Auxins and auxin transport inhibitors affect PIN-FORMED2 (PIN2) dynamics in Arabidopsis epidermal root cells through the synthetic and secretion pathway, but they do not inhibit PIN2 endocytosis.
Abstract
By using the photoconvertible fluorescence protein Dendra2 as a tag we demonstrated that neither the naturally occurring auxins indole-3-acetic acid and indole-3-butyric acid, nor the synthetic auxin analogs 1-naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid nor compounds inhibiting polar auxin transport such as 2,3,5-triiodobenzoic acid and 1-N-naphthylphthalamic acid, were able to inhibit endocytosis of the putative auxin transporter PIN-FORMED2 (PIN2) in Arabidopsis (Arabidopsis thaliana) root epidermis cells. All compounds, except Indole-3-butyric acid, repressed the recovery of the PIN2-Dendra2 plasma membrane pool after photoconversion when they were used in high concentrations. The synthetic auxin analogs 1-naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid showed the strongest inhibition. Auxins and auxin transport inhibitors suppressed also the accumulation of both newly synthesized and endocytotic PIN2 pools in Brefeldin A compartments (BFACs). Furthermore, we demonstrated that all compounds are also interfering with BFAC formation. The synthetic auxin analogs caused the highest reduction in the number and size of BFACs. We concluded that auxins and inhibitors of auxin transport do affect PIN2 turnover in the cells, but it is through the synthetic rather than the endocytotic pathway. The study also confirmed inappropriateness of the BFA-based approach to study PIN2 endocytosis because the majority of PIN2 accumulating in BFACs is newly synthesized and not derived from the plasma membrane.
Many developmental processes are dependent on the spatiotemporal distribution of active auxins, mainly on their common representative indole-3-acetic acid (IAA; Vanneste and Friml 2009; Leyser 2010; Gallavotti 2013). Auxin synthesis, degradation, and conjugation pathways determining the net balance of auxin in tissues have been intensively studied for many decades (Korasick et al., 2013; Ljung, 2013). Recently, much attention has been paid to the transport of auxins in whole plants as well as from cell to cell. Several putative auxin transporters were detected and characterized. Among those the members of the PIN-FORMED protein family (PIN proteins) are regarded as important carriers acting in the efflux of auxin from cells (for reviews, see Zažímalová et al., 2010; Grunewald and Friml, 2010; Geisler et al., 2014; Pan et al., 2015; Bennett, 2015; Strader and Zhao, 2016). Some PIN family members, such as PIN1, 2, 3, 4, and 7, are localized in the plasma membrane (PM) and determine developmental processes by regulating the distribution of auxin and its local accumulation in the plant body (Křeček et al., 2009; Vanneste and Friml, 2009; Grunewald and Friml, 2010). The PM PINs are believed to cycle rapidly and continuously between the PM and the endomembrane system (Geldner et al., 2001, 2003; Paciorek et al., 2005; Abas et al., 2006; Dhonukshe et al., 2007; Kleine-Vehn et al., 2008a, 2008b; Robert et al., 2010). Brefeldin A (BFA), a fungal lactone metabolite, was routinely used in these studies. It interacts with guanine nucleotide exchange factors and inhibits the function of its associated ADP ribosylation factor GTPase in membrane trafficking (Jackson and Casanova, 2000). According to the generally used scenario, BFA interrupts continuous cycling of PINs between the PM and endosomes by inhibiting resecretion of the endocytic PIN populations back to the cell surface [Kleine-Vehn and Friml (2008) for review]. Therefore PINs (also other plasma membrane proteins) are accumulated in distinct large multivesicular structures called BFA compartments (BFAC) or BFA bodies (Robinson et al., 2008). Because PINs gathered in BFACs were thought to originate exclusively from the PM, the occurrence of PINs in BFACs was considered as a reliable indirect indicator of their endocytosis. In fact the assessment of BFAC characteristics, such as size and number, and abundance/absence of PINs in BFACs were utilized in many studies on PINs endocytosis (Geldner et al., 2001; Paciorek et al., 2005; Dhonukshe et al., 2007; Abas et al., 2006; Sun et al., 2011; Robert et al., 2010). According to Chen et al. (2012), endocytosis of PIN proteins is mediated by a clathrin-dependent mechanism. Plant growth regulators and environmental factors were proposed to regulate this process. Notably, 1-naphthaleneacetic acid (1-NAA), a synthetic plant growth regulator of the auxin family, and also other auxins should inhibit endocytosis of PIN protein family members and some other plasma membrane proteins and consequently increase their relative abundance at the cell surface (Paciorek et al., 2005). The authors concluded that this feedback regulation was crucial for auxin-controlled growth responses. Also compounds inhibiting the polar auxin transport such as 2,3,5-triiodobenzoic acid (TIBA) and 1-N-naphthylphthalamic acid (NPA) were shown to block the intracellular accumulation of PIN1 in distinct bodies (Geldner et al., 2001), but according to the authors these compounds leave PIN1 abundance at the plasma membrane unaffected. Later on, a putative auxin receptor known as AUXIN-BINDING PROTEIN1 (ABP1) was characterized as a positive factor in clathrin recruitment to the plasma membrane, thereby promoting endocytosis (Robert et al., 2010). Consequently, binding of auxin to ABP1 should inhibit clathrin-mediated endocytosis of membrane proteins including PIN auxin transporters (Robert et al., 2010; Xu et al., 2010, 2014; Chen et al., 2012; Lin et al., 2012). However, more recently Gao et al. (2015) has found that ABP1 is not a key component in auxin signaling during Arabidopsis (Arabidopsis thaliana) development. Surprisingly, two null Arabidopsis abp1 mutants generated by the authors using ribozyme-based CRISPR and T-DNA insertion technologies do not show phenotype defects described in the case of other knockdown/knockout abp1 alleles. In addition, it has been demonstrated that phenotypes observed in the Arabidopsis abp1-1 and abp1-5 alleles might not be caused by the disruption of ABP1 (Dai et al., 2015; Enders et al., 2015). These findings call into question the results of previous publications describing a prominent role of ABP1 in embryogenesis, growth, cell division, cell expansion, and auxin signaling [see Liu (2015) and Strader and Zhao (2016) for a commentary]. It is also surprising that the inhibition effect of 1-NAA on endocytosis was observed only after a short (5 min to 60 min) but not after a long (2 h) pretreatment (Robert et al., 2010). Additionally, high concentrations of 1-NAA were applied (Paciorek et al., 2005; Abas et al., 2006; Robert et al., 2010) that may be not specific for auxin signaling. Finally, the naturally occurring auxin IAA, which logically should be the main cargo of PINs, was less effective in the endocytosis inhibition (Paciorek et al., 2005). It is worth noting that GFP tagging or immunocytochemistry methodologies, which were used in earlier studies in combination with application of pharmacological compounds, including BFA, showed only net protein abundance in the cellular compartments, but intracellular dynamics of internalized and newly synthesized protein populations were hidden. Recently we used an approach employing the green-to-red photoconvertible Dendra2 fluorescent protein developed by Gurskaya et al. (2006) that allows the simultaneous quantification of the abundance and dynamics of the currently present and the newly synthesized PIN2 populations in cell compartments (Jásik et al., 2013). We have demonstrated that the appearance of PIN2 protein in BFACs is an inappropriate way to study protein endocytosis (Jásik and Schmelzer, 2014). In fact, PIN2 is only temporarily gathered in BFACs and the majority of the protein occurring in these structures is newly synthesized. The above-mentioned discrepancies prompted us to perform a more complex study on the influence of different auxins and auxin transport inhibitors on PIN2 intracellular dynamics using the photoconvertible fluorescence protein method.
