Abstract
In this issue of Cancer Cell, Sflomos et al. (2016) describe a robust preclinical animal model of ER+ breast cancer. The authors identify the critical role of the breast microenvironment in determining hormone response of ER+ breast cancer cells and in driving the luminal phenotype of breast cancer.
Estrogen receptor positive (ER+) breast cancer is one of the most commonly diagnosed malignancies among women in the US and is rapidly becoming one of the most commonly diagnosed malignancies in women globally. Due to its prevalence, ER+ breast cancer is also a leading cause of cancer-related death around the world. ER+ breast cancer, as the name suggests, expresses estrogen receptor, which responds to estrogen by inducing, among other things, proliferation and evasion of apoptosis. Classification of breast cancer into ER+ and ER− was the earliest refinement in breast cancer diagnosis, and targeting the ER+ subset of breast cancer through endocrine therapy was one of the earliest targeted therapeutic strategies for breast cancer. While the majority of ER+ breast tumors respond to anti-estrogen therapies, a striking one-third of these tumors relapse or fail to respond. Globally, ER+ breast cancer accounts for more than 400,000 deaths each year. Therefore, research into the mechanisms of ER+ disease escaping endocrine therapy is critical to the development of more effective targeted therapies for this subset of breast cancer.
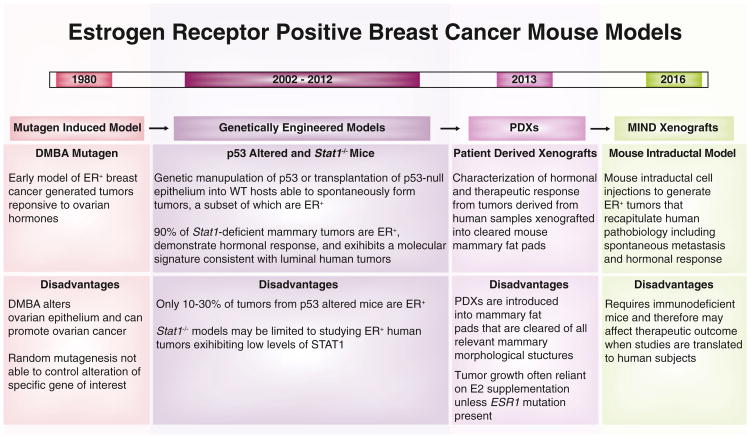
A major road block to understanding ER+ breast cancer and its resistance to endocrine therapy is the lack of physiologically relevant animal models to study this subset (Mohibi et al., 2011). The earliest animal models of ER+ breast cancer used the chemical carcinogen DMBA to induce tumorigenesis and were established in the 1980s (Medina et al., 1980) (Figure 1). The majority of DMBA-induced tumors are ER+ and respond to estrogen stimulation. While this model was extensively used to study ER+ breast cancer initially, it precluded the study of tumors initiated by specific genetic events, since tumors in this model are presumably caused by random chemical mutagenesis. This led to a search for genetically engineered mice (GEM) that could generate ER+ breast tumors.
Figure 1. Historical Perspective of Animal Models of ER+ Breast Cancer.
Timeline provides chronological context to the nearly 20-year-long refinement of animal models for the study of ER+ breast cancer, one of the leading contributors to cancer-related death worldwide. Animal models are broadly classified as mutagen-induced, genetically engineered, and xenografts, and brief descriptions of their principal characteristics and associated disadvantages are provided in the text blocks below each classification. DMBA, 7,12-dimethylbenz(A)anthracene; E2, 17-beta-estradiol; ER, estrogen receptor; ESR1, estrogen receptor 1 gene; MIND, mouse intraductal model; PDX, patient-derived xenograft.
Unfortunately, the majority of GEM models generate ER− breast tumors, suggesting that most of these mouse models fail to reproduce the steroid signaling required for the generation of ER+ breast cancer, in spite of the similarities in steroid hormone signaling between mice and humans. The best-known GEM for ER+ breast cancer is the Trp53 null mouse (Medina et al., 2002). p53 is a well-known tumor suppressor that is inactivated in almost all cancer types, albeit with various frequencies, including breast cancer, making this model system clinically relevant. However, since Trp53−/− causes development of lymphomas in mice, there are several technical challenges in using this mouse model (Figure 1). The most commonly used variation to combat the lymphoma phenotype is a transplantation of the Trp53−/− mammary epithelia into the cleared mammary fat pads of Trp53+/+ mice. However, only 11% of these transplants result in ER+ breast cancer, making this model system difficult to use for preclinical studies of ER+ breast cancer. Moreover, TP53 is frequently inactivated in ER− human breast cancer but is only inactivated in ∼30% of human ER+ breast tumors, highlighting the need to develop a more representative and reliable ER+ breast cancer model.
In 2012, theStat1−/− mouse model was reported to reliably generate ER+ breast tumors that are hormone responsive and depend on steroid signaling for their growth (Chan et al., 2012). Moreover, gene expression profiling of these tumors indicated that they closely resemble human luminal breast cancer. This recently developed mouse model is the closest approximation to a sound preclinical animal model for ER+ breast cancer, but it may be limited by the biology “locked in” by the STAT1 deficiency (Figure 1).
