ±32.1 | .54 |
| sTfR, nmol/L | 33.0 ±11.6c | 22.2 ±8.3d | 29.6 ±18.2c | 21.0 ±7.1d | .01 |
| ZPP/H, μmol/mol | 146.6 ±33.0c | 97.7 ±29.9d | 141.8 ±67.9c | 96.3 ±33.1d | .00 |
ID, iron deficiency.
Values are expressed as means ±SD, and p-values are based on ANOVA.
N varies slightly due to occasional missing data for some measures.
Maternal hemoglobin in late pregnancy (36–37 weeks).
Superscript letters c and d: groups with the same superscript do not differ; those with different letters differ significantly (p <.05).
EEG Outcomes
GLM mixed model analysis for frontal EEG asymmetry scores in the four iron groups and three experimental conditions showed a significant main effect for iron group (F(3,76) = 2.65; p =.05; partial η2 =.10). Over all conditions, the pre- and postnatal ID group had negative EEG asymmetry scores (mean [SD] baseline =−.18 [.21], peek-a-boo =−.14 [.19], stranger approach =−.23 [.26]), which differed significantly from the prenatal ID only group (mean [SD] baseline =−.01 [.24], peek-a-boo =−.05 [.27], stranger approach =.04 [.20]), the postnatal ID only group (mean [SD] baseline =.02 [.28], peek-a-boo =.01 [.28], stranger approach =.05 [.22]), and the not ID group (mean [SD] baseline =.01 [.24], peek-a-boo =.04 [.17], stranger approach =.02 [.23]). These results reflect right frontal asymmetry in the pre-and postnatal ID group, whereas the other three groups generally showed a slight left frontal EEG asymmetry.
There was no main effect for condition, meaning that there were no significant differences in frontal EEG asymmetry scores between baseline, peek-a-boo, and stranger approach conditions (F(2,75) = .12; p =.89; partial η2 =.00). The interaction between iron group and condition was not statistically significant, but the partial η2 indicated a low-medium effect (F(6,150) = 1.27; p =.27; partial η2 =.05).
GLM mixed model analysis with covariate control showed similar results. There was a significant main effect for iron group (F(3,74) = 3.15; p <.05; partial η2 =.11); infant birth weight and maternal depressed mood were the only background factors that met the criterion for inclusion in the final model. Adjusted means, standard errors, and p-values are presented in Figure 1. There was no main effect for condition (F(2,73) = .05; p =.95; partial η2 =.00). Once again, the interaction between iron group and condition was not statistically significant, but the partial η2 indicated a low-medium effect (F(6,148) = 1.28; p =.27; partial η2 =.05). Finally, we tested for a moderation of maternal depressed mood on the effect of iron group on frontal EEG asymmetry. The interaction of iron group and maternal depressed mood was not statistically significant, with a low-medium effect size (F(3,71) = 1.28, p =.29; partial η2 =.05).
FIGURE 1.
Frontal EEG asymmetry by four iron groups [pre- and postnatal ID (white), prenatal ID only (black), postnatal ID only (gray), and not ID (haskmark)] in three experimental conditions. Negative values indicate right frontal EEG asymmetry. *p <.05.
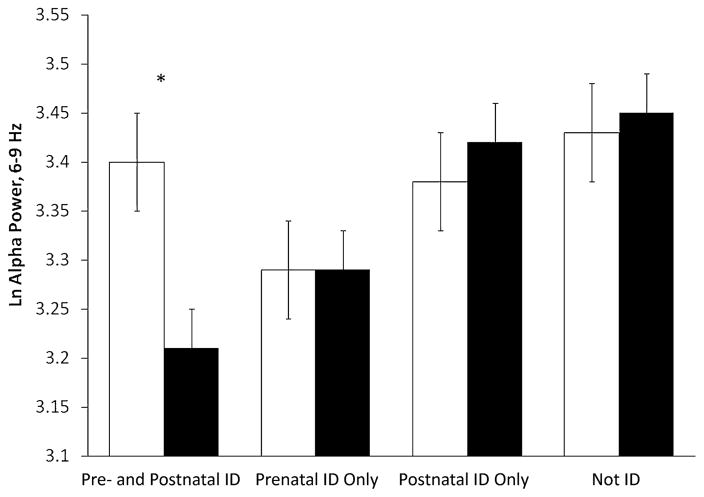
An additional GLM mixed model analysis was performed for differences in alpha power scores by group, condition, and hemisphere with covariate control (infant birth weight and maternal depressed mood). There was a significant interaction between iron group and hemisphere (F(3,74) = 3.17; p <.05; partial η2 =.11). Therefore, left and right hemisphere data were analyzed separately. There were no significant group differences for the left hemisphere, and the effect size was small (η2 =.02). For the right hemisphere, there was a suggestive group difference with a medium effect size η2 =.06. Pairwise comparisons were also suggestive (p =.08) for the difference between pre- and postnatal ID and not ID groups (mean [SD] =3.21 [.40] vs. 3.45 [.42]) with a medium Cohen’s effect size of .59 SD. In addition, we compared frontal alpha power in left and right hemisphere within iron group. Greater left than right frontal alpha power was found only in the pre- and postnatal ID group. Adjusted means, standard errors, and p-values are presented in Figure 2. There were no other statistically significant or marginal interactions, but the partial η2 indicated low to low-medium effects: iron group ×condition (η2 =.04) and iron group ×condition ×hemisphere (η2 =.05).