RESULTS
1-NAA Pretreatment Does Not Increase PIN2 Abundance in the PM but Interferes with Development of BFACs
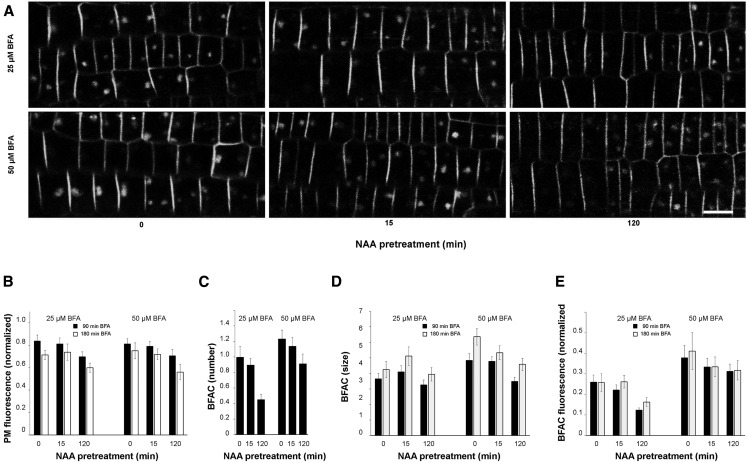
In our earlier study (Jásik et al., 2013), we demonstrated that Dendra2 as a tag is not photoconverted under standard confocal imaging conditions using the 488 nm line of the Argon laser for excitation. Therefore, Dendra2 can be used as a classical fluorescence protein tag similar to GFP. In the pivotal testing, we used the PIN2-Dendra2 line of Arabidopsis in experiments without photoconversion of the PIN2-Dendra2 fusion protein to confirm previous results with 1-NAA. We pretreated seedlings with a dose of 10 μM 1-NAA, which was used also in earlier studies with PIN2 (Paciorek et al., 2005; Robert et al., 2010) for 15 min or 120 min and then kept them on medium containing 25 μM or 50 μM BFA. Representative images taken after 1.5 h and 3 h BFA treatment are shown in Figure 1A. The results demonstrate that, under our experimental conditions, 1-NAA pretreatment does not increase the PIN2 amount in the PM when compared with untreated samples and that BFACs, visualized by PIN2-Dendra2, are apparent in the cells. We quantified the PIN2-Dendra2 protein abundance in the PM by measuring the fluorescence intensity and monitored the development of BFACs and the occurrence of PIN2-Dendra2 inside them. We did not record a relative increase of abundance of PIN2 in the PM of root epidermal cells, but in comparison with seedlings not treated with 1-NAA, instead detected a slight reduction of fluorescence signal intensity. This effect was obvious mainly after long 1-NAA pretreatment (Fig. 1B). When we analyzed the development of BFACs, we found that numerous well-formed PIN2-Dendra2 positive bodies were always present in root epidermal cells despite 1-NAA pretreatment. However, quantitative analysis showed that 1-NAA decreased the number of BFACs per cell (Fig. 1C), the size of BFACs (Fig. 1D), and the abundance of PIN2 protein in BFACs (Fig. 1E). In all cases, 120 min 1-NAA pretreatment resulted in a stronger inhibitory effect than the shorter 15 min 1-NAA application. For comparison, we used the PIN2-GFP line (Xu and Scheres, 2005), which was employed in earlier studies. The results were very similar to those obtained with the PIN2-Dendra2 line (Supplemental Fig. S1).
Figure 1.
Influence of 1-NAA of PIN2 dynamic in PM and BFACs. Seedlings were pretreated with 10 µM 1-NAA for the indicated period, imaged and placed on the medium with BFA and reimaged after 1.5 h and 3 h. Representative images captured after 3 h BFA treatment are shown in (A). Note that 1-NAA pretreatment does not increase the abundance of PIN2 in the PM (B) but inhibits slightly the BFAC development and PIN2 accumulation in the BFACs.
