Abstract
Integrating knowledge from physiological ecology, evolutionary biology, phylogenetics, and paleobiology provides novel insights into factors driving plant physiological responses to both past and future climate change.
Since the Industrial Revolution began approximately 200 years ago, global atmospheric carbon dioxide concentration ([CO2]) has increased from 270 to 401 µL L−1, and average global temperatures have risen by 0.85°C, with the most pronounced effects occurring near the poles (IPCC, 2013). In addition, the last 30 years were the warmest decades in 1,400 years (PAGES 2k Consortium, 2013). By the end of this century, [CO2] is expected to reach at least 700 µL L−1, and global temperatures are projected to rise by 4°C or more based on greenhouse gas scenarios (IPCC, 2013). Precipitation regimes also are expected to shift on a regional scale as the hydrologic cycle intensifies, resulting in greater extremes in dry versus wet conditions (Medvigy and Beaulieu, 2012). Such changes already are having profound impacts on the physiological functioning of plants that scale up to influence interactions between plants and other organisms and ecosystems as a whole (Fig. 1). Shifts in climate also may alter selective pressures on plants and, therefore, have the potential to influence evolutionary processes. In some cases, evolutionary responses can occur as rapidly as only a few generations (Ward et al., 2000; Franks et al., 2007; Lau and Lennon, 2012), but there is still much to learn in this area, as pointed out by Franks et al. (2014). Such responses have the potential to alter ecological processes, including species interactions, via ecoevolutionary feedbacks (Shefferson and Salguero-Gómez, 2015). In this review, we discuss microevolutionary and macroevolutionary processes that can shape plant responses to climate change as well as direct physiological responses to climate change during the recent geologic past as recorded in the fossil record. We also present work that documents how plant physiological and evolutionary responses influence interactions with other organisms as an example of how climate change effects on plants can scale to influence higher order processes within ecosystems. Thus, this review combines findings in plant physiological ecology and evolutionary biology for a comprehensive view of plant responses to climate change, both past and present.
Figure 1.
A, Abiotic conditions directly affect plant physiological traits. Also, the probability that a given species persists with climate change (both in the past and future) is influenced by the degree of phenotypic plasticity in these traits, the ability of populations to migrate and track environmental conditions in space, and the potential for populations to evolve traits that are adaptive in the novel environment. Interactions between plants and other organisms also affect plant physiology, the strength of selection on plant traits, and the probability of persistence. Climate change alters species interactions via direct effects on plant antagonists and mutualists and via changes in plant traits that influence the dynamics of these interactions. B, Following an environmental perturbation (vertical dashed line), plant populations with low genetic and/or phenotypic variability are unlikely to persist (red line). Phenotypic plasticity can facilitate the tolerance of environmental change over the short term (blue line). Migration to a more favorable environment and/or the evolution of adaptive traits (including greater plasticity) can facilitate long-term responses to environmental change (orange line).
Due to rapid climate change, plants have become increasingly exposed to novel environmental conditions that are outside of their physiological limits and beyond the range to which they are adapted (Ward and Kelly, 2004; Shaw and Etterson, 2012). Plant migration may not keep pace with the unprecedented rate of current climate change (Loarie et al., 2009); therefore, rapid evolutionary responses may be the major process by which plants persist in the future (Franks et al., 2007; Alberto et al., 2013). In addition, although plants may have evolved physiological plasticity that produces a fitness advantage in novel environments, climate change may be so extreme as to push plants beyond tolerance ranges even in the most plastic of genotypes (Anderson et al., 2012).
ADVANCES
Rapid climate change is disrupting long-standing patterns of natural selection on plant physiological traits. Microevolutionary responses to these changes can occur over time scales relevant to ecological processes.
Emerging macroevolutionary analyses using large, time-calibrated phylogenies provide insight into evolutionary changes in plant physiology and species diversification rates following past climate change events.
Past conditions, such as low [CO2] during glacial cycles, likely produced lingering adaptations that could limit plant physiological responses to current and future climate change.
Climate change can affect plant traits, fitness, and survival indirectly via shifts in biotic interactions. The ecoevolutionary consequences of altered species interactions can be as important as the direct effects of climate change on plant physiology.
Understanding the potential for evolutionary responses at the physiological level is a key challenge that must be met in order to improve predictions of plant response to climate change. A focus on physiology is critical because these processes scale from individual to ecosystem levels. For example, [CO2] rise and climate change that alter photosynthetic rates may shift plant growth rates, overall productivity, and resource use (Ainsworth and Rogers, 2007; Norby and Donald, 2011; Medeiros and Ward, 2013). Other physiological responses to altered climate include increasing leaf sugars with elevated [CO2], which may influence major life history traits such as flowering time and fitness via sugar-sensing mechanisms (Springer et al., 2008; Wahl et al., 2013). At higher scales, shifts in source/sink relationships of photosynthate can influence seedling survival, whole-plant growth, competitive ability within the broader plant community, symbiotic interactions, and fitness. Therefore, the potential for physiological functioning to evolve in response to climate change will be a key indicator of plant resiliency (or lack thereof) in future environments. Defining physiological components that correlate with fitness, particularly in newly emerging environments, will allow us to identify candidate processes that may be under strong selection in future environments and to predict the composition and functioning of future plant populations and communities (Kimball et al., 2012).
