Multielement imaging of ion transport and distribution in Arabidopsis roots shows distinct spatial and dynamic gradients.
Abstract
Better understanding of root function is central for the development of plants with more efficient nutrient uptake and translocation. We here present a method for multielement bioimaging at the cellular level in roots of the genetic model system Arabidopsis (Arabidopsis thaliana). Using conventional protocols for microscopy, we observed that diffusible ions such as potassium and sodium were lost during sample dehydration. Thus, we developed a protocol that preserves ions in their native, cellular environment. Briefly, fresh roots are encapsulated in paraffin, cryo-sectioned, and freeze dried. Samples are finally analyzed by laser ablation-inductively coupled plasma-mass spectrometry, utilizing a specially designed internal standard procedure. The method can be further developed to maintain the native composition of proteins, enzymes, RNA, and DNA, making it attractive in combination with other omics techniques. To demonstrate the potential of the method, we analyzed a mutant of Arabidopsis unable to synthesize the metal chelator nicotianamine. The mutant accumulated substantially more zinc and manganese than the wild type in the tissues surrounding the vascular cylinder. For iron, the images looked completely different, with iron bound mainly in the epidermis of the wild-type plants but confined to the cortical cell walls of the mutant. The method offers the power of inductively coupled plasma-mass spectrometry to be fully employed, thereby providing a basis for detailed studies of ion transport in roots. Being applicable to Arabidopsis, the molecular and genetic approaches available in this system can now be fully exploited in order to gain a better mechanistic understanding of these processes.
Investigations of the localization of inorganic elements in young plant roots may answer a range of important and unresolved questions with respect to root functionality and plant nutrient transport. To date, our understanding of how plants control the radial root transport of essential plant nutrients and toxic elements is mainly circumstantial, relying on changes in shoot or shoot-to-root concentration ratios or analyses of xylem sap composition. Roots of Arabidopsis (Arabidopsis thaliana) have a simple cellular organization and are unrivaled in their ability to be imaged by confocal microscopy, as they are very thin (diameter approximately 120 µm) and have a low background fluorescence. This has led to an amazingly detailed understanding of the growth and development of roots. Unfortunately, the fragile nature of these roots constitutes a major challenge when trying to understand the processes that drive nutrient uptake at the same level of detail. The method we present here for element bioimaging of Arabidopsis roots is a critical step in utilizing the potential of combining targeted genetic modifications and bioimaging at the cellular level in order to unravel the complexities of how roots selectively acquire and translocate mineral nutrients from the soil.
The uptake and radial transport of inorganic ions is tightly controlled by transport proteins varying in selectivity, affinity, and capacity. However, it was demonstrated recently that physical barriers in the root systems also play a pivotal role in regulating ion uptake, for example by the epidermis at the root surface as well as by the lignin and suberin barriers adjacent to the endodermis (Hosmani et al., 2013; Pfister et al., 2014; Kamiya et al., 2015; Barberon et al., 2016). The cells of the endodermis are sealed by the Casparian strip, a lignin-based barrier that restricts the apoplastic movement of ions and water into the vascular bundles. Further control of ion movement into the stele (and ultimately to the leaves via the xylem) is achieved by suberin deposition along the cell walls of the endodermis (Baxter et al., 2009; Geldner, 2013). Radial transport of inorganic ions may also be impeded by the lack of proper ligands, for example by nicotianamine (NA) or other organic acids. The functional effects that root barriers and ligands have on nutrient acquisition are still elusive, and they probably constitute a combined response to various genetic determinants that are yet to be understood at the mechanistic level. A better understanding of the radial root transport of essential nutrients is a prerequisite to improve nutrient uptake efficiency in crops and optimize the use of natural resources in agriculture. Likewise, better knowledge about the transport of potentially toxic trace elements such as cadmium (Cd) and arsenic (As) will help improve food safety.
Different techniques are available for elemental imaging, typically divided into mass spectrometry (MS)-based techniques (e.g. nano-secondary ion mass spectrometry [nano-SIMS] or laser ablation-inductively coupled plasma-mass spectrometry [LA-ICP-MS]) and synchrotron x-ray-based techniques (e.g. energy-dispersive x-ray microanalysis, proton-induced x-ray emission, x-ray fluorescence, or x-ray absorption spectrometry; Zhao et al., 2014). Relative to SIMS and the synchrotron x-ray-based techniques, LA-ICP-MS offers a range of advantages in terms of low detection limits and high sensitivity for many elements (Becker et al., 2010b; for details, see “Discussion”). LA-ICP-MS also is much more accessible and has substantially lower running costs than any of the competitive techniques.
Because of the requirement for dry samples, the usefulness of LA-ICP-MS for root analysis has long been hampered. For this reason, LA-ICP-MS analysis of plant materials has to date mostly been applied to naturally dry samples, like seeds and cereal grains (Lombi et al., 2011b; Olsen et al., 2016). The analysis of hydrated samples, like roots, poses major sample preparation challenges, with respect to maintaining biological structure and the native composition of the elements therein, during drying. Uncontrolled drying of cross sections or longitudinal sections of young roots causes the disruption of most cells, mainly due to the sudden loss of turgor and the resulting ion leakage. In order to preserve sample integrity, specimens for microscopy are typically dehydrated with a slow, gradual exchange of water with ethanol, acetone, or tetrabutyl alcohol (Feder and Obrien, 1968; Beeckman and Viane, 2000). Following dehydration, it is a common practice to embed the specimens in a block, typically a resin or paraffin block, prior to sectioning. However, young plant roots, like the ones of the genetic model plant Arabidopsis, are extremely fragile, and they easily disintegrate during such sample preparation. In addition, for elemental bioimaging, these procedures are problematic, since some ions will leak out of the tissue during prolonged soaking in organic solvents (Fourie and Peisach, 1977; Davies et al., 1991). It is unclear, however, how dehydration affects the leakage and displacement of different elements and to what degree. In theory, ions with a low valence and little or no interaction with other compounds (e.g. potassium [K+] and sodium [Na+]) should be highly diffusible and easily lost from the tissue, whereas divalent and trivalent cations such as manganese Mn2+, zinc (Zn2+) and iron (Fe2+ and Fe3+) should be less prone to leakage due to covalent bonding or coordination with various ligands.
In order to maintain not only the elements present in the root tissue but also the integrity of various chemical components (i.e. ligands, proteins, nucleic acids, and metabolites), rapid freezing of the root is an attractive alternative to dehydration. After freezing, samples can be sectioned on a cryotome and then freeze-dried prior to analysis; alternatively, they can be freeze-dried first and then sectioned (Bhatia et al., 2004). In order to be able to prepare very thin sections (less than 5 µm) while still maintaining cell structures and element composition, freeze-substitution also has been employed (Siegele et al., 2008; Smart et al., 2010). This technique is based on ultra-rapid freezing followed by slow substitution of the ice with acetone, then chemical fixatives like osmium tetroxide (Smart et al., 2010) or tetrahydrofuran (Pålsgård et al., 1994). It has been shown, however, that the localization of highly diffusible ions, like K+ and Na+, may be altered significantly during freeze substitution (Smart et al., 2010).
For ordinary cryo-sectioning, the initial freezing and subsequent sectioning are typically done in OCT (Optimal Cutting Temperature) medium (Tissue-Tek; Sakura Finetek), which is a glycol-based freezing medium (containing polyvinyl alcohol and polyethylene glycol) that facilitates fast freezing and mechanical support of the specimen during the following sectioning. Upon transfer of the sections to glass slides, the section melts briefly, thereby adhering to the surface of the glass slide. In this critical process, the melted OCT medium may cover small specimens, partly or fully. OCT medium is highly water soluble and can be washed off easily, although with the risk of also washing off leachable ions. Also, the hygroscopic OCT medium has a high osmotic potential, which means that it may cause water and/or ion diffusion upon direct contact with the root during the time that passes from excision to freezing in liquid nitrogen, with the risk of ion displacement inside and/or outside of the specimen.