1-NAA Suppresses Delivery of Newly Synthetized PIN2 to the PM and Accumulation of PIN2 Populations in BFACs
In our earlier study (Jásik and Schmelzer, 2014), we showed that both the newly synthesized and the internalized PIN2 population were gathered in BFACs and that the newly synthesized PIN2 form was even predominant. As 1-NAA pretreatment reduces the abundance of PIN2 in the PM as well as inhibits the accumulation of PIN2 within BFACs, we wished to find out which population, the newly synthesized or the one of plasma membrane origin, is responsible for this decrease in abundance of PIN2 in the PM and BFACs. Because photoconversion of Dendra2 is an irreversible process (Chudakov et al., 2007), this fluorescent protein is a suitable tool for quantitative monitoring of the two PIN2 populations in the PM and in BFACs (Jásik et al., 2013; Jásik and Schmelzer, 2014). The speed of PIN2-Dendra2 fusion protein internalization can be estimated by measuring the red signal intensity in the PM and, vice versa, the degree of the PIN2 PM pool recovery can be determined by evaluating the green fluorescence intensity in the PM. We pretreated seedlings again with 10 μM 1-NAA for either 15 min or 120 min and then, after photoconversion of the fusion PIN2-Dendra2 protein, we kept them on medium with 50 μM BFA and without or with 10 μM 1-NAA. We failed to observe a delay in the disappearing of the red signal in the PM after 1-NAA pretreatment, or even an increase in its intensity in the PM as a consequence of expected inhibition of PIN2 endocytosis (Fig. 2). We actually noted a relative slight decrease of signal strength mainly when seedlings were kept permanently on the medium supplemented with 1-NAA. When we analyzed the green signal in the PM we found inhibition of green signal recovery in all experiments. This effect was evident mainly when seedlings were permanently growing on the medium with 1-NAA or pretreated with 1-NAA for the long 2 h period (Fig. 2). When we analyzed signal intensities in BFACs, we found a similar tendency i.e. reducing of both green and red signal intensities in comparison with 1-NAA untreated samples (Fig. 2). Permanent 1-NAA treatment and long 2 h 1-NAA pretreatment showed more obvious effects than short 15 min 1-NAA pretreatment. The finding that 1-NAA is not able to inhibit endocytosis of PIN2, was confirmed in a modified photoconversion experiment. In this case, we firstly photoconverted PIN2-Dendra2 in roots and after that, we kept seedlings under standard cultivation conditions for 2 h. As expected in the context of our previous studies (Jásik et al., 2013; Jásik and Schmelzer, 2014), after 2 h, a significant part of the PM PIN2 population was already replaced by the newly synthesized green fluorescing form (Fig. 3). However, the red fluorescing population was still predominant because the PM half-life time of PIN2 is approximately 3.5 h (Jásik et al., 2013). These seedlings were treated with 10 μM 1-NAA for 15 min, then imaged and cultivated on medium with 50 µM BFA. When we analyzed in this experiment the red and green fluorescence signal intensities in the PM after 1.5 h and 3 h BFA treatment, we found a similar decrease of the red signal intensity in 1-NAA-treated and control samples and a slight inhibition of the green signal recovery in the PM when 1-NAA was applied (Fig. 3). When we examined the green and red signal intensities in BFACs of the control samples not treated with 1-NAA, we found a clear predominance of the green over the red signal (Fig. 3) despite the opposite situation in the PM at time point 0 h. This experiment confirms our previous finding (Jásik and Schmelzer, 2014) that BFA compartments accumulate mainly the newly synthesized PIN2 population. In 1-NAA-treated seedlings, the fluorescence intensities of both red and green signals in BFACs were significantly reduced (Fig. 3) when compared with control seedlings.
Figure 2.
Effect of 1-NAA on dynamics of green and red PIN2 populations in the PM and BFACs. Seedlings were pretreated with 10 µM 1-NAA for either 15 min or 120 min. Roots were imaged, then they were illuminated to photoconvert the PIN2-Dendra2 fusion protein at which point they were imaged again and treated with 50 μM BFA in the presence or absence of 10 µM 1-NAA. Samples were reimaged again after 1.5 h and 3 h. 1-NAA was not able to suppress PIN2 endocytosis (red signal intensity falling in the PM) but inhibits PIN2 delivery to the PM (green signal recovery in PM). 1-NAA inhibits also an accumulation of both PIN2 populations in BFACs.
Figure 3.
Dynamics of red and green PIN2 populations in the PM and BFACs after a short 1-NAA treatment. At the beginning of the experiment roots were illuminated to photoconvert PIN2-Dendra2 fusion protein, then seedlings were cultivated on the 1/2 MSMO medium and after 2 h they were treated with 10 µM 1-NAA for 15 min. The roots were imaged (time point 0), then seedlings were placed on the medium with 50 µM BFA and after 1.5 h and 3 h BFA treatment the roots were reimaged. Note that short 1-NAA treatment does not cause inhibition of red signal vanishing from the PM, but it does suppress accumulation of both PIN2 populations in BFACs.
1-NAA Is Not Affecting PIN2 Endocytosis at Any Concentration
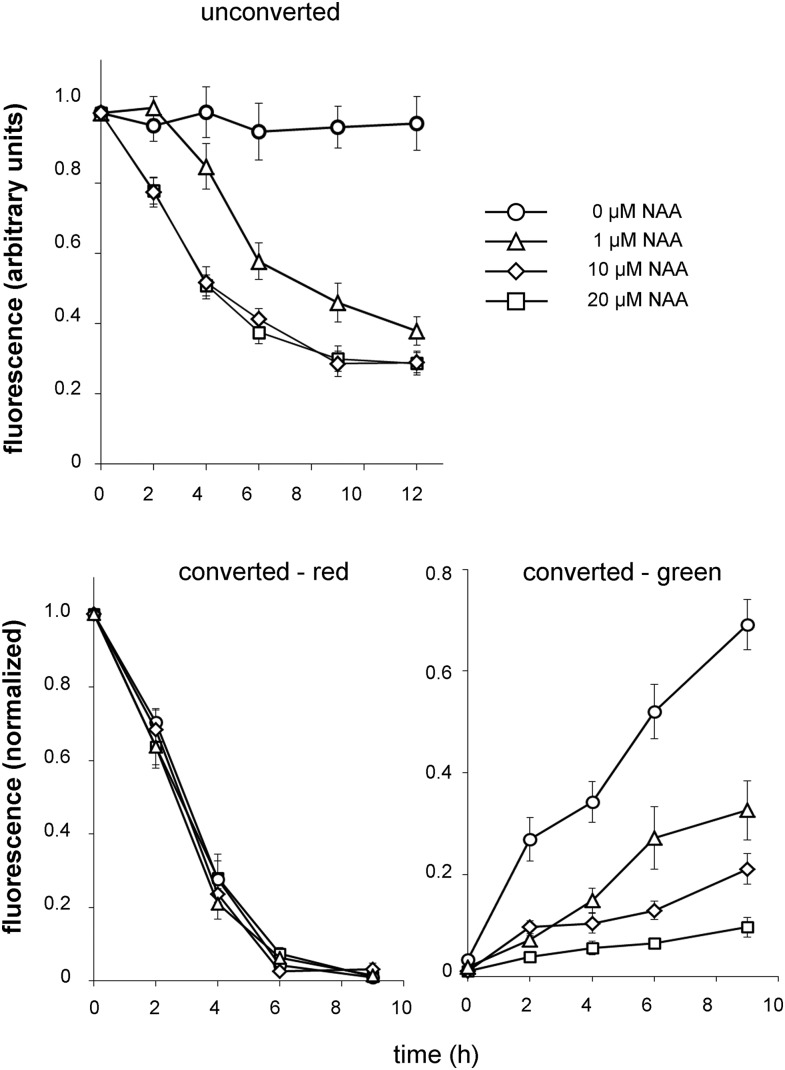
For complementation, we wished to test whether the inhibitory effect of 1-NAA on endocytosis occurs at other 1-NAA concentrations. Because 1-NAA pretreatment showed only a weak effect on the PIN2 dynamic in the PM and BFACs, we kept, in further experiments, seedlings permanently on auxins. At first, we performed an experiment without photoconversion by using 0,1, 10, or 20 μM 1-NAA. We observed a clearly decreased signal intensity in the PM on media supplemented with 1-NAA and this decrease was 1-NAA concentration-dependent (Fig. 4). Then, we carried out the same experiment, but in the photoconversion mode. In the time-lapse experiment, we analyzed the red and green signal intensities in the PM. The dose-response curves for the red signal were almost superimposable, indicating that there was not a significant difference in the response to different 1-NAA concentrations (Fig. 4). This result suggests that 1-NAA was not affecting PIN2 endocytosis at any concentration. When we analyzed recovery of the green signal in the PM, we observed a strong inhibition of this process, which was dependent on the 1-NAA concentration (Fig. 4).