It is clear that long-term changes in the environment spanning millions of years of plant evolution have shaped the major physiological pathways that are present in modern plants (Edwards et al., 2010; Sage et al., 2012), and these pathways will determine the range of physiological tolerances for the response to novel environments of the future. In addition, relatively recent conditions in the geologic record have shaped selective pressures on plant physiology (Ward et al., 2000) and may influence the ability of plants to respond to future conditions. For example, the peak of the last glacial period (20,000 years ago) represents a fascinating time when low [CO2] (180–200 µL L−1) likely constrained the physiological functioning of C3 plants. During that period, [CO2] was among the lowest values that occurred during the evolution of land plants (Berner, 2006). Modern C3 annuals grown at glacial [CO2] exhibit an average 50% reduction in photosynthesis and growth as well as high levels of mortality and reproductive failure relative to plants grown at modern [CO2] (Polley et al., 1993; Dippery et al., 1995; Sage and Coleman, 2001; Ward and Kelly, 2004). Thus, this period likely imposed strong selective pressures on plants, as evidenced directly by artificial selection experiments (Ward et al., 2000) and in the recent geologic record (Gerhart and Ward, 2010).
A series of key questions have now emerged. (1) How will plants evolve in response to rapid climate change? (2) How will evolutionary history and species interactions influence this evolutionary trajectory? (3) How have past responses to climate change in the geologic record influenced current and potentially future responses to a rapidly changing environment? To address these questions, we report on emerging concepts in the broad field of evolutionary physiology, paying specific attention to processes ranging from microevolution to macroevolution, the influence of species interactions on these processes, and insights from paleobiology (where we provide new findings). This review is not intended to cover all of the current ground-breaking work in this area but rather to provide an overview of how a multitude of approaches can influence our overall understanding of how plant physiological evolution has altered past ecosystems as well as those that will emerge during the Anthropocene Epoch.
MICROEVOLUTIONARY RESPONSES OF PLANT PHYSIOLOGY TO CLIMATE CHANGE
By altering thermal and precipitation regimes and [CO2], climate change is disrupting long-standing patterns of natural selection on plant physiology, morphology, and life history. Novel environmental pressures could reduce germination success, plant viability, and fecundity in the short term as mediated through effects on physiology (Anderson, 2016). Phenotypic plasticity can temporarily alleviate the effects of directional selection pressures that are expected to arise with climate change (Fig. 1; Nicotra et al., 2010) but may not enable long-term population persistence as conditions fall outside of the bounds of historical variability. Species will ultimately have to evolve or migrate in pace with climate change to avoid extinction (Fig. 1). Many species already have shifted their distributions to higher latitudes and elevations (Perry et al., 2005; Lenoir et al., 2008), yet evidence for evolution in response to climate change remains sparse at best (Franks et al., 2007; Merilä, 2012). Here, we discuss conditions that may promote or impede physiological and morphological adaptation to climate change in plants.
Phenotypic Plasticity
Phenotypic plasticity is a fundamental mechanism by which species respond to a changing environment. Climate change has prompted plastic responses in physiological traits for a wide variety of plant taxa (Gunderson et al., 2010; Liancourt et al., 2015), yet few studies examine the fitness consequences of plastic responses. The direction and adaptive value of plasticity can be assessed experimentally, where common genotypes are exposed to contrasting conditions designed to simulate a changing climate (Fig. 2). In the context of climate change, adaptive plasticity results in an equivalent or higher fitness of induced phenotypes relative to the original phenotype in the novel environment. The response to selection depends on the strength of selection on plasticity, the degree of heritable variation in plasticity, and the strength and direction of selection on other traits that are genetically correlated with the plastic response (Lande and Arnold, 1983).
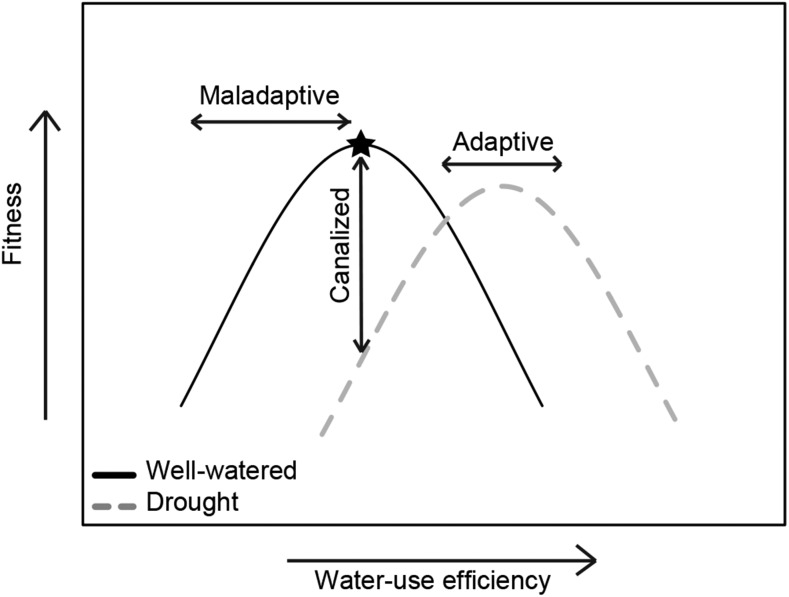
Figure 2.