In order to meet these challenges, we have developed a novel sample preparation method. We show that encapsulation of the fresh tissue with paraffin prior to freezing and cryo-sectioning is an essential step in order to avoid the displacement of elements. Furthermore, we also show that the method is applicable to the very small and fragile roots of Arabidopsis. The method was tested using the NA synthase quadruple mutant nas1nas2nas3nas4 (nas4x), which is unable to synthesize the metal chelator NA. Upon cultivation in hydroponics, the leaves of these mutants display symptoms typical for Fe deficiency (Schuler et al., 2012). Total element concentration and xylem sap analyses, in combination with elemental bioimaging of the roots, showed that NA deficiency has different effects on radial and long-distance transport of Fe compared to Zn and Mn, which in turn appears to be related to xylem-loading processes as well as the affinity of NA and other ligands to these different metal ions.
By enabling the examination of Arabidopsis roots, the genetic model plant of choice in plant science, the methodological developments described here pave the way for a range of new possibilities for investigating ion uptake, transport, and compartmentation in root tissues. Moreover, the method works for any element present in the tissue, including any added isotope, be it an essential plant nutrient or any other element taken up by plant roots.
RESULTS
The Effect of Dehydration on the Loss of Nutrient Ions
Initially, we attempted to perform elemental bioimaging analyses on cross sections prepared from Arabidopsis roots that had been dehydrated with ethanol, using a standard protocol for microscopy sample preparation (Feder and Obrien, 1968; Beeckman and Viane, 2000). We observed that the resulting ion intensities, especially for 39K and 24Mg, were surprisingly weak and reasoned that substantial losses of these ions probably had occurred during the prolonged dehydration procedure in ethanol. To confirm this, roots of 6-week-old wild-type Arabidopsis were subjected to dehydration with ethanol (see “Materials and Methods”). After dehydration, the roots were freeze-dried, digested, and analyzed by Inductively Coupled Plasma-Optical Emmission Spectrometry (ICP-OES). Control samples were freeze-dried immediately after harvest, digested, and analyzed by ICP-OES. As can be seen in Table I, the dehydration treatment induced a substantial loss of particularly K+, but also more than 70% of divalent ions like Ca2+ and Zn2+ were lost. This clearly shows that conventional dehydration protocols are not suitable for elemental bioimaging analyses.
Table I. Total element concentrations in the roots of 6-week-old Arabidopsis wild-type plants, sequentially dehydrated with ethanol, then dried, digested, and analyzed by ICP-OES.
The right column shows the concentration changes compared with roots from control plants (i.e. plants that were not dehydrated with ethanol; n = 4).
Cryo-Sectioning to Maintain Tissue Structure during Sample Preparation
We reasoned that instant freezing of the Arabidopsis roots would maintain the native ionic composition, and if kept frozen throughout sectioning and the subsequent drying, no ions would change place within the tissues or be lost from the sample (i.e. leak out of the root). After sectioning, during the transfer to glass slides, we observed that the specimens were covered, partly or fully, by the OCT embedding medium (Fig. 1C). When analyzing these OCT medium-covered samples with LA-ICP-MS, very low sensitivity was obtained, and it was very difficult to assign the ion signals to specific tissue structures within the specimens (data not shown).
Figure 1.
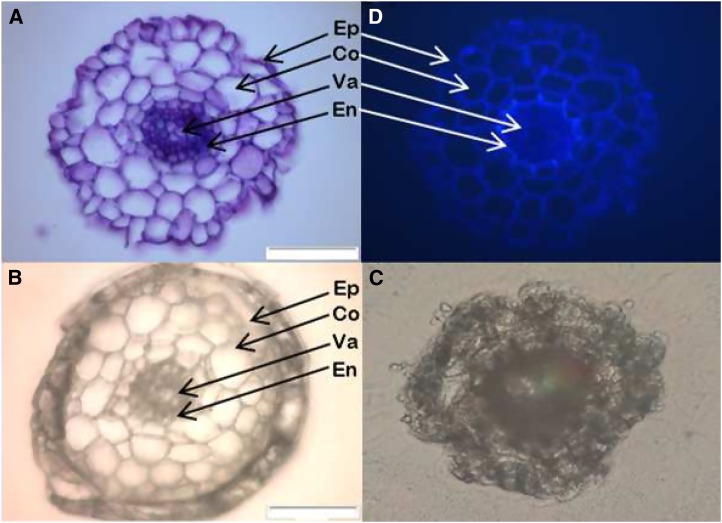
Cross sections from Arabidopsis roots. A, Fresh cross section that was first encapsulated in paraffin, frozen and cryo-sectioned, then stained with Toluidine Blue and viewed with a microscope in the wet condition. B and C, Cross sections that were either encapsulated in paraffin (B) or not (C) prior to freezing, cryo-sectioning and freeze-drying. D, UV light image of a fresh section showing suberized and lignified tissues. A, B, and C were captured with bright field microscopy, and D was captured with UV light microscopy (450–490 nm). Ep, Epidermis; Co, cortex; Va, vascular tissues; En, endodermis. Bars = 50 µm.
In order to solve this, paraffin was used to encapsulate the whole roots, preventing the ions from leaking out of the tissues and at the same time keeping the hygroscopic OCT medium physically separated from the sample, which appeared to be highly critical in order to avoid osmotic movement of water and ions from the tissue. The paraffin coating also was a successful way to keep the surface of the specimen free from OCT medium while maintaining the turgor and integrity of the root cells. The microscopy images clearly showed that the structure of the root was nicely preserved after drying, with the major tissues types, including the epidermis, cortex cells, endodermis, and vascular tissues, still being clearly visible (compare Fig. 1, A and B). The paraffin ring coating the root also could be seen, as well as the surrounding OCT medium, efficiently kept away from the surface of the specimen (Fig. 1B). Note that the cross section in Figure 1C also was first frozen, then sectioned and dried, although without the paraffin encapsulation. The UV light image (Fig. 1D) shows lignified and suberized tissues, thickenings characteristic of the Casparian strip and suberization observed in the endodermis.
Preparation and Application of an Internal Standard
An internal standard based on an element that is not normally found in root tissues is important for comparison of ion intensities between samples and analytical runs and to validate that the tissue is quantitatively ablated. Ideally, the applied internal standard should have a similar ionization potential to the target analyte. For this reason, rubidium (Rb; ionization potential, 4.18 eV) was chosen, since it has a similar ionization potential to K (4.34 eV), which is the element we focused on during method development, as K is an ideal proxy for highly diffusible ions because it does not bind strongly to any ligands and adsorbs poorly to the negative charges in the cell walls. We tested many different strategies for the application of an internal Rb standard. These included a couple of approaches for applying an Rb-containing solution to a glass slide, for example by drying a drop onto the glass slide, applying it with a brush and then drying it, or spraying it onto the surface. In order to reduce the surface tension of the water droplets, we also tried different mixtures of water and ethanol (20%–60%) in the solution. All of these attempts were more or less unsuccessful, since the standard solution applied in these ways was not evenly distributed on the surface (data not shown). Next, we tried injecting the Rb solution into a marker pen, allowing it to equilibrate overnight, and then using it to draw a line onto the surface of the polyethylene naphthalate (PEN) membrane-covered glass slide. When ablating this colored line with LA-ICP-MS, we clearly detected Rb as both its 85Rb and 87Rb isotopes. The distribution was much better than in our previous attempts (i.e. by drying, brushing, or spraying). However, there was still a 15% to 20% relative sd of the 85Rb signal across the ablated lines containing the internal standard (data not shown). In order to apply a more uniform amount of Rb standard below the sample, we finally developed a method based on injection of the Rb solution directly into an inkjet cartridge (Fig. 2; see “Materials and Methods”). The standard was then printed five times on a transparent plastic sheet, ensuring a thick, homogenous layer. When ablating this surface, the 85Rb signal was very stable, and we consistently recorded relative sd values between 4% and 6% across the whole rectangle (data not shown).