Figure 4.
Concentration-dependent effect of 1-NAA on PIN2 abundance in the PM. In the photoconverted experiment seedlings were pretreated with 1-NAA of different concentrations for 30 min, then the roots were imaged, illuminated to photoconvert Dendra2, again imaged, and subsequently seedlings were kept on medium with the 1-NAA at the concentration used during pretreatment for the time-lapse experiment. Note that an evident decreasing of a PIN2 abundance in PM observed in an experiment without conversion (unconverted) is due to a suppression of a delivery of the newly synthesized green PIN2-Dendra2 population into the PM (converted - green), while releasing of the red PIN2-Dendra2 population from the PM (converted - red) is not affected.
Auxins and Inhibitors of Polar Auxin Transport Affect Root Growth
In earlier studies, the synthetic auxins 1-NAA and 2,4-dichlorophenoxyacetic acid (2,4-D) showed strong inhibitory effects, but the naturally occurring auxin IAA was less effective in blocking PIN2 endocytosis (Paciorek et al., 2005). The authors suggested that the IAA was quickly degraded. Furthermore, in the case of PIN FORMED1 (PIN1), which is a close homolog of PIN2 but with a different expression pattern, auxin transport inhibitors also suppressed the protein cycling. Therefore, we examined other auxins, i.e. naturally occurring auxins such as indole-3-butyric acid (IBA) and IAA, the synthetic analogs 2,4-D and 1-NAA, and the inhibitory compounds NPA and TIBA. In the root elongation assay, we could show that all auxins and both inhibitors of polar auxin transport had an expected effect on root growth (Supplemental Fig. S2). We observed a very strong inhibition effect on root growth during the experiment mainly in the case of 1-NAA and 2,4-D, while IBA, NPA, and TIBA showed intermediate effects. IAA was strongly inhibitory on d 1 of treatment, but from d 2 on, the root growth started to accelerate. Such a pattern of IAA action is expected as it has been well documented that active IAA is gradually degraded, conjugated, or stored in vacuoles over time (de Klerk et al., 2008; Novák et al., 2012; Ranocha et al., 2013; Pěnčík et al., 2013). TIBA and NPA showed agravitropic effects.
Auxins and Auxin Transport Inhibitors Do Not Affect PIN2 Endocytosis
In the experiment on the inhibition effects of compounds concerning PIN2 cycling we pretreated seedlings for 30 min with auxins or auxin transport inhibitors and after photoconversion of the PIN2-Dendra2 we kept them on medium supplemented with the same compound and 50 µM BFA. Simultaneously the test was carried out without BFA. We have used high concentrations of the substances as such have been used in earlier studies on the inhibition of PIN protein endocytosis. Results of a time-lapse experiment for all auxins and inhibitors of polar auxin transport are summarized in Figure 5. In the experiment without BFA, we observed no effect of any auxin or inhibitors of polar auxin transport on the decreasing of red signal intensity in the PM. When we analyzed the recovery of green signal in the PM, we observed significant differences between compounds. The synthetic auxin 2,4-D inhibited green signal recovery in the PM almost completely (Figs. 5B and 6B), 1-NAA was also strongly inhibitory (Fig. 5B) followed by the natural auxin IAA (Fig. 5C) and the inhibitors TIBA and NPA (Fig. 5D), while another natural auxin, IBA, showed no effect (Fig. 5C). In the experiments with BFA, we obtained similar results, but the inhibitory effect of auxins and inhibitors of auxin transport on green signal recovery in the PM was augmented due to the additive inhibitory effect of BFA. When we analyzed intensities of green and red signals in BFACs, we observed a slight effect of IBA (Fig. 5B), but other auxins, such as IAA (Fig. 5B), 1-NAA and 2,4-D (Figs. 5C and 6D), and the inhibitor TIBA (Fig. 5D), suppressed very strongly the accumulation of both PIN2 populations while NPA showed an intermediate effect (Fig. 5D). Furthermore, we investigated whether auxins and auxin transport inhibitors influence BFAC development. All compounds caused an intermediate reduction of the BFAC size (Fig. 7A) and a considerable decrease of BFAC abundance within the cells (Fig. 7B). Especially the synthetic auxins 2,4-D and 1-NAA strongly reduced the number of BFACs per cell; both auxin transport inhibitors showed an intermediate effect and the natural auxins IAA and IBA had the smallest influence.
Figure 5.
Influence of different auxins and inhibitors of auxin transport on PIN2 dynamics in the PM and BFACs. In general, auxins (except IBA) and auxin transport inhibitors inhibited the restoration of the PIN2 population in the PM and repressed the accumulation of both PIN2 populations in BFACs, but did not affect the release of PIN2 from the PM. Auxins and NPA were used at a concentration of 10 μM; TIBA at a concentration of 20 μM. Seedlings were treated with compounds for 30 min. Roots were imaged, then illuminated to photoconvert Dendra2 in the PIN2-Dendra2 fusion protein, at which point they were reimaged and seedlings were moved onto the medium with the same compound and 50 µM BFA for the time-lapse experiment.