Consider a hypothetical population that is experiencing increasing aridity owing to climate change. Adaptive plasticity in water-use efficiency (WUE) may allow the population to withstand changing conditions. To examine the adaptive value of plasticity, researchers quantify WUE in well-watered and drought treatments. In well-watered historical conditions, stabilizing selection favors intermediate WUE because plants with low WUE risk desiccation and plants with high WUE have reduced growth. Drought stress shifts the fitness function, such that optimal fitness now occurs at higher levels of WUE. Plasticity is adaptive when the novel trait values produce similar or higher fitness than the former trait values could have achieved under drought conditions. If WUE does not change in drought, then trait canalization could restrict population persistence. Maladaptive plasticity reduces fitness and could lead to population declines.
The cumulative effects of plasticity throughout a plant’s life cycle can be extensive. For example, a plant that is being shaded by a canopy will sense a red-to-far-red light ratio below optimum, triggering physiological, molecular, and developmental adjustments that enhance light capture (Keuskamp et al., 2010). This shade-avoidance syndrome (Schmitt and Wulff, 1993) can induce plastic responses in traits expressed later in life history (e.g. accelerations in the onset of flowering) and indirectly influence the strength or form of selection on these traits (Donohue, 2003). Furthermore, these types of plastic responses can be far reaching, as the maternal environment can influence offspring phenotype and fitness (transgenerational plasticity or maternal effects; Galloway and Etterson, 2007).
Plasticity can facilitate evolution by alleviating the immediate selection pressures imposed by climate change, providing more time for evolutionary responses (Chevin et al., 2010). For instance, adaptive plasticity in WUE (carbon uptake per water loss) enabled plants from three genetically differentiated populations of the annual Polygonum persicaria to maintain high fitness in both drought and well-watered environments (Heschel et al., 2004). The maintenance of fitness under drought stress may allow this species more time to respond to other stressors. Plasticity also can promote genetic change if the phenotypes exposed to selection become fixed through genetic assimilation (Badyaev, 2005). Additionally, increased environmental variation projected under climate change may favor the evolution of higher levels of plasticity in physiological traits (Nicotra et al., 2010). Nevertheless, costs or limits to producing plastic responses can constrain the ongoing evolution of plasticity, ironically (DeWitt et al., 1998). Despite the ubiquity with which climate change is eliciting plastic responses in plant physiology, the potential contributions of plasticity to evolutionary processes remain largely underexplored in natural systems.
Adaptive Evolution
For adaptive evolution to occur, a population must have sufficient genetic variation in traits targeted by selection, including physiological traits. Estimates of heritability for physiological traits can vary widely by trait type (Geber and Griffen, 2003; Johnson et al., 2009), with lower heritability in physiological traits that are instantaneously measured than in those that represent broader temporal integration (Ackerly et al., 2000). The strength and form of selection, coupled with rates of gene flow and mutation, ultimately determine whether genetic variation in a population is replenished or depleted over time (Mitchell-Olds et al., 2007). Small fragmented plant populations are particularly susceptible to diminished genetic variation and, consequently, may undergo increased extinction risks associated with climate change (Jump and Peñuelas, 2005; Leimu et al., 2006). To improve our ability to assess the capacity for rapid evolution in plant physiology, additional investigations must estimate the degree of genetic variation and the strength of selection under simulated climate change.
Genetic correlations can constrain evolution if the direction of the correlation opposes that of selection. For example, Etterson and Shaw (2001) detected additive genetic correlations that were antagonistic to the direction of selection in the annual legume Chamaecrista fasciculata and concluded that these correlations would likely impede adaptation to climate change. Furthermore, recent evolution of drought avoidance via early flowering increased Brassica rapa’s vulnerability to pathogens (O’Hara et al., 2016), demonstrating that climate change can restrict the joint evolution of plant physiological traits. Genetic correlations generated by pleiotropy (the influence of a single gene on multiple traits) generally are stable and can restrict the rate of evolution (Mitchell-Olds, 1996). The same is true of genetic correlations maintained by linkage disequilibrium when loci are in close proximity (Falconer and Mackay, 1996). However, artificial selection studies have demonstrated that rapid evolution is still possible in spite of pleiotropic genetic correlations (Conner et al., 2011), and linkage disequilibrium decays quickly in large, outcrossing populations with high recombination rates (Flint-Garcia et al., 2003). Genetic constraints have been invoked as a considerable barrier to adaptive evolution in response to climate change, and characterizing the genetic architecture of functional traits in natural populations is paramount for predicting evolutionary change.