Figure 2.
Schematic illustration of the procedure for the application of an Rb standard underneath a cross section from Arabidopsis roots. The cross section was first encapsulated in paraffin, cryo-sectioned, and then freeze-dried. The Rb standard was applied on a transparent polymer with an ink-jet printer and then manually inserted in the PEN membrane envelope, under the cross section.
Optimization of Laser Ablation Settings
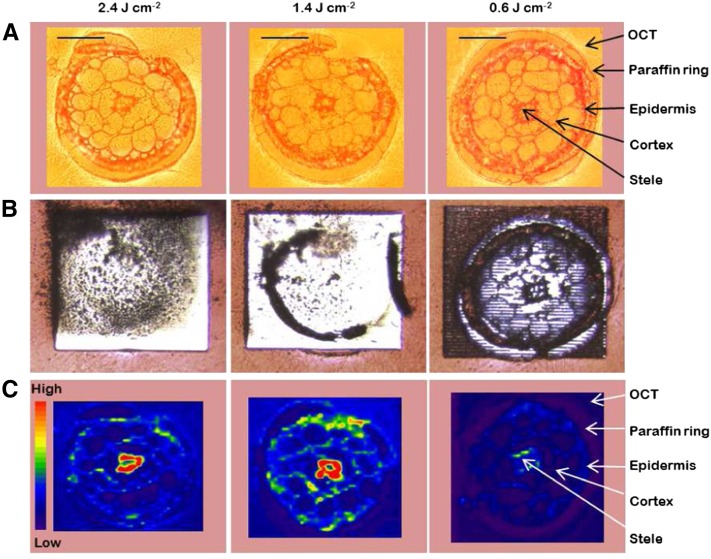
The next step was to optimize the laser ablation (LA) settings for the analysis of the paraffin-coated, cryo-sectioned, and dried specimens, which now had an internal Rb standard placed underneath them. In LA, there is a tradeoff between resolution and sensitivity, both with respect to the energy level of the laser beam and also the thickness of the root slice. Using a high energy setting for the laser beam (50% or more of the maximum energy; corresponding to more than 3 J cm−2) might result in inferior resolution, since a high-energy pulse will spread out more than a corresponding low-energy pulse. Conversely, a lower energy would minimize this spread, helping to maintain a high spatial resolution. However, using too low an energy would result in less material being ablated, which of course reduces the intensity of the signal, since less material would reach the plasma of the inductively coupled plasma-mass spectrometry (ICP-MS) device. Roots are composed of structures with very different hardness. Typically, cell walls and cells in the stele are much more dense and rigid than cells in the cortex. If tissue removal during LA was not complete, variations in hardness might bias the results, as it would be impossible to tell whether differences in the obtained signal intensities are due to differences in tissue hardness or in absolute ion concentrations. For this reason, we optimized the LA settings for a complete removal of the tissue in the cross sections but using the lowest possible laser energy, in order to maximize spatial resolution. We tried different thicknesses of the sections, in combination with different laser energies. As can be seen in Figure 3, 16-µm-thick cross sections of the roots were quantitatively removed using laser energy levels of 2.36 or 1.4 J cm−2 (corresponding to 40% and 30% of the maximum energy), whereas 0.59 J cm−2 (corresponding to 20% of the maximum energy) was not sufficient to obtain a quantitative ablation of the tissue.
Figure 3.
Optimization of LA-ICP-MS settings in order to obtain a quantitative ablation and to achieve the best possible spatial resolution when analyzing cross sections from Arabidopsis roots. All three cross sections were 16 µm thick and were analyzed with decreasing energy levels (left to right) and otherwise identical settings. The energy levels in the laser beam were 2.36, 1.4, and 0.59 J cm−2, respectively. A shows bright-field microscopy images, B shows microscopy images taken after the ablation, and C shows the distribution and ion intensity of K, analyzed as 39K, with LA-ICP-MS. The red-to-blue color spectrum in C represents high to low intensities, respectively (range, 2,000–80,000 counts). Bars = 50 µm.
Next, we investigated the influence of sample thickness on the sensitivity (Fig. 4). From the same root, 20-, 16-, or 12-µm-thick sections were ablated using approximately 1.4 J cm−2 energy in the laser beam. All the sections were quantitatively ablated using these settings (see insert of the 20-µm-thick section before and after ablation; Fig. 4). However, as expected, the signal intensity of 39K was much stronger in the 16- and 20-µm-thick sections compared with the 12-µm thickness. Hence, in order to get the best possible resolution (from a quantitative ablation) and the maximum signal intensity from the same ablation, sections should be 16 to 20 µm thick.
Figure 4.
Impact of the thickness of cross sections from Arabidopsis roots on ion intensity. Sections with 20, 16, and 12 µm thickness were analyzed with 1.4 J cm−2 energy in the laser beam. All samples, including the 20-µm-thick section, were quantitatively ablated (top left, before ablation; bottom left, after ablation). The color spectrum represents an ion intensity map of K, analyzed as 39K (range, 2,000–80,000 counts). Bars = 50 µm.
Since we applied the internal standard underneath the cross sections, another criterion for a successful ablation was that the laser beam should penetrate into the internal standard below the root section without hitting the glass slide.
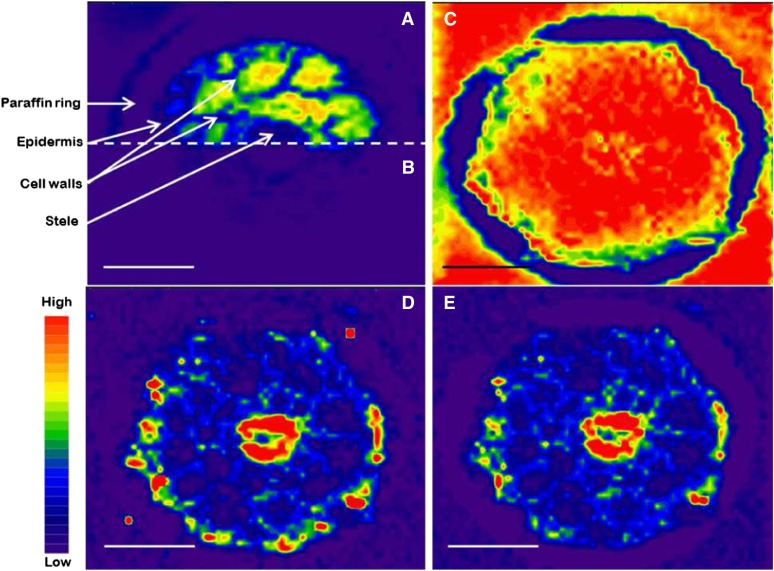
A nonquantitative ablation is seen in Figure 5B, where the low energy (0.6 J cm−2) ablated some root material but nothing of the underlying Rb standard, analyzed as the 85Rb isotope. When using 1.4 J cm−2 energy in the laser beam, all the soft tissues of the root were ablated, together with the Rb standard below these areas (Fig. 5A). However, in the areas with hard structures (e.g. cell walls and stele), only part of the root tissue was ablated; hence, the laser beam did not penetrate into the Rb standard below these structures, leaving them without any 85Rb signal (Fig. 5A). The higher energy used in Figure 5C (2.4 J cm−2) ablated all the root tissues quantitatively and penetrated down to the Rb standard below, now generating an even 85Rb signal throughout the area of the cross section. Therefore, in order to obtain a quantitative ablation and at the same time reach down to the Rb standard, we had to use this higher energy level. The 85Rb signal could now be used to correct the obtained values, where the bottom left image (Fig. 5D) is the raw 66Zn signal from the same section as in Figure 5C and the bottom right image is the 66Zn signal, again from the same section as in Figure 5C, now corrected on the basis of the 85Rb signal (Fig. 5E). It should be noted that Zn has an ionization potential of 9.39 eV, which differs considerably from that of Rb (4.18 eV). However, the internal standard was not used for absolute quantification in this case but only to account for possible signal drift and to document a quantitative ablation (viz. where all tissue materials are transported as an aerosol to the ICP-MS device for detection).