FIGURE 2.
Frontal alpha power [Left: F3 (white), Right: F4 (black)] by four iron groups over three experimental conditions. *p <.05.
DISCUSSION
This study evaluated the relations between timing and duration of ID in infancy and frontal EEG asymmetry in 9-month-old infants. We found that infants with pre-and postnatal ID had greater relative right frontal EEG asymmetry compared to the other iron groups. Right frontal asymmetry in the pre- and postnatal ID group was related to relatively lower frontal alpha power in the right hemisphere. This finding indicates greater brain activation in the right hemisphere in infants who were ID at both birth and 9 months.
As right frontal EEG asymmetry is considered a marker of withdrawal-like behaviors (Davidson, 1998; Fox, 1991, 1994), our results are consistent with previous studies linking ID in infancy with poorer social–emotional behavior, such as more shyness, less engagement, decreased positive affect, and less optimal mother-infant interaction (Lozoff, 2011). In addition, the results are consistent with the only previous study of EEG asymmetry, which showed right frontal EEG asymmetry in adults with low serum ferritin levels (Tucker et al., 1984).
According to the approach/withdrawal model, right frontal EEG asymmetry is associated with greater fear and more negative emotional reactivity (Davidson & Fox, 1989). The pattern of resting frontal brain EEG has been suggested as a “trait-like” correlate of individual differences in affective style in adults and children (Fox, 1991). This physiological index is believed to measure emotional and temperamental personality predispositions. As such, during resting EEG, individuals displaying left frontal EEG asymmetry often exhibit approach motivation and positive affect, while individuals displaying right EEG asymmetry have been found to exhibit withdrawal behaviors and negative affect (Fox, 1991). Interpreting our results in light of this model would suggest that having ID as a fetus/neonate and infant relates to social–emotional changes reflecting personality predispositions/alterations, such as negative reactivity.
Along with interpreting and discussing our results in light of an affective style and emotional reactivity theory, it is important to note that frontal EEG asymmetry was assessed at only one time-point and the sample was relatively small, especially in the pre- and postnatal ID group. We did not find statistically significant differences between conditions or interactions with ID group. These negative findings should also be interpreted cautiously, as statistical power was limited and several partial η2 values for interactions indicated non-trivial effect sizes. The measures and experimental conditions were designed based on studies in Western cultures (Dawson et al., 1992; Jones et al., 2001). Research demonstrating cultural differences in social–emotional development raises questions about cross-cultural validity of the experimental conditions for Chinese infants (Chen & Rubin, 2011; Gartstein et al., 2006). As reported, peek-a-boo game was not so positive for some of the infants. In light of these limitations, a corroborating multi-method approach combining neurophysiological along with behavioral observations would be informative, along with cross-cultural studies.
The postulated mechanisms for ID-related social–emotional alterations generally involve ID effects on neurotransmitter function. Rodent models of ID show altered dopamine metabolism and function, as well as other monoamine neurotransmitters, such as serotonin and norepinephrine (Kim & Wessling-Resnick, 2014). Findings in humans are consistent with such neurotransmitter changes (Lozoff, 2011). However, iron is involved in various brain processes related to emotional behaviors, making it difficult to specify the mechanism-(s) for the EEG asymmetry findings. It seems likely that the influence of ID on emotional behavior is multifactorial (Kim & Wessling-Resnick, 2014). Further studies are needed to evaluate the linkage between frontal EEG asymmetry, emotional behaviors, and brain mechanisms of pre- and postnatal ID.
Greater right frontal EEG asymmetry only in the pre- and postnatal ID group suggests that changes in frontal EEG occur when infants are ID during both peak times of ID—late fetal period and infancy. We speculate that infants in the pre- and postnatal ID group suffered from early chronic ID, but iron status was evaluated at only two time-points. Thus, we cannot determine whether infants had ID throughout the period from birth to 9 months. However, it seems likely that infants with ID both at birth and 9 months had ID for a longer duration than infants with ID at only one time-point. Further studies are needed to extend our understanding about longitudinal effects of ID in infancy on EEG activity.