Gene Flow
Plant populations are connected over spatial scales by pollen and seed dispersal. If local populations lack sufficient genetic variation to respond to novel selection, gene flow can expand genetic variation, reduce inbreeding, and facilitate evolutionary responses to selection (Frankham, 2005). For instance, the budburst phenology of two Scottish birch (Betula) species may not evolve in pace with climate change without gene flow from populations with earlier phenologies (Billington and Pelham, 1991). Some plant species, including trees in the genera Quercus and Eucalyptus, display genetically based clinal variation across climatic gradients in physiological traits such as stomatal conductance and drought and frost tolerance (Marchin et al., 2008; Kremer et al., 2014). Under climate change, gene flow from central populations may benefit peripheral populations at the leading edge of the range by introducing alleles preadapted to warm conditions (Aitken and Whitlock, 2013; Kremer et al., 2014).
Gene flow also can restrict evolutionary responses to climate change by introducing maladapted alleles into populations that are already lagging in their adaptive responses to changing conditions (Lenormand, 2002). High rates of gene flow from central populations may overwhelm selection in the trailing edge populations, preventing adaptation to novel conditions (Kirkpatrick and Barton, 1997; Bridle and Vines, 2007). The potential evolutionary consequences of gene flow for adaptation to climate change are variable and require further examination in appropriate ecological contexts. This is especially true in the context of plant physiology, for which we need additional data on genetic variation in natural populations and more information about the extent to which populations are connected by gene flow.
Microevolution: Unanswered Questions and Future Directions
Experiments that simultaneously manipulate multiple climate change factors hold great promise for elucidating the physiological processes that underlie climate change responses (Eller et al., 2011) and for improving our ability to predict plant evolution. However, few empirical studies directly evaluate the microevolution of physiology under climate change. Future efforts should quantify multiple physiological traits and fitness components in plants of known origin to assess genetic constraints on climate change response and to evaluate the adaptive nature of physiological plasticity. Additionally, common garden experiments across spatial climatic gradients can reveal whether climate change disrupts local adaptation in physiology (Marchin et al., 2008; Wang et al., 2010; Wilczek et al., 2014). Studies that integrate population and quantitative genetics can test whether gene flow hastens physiological adaptation through the introgression of alleles from populations that have evolved under conditions that reflect climate projections. Finally, field studies can illuminate the role of biotic interactions in shaping physiological plasticity and evolution in natural systems.
MACROEVOLUTIONARY RESPONSES OF PLANT PHYSIOLOGY TO CLIMATE CHANGE
Climate change expands certain ecological niches at the expense of others. The availability of ecological opportunities and the ability of species to exploit these opportunities can dictate the tempo of species diversification as well as patterns of phenotypic evolution (Simpson, 1953). Therefore, climate change has the potential to alter patterns of species diversification and generate macroevolutionary trends in plant physiology. Comparative work has established that physiological traits can provide an evolutionary advantage in a novel environment (Givnish, 1987), potentially allowing access to a new ecological niche or improving competitive advantage in an expanded niche. Either of these situations may boost population density, geographic range size, or the success of peripherally isolated populations. These changes, in turn, can decrease the probability of extinction or increase the rate of speciation (Heard and Hauser, 1995), stimulating plant species diversification.
A phylogenetic approach can identify associations between environmental change, trait evolution, and macroevolutionary patterns of species diversification. This approach relies on fossil-calibrated phylogenetic trees that estimate divergence events in absolute time. Using trait data for each tip in the phylogenetic tree and a model for trait evolution, the evolutionary history of traits can be reconstructed on the time-calibrated tree (Schluter et al., 1997). When the evolution of more than one trait is modeled on the tree, phylogenetic comparative methods can test for patterns of correlated evolution between traits (Pagel, 1994). These correlations may signal constraints on the evolution of key traits, where their origin is contingent on the presence of preexisting enabling traits. Model-based approaches can identify shifts in diversification rate on time-calibrated trees (Rabosky, 2014) and test whether diversification rates are influenced by trait evolution (Maddison et al., 2007). As a case study, we discuss how a phylogenetic approach has connected innovations in plant photosynthesis to species diversification following climate change during the Miocene.
Miocene Climate Change and Innovations in Photosynthesis
A significant decline in [CO2] that began in the early Oligocene (approximately 32 million years ago) coincided with global cooling and aridification in the mid-Miocene (approximately 14 million years ago; Tripati et al., 2009); these environmental changes imposed physiological stress on plants, particularly those living in warm or arid habitats (Ehleringer and Monson, 1993). As atmospheric [CO2]:[O2] declines and temperatures rise, the oxygenation reaction with Rubisco increases relative to carboxylation (Ehleringer and Monson, 1993), reducing the efficiency of photosynthesis. Photorespiration scavenges some of the lost carbon from this process, but net losses of carbon and energy still occur. Evaporative water loss increases with photorespiration rates because greater stomatal conductance is necessary to make up for carbon losses (Monson et al., 1983).