Figure 5.
A, An ion intensity image of 85Rb in the top half of a 20-µm-thick cross section from Arabidopsis roots, analyzed by LA-ICP-MS with 1.4 J cm−2 in the laser beam. B, An ion intensity map of 85Rb in the bottom half of the same section as in A, analyzed with 0.6 J cm−2 in the laser beam. C, An 85Rb ion intensity map analyzed with 2.4 J cm−2 in the laser beam. D, An ion intensity map of 66Zn from the same cross section as in C. E, The distribution and ion intensity of 66Zn in the same cross section as in C, where the values have been corrected, using the 85Rb signal as an internal standard. The color spectrum represents high to low ion intensities (for 85Rb, range, 10–5,000 counts [A–C]; for 66Zn, range, 0–150 counts [D and E]). Bars = 50 µm.
Multielement Bioimaging with LA-ICP-MS
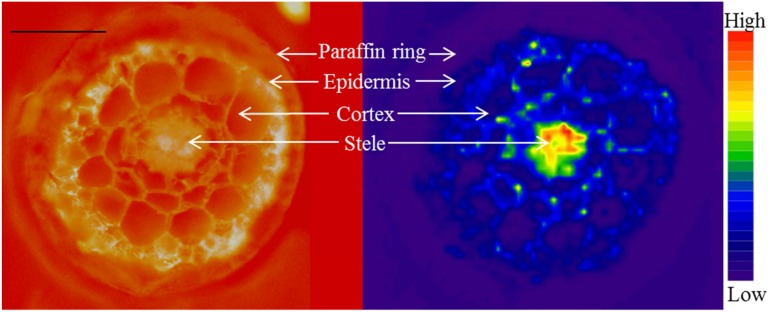
Following the method optimization described above, a cross section of a wild-type Arabidopsis root was analyzed by LA-ICP-MS using a 5-µm resolution (spot size). In Figure 6, a microscopy image and the corresponding distribution of 39K are shown, revealing its relative concentrations in the different tissues. The 39K signal in this image was not corrected relative to the 85Rb signal, since it was not used for any intercomparisons. Zn and Mn also were analyzed in the same root section, underlining the multielement capacity possible with LA-ICP-MS (data not shown). Any other elements present in the tissue also can be analyzed in principle, given that they have a concentration above the detection limit. Depending on ionization potentials and isotopic abundances of the elements, five to 10 elements may be monitored in the same analytical run. All essential plant nutrients, except for nitrogen (N) and chlorine (Cl), can be analyzed. In addition, other relevant elements, like Na, Cd, As and Al, all can be studied with the method.
Figure 6.
A 20-µm-thick cross section from Arabidopsis roots encapsulated in paraffin, cryo-sectioned, and freeze-dried prior to analysis. The image at left is a UV fluorescence image (450–490 nm) taken before ablation, where the red background color originates from an Rb standard placed below the cross section. The image at the right shows the distribution and ion intensity of 39K ions in the same sample, analyzed by LA-ICP-MS, where the color spectrum represents high to low ion intensities (range, 2,000–80,000 counts). Bar = 50 µm.
Bioimaging of NAS Quadruple Mutants
The applicability of the method was tested with a case study using 6-week-old quadruple Arabidopsis nas4x mutants and wild-type plants. The nas4x mutants displayed severe deficiency symptoms of interveinal chlorosis, especially in the youngest leaves, even though both mutant and wild-type plants were grown with the full spectrum of mineral nutrients (Fig. 7).
Figure 7.
The large image shows wild-type (WT) and nas4x quadruple mutant Arabidopsis plants, 6 weeks old, cultivated in a hydroponic system with standard nutrient conditions. The mutant (left) displays interveinal chlorosis, which is a known symptom of iron deficiency. The small image shows a full-size image of the wild-type and nas4x mutant plants.
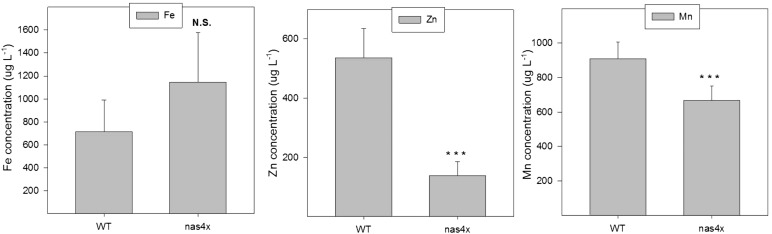
The total concentrations of Zn and Mn in the leaves clearly showed that the nas4x mutant was very inefficient in the long-distance, root-to-shoot transport of these two elements (Table II). In nas4x, the shoot concentration of Zn was approximately half that observed in the wild type, and for Mn, the decrease was 22% (P < 0.01 for both elements). Interestingly, no significant difference in shoot Fe concentration was found between the wild type and the nas4x mutant (P > 0.1), even though nas4x displayed what could be interpreted as Fe deficiency symptoms. Hence, with respect to root-to-shoot transport of Fe, the nas4x mutant was similar to the wild type.
Table II. Total element concentrations in shoots and roots of 6-week-old wild-type and nas4x mutant plants of Arabidopsis, cultivated under normal nutrient conditions in a hydroponic system.
All samples were dried, digested, and then analyzed by ICP-OES (n = 3).
| Element | Wild-Type Shoot | nas4x Shoot | nas4x Shoot Percentage Difference from the Wild Type | P | Wild-Type Root | nas4x Root | nas4x Root Percentage Difference from the Wild Type | P |
|---|---|---|---|---|---|---|---|---|
| µg g−1 dry wt | µg g−1 dry wt | |||||||
| Mn | 412 ± 14 | 322 ± 5 | −21.9 | <0.001 | 182 ± 28 | 272 ± 87 | 49.2 | 0.167 |
| Fe | 198 ± 86 | 168 ± 46 | −15.4 | 0.618 | 9,890 ± 528 | 15,400 ± 2,720 | 55.7 | 0.026 |
| Zn | 156 ± 12 | 73 ± 5 | −53.0 | <0.001 | 1,000 ± 262 | 1,630 ± 182 | 63.1 | 0.027 |
The elemental composition of the xylem sap in Figure 8 confirmed the shoot data in Table II, with Zn and Mn concentrations being significantly lower in the xylem sap of nas4x compared with the wild type (P < 0.05). Again, Fe was different from the other three elements, showing no significant difference in xylem sap concentration between the wild type and the mutant (P > 0.05). Furthermore, there were no significant differences in the K or Mg concentrations in the xylem sap of the nas4x and wild-type plants, supporting that the xylem sap flow rates were similar (data not shown).
Figure 8.
Xylem sap concentrations of micronutrients in 6-week-old wild-type (WT) and nas4x mutant plants of Arabidopsis (n = 3). The plants were cultivated under standard nutrient conditions in a hydroponic system.
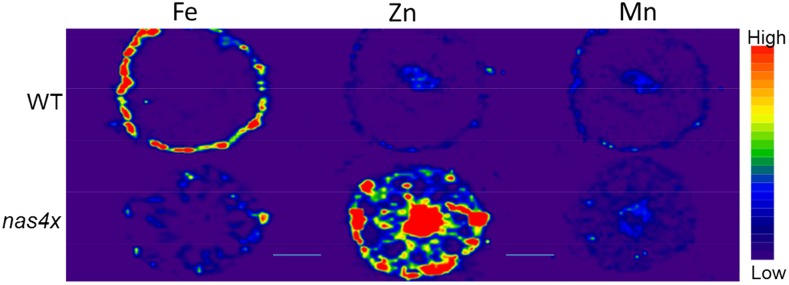
Using LA-ICP-MS, we observed that the nas4x mutant accumulated substantially more Zn than the wild type in all root tissues, including in the stele, endodermis, cortex, and epidermis (Fig. 9, middle). This indicates a severely reduced capacity for radial ion transport and depressed xylem loading of Zn in the nas4x mutant, which also was corroborated by the lower Zn concentrations in the xylem sap and in the shoots (Table II; Fig. 8). The total root concentration of Zn was 63% higher in the roots of nas4x compared with the wild type (P < 0.05; Table II), which is consistent with the LA-ICP-MS imaging. In agreement with the xylem sap data in figure 8, the Mn image (Fig. 9, right) were much less contrasting. However, slightly more Mn seemed to accumulate in the cortex and around the vascular tissues in the nas4x mutant compared to the wild type.