Figure 6.
An example of seedling root treated by 2,4-D as described in Fig. 5.
Figure 7.
Sizes and numbers of BFA compartments after the treatment of seedlings with different auxins and auxin transport inhibitors. Seedlings were pretreated with auxins (10 μM) or auxin transport inhibitors, either NPA (10 μM) or TIBA (20 μM) for 30 min and then kept on the medium with the same compound and 50 μM BFA. Sizes of BFACs were estimated after 1.5 h and 5 h BFA treatment (A), numbers of BFACs after 1.5 h treatment (B).
1-NAA Shows Only a Slight Effect on PIN2 Transcript Abundance
Finally, we checked whether the synthetic auxin 1-NAA, which is traditionally used in such studies, and which, in our experiments, showed a strong inhibitory effect on the recovery of the plasma membrane PIN2 pool, had an inhibitory effect already at the transcriptional level. By RT-PCR using three different combinations of PIN2-specific primers, we found that 5 μM 1-NAA caused a slight reduction of PIN2 transcript abundance (Fig. 8). PIN2 expression was suppressed already after 90 min of 1-NAA treatment, but later the RNA transcription remained on the same level.
Figure 8.
RT-PCR comparison of extracts from control and 1-NAA-treated roots using three combinations of PIN2-specific primers. Intensities of bands obtained by RT-PCR were related to band intensity of the reference gene POLYUBIQUITIN10 [At4G05320, Czechowski et al. (2005)]. Note relatively slight decreasing PIN2 transcript abundance in roots treated with 1-NAA.
DISCUSSION
This study, together with our recent articles in which we employed a photoconvertible fluorescence protein technique (Jásik et al., 2013; Jásik and Schmelzer, 2014), provides (to our knowledge) new insights on trafficking of PIN2 protein. Previous opinions and our findings are compared in Figure 9. Earlier studies have suggested that auxins block endocytosis of PIN and other PM proteins (Paciorek et al., 2005; Robert et al., 2010). It was postulated that auxins regulate PIN abundance and activity at the cell surface by modulating protein trafficking, and in this way provide mechanisms for the feedback regulation of auxin transport (Paciorek et al., 2005). Inhibition of endocytosis was demonstrated by direct measurement of PIN2 protein abundance at the PM and indirectly by monitoring of BFAC appearance (Paciorek et al., 2005; Robert et al., 2010). In addition, genetic approaches by altering auxin metabolic or signaling pathways were utilized to support this idea. Indeed Paciorek et al. (2005) stated that the PM pool of PIN2 increased from 62% of the whole PIN2 cell amount to 82% after 1-NAA pretreatment for 30 min. We applied a methodology using the green to red photoconvertible fluorescence protein Dendra2 and monitored the red signal intensity in the PM after photoconversion of the fusion PIN2-Dendra2 protein. In this procedure, the changes of red signal intensity in the PM specifically reflect the internalizing dynamics of the tagged protein PIN2 (Jásik et al., 2013; Jásik and Schmelzer, 2014). In our experiments with PIN2-Dendra2, we were not able to corroborate previous findings on inhibition of PIN endocytosis by auxin. In all experiments with different auxins and auxin transport inhibitory compounds, we failed to observe suppression of red signal disappearance from the PM regardless of whether BFA was applied or not. In addition, after 1-NAA treatment at different concentrations we did not observe such an event of endocytosis inhibition. There are, however, several differences in experiment design, which may be responsible for this discrepancy in the results. Paciorek et al. (2005), claimed that they analyzed cortex cells while in our study epidermal cells were studied. To exclude effects on PIN2 expression, Paciorek et al. (2005) used in their whole study the general inhibitors cordycepin and cycloheximide. These drugs may have side effects, e.g. in our experiments cycloheximide accelerated the PIN2 internalization process in the photoconverted as well as unconverted approaches (Jásik et al., 2013; Jásik and Schmelzer, 2014). The photoconversion technique does not require blocking of the synthesis pathway because newly synthesized PIN2 is emitting green fluorescence and this signal can be recorded simultaneously with the red signal (Jásik et al., 2013). Finally, in the previous study, the authors used immunohistology as a method. Interpretation of data as proportion of signal intensities may be problematic in this procedure. On the other side, our findings are in very good agreement with the results obtained by Sieberer et al. (2000) and Leitner et al. (2012). The authors, by using a translational fusion PIN2-GUS construct (Sieberer et al., 2000) and by comprehensive quantitative western-blot analysis of membrane protein fractions (Leitner et al., 2012), showed a significant reduction of PIN2 in the cells/membranes caused by 1-NAA. PIN2 protein levels started to decrease in membrane protein fractions considerably after 60 min of plant growth regulator treatment, which coincided with an increase of the vacuolar PIN2 signal. This indicates vacuolar targeting of PIN2 and its degradation in response to the 1-NAA treatment. Our mapping of a newly synthesized pool of PIN2 after photoconversion represents, to our knowledge, a new important finding. We demonstrated that 1-NAA and other auxins suppress not only membrane PIN pool recovery, but also significantly reduces the amount of the green PIN2 population in BFACs. Auxins most likely affect some pathway upstream of the gathering of the green PIN2 population in BFACs, i.e. they might induce PIN2 degradation shortly after its proteosynthesis or simply decelerate a step in the PIN2 synthetic pathway. In addition to the impairment of exocytosis pathways by BFA, very strong suppression of the PM PIN2 pool recovery in our experiment with BFA and auxins may also be explained by an additional inhibition effect of BFA on PIN2 synthesis. According to an earlier study by Pan et al. (2009), BFA suppressed PIN2 synthesis approximately 25% at the transcriptional level. Whether auxins and auxin transport inhibitors influence PIN2 transcription (and if so, at which concentrations) is not well understood. 1-NAA at high doses does not seem to regulate PIN2 at this level. We and other authors (Sieberer et al., 2000; Abas et al., 2006) were not able to demonstrate a substantial decrease of PIN2 transcript abundance in the roots after 1-NAA treatment, which could explain the strong reduction of PIN2 protein amount in the PM. Peer et al. (2004) showed strong up-regulation of PIN2 with TIBA and NPA in roots of the Arabidopsis Ler ecotype. The native auxin IAA, however, in a more natural concentration (1 μM) than used in experiments on PIN2 endocytosis inhibition, apparently slightly upregulates PIN2 transcription [Peer et al. (2004) and also the Genevestigator https://genevestigator.com database].