CO2-concentrating mechanisms (CCMs) are physiological pathways that increase the ratio of [CO2] to [O2] near the site of CO2 fixation, thus reducing photorespiration (Hatch, 1987; Winter and Smith, 1996). There are two main types of CCMs: Crassulacean acid metabolism (CAM) and C4 photosynthesis. Both separate initial carbon fixation from the rest of photosynthesis by using phosphoenolpyruvate carboxylase (PEPC) rather than Rubisco to fix atmospheric CO2 into a four-carbon (C4) acid. The C4 acid is later decarboxylated to release CO2 within photosynthetic cells, where Rubisco refixes it in the standard Calvin cycle in the absence or near absence of photorespiratory carbon losses. In CAM plants, the diurnal pattern of stomatal opening is inverted, such that PEPC fixes CO2 at night and the C4 acids are decarboxylated during the day, allowing Rubisco to refix CO2. Since stomata are closed during the day, CAM greatly improves WUE in arid habitats (Winter and Smith, 1996). In C4 plants, PEPC and Rubisco function during the day, but PEPC is active in mesophyll cells and C4 acids are transported to bundle sheath cells where Rubisco and the Calvin cycle operate (Hatch, 1987).
CCMs have evolved numerous times in higher plants (Edwards and Ogburn, 2012) and are key traits that increased the diversification of certain lineages following the Miocene climate change and ultimately contributed to the dominance of these groups in arid landscapes (Sage et al., 2012). Recent studies have used a phylogenetic approach to examine the relationship between Miocene climate change, CCM evolution, and diversification rate.
Phylogenetic Patterns of CCM Evolution and Diversification Rate
The evolution of CCMs has been reconstructed for several plant groups. In grasses, sedges, and eudicots, the origins of C4 photosynthesis date to the Oligocene through the Miocene (Besnard et al., 2009; Christin et al., 2011; Spriggs et al., 2014). In bromeliads, orchids, and Euphorbia spp., origins of CAM photosynthesis date from the early Miocene to the late Pliocene (Horn et al., 2014; Silvestro et al., 2014; Bone et al., 2015). The timing of CCM origins in these groups is consistent with the hypothesis that CCM evolution is associated with declining [CO2].
Based on current distributions, the evolution of CCMs appears to occur most often in semiarid to arid regions (Sage et al., 2011). Phylogenetic studies demonstrate that the evolution of C4 photosynthesis in grasses is correlated significantly with shifts to open and drier habitats (Edwards and Smith, 2010). The evolution of CAM in terrestrial Eulophiinae orchids is associated with shifts from the occupation of humid habitats to hot and dry habitats (Bone et al., 2015), as is true for orchids and bromeliads that evolve an epiphytic habit in forest canopies where water availability is diurnally and seasonally intermittent (Silvestro et al., 2014; Givnish et al., 2015). These patterns are consistent with the hypothesis that CCMs confer the greatest advantage in water-limited habitats, particularly with respect to CAM.
Phylogenetic studies have identified significant shifts toward increased diversification rates in clades that evolved CCMs, predominantly in the Miocene following the initial evolution of CCMs (Arakaki et al., 2011; Table I). Thus, the Miocene climate change appears to have created an ecological opportunity allowing species that evolved CCMs to diversify. Recent studies find that the evolution of CCMs is associated with elevated net diversification rates compared with C3 plants (Table I). In these studies, the evolution of CCMs increases both speciation and extinction rates, suggesting that the evolution of CCMs is associated with greater species turnover.
Table I. Estimated speciation rates (λ), extinction rates (μ), and net diversification rates (r = λ − μ) associated with C3 photosynthesis and CCM-based photosynthesis.
Missing values (NA) indicate rates that were not reported in the original study.
| Clade | λC3 | λCCM | μC3 | μCCM | rC3 | rCCM | Reference |
|---|---|---|---|---|---|---|---|
| Poales | 0.996 | 1.454 | 0.954 | 1.242 | 0.042 | 0.212 | Bouchenak‐Khelladi et al. (2014) |
| Poaceaea | 0.6924 (0.3627) | 0.5667 (0.3011) | 0.5539 (0.2857) | 0.3267 (0.1667) | 0.1386 (0.077) | 0.24 (0.1344) | Spriggs et al. (2014) |
| Euphorbia | NA | NA | NA | NA | 0.062 | 0.177 | Horn et al. (2014) |
| Orchidaceae | 0.362 | 1.482 | 0.381 | 1.356 | −0.019 | 0.13 | Givnish et al. (2015) |
| Bromelioideae | 0.52 | 1.249 | 0.115 | 0.482 | 0.405 | 0.767 | Silvestro et al. (2014) |
Spriggs et al. (2014) included analyses based on two different dating hypotheses. Parentheses show values for the second phytolith-based dating hypothesis.
CCMs evolved repeatedly in some clades inhabiting warm and arid environments, yet genetic and developmental factors may have constrained the evolution of this innovation in other clades. The evolution of CCMs may be contingent on prior physiological adaptations: in grasses, C4 photosynthesis evolves from species that already have increased proportions of bundle sheath cells (Christin et al., 2013b). CAM photosynthesis may evolve in species with succulence, as this trait enables a greater capacity for storing water and C4 acids at night (Edwards and Ogburn, 2012). The evolution of CCMs also may be contingent on the presence of extra copies of genes encoding enzymes such as PEPC that are recruited into the CCM biochemical pathway. These extra copies may be obtained through gene duplication followed by neofunctionalization (Christin et al., 2013a) or introgression (Besnard et al., 2009; Christin et al., 2012).