In the present study, prenatal iron status as measured from cord blood reflects fetal iron status in late gestation. It is important to note that fetal iron status earlier in gestation and maternal iron status during pregnancy were unknown. According to our previous pilot study, maternal ID was common in this region (Shao et al., 2012). Thus, likely explanations for ID in the infants include maternal ID, rapid infant weight gain pre- and postnatally, no routine iron supplementation for mothers or infants, and insufficient dietary sources of bioavailable iron, but lack of data on maternal iron status limits the study’s ability to determine the causes of infant ID. Other study limitations include lack of information on cord clamping and micronutrient deficits other that ID and data on illnesses for only half the infants. To facilitate comparisons across studies, the cutoff approach we used to define ID at 9 months is similar to what we used previously. However, there is debate about appropriate cutoffs (Domellöf, Dewey, Lönnerdal, Cohen, & Hernell, 2002) and studies vary. Despite these limitations, our results point to effects of prenatal ID on fetal brain maturation and recurrent ID on subsequent development. The present study adds an electrophysiological measure to the growing body of behavioral data on alterations in social–emotional outcomes in relation to ID in infancy.
Acknowledgments
This study was part of a NIH-supported cross-species program project (Brain and Behavior in Early Iron Deficiency, P01 HD039386, Betsy Lozoff, Principal Investigator); and a National Natural Science Foundation of China (NSFC) supported grant (Effects of early iron deficiency on child brain and development, No. 81273085, Jie Shao, Principal Investigator).
Contract grant sponsor: NIH
Contract grant number: P01 HD039386
Contract grant sponsor: National Natural Science Foundation of China (NSFC)
Contract grant number: 81273085
The authors thank the infants and families who participated in the study and all team members in Children’s Hospital of Zhejiang University School of Medicine and at the Center for Human Growth and Development for their assistance with data collection and data reduction.
Footnotes
Disclosure: None of the authors had any personal or financial conflicts of interest.
References
- Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in >= 35 weeks gestational age infants. Journal of Pediatrics. 2013;163:1267–1271. doi: 10.1016/j.jpeds.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. Journal of Perinatology. 2004;24:757–762. doi: 10.1038/sj.jp.7211178. [DOI] [PubMed] [Google Scholar]
- Armony-Sivan R, Kaplan-Estrin M, Jacobson SW, Lozoff B. Iron-deficiency anemia in infancy and mother-infant interaction during feeding. Journal of Developmental and Behavioral Pediatrics. 2010;31:526–532. doi: 10.1097/DBP.0b013e3181dc525d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J. Iron deficiency alters brain development and functioning. Journal of Nutrition. 2003;133:1468S–1472S. doi: 10.1093/jn/133.5.1468S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behavioral Brain Research. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Buss KA, Schumacher JRM, Dolski I, Kalin NH, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Healthy People—2000 National Health Promotion and Disease Prevention Objectives Final Review. Hyattsville, MD: Department of Health and Human Services; 2001. [Google Scholar]
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morbidity and Mortality Weekly Report. 1998;47:1–29. [PubMed] [Google Scholar]
- Chang S, Wang L, Wang Y, Brouwer ID, Kok FJ, Lozoff B, Chen C. Iron-deficiency anemia in infancy and social emotional development in preschool-aged Chinese children. Pediatrics. 2011;127:e927–e933. doi: 10.1542/peds.2010-1659. [DOI] [PubMed] [Google Scholar]
- Chen X, Rubin KH. Culture and socioemotional development. In: Chen X, Rubin KH, editors. Socio-emotional development in cultural context. New York, NY: Guilford Press; 2011. pp. 1–8. [Google Scholar]
- Cook JD, Finch CA. Assessing iron status of a population. American Journal of Clinical Nutrition. 1979;32:2115–2119. doi: 10.1093/ajcn/32.10.2115. [DOI] [PubMed] [Google Scholar]
- Corapci F, Calatroni A, Kaciroti N, Jimenez E, Lozoff B. Longitudinal evaluation of externalizing and internalizing behavior problems following iron deficiency in infancy. Journal of Pediatric Psychology. 2010;35:296–305. doi: 10.1093/jpepsy/jsp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition & Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Dawson G, Klinger LG, Panagiotides H, Hill D, Spieker S. Frontal lobe activity and affective behavior of infants of mothers with depressive symptoms. Child Development. 1992;63:725–737. [PubMed] [Google Scholar]
- Domellöf M, Dewey KG, Lönnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. Journal of Nutrition. 2002;13:3680–3686. doi: 10.1093/jn/132.12.3680. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right. Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. Monographs of the Society for Research in Child Development. 1994;59:152–166. [PubMed] [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10-month-old infants. Developmental Psychology. 1988;24:230–236. [Google Scholar]