Figure 9.
Twenty-micrometer-thick cross sections from roots of wild-type (WT; top) and nas4x (bottom) Arabidopsis plants analyzed by LA-ICP-MS. The plants were 6 weeks of age and were cultivated under normal nutrient conditions in a hydroponic system. The color spectrum represents high to low ion intensities of 56Fe (left), 66Zn (middle), and 55Mn (right), in the ranges 4,000 to 20,000, 200 to 500, and 40 to 400 counts, respectively. Bars = 50 µm.
Similar to Zn, the total root Fe was increased significantly in nas4x compared with the wild type (P < 0.05; Table II). However, unlike Zn, this increase in root Fe was due mainly to increased accumulation of Fe in the cortex and cortical cell walls. This contrasts with Fe accumulation in the wild type, which primarily occurs in the epidermis. The images in Figure 9 seem to indicate a higher total Fe concentration in the wild type, in contrast to the results in Table II, showing 56% higher Fe concentration in the nas4x roots. However, the accumulated signal counts (the sum of all counts throughout the cross section) was, in fact, slightly higher in the mutant; additionally, these counts appeared in a smaller tissue area than in the wild type. Interestingly, neither nas4x nor the wild type accumulated Fe in and around the stele, which could explain the similar whole-shoot and xylem sap concentrations.
DISCUSSION
Elemental imaging can be performed with many different analytical techniques. All of these techniques have their benefits and shortcomings with respect to sample preparation, element coverage, sensitivity, and spatial resolution.
The x-ray-based techniques act very differently on a sample compared with the MS-based techniques, since the x-rays penetrate into the sample and any surrounding material whereas the MS-based techniques ablate only the top layer of the specimen. Thus, in x-ray-based techniques, it is important not to smear the signals from the top cell layers with signals from the deeper layers of the sample. In order to avoid this, very thin sections have to be prepared (less than 5 µm thick), which typically compromise sensitivity. However, new approaches have enabled direct analysis of hydrated roots, with a minimum of sample preparation, using synchrotron x-ray fluorescence (Lombi et al., 2011a). However, prolonged exposure to x-rays causes damage to the tissue sample, which might alter the distribution of elements (Zhao et al., 2014).
With respect to spatial resolution, nano-SIMS outcompetes all other imaging techniques, reaching as low as a 50-nm spatial resolution (Moore et al., 2012), whereas x-ray-based techniques reach between 1 and 2 µm and LA-ICP-MS systems typically range from 5 to 200 µm (Becker et al., 2010b). New LA designs, however, like the near-field LA type, can go below 5 µm, and substitution of the LA unit with a laser microdissection unit has been used to improve resolution down to 1 µm (Becker et al., 2010a).
Since MS-based techniques mainly rely on the first ionization potential for each element, these techniques offer a wide range of elements to be tested, including both light and heavy atoms. Additionally, MS-based techniques offer the analysis of stable isotopes. Due to technical improvements, including the development of the triple quadrupole ICP-MS method, traditionally problematic atoms with high ionization energies or atoms affected by strong interferences, such as sulfur and phosphorus, can now be analyzed with low background noise, offering greatly improved sensitivities (Balcaen et al., 2015). With x-ray-based techniques, sensitivity generally decreases with decreasing atomic number. Since the lighter elements (e.g. K, N, Mg and Al) have low emission energies they are difficult to detect, even at relatively small depths within the sample. Heavier elements (e.g. Cu, Ag and Au) have much higher emission energies, which can pass through greater distances within a sample, resulting in a higher sensitivity (Zhao et al., 2014). One of the main limitations with SIMS is the poor sensitivity for detection of certain elements, such as Zn, Mn, and cd. This is due to a low secondary ion yield of these particular elements (Moore et al., 2012). With detection limits observed in the sub-µg g−1 level for many elements, LA-ICP-MS is more sensitive than either SIMS or x-ray-based techniques (Becker et al., 2010b). Relative to SIMS and synchrotron x-ray-based techniques, LA-ICP-MS is also much more accessible and has substantially lower running costs.
The combined use of synchrotron x-ray-based techniques and tomography analyses has enabled the direct analysis of hydrated roots (Lombi et al., 2011a). The concentrations of seven different elements were analyzed in roots of cowpea (Vigna unguiculata), and using a mathematical model, images and estimated concentrations in virtual cross sections could be displayed (Wang et al., 2013). As mentioned above, one of the main challenges with this analytical approach has been the tissue damage from x-ray treatment during prolonged analyses. However, with new, fast detectors, much progress has been made in recent years, reducing the exposure times from hours to minutes (Lombi et al., 2011a). This approach reduces the radiative damage of highly sensitive hydrated tissue samples and allows the construction of ultra-thin two-dimensional images and might enable three-dimensional imaging using confocal detection (Lombi et al., 2011a) The experiments by Lombi et al. (2011a) and Wang et al. (2013) were conducted in concentration ranges relevant for environmental toxicology, but it should be noted that these were far higher than the physiological concentration ranges normally used for cultivating plants. The Zn supply of Wang et al. (2013), for example, was 40 times higher than that considered to be a normal Zn supply in hydroponics. Thus, to understand ion transport processes under physiological nutrient concentrations, the high sensitivity of LA-ICP-MS offers a number of advantages relative to state-of-the-art synchrotron techniques.
Sample Preparation and LA-ICP-MS Analysis
Analyzing hydrated samples imposes major challenges with respect to sample preparation, since the sample needs to be dried prior to LA-ICP-MS analysis. After drying, both the biological structures and the elemental ion composition of the specimen need to be maintained in order for the analysis to be meaningful (Moore et al., 2012). Also, absolutely planar sections need to be obtained, since LA-ICP-MS is sensitive to sample topography and matrix composition. Hence, with respect to sample preparation, LA-ICP-MS analyses face similar challenges as nano-SIMS (Moore et al., 2012, 2014). As was shown here and elsewhere, existing protocols for microscopy are not designed to maintain the ionic composition of plant samples. In roots of the grass Festuca rubra, an average of 78% of the Zn was lost during dehydration and fixation processes (Davies et al., 1991), which is consistent with the findings presented here (Table I). Hence, we conclude that the use of standard protocols for microscopy drains ions out of the root tissues, leading to erroneous conclusions about ion distribution and concentration.
Freezing is an attractive alternative to dehydration, fixing both cellular structures and ions in their native states. Some SIMS and LA-ICP-MS instruments have the capability to analyze samples in their frozen, hydrated state, thus avoiding any sample preparation artifacts (Metzner et al., 2010). However, apart from the fact that very few instruments have this highly specialized facility, it has been reported that such analyses typically are cumbersome and technically challenging to perform, since they suffer from serious analytical artifacts related to hardness, background noise, and interference (Metzner et al., 2010; Moore et al., 2012). Hence, for most element bioimaging techniques, freezing followed by water removal is still the preferred method of sample preparation (Smart et al., 2010; Moore et al., 2014).
During freezing, there is a risk of ice crystal formation, which may rupture the tissues, causing ion leakage and displacement, resulting in poor-quality imaging. Fast, high-pressure freezing, typically at approximately 200 MPa and −196°C, is frequently used to overcome such problems, as it decreases ice crystal formation, also in the deeper layers of the sample (Moore et al., 2012). Here, we did not use high-pressure freezing, yet we observed little or no ice formation in the specimens after plunge freezing in liquid nitrogen. Most likely, this was due to the small size of the Arabidopsis roots, which enabled rapid freezing. We also precooled the OCT medium in order to facilitate the fastest possible freezing, minimizing ice crystal formation.