Insights from Phylogenetic Patterns of CCM Evolution
The case of CCM evolution is a particularly compelling example where diverse yet complementary approaches are focused on understanding macroevolutionary patterns in plant physiology and the underlying mechanisms for these patterns. An emerging consensus from these studies is that the evolution of CCMs following climate change alters patterns of species diversification in similar ways across diverse angiosperm clades, yet the origin of CCMs may depend on the ancestral ecological niche or even the ancestral genomic content. These general themes may be true for other plant physiological traits that mediate plant responses to environmental change. For example, recent work using a phylogenetic approach suggests that adaptation in leaf stomatal ratio is associated with environmental conditions and selection for fast growth rate, yet it is also subject to constraints mediated by tradeoffs between photosynthetic rate and biotic interactions (Muir, 2015). As studies on evolutionary patterns in plant physiology accumulate, an important goal will be to synthesize mechanistic and phylogenetic studies and fossil evidence in order to characterize and predict macroevolutionary responses to climate change (Rothwell et al., 2014).
PLANT EVOLUTIONARY PHYSIOLOGY INFERRED FROM THE FOSSIL RECORD
Investigations that reconstruct plant physiological functioning of the past using ancient plant specimens enhance our understanding of plant evolutionary physiology, provide powerful information on how plants responded to long-term changes in climate, and generate insights into how past environments have shaped the current physiological structure of plants. The study of ancient plant specimens allows for a direct assessment of physiological responses across time scales where physiological traits may have been responding to selective agents. These studies are particularly powerful when (1) modern plant equivalents exist for comparison, (2) specimens are compared in controlled locations where local climates are known over time, and (3) preservation is high enough to allow for measurements in organic tissue (e.g. stable isotopes and DNA analyses) and/or high resolution of anatomical structures. Additionally, ancient plants that have no modern analogs can provide important examples of physiologies that did not persist in response to climate shifts as well as physiologies that evolved in extreme environments. Approaches that allow for the study of ancient plant physiology in the fossil record can involve plants that perished thousands or even millions of years ago (Gulbranson and Ryberg, 2013). Below, we discuss examples where stable isotope analyses of ancient tissue and assessments of structure-function relationships in the geologic record have advanced our understanding of evolutionary patterns of plant physiology. We focus on plant responses to low [CO2] during the last glacial period, which is likely to have been a strong selective agent due to limiting carbon for photosynthesis (Ward et al., 2000; Gerhart and Ward, 2010).
Measurements of stable carbon isotope ratios are an excellent technique for assessing plant physiology over time in an evolutionary context and are commonly expressed relative to an international standard using per mil notation: δ = (Rsample/Rstandard – 1) × 1, 000, where R is the ratio of the heavy isotope (13C) to the lighter isotope (12C) and the standard is Pee Dee Belemnite. The carbon isotope ratio of leaf tissue (or other tissue types corrected to leaf values) is a function of (1) the different diffusion rates of 13CO2 versus 12CO2, (2) the fractionation effect of Rubisco, and (3) leaf ci/ca, representing the ratio of leaf intercellular [CO2] (ci) to atmospheric [CO2] (ca; Farquhar et al., 1989). Since the first two components are constants, leaf ci/ca can be calculated from carbon discrimination values (1) when the carbon isotope ratio of source air (for photosynthesis) is known:
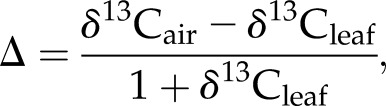 |
(1) |
where δ13Cair is adjusted for the age of the ancient specimen. From Δ, ci/ca can be calculated as:
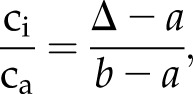 |
(2) |
where a and b are constant fractionation factors that account for the slower diffusion of 13CO2 relative to 12CO2 (4.4‰) and the net discrimination effects of Rubisco (27‰–30‰), respectively (Farquhar et al., 1989). Additionally, ci can be determined if ca is known (Ward, 2005).
ci/ca ratios are dependent on the dual effects of leaf stomatal conductance that influences the supply of CO2 to the leaf and the demand for CO2 that is determined via photosynthetic capacity (see Figure 4 in Ehleringer and Cerling, 1995). Higher ci/ca is indicative of higher stomatal conductance and/or lower photosynthetic capacity, which serve to reduce 13C in leaf tissue. This effect is indicated by reductions in carbon isotope values (Eq. 1) or increases in carbon discrimination (Eq. 2). Measures of ci/ca over evolutionary time indicate how incoming carbon through stomata is balanced with water loss and provide information on responses of photosynthetic capacity to environmental stimuli (e.g. light and nutrient availability; Ehleringer et al., 1997). Moreover, stable carbon isotope ratios have provided evidence of the first CO2-assimilating mechanisms to arise during early autotrophic evolution in the Earth’s history (3.8 billion years ago) and influenced our understanding of the effects of anthropogenic climate change on plant physiological functioning (Battipaglia et al., 2013).