Figure 9.
Schematic illustration of effects of BFA and 1-NAA on intracellular dynamics of PIN2. Previous opinions on PIN2 trafficking are summarized in (A, C, E, and G); our findings obtained by using a photoconvertible fluorescence protein approach are shown in (B, D, F, and H). According to earlier studies, PIN2 cycles quickly and continuously between endosomes and the PM [A, reviewed by Kleine-Vehn and Friml (2008), Vanneste and Friml (2009), and Grunewald and Friml (2010)] but we have failed to confirm the constitutive endocytic recycling of the PIN2 protein [B, Jásik et al. (2013) and Jásik and Schmelzer (2014)]. BFA was believed to block continuous cycling of PIN2 protein by inhibition of the resecretion of the endocytic PIN2 population back to the PM [C, Kleine-Vehn and Friml (2008), Vanneste and Friml (2009), and Grunewald and Friml (2010)]. We have demonstrated that the newly synthesized protein PIN2 population prevails over the endocytic PIN2 pool in BFACs and therefore the appearance of PIN2 in these structures is not a sign of PIN2 endocytosis [D, Jásik and Schmelzer (2014) and this study]. 1-NAA and other auxins should block the PIN2 internalization process and the protein should remain in the PM [E, Paciorek et al. (2005) and Robert et al. (2010)]. BFA compartments that were believed to accumulate exclusively the endocytotic PIN2 population are normally differentiated but not detectable as PIN2 is absent [G, Paciorek et al. (2005) and Robert et al. (2010)]. In this study, we are demonstrating that neither 1-NAA, nor other auxins, nor auxin transport inhibitors, affect PIN2 endocytosis (F); however, delivery of the newly synthesized PIN2 population to the PM (G), development of BFACs, and accumulation of both PIN2 populations inside the BFACs, is impaired (H). Arrows show intracellular trafficking of PIN2; decreased degree of PIN2 trafficking in (D, F, and H) is illustrated by thinner arrow lines. Supposed constitutive cycling of PIN2 between TGN/EE and the PM is shown by arrows forming an ellipse. The x means blocking of process by a compound treatment. BFAC, Brefeldin A compartment; GA, Golgi apparatus; MVB/PV, multivesicular body/provacuole; TGN/EE, trans Golgi network/early endosome.
The second proof of an auxin inhibitory effect on PIN2 endocytosis is provided by the inability to detect BFACs in the cells after auxin application (Paciorek et al., 2005; Robert et al., 2010). Indeed, in many articles on the endocytosis inhibition by various compounds that involve the BFA-based approach, the reduction of BFAC numbers, sizes, or relative average surfaces, altogether with reduction of protein abundance in BFACs, was traditionally considered as evidence of endocytosis inhibition (Paciorek et al., 2005; Abas et al., 2006; Robert et al., 2010; Sun et al., 2011; Shibasaki et al., 2009; Du et al., 2013). We would like to note that the ultrastructural analyses demonstrate that BFACs induced in the roots by relatively high concentrations of BFA (25 μM and more) are rather complex structures apparently not formed only by endocytic vesicles (Robinson et al., 2008) and, furthermore, that cells show apparently a broad spectrum of BFA responses (Nebenführ et al., 2002; Takáč et al., 2011). According to the earlier study by Paciorek et al. (2005), the endocytosis of PIN2 is strongly suppressed by auxins, mainly by synthetic members such as 1-NAA or 2,4-D. Consequently, BFACs that were believed to accumulate specifically the PIN2 pool internalized from the PM, were not visible in the cells. Additionally, the authors state, auxins interfere neither with the BFAC formation nor other subcellular trafficking processes. In our previous study (Jásik and Schmelzer, 2014), we demonstrated that the BFA-based approach is not suitable for studying PIN2 endocytosis because newly synthesized PIN2 populations prevailed over the endocytic pool in BFACs. In view of the foregone, we should be able to monitor BFACs formation by analyzing the newly synthesized PIN2 population, even when endocytosis of PIN2 is completely stopped by auxins or other compounds. We failed to confirm this assumption, as we could not find BFACs accumulating exclusively the green PIN2-Dendra2 population. After application of different auxins or auxin transport inhibitors, all BFACs at every time point in the time-lapse experiment contained both red and green PIN2 pools, but at considerably reduced levels. The decrease of abundances of PIN2 populations was dependent on applied compounds. Reduced abundance of red population of PIN2 in BFACs was not due to the inhibition of PIN2 endocytosis, as this reduction was not accompanied by delayed disappearance of red PIN2 population from the PM.
In conclusion, our results indicate that auxins in high concentrations show more complex effects than previously supposed. Obviously, auxins and auxin transport inhibitors do not inhibit PIN2 endocytosis but affect proteosynthesis and/or downstream processes and interact directly with formation of BFACs. Mainly synthetic auxin analogs such as 1-NAA and 2,4-D very strongly decrease the number and size of BFACs and suppress accumulation of PIN2 inside them. This study again confirms that the appearance of PIN2 protein in BFACs is not a sign of its endocytosis as it was previously stated. Reduced abundance of PIN2 in BFACs is caused mainly by the absence of newly synthesized PIN2 and only in part by the decreased amount of the PIN2 pool delivered from the PM. It is possible that acidic auxins applied to cells at unnaturally high concentrations and their accumulation inside cells simply cause total disruption of the inner cellular environment and slow down life processes in the cells. The inhibited BFA uptake may be one of the consequences. Although Paciorek et al. (2005) deny such a possibility, the direct measurement of BFA concentration in the cells after auxin treatments has not been made.
MATERIALS AND METHODS
PIN2-Dendra2 Transgenic Line
Generation of the PIN2-Dendra2 fusion was described in detail in Jásik et al. (2013). DNA fragments of PIN2 were multiplied by PCR from Arabidopsis (Arabidopsis thaliana Col-0) genomic DNA. Dendra2 was amplified by PCR from a GatewayDendra2-At-N vector (Evrogen). DNA fragments were cloned into the pAMPAT-MSC vector (GenBank: AY436765.1) so that Dendra2 was positioned in the large intracytoplasmic loop. Primers used for cloning are listed in the Supplemental Table S1. The construct was transformed into the Arabidopsis Col-0 accession using Agrobacterium tumefaciens GV3101 (pMP90RK, Koncz and Schell, 1986) by the floral-dip method. Homozygous plants were selected on the 1/2 MSMO medium (half-concentration of MSMO salts purchased from Sigma-Aldrich, Cat. no. M6899, 0.7% agar, 1% Suc, pH 5.7) supplemented with 7.5 mg/L phosphinothricin (Duchefa).