In order to perform cryo-sectioning, the use of a cutting medium is needed for thin, fragile roots. For larger roots, direct freezing without support can be done in liquid propane, cooled by liquid nitrogen, and subsequently cut in cross sections. (Schneider et al., 2002).
An alternative to traditional cryo-sectioning with OCT medium is freeze substitution. Freeze substitution replaces the water with an organic solvent, typically acetone, after which the acetone is substituted with a resin or another fixative. Once embedded, the sample can be sliced into thin sections, down to a thickness of 1 µm. Even though this technique is regarded as the state-of-the-art sample preparation technique (Moore et al., 2012; Zhao et al., 2014), it has some disadvantages, and the localization of highly diffusible ions like K+ and Na+ may still be significantly altered during the acetone substitution and the following chemical fixation (Smart et al., 2010). In that study, the Ca signal was stronger than the K signal in the cytosol, suggesting misplacement of these elements during sample preparation (Smart et al., 2010). In another study, about 90% of the nickel ions in leaf tissues were lost during freeze substitution with tetrahydrofuran (Budka et al., 2005). Here, we have shown, using the highly mobile K+ ion as a proxy for ions that are easily lost from the tissue, that the elemental ion composition can be maintained with a simple encapsulation of the root tissue with paraffin, prior to freezing and sectioning on a cryotome. After cryo-sectioning, the cross sections were put in an ordinary freezer overnight. During storage for 16 h, we found that ice sublimation occurred, which dried the samples completely. Hence, dry cross sections were obtained without having to move them to a freeze-drier facility, without adding solvents, and without pressure or temperature changes imposed on the samples, which all could alter the distribution of elements. The method is applicable to other plants species also, e.g. barley (Hordeum vulgare; Fig. S1).
The application of an internal standard proved useful in order to record reproducible images and enable comparisons between the results recorded in separate analytical runs. For the normalization of topographic differences or differences in tissue hardness, an element already present in the tissue can be used. Carbon (13C) has been used frequently in other LA-ICP-MS studies (Becker et al., 2010a, 2010b). In our root sections, the signal from 13C was very weak, which made this approach unreliable. Also, roots may have large differences in suberin content, depending on their nutrient status (Barberon et al., 2016), and since suberin is a carbon-rich compound, the use of this element for normalization could be a significant source of error. Here, we decided to apply Rb as an internal standard, since we knew that it was not present in our nutrient solutions. Rb was chosen due to its similarity to K in terms of its ionization potential (first ionization potentials: K = 4.34 eV and Rb = 4.18 eV). In practice, and in future analyses, any element or isotope can be used in the way described here, which enables comprehensive multielement fingerprinting analyses. The normalization shown in Figure 5, D and E, indicates that only minor differences in the images occur upon normalization with the Rb signal, yet for day-to-day comparisons, it is a very valuable tool, since there are typically quite large variations in ICP-MS sensitivity on a day-to-day basis. As stated above, Zn has a different ionization potential (9.39 eV) than Rb (4.18 eV); hence, it can only be used for monitoring instrumental changes in signal strength and, in this case, also to document a quantitative ablation process. For semiquantification or absolute quantification, an element with an ionization potential closer to Zn would have to be used.
Method Validation Using NAS Mutants
We tested the developed method on the nas4x mutant, which is unable to synthesize NA, an essential ligand for metal ion homeostasis in plants (von Wiren et al., 1999; Takahashi et al., 2003). Changes in NAS gene expression have been shown to strongly affect the transport and distribution of Fe, Zn, Cu, and Mn (Klatte et al., 2009; Deinlein et al., 2012). Consistent with these previous observations, the nas4x quadruple mutants showed interveinal chlorosis, mainly present in the youngest leaves, which is a well-known characteristic of mineral nutrient deficiencies (Fig. 7; Takahashi et al., 2003; Schuler et al., 2012). However, our analysis of the total element concentrations in the shoots revealed deficiencies of Zn and Mn but not of Fe (Table II; Klatte et al., 2009; Schuler et al., 2012). It should be noted that we did not separate the shoot into young and old leaves, so these observations are based on the average concentration of the whole shoot. Schuler et al. (2012) observed that the nas4x mutant seemed to have a defect with respect to Fe delivery to sink organs, which may explain the marked interveinal chlorosis observed in the young leaves of our plants (Fig. 7).
Xylem sap data confirmed that the root-to-shoot transport of Fe was less affected by NA deficiency than that of Zn and Mn (Fig. 8). Substantial increases in citrate levels have been reported for nas mutants and might be the reason for the absence of a reduction in shoot Fe concentration, since citrate is the preferred Fe ligand at the low pH of the xylem sap (von Wiren et al., 1999; Rellán-Alvarez et al., 2008; Schuler et al., 2012). The low xylem concentrations of Zn and Mn point toward a pivotal role of NA in xylem loading of these elements but not for Fe. A significant decrease in xylem Zn concentration was observed in Arabidopsis halleri RNA interference lines with depressed NA content by Cornu et al. (2015). Speciation analyses of the xylem sap revealed that the main Zn ligands, both in the wild type and RNA interference lines, were malate and/or citrate, not NA (Cornu et al., 2015). Using LA-ICP-MS bioimaging, we show that Zn accumulated particularly in and around the stele in the nas4x mutant (Fig. 9, middle), which is consistent with the obtained xylem and shoot data (Table II; Fig. 8) and the proposed primary role for NA in loading Zn into the xylem. Fe in the same root section from nas4x showed no such accumulation in and around the stele (Fig. 9, left), suggesting that NA is not required for xylem loading of Fe. The Arabidopsis mutant frd3, which lacks a functional citrate transporter, was reported to have approximately 40% lower citrate concentrations in the xylem sap and showed Fe accumulation in the root central cylinder (Schuler et al., 2012), which underlines the importance of citrate for xylem-mediated root-to-shoot Fe transport. The fact that nas4x mutant plants developed symptoms of interveinal chlorosis indicative of Fe deficiency, despite having similar whole-shoot Fe concentrations to the wild type, suggests that NA is involved in phloem-based partitioning of Fe after unloading from the xylem. Consequently, insufficient amounts of Fe reach the chloroplasts in the interveinal cells of the young, newly developed leaves, leading to chlorosis. Thus, using LA-ICP-MS to image the steady-state distribution of Fe, Zn, and Mn in the nas4x mutant has provided in planta evidence of a role for NA in loading Zn and Mn, but not Fe into the xylem. Our data clearly show that LA-ICP-MS is a powerful technique to study the consequences of changes in available ligands for ion transport and xylem loading at the cellular level. As such, the method can reveal important processes that would have been otherwise overlooked.
CONCLUSION
We conclude that LA-ICP-MS constitutes a powerful technique for biological investigations of intracellular and intercellular ion transport. Moreover, ICP-MS offers a wide range of elements to be analyzed, including light elements and biologically important elements such as Zn and Mn, which have poor sensitivity in synchrotron x-ray fluorescence-based techniques and in SIMS, respectively. Also, since the presented method is applicable to the small and fragile roots of Arabidopsis, the full range of molecular, genetic, and cell biological approaches available in this genetic system can be utilized. For example, a broad selection of mutants with alterations in ion transporter function or root cellular morphology can be studied, allowing a deeper understanding of the functional role of different cell types, extracellular barriers such as Casparian strips and suberin lamellae, and a cell-level resolution of the major ion transport pathways. As such, research on agricultural nutrient use efficiency and plant adaptability to adverse environmental conditions will benefit from the possibilities that the ICP-MS-based bioimaging method offers. Since the method can be further developed to also maintain the native composition of proteins, enzymes, RNA, and DNA, the protocol may be useful in combination with other omics techniques.