Ehleringer and Cerling (1995) proposed that ci/ca might serve as a physiological and possibly evolutionary set point for photosynthesis within C3 plants (see highlighted box for an original case study of this from our own research). This ratio does not express absolute gas flux rates but, rather, indicates overall plant functioning as integrated from the dual influences of stomatal regulation (CO2 supply to the leaf) and investment in photosynthetic machinery (CO2 demand in photosynthesis; Farquhar et al., 1989). Furthermore, leaf carbon isotope ratios provide a time-integrated measure of physiological responses, since they capture carbon fixation over the course of leaf development and, therefore, serve as an excellent phenotypic proxy for physiology in evolutionary studies. Plants with shorter life histories and faster growth rates exhibit higher ci/ca values compared with perennials (Dawson et al., 2002). In addition, carbon isotope ratios tend to exhibit high levels of heritability within species (Dawson et al., 2002). Hausmann et al. (2005) mapped five quantitative trait loci that influence carbon isotope ratios in Arabidopsis (Arabidopsis thaliana), which colocalized with quantitative trait loci controlling flowering time. Furthermore, genotypes of crops and natural species have shown stability in ci/ca across differing environments, and the rank order of ci/ca often is maintained among plant genotypes across different weather extremes through time (Sandquist and Ehleringer, 2003). Such a response is not surprising given that stomatal conductance and photosynthetic capacity are linearly and positively related across a wide range of taxa from clubmosses to herbaceous forbs and grasses (Franks and Beerling, 2009). In this sense, increases in both stomatal conductance and photosynthetic capacity have opposing effects on ci/ca that may serve to stabilize this ratio across wide-ranging conditions. Such responses also have been observed within species, allowing for a balance in stomatal and nonstomatal limitations on leaf-level physiology in response to climate change and shifts in resource availability across contemporary and geologic time scales (Ehleringer and Cerling, 1995; Ward, 2005; Gerhart and Ward, 2010; Gerhart et al., 2012; Easlon et al., 2015). These findings suggest that ci/ca may have interesting evolutionary pathways, whereby this trait appears to be evolutionarily homeostatic in some cases. The combination of alleles that maintain this response will be important to understand in future studies.
BOX 1: CASE STUDY TESTING THE SET-POINT HYPOTHESIS FOR ci/ca
To test the set-point hypothesis for ci/ca proposed by Ehleringer and Cerling (1995) in controlled locations over geologic time scales, we compared the physiological patterns of modern and glacial trees preserved within the La Brea tar pits in southern California (Juniperus spp.) and peat bogs in the North Island, New Zealand (Agathis australis; new data). This allowed us to evaluate the responses of two coniferous species from different hemispheres that experienced different environmental changes since the last glacial period. Juniperus spp. experienced climate conditions that were cooler and wetter than at present. Modern high-elevation trees serve as an environmental control and allowed us to isolate the effects of changing [CO2] from other environmental changes (Gerhart et al., 2012). Unlike the Juniperus spp. in our study, glacial Agathis spp. experienced warmer and wetter conditions during the last glacial period compared with modern climates (Elliot et al., 2005; Horrocks et al., 2007; D’Costa et al., 2008). We examined the effects of increasing [CO2] in controlled locations in both study systems; the full Juniperus spp. sampling scheme additionally enabled us to determine the independent effects of rising [CO2] from glacial to present periods.
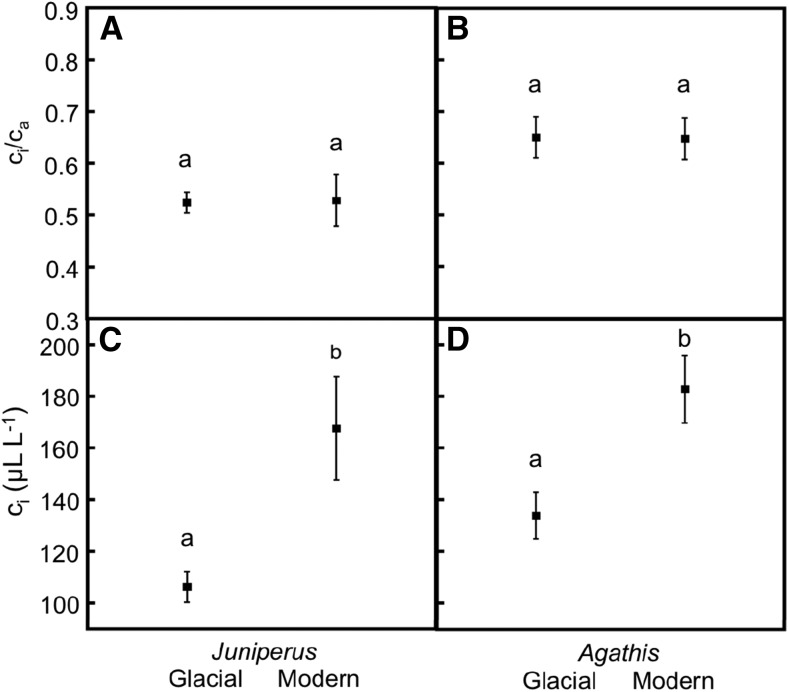
Interestingly, both Juniperus and Agathis spp. showed constant ci/ca throughout the last 50,000 years (Box 1 Fig.), supporting the set-point hypothesis proposed by Ehleringer and Cerling (1995). Constant ci/ca likely resulted from decreases in both stomatal conductance and photosynthetic capacity as [CO2] increased from past to present. Furthermore, constant ci/ca, coupled with reduced [CO2] in glacial periods, resulted in dramatic reductions in ci for glacial trees. For both Juniperus and Agathis spp., glacial ci values were on average 50 to 60 µL L−1 below modern values. Additionally, glacial ci values exhibited only a narrow overlapping window with modern values of 3 to 6 µL L−1, with less than 1% of all annual rings (glacial and modern) falling in this range. Therefore, despite experiencing different environmental changes, both Juniperus and Agathis spp. show stability in ci/ca with increasing [CO2]. Additionally, both species show unprecedented low levels of ci during the last glacial period relative to modern plants, suggesting the likelihood of physiological carbon starvation in these trees (Gerhart et al., 2012). Minimum ci values of each species (95 µL L−1 for glacial Juniperus spp. and 110 µL L−1 for glacial Agathis spp.) may represent a physiological carbon compensation point for survival, below which trees may not be able to maintain a positive carbon balance for the maintenance of respiration, growth, and survival (Gerhart et al., 2012).