- Gartstein MA, Gonzalez C, Carranza JA, Ahadi SA, Ye R, Rothbart MK, Yang SW. Studying cross-cultural differences in the development of infant temperament: People’s Republic of China, the United States of America, and Spain. Child Psychiatry and Human Development. 2006;37:145–161. doi: 10.1007/s10578-006-0025-6. [DOI] [PubMed] [Google Scholar]
- Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutrition Reviews. 2011;69:S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Henderson HA, Marshall PJ. Behavioral reactivity and approach-withdrawal bias in infancy. Developmental Psychology. 2008;44:1491–1496. doi: 10.1037/a0012855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS statistics for Windows, version 21. 0. Armonk NY: IBM Corp; 2012. [Google Scholar]
- Jones NA, Field T, Almeida A. Right frontal EEG asymmetry and behavioral inhibition in infants of depressed mothers. Infant Behavior and Development. 2009;32:298–304. doi: 10.1016/j.infbeh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Jones NA, Field T, Fox NA, Davolos M, Gómez C. EEG during different emotions in 10-month-old infants of depressed mothers. Journal of Reproductive and Infant Psychology. 2001;19:295–312. [Google Scholar]
- Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. Journal of Nutritional Biochemistry. 2014;25:1101–1107. doi: 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. Journal of Nutrition. 2011;141:740S–746S. doi: 10.3945/jn.110.131169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Castillo M, Clark KM, Smith JB, Sturza J. Iron supplementation in infancy contributes to more adaptive behavior in 10-year-old children. Journal of Nutrition. 2014;144:838–845. doi: 10.3945/jn.113.182048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. Journal of Pediatrics. 2008;152:696–702. doi: 10.1016/j.jpeds.2007.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Smith J, Clark KM, Perales CG, Rivera F, Castillo M. Home intervention improves cognitive and social-emotional scores in iron-deficient anemic infants. Pediatrics. 2010;126:e884–e894. doi: 10.1542/peds.2009-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: Outcomes at 25 years. Journal of Pediatrics. 2013;163:1260–1266. doi: 10.1016/j.jpeds.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlab and Statistics Toolbox. Natick, Massachusetts: The MathWorks, Inc; 2012. [Google Scholar]
- McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer B, Kling PJ. Impact of multiple prenatal risk factors on newborn iron status at delivery. Journal of Pediatric Hematology/Oncology. 2013;35:473–477. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarinen UM, Siimes MA. Serum ferritin in assessment of iron nutrition in healthy infants. Acta Paediatrica. 1978;67:745–751. doi: 10.1111/j.1651-2227.1978.tb16254.x. [DOI] [PubMed] [Google Scholar]
- Shao J, Lou J, Rao R, Georgieff MK, Kaciroti N, Felt BT, … Lozoff B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. Journal of Nutrition. 2012;142:2004–2009. doi: 10.3945/jn.112.162362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Miller M, Soldin SJ. Pediatric reference ranges for zinc protoporphyrin. Clinical Biochemistry. 2003;36:21–25. doi: 10.1016/s0009-9120(02)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus RJ. Defining iron-deficiency anemia in public health terms: A time for reflection. Journal of Nutrition. 2001;131:565S–567S. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. Journal of Pediatrics. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Sandstead HH, Penland JG, Dawson SL, Milne DB. Iron status and brain function: Serum ferritin levels associated with asymmetries of cortical electrophysiology and cognitive performance. The American Journal of Clinical Nutrition. 1984;39:105–113. doi: 10.1093/ajcn/39.1.105. [DOI] [PubMed] [Google Scholar]
- Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, Connor JR, … Felt B. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. Journal of Nutrition. 2012;142:2040–2049. doi: 10.3945/jn.112.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. Journal of Nutrition. 2007;137:118–124. doi: 10.1093/jn/137.1.118. [DOI] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, Kovacs M, George CJ. Long-term stability of electroencephalographic asymmetry and power in 3 to 9 year-old children. International Journal of Psychophysiology. 2008;67:70–77. doi: 10.1016/j.ijpsycho.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Developmental Psychobiology. 2005;46:141–153. doi: 10.1002/dev.20049. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. Geneva, Switzerland: WHO Press; 2008. [Google Scholar]
- Youdim MB, Yehuda S. The neurochemical basis of cognitive deficits induced by brain iron deficiency: Involvement of dopamine-opiate system. Cellular and Molecular Biology. 2000;46:491–500. [PubMed] [Google Scholar]