MATERIALS AND METHODS
Plant Cultivation
Arabidopsis (Arabidopsis thaliana) accession Columbia-0 and the Arabidopsis nas4x quadruple mutant were used for all experiments. The nas4x mutant was obtained by crossing homozygous T-DNA insertion mutants for nas1 (SALK_082174), nas2 (SALK_066962), nas3 (SALK_106467), and nas4 (SALK_135507; The Arabidopsis Information Resource; www.arabidopsis.org) in different combinations to obtain initially double and subsequently triple mutants, two of which were crossed to obtain the quadruple mutant. Seeds were sterilized in 50% ethanol for 1 min and in 2.5% NaClO (Klorin original; Colgate-Palmolive) with 0.05% (v/v) Triton X-100 for 10 min, then rinsed three times with Milli-Q water. Then, they were stratified for 48 h at 4°C in the dark. Seeds were germinated in distilled water, and 1-week-old plants were transferred to a hydroponic system containing 0.25 mm CaCl2, 1 mm KH2PO4, 0.05 mm KCl, 0.25 mm K2SO4, 1 mm MgSO4, 0.1 mm NaFe-EDTA, 2 mm NH4NO3, 30 μm H3BO3, 5 μm MnSO4, 1 μm ZnSO4, 1 μm CuSO4, 0.7 μm NaMoO4, and 1 μm NiSO4. The pH of the solution was adjusted to 5.8 by KOH (2 m). Nutrient solutions were replaced every 2 d. All plants were grown in a controlled environment with 8 h of light (90 μmol m–2 s–1), 22°C/19°C (day/night), and 75% humidity.
Total Element Concentration
Whole roots were gently dried on a napkin and then freeze-dried at −45°C, 1 mbar for 36 h together with the shoots. Then, each sample was weighed. All leaves of the shoot were treated as the same sample (i.e. we did not separate young and old leaves). All samples were digested with 500 µL of 70% HNO3 and 250 µL of 15% H2O2 and subsequently diluted to 10 mL. Elemental analysis was performed with an ICP-OES device (Agilent 5100) using external calibration, drift check samples, and certified reference material for optimal data quality (Olsen et al., 2016).
Xylem Sap Analysis
At 0.5 h before the onset of light in the climatic chamber, the plants were cut just below the leaf rosette with a ceramic knife. The first 1 µL of xylem sap was discarded, then 3 to 6 µL was collected from each replicate. Each sample was diluted one time with 7% HNO3 (2 µL of xylem sap + 2 µL of HNO3), giving a final volume of 4 µL with an acid concentration of 3.5%. Two microliters of this sample was injected directly into the ICP-MS device in flow injection analysis mode using an inert HPLC instrument (Ultimate 3000; Thermo Scientific) as autosampler and injector, 3.5% HNO3 as mobile phase, and a flow rate of 0.3 mL min−1 (Olsen et al., 2016). The concentration was measured using external calibration, injected using the same conditions as described for the samples.
Conventional Root Tissue Dehydration
The entire root was cut off from the shoot and put into a 50-mL Eppendorf tube filled with FAA fixative, where it was left in the fridge overnight (+6°C). The FAA fixative contained (for 100 mL) 52 mL of 96% ethanol, 5 mL of acetic acid, 5 mL of 37% formaldehyde, and 28 mL of Milli-Q water. The next day, all the FAA fixative was discarded, and the sample was washed twice in Milli-Q water and then soaked in 50% ethanol for 30 min. Then, the sample was transferred to 70% ethanol, 96% ethanol, and 100% ethanol, each time for 30 min. The last step was a transfer to a new solution of 100% ethanol, where the sample was kept for 3 h. All the steps were performed at room temperature.
Sample Preparation for LA-ICP-MS Analysis
The root samples for LA-ICP-MS analyses were all cut approximately 2 cm from the tip of the primary root. The length of each piece of primary root was between 6 and 8 mm, and any lateral roots were cut off from this root piece. After gentle drying on a napkin, the root piece was dipped in melted paraffin (70°C), which was then allowed to harden for 5 s. The paraffin-coated root was then put in a handmade aluminum foil mold containing nonfrozen but precooled OCT medium (4°C; Tissue-Tek), in which the root segment was aligned horizontally and submerged in OCT medium. The mold with its contents was then put in liquid nitrogen in order to freeze it instantaneously. After freezing, the solid OCT medium block was transferred to a cryotome (Leica CM050S), precooled to −25°C, where it was mounted for sectioning. Cross sections 12 to 20 µm thick were cut and transferred with a thin paintbrush onto membrane-covered glass slides (MembraneSlide 1.0 PEN; Carl Zeiss Microscopy). For transfer of the sections, the down side of the glass was warmed slightly with a finger, allowing the section to briefly melt onto the glass slide, ensuring uniform adhesion to the membrane in a horizontal position. Immediately after this critical step, the section was deep frozen again inside the cryotome. In order to evaluate the quality of the root sections, some samples were visualized with a light microscope. These samples were always discarded, since they were thawed completely and, hence, considered unfit for further analysis. If the sample check in the microscope was satisfactory, new sections were cut and immediately left to dry in the freezer overnight.
Preparation and Application of an Internal Standard
In order to apply an internal standard, we used an inkjet printer (Brother; model DCP-J412). Briefly, a small hole was drilled in the magenta ink cartridge and 0.5 mL of ink was removed using a syringe with a needle. Then, 0.5 mL of an Rb solution (10,000 mg L−1; Peak Performance-certified reference material; CPI International) was injected, and the hole was sealed with glue. The ink cartridge was left to equilibrate overnight, and then it was installed into the inkjet printer. A magenta-colored rectangle was printed on a regular transparent plastic sheet. The same rectangle was printed on the same area of the plastic sheet five times, ensuring a thick, very homogenous application of Rb-containing ink. An approximately 1- × 2.5-cm rectangle was cut from the plastic sheet and carefully inserted under the PEN membrane of the membrane-covered PEN microscopy slide, which had been opened carefully on one side with a scalpel (Fig. 2).
LA-ICP-MS Analysis
The LA-ICP-MS analyses were performed with a nanosecond LA unit (NWR193; New Wave Research) equipped with an argon fluoride (ArF) excimer laser source operating at 193 nm using the following key settings: energy, 1.4 to 2.3 J cm−2 (30%–40% of maximum energy); scan speed, 10 µm s−1; repetition rate, 40 to 60 Hz; and spot size, 5 µm. All elemental signals were obtained with an Agilent Technologies7900 ICP-MS device operated in helium mode. The isotopes analyzed were 85Rb, 39K, 24Mg, 55Mn, 56Fe, and 66Zn, using an integration time of 0.1 s. The key settings on the ICP-MS device were as follows: sample cone depth, 5 mm; carrier gases, 1 mL min−1; and octopole collision gas (helium), 1 mL min−1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Twenty-micrometer-thick cross sections from roots of barley.
Supplementary Material
Acknowledgments
We thank Thomas H. Hansen and Lena Byrgesen for excellent technical assistance; Thomas C. de Bang for valuable inputs and comments; and Judith van de Mortel and Huilan Wu for construction and functional confirmation of the nas4x quadruple T-DNA insertion knockout mutant.
Glossary
- NA
nicotianamine
- nano-SIMS
nano-secondary ion mass spectrometry
- LA-ICP-MS
laser ablation-inductively coupled plasma-mass spectrometry
- SIMS
secondary ion mass spectrometry
- MS
mass spectrometry
- Fe
iron
- Zn
zinc
- Mn
manganese
- Ca
calcium
- K
potassium
- Mg
magnesium
- Rb
rubidium
- PEN
polyethylene naphthalate
- LA
laser ablation
- ICP-MS
inductively coupled plasma-mass spectrometry
Footnotes
This work was carried out within the ERA-NET Coordinating Action in Plant Sciences program project ERACAPS13.089_RootBarriers, with support from the Innovation Fund Denmark (grant no. 4084–00001B to J.K.S.), the Biotechnology and Biological Sciences Research Council (grant no. BB/L027739/1 to D.E.S.), and the Earth and Life Sciences division of the Netherlands Organization for Scientific Research (grant no. 849.13.003 to M.G.M.A.), as well as by the China Scholarship Council (grant no. 201406170047).