In parallel to the generation of these various GEM models, several researchers attempted the use of intraductal injection of oncogenes in viral constructs into the mouse mammary duct as a mean of producing clinically relevant models of breast cancer (Fisher et al., 1999). One of the chief concerns with GEM models is the prevalence of the oncogenic insult in almost every cell of the mouse mammary gland, a phenomenon that is not reflective of sporadic human breast tumorigenesis, where a few cells provide the initiating impetus for the tumor. Using intraductal injections of constructs expressing the well-known oncoprotein HER2, ER+ hormone-responsive breast tumors can be consistently generated from immune-proficient mice (Du et al., 2006). However, ER+/HER2+ breast cancers are a minority subset of human ER+ breast cancers.
Cell lines from human breast tumors have long been used for studying ER+ breast cancer. However, the artificial environment in which these cells are grown and the long time that these cells have spent in culture conditions precludes them from being used as a gold standard for therapeutics or translational breast cancer research. The use of xeno-graft models, in which ER+ human breast cancer cells are injected either under the skin or into the mouse mammary stroma, has been adopted as a more convincing readout for clinically relevant phenotypes. However, these ER+ breast cancer cells do not grow in mouse mammary stroma without the implantation of estrogen pellets that produce 18–40 times the physiological levels of estrogen in mice. The reason why ER+ breast cancer cells do not grow under physiological hormone levels in mice has remained elusive until the study by Sflomos and colleagues published in this issue of Cancer Cell (Sflomos et al., 2016). Their discovery that human ER+ breast cancer cells implanted into the mouse mammary stroma respond to the high levels of TGF-β through basal reprogramming provides an answer to this puzzle. Most importantly, the results of this paper indicate a means to overcome this problem by using intraductal injections to introduce ER+ breast cancer cells directly into the mouse mammary duct, to more closely approximate the natural environment of these cells. Additionally, the MIND model system is characterized by spontaneous metastasis from ER+ breast cancer cells, opening yet another avenue for preclinical research (Figure 1).
Sflomos and colleagues then extend this technique to generate patient-derived xenografts (PDXs) by injecting primary human ER+ breast tumor tissue directly into the mouse mammary duct. Breast PDXs are considered a breakthrough in clinically relevant breast cancer research (Li et al., 2013) (Figure 1). These PDXs, implanted in the mouse mammary stroma, are demonstrably faithful to the primary tumors that they arise from and are heralded as useful real-time determinants of therapeutic efficacy for patients in the near future. However, most successful PDX tumors so far have been from ER− primary breast tumor tissue. In contrast, ER+ PDX have been largely generated from treatment-resistant, estrogen-independent metastatic tumors. Sflomos et al. identify the reason for this discrepancy and demonstrate that ER+ PDXs from primary tissue can be transplanted without estrogen supplementation into immunocompromised mice via intraductal injections, allowing them to maintain ER signaling and response to steroid hormones and endocrine therapy. This finding is a potential game-changer for breast cancer research, and we predict that it will likely translate into new therapeutic strategies for ER+ breast cancer in the near future.
References
- Chan SR, Vermi W, Luo J, Lucini L, Rickert C, Fowler AM, Lonardi S, Arthur C, Young LJ, Levy DE, et al. Breast Cancer Res. 2012;14:R16. doi: 10.1186/bcr3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Podsypanina K, Huang S, McGrath A, Toneff MJ, Bogoslovskaia E, Zhang X, Mo-raes RC, Fluck M, Allred DC, et al. Proc Natl Acad Sci USA. 2006;103:17396–17401. doi: 10.1073/pnas.0608607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Orsulic S, Holland E, Hively WP, Li Y, Lewis BC, Williams BO, Varmus HE. Oncogene. 1999;18:5253–5260. doi: 10.1038/sj.onc.1203087. [DOI] [PubMed] [Google Scholar]
- Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, He X, Liu S, Hoog J, Lu C, et al. Cell Rep. 2013;4:1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D, Butel JS, Socher SH, Miller FL. Cancer Res. 1980;40:368–373. [PubMed] [Google Scholar]
- Medina D, Kittrell FS, Shepard A, Stephens LC, Jiang C, Lu J, Allred DC, McCarthy M, Ullrich RL. FASEB J. 2002;16:881–883. doi: 10.1096/fj.01-0885fje. [DOI] [PubMed] [Google Scholar]
- Mohibi S, Mirza S, Band H, Band V. J Carcinog. 2011;10:35. doi: 10.4103/1477-3163.91116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sflomos G, Dormoy V, Metsalu T, Jeitziner T, Battista L, Treboux A, Delaloye JF, Fiche M, Vilo J, Ayyanan A, et al. Cancer Cell 29. 2016;29:407–422. doi: 10.1016/j.ccell.2016.02.002. this issue. [DOI] [PubMed] [Google Scholar]