Experimental Conditions and Media
Seeds were surface-sterilized with 1% (w/v) sodium hypochlorite and germinated on 1/2 MSMO containing agar. Petri dishes were kept in a vertical position at 21°C and under continuous light (100 μmol m−2 s−1). Before the experiments, 4-d-old seedlings were transferred onto fresh medium for 12 h. Treatments of seedlings were done on microscope slides covered with 1.5 mL solid culture medium. Slides were placed on the culture medium in petri dishes. BFA, auxins, and auxin transport inhibitors were dissolved in ethanol. The final concentration of ethanol in the medium was 0.05% (v/v). We used relatively high doses of auxins and auxin transport inhibitors because similar concentrations have been applied in previous studies on the PIN2 endocytosis inhibition by these compounds. Medium pH was rechecked after drug supplementation. Chemicals were from Sigma-Aldrich. Seedlings were pretreated for 30 min with auxins or auxin transport inhibitors before first imaging (time point 0) if not otherwise stated.
Microscopy
Roots were imaged as described previously in Jásik et al. (2013) and Jásik and Schmelzer (2014) in green and red fluorescence channels. Images were acquired with a Zeiss LSM-510 META confocal laser scanning microscope equipped with an inverted Zeiss Axiovert 200M microscope. A 20×/0.75 Plan-Apochromat objective was applied and the pinhole was set to 180 μm. Green (excitation with the 488 nm line of the argon laser, signal collection with a 505 nm to 530 nm band-pass filter) and red (excitation with the 543 nm line of the HeNe laser, signal collection with a 565 nm to 615 nm band-pass filter) signals were acquired sequentially in a multitrack mode. The HFT UV/488/543/633 main dichroic mirror was used for both channels. Identical gain and offset parameters of the LSM detector and laser line power were kept during experiments. To photoconvert Dendra2 in PIN2-Dendra2 fusion protein, root tips were illuminated for 15 s using the 100 W mercury lamp of the inverted microscope, the 20×/0.75 Plan-Apochromat objective, and an excitation filter BP 405/5. After illumination, the roots were again imaged and then reimaged at different time points during the time-lapse experiment. Because we demonstrated in our earlier study (Jásik et al., 2013) that recovery of the plasma membrane pool of PIN2 was suppressed in covered samples due to anaerobic conditions, we routinely removed the coverslip after every imaging.
Data Evaluation
Fifteen cells were analyzed in every root. Mean fluorescence intensities of transversal epidermal PMs were evaluated by the software ImageJ (National Institutes of Health) using the “straight line” selection mode, and sizes of BFACs were measured with the same program using the “freehand” selection mode. Five seedlings were used per slide and the experiments were repeated three times. If not otherwise stated, results in graphs are presented as normalized data. In photoconversion experiments, green signal intensities of the PM and BFACs in individual roots were normalized at every time point with respect to the mean of green signal intensities emitted by the PM of the same root before photoconversion of PIN2-Dendra2 fusion protein. Time point 0 represents the relative green signal intensity recorded immediately after photoconversion of the fusion protein. The means of red signal intensities were normalized with respect to the mean red fluorescence intensity measured immediately after photoconversion (time point 0). The values of the time point 0 were set to 1. In experiments without photoconversion, signal intensities of the PM and BFACs in individual roots were normalized at every time point with respect to the mean of signal intensities emitted by the PM of the same root at time point 0 (first imaging). Time points displayed on the graphs prepared in Microsoft Excel represent means of normalized values of 15 roots; bars correspond to ses of the means (se). Images in figures were processed with Adobe Photoshop CS2 and Microsoft Publisher software.
cDNA Synthesis and RT-PCR
Total RNA was extracted from 50 mg of roots using the RNeasy Plant Mini Kit (Qiagen). After elimination of the genome DNA contamination with RNA-free DNase I (Thermo Fisher Scientific), the cDNA was prepared from 1 μg total RNA using the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) and anchored oligo(dT) primer. PCR analysis was performed in a total volume of 15 μL containing 0.5 μL cDNA templates using the GoTaq Hot Start Polymerase (Promega) on an Eppendorf Mastercycler Pro instrument (Eppendorf). The following amplification program was applied: one cycle at 95°C for 3 min, 30 cycles at 95°C for 15 s, 56°C for 30 s, 73°C for 30 s, and then one cycle at 73°C for 5 min. The primers are listed in the Supplemental Table S1. POLYUBIQUITIN10 (At4G05320) was used as a reference gene (Czechowski et al., 2005). The experiment was independently repeated twice.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Influence of 1-NAA on PIN2 dynamics in the PM and BFACs.
Supplemental Figure S2. Effect of different auxins and auxin transport inhibitors on root growth.
Supplemental Table S1. Primers used for RT PCR and for cloning.