References
- Balcaen L, Bolea-Fernandez E, Resano M, Vanhaecke F (2015) Inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS): a powerful and universal tool for the interference-free determination of (ultra)trace elements. A tutorial review. Anal Chim Acta 894: 7–19 [DOI] [PubMed] [Google Scholar]
- Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459 [DOI] [PubMed] [Google Scholar]
- Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JS, Niehren S, Matusch A, Wu B, Hsieh HF, Kumtabtim U, Hamester M, Plaschke-Schlutter A, Salber D (2010a) Scaling down the bioimaging of metals by laser microdissection inductively coupled plasma mass spectrometry (LMD-ICP-MS). Int J Mass Spectrom 294: 1–6 [Google Scholar]
- Becker JS, Zoriy M, Matusch A, Wu B, Salber D, Palm C, Becker JS (2010b) Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom Rev 29: 156–175 [DOI] [PubMed] [Google Scholar]
- Beeckman T, Viane R (2000) Embedding thin plant specimens for oriented sectioning. Biotech Histochem 75: 23–26 [DOI] [PubMed] [Google Scholar]
- Bhatia NP, Walsh KB, Orlic I, Siegele R, Ashwath N, Baker AJM (2004) Studies on spatial distribution of nickel in leaves and stems of the metal hyperaccumulator Stackhousia tryonii using nuclear microprobe (micro-PIXE) and EDXS techniques. Funct Plant Biol 31: 1061–1074 [DOI] [PubMed] [Google Scholar]
- Budka D, Mesjasz-Przybylowicz J, Tylko G, Przybylowlicz WJ (2005) Freeze-substitution methods for Ni localization and quantitative analysis in Berkheya coddii leaves by means of PIXE. Nucl Instrum Methods Phys Res B 231: 338–344 [Google Scholar]
- Cornu JY, Deinlein U, Höreth S, Braun M, Schmidt H, Weber M, Persson DP, Husted S, Schjoerring JK, Clemens S (2015) Contrasting effects of nicotianamine synthase knockdown on zinc and nickel tolerance and accumulation in the zinc/cadmium hyperaccumulator Arabidopsis halleri. New Phytol 206: 738–750 [DOI] [PubMed] [Google Scholar]
- Davies KL, Davies MS, Francis D (1991) Zinc-induced vacuolation in root meristematic cells of Festuca rubra L. Plant Cell Environ 14: 399–406 [Google Scholar]
- Deinlein U, Weber M, Schmidt H, Rensch S, Trampczynska A, Hansen TH, Husted S, Schjoerring JK, Talke IN, Krämer U, et al. (2012) Elevated nicotianamine levels in Arabidopsis halleri roots play a key role in zinc hyperaccumulation. Plant Cell 24: 708–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder N, Obrien TP (1968) Plant microtechnique: some principles and new methods. Am J Bot 55: 123 [Google Scholar]
- Fourie HO, Peisach M (1977) Loss of trace elements during dehydration of marine zoological material. Analyst (Lond) 102: 193–200 [DOI] [PubMed] [Google Scholar]
- Geldner N. (2013) The endodermis. Annu Rev Plant Biol 64: 531–558 [DOI] [PubMed] [Google Scholar]
- Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE (2013) Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc Natl Acad Sci USA 110: 16283 erratum Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE (2013) Proc Natl Acad Sci 110: 14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya T, Borghi M, Wang P, Danku JMC, Kalmbach L, Hosmani PS, Naseer S, Fujiwara T, Geldner N, Salt DE (2015) The MYB36 transcription factor orchestrates Casparian strip formation. Proc Natl Acad Sci USA 112: 10533–10538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P (2009) The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol 150: 257–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, de Jonge MD, Donner E, Kopittke PM, Howard DL, Kirkham R, Ryan CG, Paterson D (2011a) Fast x-ray fluorescence microtomography of hydrated biological samples. PLoS ONE 6: e20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombi E, Smith E, Hansen TH, Paterson D, de Jonge MD, Howard DL, Persson DP, Husted S, Ryan C, Schjoerring JK (2011b) Megapixel imaging of (micro)nutrients in mature barley grains. J Exp Bot 62: 273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R, Thorpe MR, Breuer U, Blümler P, Schurr U, Schneider HU, Schroeder WH (2010) Contrasting dynamics of water and mineral nutrients in stems shown by stable isotope tracers and cryo-SIMS. Plant Cell Environ 33: 1393–1407 [DOI] [PubMed] [Google Scholar]
- Moore KL, Chen Y, van de Meene AML, Hughes L, Liu W, Geraki T, Mosselmans F, McGrath SP, Grovenor C, Zhao FJ (2014) Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol 201: 104–115 [DOI] [PubMed] [Google Scholar]
- Moore KL, Lombi E, Zhao FJ, Grovenor CRM (2012) Elemental imaging at the nanoscale: NanoSIMS and complementary techniques for element localisation in plants. Anal Bioanal Chem 402: 3263–3273 [DOI] [PubMed] [Google Scholar]
- Olsen LI, Hansen TH, Larue C, Østerberg JT, Hoffmann RD, Liesche J, Krämer U, Surblé S, Cadarsi S, Samson VA, et al. (2016) Mother-plant-mediated pumping of zinc into the developing seed. Nat Plants 2: 16036. [DOI] [PubMed] [Google Scholar]
- Pålsgård E, Lindh U, Roomans GM (1994) Comparative study of freeze-substitution techniques for x-ray microanalysis of biological tissue. Microsc Res Tech 28: 254–258 [DOI] [PubMed] [Google Scholar]
- Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JEM, Yamazaki M, Li G, Maurel C, Takano J, et al. (2014) A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3: e03115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Alvarez R, Abadía J, Alvarez-Fernández A (2008) Formation of metal-nicotianamine complexes as affected by pH, ligand exchange with citrate and metal exchange: a study by electrospray ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 22: 1553–1562 [DOI] [PubMed] [Google Scholar]
- Schneider T, Strasser O, Gierth M, Scheloske S, Povh B (2002) Micro-PIXE investigations of apoplastic iron in freeze-dried root cross-sections of soil grown barley. Nucl Instrum Methods Phys Res B 189: 487–493 [Google Scholar]
- Schuler M, Rellán-Álvarez R, Fink-Straube C, Abadía J, Bauer P (2012) Nicotianamine functions in the phloem-based transport of iron to sink organs, in pollen development and pollen tube growth in Arabidopsis. Plant Cell 24: 2380–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele R, Kachenko AG, Bhatia NP, Wang YD, Ionescu M, Singh B, Baker AJM, Cohen DD (2008) Localisation of trace metals in metal-accumulating plants using mu-PIXE. X-Ray Spectrom 37: 133–136 [Google Scholar]
- Smart KE, Smith JAC, Kilburn MR, Martin BGH, Hawes C, Grovenor CRM (2010) High-resolution elemental localization in vacuolate plant cells by nanoscale secondary ion mass spectrometry. Plant J 63: 870–879 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003) Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15: 1263–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wiren N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC, (1999) Nicotianamine chelates both FeIII and FeII: implications for metal transport in plants. Plant Physiol 119: 1107–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Menzies NW, Lombi E, McKenna BA, de Jonge MD, Donner E, Blamey FPC, Ryan CG, Paterson DJ, Howard DL, et al. (2013) Quantitative determination of metal and metalloid spatial distribution in hydrated and fresh roots of cowpea using synchrotron-based x-ray fluorescence microscopy. Sci Total Environ 463-464: 131–139 [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Moore KL, Lombi E, Zhu YG (2014) Imaging element distribution and speciation in plant cells. Trends Plant Sci 19: 183–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.