Supplementary Material
Acknowledgments
We thank Professor C. Koncz for providing laboratory support, and Professor B. Scheres for providing the PIN2-EGFP line.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C (2006) Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256 [DOI] [PubMed] [Google Scholar]
- Bennett T. (2015) PIN proteins and the evolution of plant development. Trends Plant Sci 20: 498–507 [DOI] [PubMed] [Google Scholar]
- Chen X, Naramoto S, Robert S, Tejos R, Löfke C, Lin D, Yang Z, Friml J (2012) ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol 22: 1326–1332 [DOI] [PubMed] [Google Scholar]
- Chudakov DM, Lukyanov S, Lukyanov KA (2007) Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc 2: 2024–2032 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhang Y, Zhang D, Chen J, Gao X, Estelle M, Zhao Y (2015) Embryonic lethality of Arabidopsis abp1-1 is caused by deletion of the adjacent BSM gene. Nat Plants 1: 15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klerk GJ, Hanecakova J, Jásik J (2008) Effect of medium-pH and MES on adventitious root formation from stem disks of apple. Plant Cell Tissue Organ Cult 95: 285–292 [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Du Y, Tejos R, Beck M, Himschoot E, Li H, Robatzek S, Vanneste S, Friml J (2013) Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc Natl Acad Sci USA 110: 7946–7951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC (2015) Genome sequencing of Arabidopsis abp1-5 reveals second-site mutations that may affect phenotypes. Plant Cell 27: 1820–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallavotti A. (2013) The role of auxin in shaping shoot architecture. J Exp Bot 64: 2593–2608 [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y (2015) Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci USA 112: 2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Wang B, Zhu J (2014) Auxin transport during root gravitropism: transporters and techniques. Plant Biol (Stuttg) 16(Suppl 1): 50–57 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K (2001) Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Grunewald W, Friml J (2010) The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J 29: 2700–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurskaya NG, Verkhusha VV, Shcheglov AS, Staroverov DB, Chepurnykh TV, Fradkov AF, Lukyanov S, Lukyanov KA (2006) Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat Biotechnol 24: 461–465 [DOI] [PubMed] [Google Scholar]
- Jackson CL, Casanova JE (2000) Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol 10: 60–67 [DOI] [PubMed] [Google Scholar]
- Jásik J, Boggetti B, Baluška F, Volkmann D, Gensch T, Rutten T, Altmann T, Schmelzer E (2013) PIN2 turnover in Arabidopsis root epidermal cells explored by the photoconvertible protein Dendra2. PLoS One 8: e61403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jásik J, Schmelzer E (2014) Internalized and newly synthesized Arabidopsis PIN-FORMED2 pass through brefeldin A compartments: a new insight into intracellular dynamics of the protein by using the photoconvertible fluorescence protein Dendra2 as a tag. Mol Plant 7: 1578–1581 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, Benková E, Friml J (2008a) ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr Biol 18: 526–531 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J (2008) Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol 24: 447–473 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Leitner J, Zwiewka M, Sauer M, Abas L, Luschnig C, Friml J (2008b) Differential degradation of PIN2 auxin efflux carrier by retromer-dependent vacuolar targeting. Proc Natl Acad Sci USA 105: 17812–17817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls tissue-specific expression of chimeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204: 383–396 [Google Scholar]
- Korasick DA, Enders TA, Strader LC (2013) Auxin biosynthesis and storage forms. J Exp Bot 64: 2541–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Křeček P, Skůpa P, Libus J, Naramoto S, Tejos R, Friml J, Zažímalová E (2009) The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol 10: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J, Petrášek J, Tomanov K, Retzer K, Pařezová M, Korbei B, Bachmair A, Zažímalová E, Luschnig C (2012) Lysine63-linked ubiquitylation of PIN2 auxin carrier protein governs hormonally controlled adaptation of Arabidopsis root growth. Proc Natl Acad Sci USA 109: 8322–8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser O. (2010) The power of auxin in plants. Plant Physiol 154: 501–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Nagawa S, Chen J, Cao L, Chen X, Xu T, Li H, Dhonukshe P, Yamamuro C, Friml J, Scheres B, Fu Y, et al. (2012) A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol 22: 1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM. (2015) Auxin Binding Protein1 (ABP1): a matter of fact. J Integr Plant Biol 57: 234–235 [DOI] [PubMed] [Google Scholar]
- Ljung K. (2013) Auxin metabolism and homeostasis during plant development. Development 140: 943–950 [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Hényková E, Sairanen I, Kowalczyk M, Pospíšil T, Ljung K (2012) Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J 72: 523–536 [DOI] [PubMed] [Google Scholar]
- Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof Y-D, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, Friml J (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Pan J, Fujioka S, Peng J, Chen J, Li G, Chen R (2009) The E3 ubiquitin ligase SCFTIR1/AFB and membrane sterols play key roles in auxin regulation of endocytosis, recycling, and plasma membrane accumulation of the auxin efflux transporter PIN2 in Arabidopsis thaliana. Plant Cell 21: 568–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Chen J, Yang Z (2015) Auxin regulation of cell polarity in plants. Curr Opin Plant Biol 28: 144–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pěnčík A, Simonovik B, Petersson SV, Henyková E, Simon S, Greenham K, Zhang Y, Kowalczyk M, Estelle M, Zažímalová E, Novák O, Sandberg G, et al. (2013) Regulation of auxin homeostasis and gradients in Arabidopsis roots through the formation of the indole-3-acetic acid catabolite 2-oxindole-3-acetic acid. Plant Cell 25: 3858–3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranocha P, Dima O, Nagy R, Felten J, Corratgé-Faillie C, Novák O, Morreel K, Lacombe B, Martinez Y, Pfrunder S, Jin X, Renou JP, et al. (2013) Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat Commun 4: 2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Čovanová M, Hayashi K, Dhonukshe P, et al. (2010) ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG, Langhans M, Saint-Jore-Dupas C, Hawes C (2008) BFA effects are tissue and not just plant specific. Trends Plant Sci 13: 405–408 [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Uemura M, Tsurumi S, Rahman A (2009) Auxin response in Arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21: 3823–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer T, Seifert GJ, Hauser MT, Grisafi P, Fink GR, Luschnig C (2000) Post-transcriptional control of the Arabidopsis auxin efflux carrier EIR1 requires AXR1. Curr Biol 10: 1595–1598 [DOI] [PubMed] [Google Scholar]
- Strader LC, Zhao Y (2016) Auxin perception and downstream events. Curr Opin Plant Biol 33: 8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen Q, Qi L, Jiang H, Li S, Xu Y, Liu F, Zhou W, Pan J, Li X, Palme K, Li C (2011) Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol 191: 360–375 [DOI] [PubMed] [Google Scholar]
- Takáč T, Pechan T, Richter H, Müller J, Eck C, Böhm N, Obert B, Ren H, Niehaus K, Šamaj J (2011) Proteomics on brefeldin A-treated Arabidopsis roots reveals profilin 2 as a new protein involved in the cross-talk between vesicular trafficking and the actin cytoskeleton. J Proteome Res 10: 488–501 [DOI] [PubMed] [Google Scholar]
- Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Xu J, Scheres B (2005) Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR1 function in epidermal cell polarity. Plant Cell 17: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H, Wang W, Jones AM, et al. (2014) Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zažímalová E, Murphy AS, Yang H, Hoyerová K, Hošek P (2010) Auxin transporters—why so many? Cold Spring Harb Perspect Biol 2: a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.