Box 1 Figure.
Physiological and growth responses of glacial and modern Juniperus spp. and Agathis spp. A, Juniperus spp. ci/ca. B, Agathis spp. ci/ca. C, Juniperus spp. ci. D, Agathis spp. ci. Data are shown as group means with error bars representing 1 sd. Letters above the error bars represent significance, with different letters indicating P < 0.0003. Juniperus spp. data in A and C are reproduced in summary from Gerhart et al. (2012). Glacial Agathis spp. were excavated from peat bogs surrounding Lake Ngatu near Awanui in Northland (n = 8) and 14C dated from 52.2 thousand years ago to more than 52.8 thousand years ago. Modern Agathis spp. were obtained from remnants of old buildings and piers throughout the Awanui region (n = 8). Consequently, modern specimens ranged in age from 0.9 to 3.7 kyr BP.
With regard to changing atmospheric [CO2], Gerhart et al. (2012) found that interannual variability in ci/ca (from annual tree rings) was significantly higher in modern versus glacial Juniperus spp., despite similar levels of climatic variability in these time periods (Mayewski et al., 2004). Significantly, reduced interannual variation in Juniperus spp. during the last glacial period was attributed to the constraints of low [CO2] on physiological function, while high variation in modern Juniperus spp. was attributed to the effects of water availability that differ on an annual basis (Gerhart et al., 2012). Thus, Juniperus spp. show evidence of physiological shifts that appear to reflect changes in limiting factors that likely influenced evolutionary processes across geologic time.
When surveying studies with ancient plants as well as modern plants, it has been noted that ci/ca is maintained across [CO2] gradients in the majority of cases, as was shown in the examples above (Gerhart and Ward, 2010). However, there are a number of notable exceptions. For example, Becklin et al. (2014) measured ci/ca in an intact plant community in the southwestern United States between the last glacial period and the present (185–400 µL L−1 CO2 gradient) by sampling packrat middens. The authors found some evidence of stability in ci/ca during limited time periods but more pronounced evidence of increasing ci/ca from past to present in the majority of species. Decreases in both stomatal conductance and photosynthetic capacity from past to present could explain such a result. Specifically, photosynthetic capacity may have been proportionally more reduced in response to declining nitrogen availability from past to present, as evidenced by lower leaf nitrogen in modern specimens relative to glacial ones. In response to these and other exceptions, Voelker et al. (2016) conducted a modeling analysis to describe the homeostatic leaf gas-exchange response to glacial through future changes in [CO2]. Those authors concluded that plants may not directly maintain constant ci/ca per se but may be modulating their physiologies to maximize carbon gain at low [CO2] (glacial periods) with a shift toward reducing water loss as photosynthesis approaches CO2 saturation at elevated [CO2] (future levels). In support of this idea, glacial plants often have higher stomatal density/index relative to modern plants (for review, see Royer, 2001); enhanced CO2 diffusion into leaves at the expense of additional water loss may have been a beneficial tradeoff during periods when [CO2] was highly limiting. In one example, Beerling et al. (1993) found that Salix herbacea exhibited some of the highest stomatal densities in the fossil record during the Wolstonian and most recent glacial stages. Beerling (2005) also found that Selaginella selagenoides and Selaginella kraussiana showed a 30% reduction in stomatal density from the last glacial period to the present as [CO2] rose from 280 to 400 µL L−1. However, Becklin et al. (2014) did not find evidence for shifts in stomatal index or stomatal pore size in Juniperus osteosperma or Pinus longaeva in a controlled location in the Great Basin across 20,000 years of evolutionary time. Nonetheless, these empirical and modeling efforts highlight the diverse evolutionary strategies of plants to overcome carbon, water, and nutrient limitations through the modulation of leaf-level characteristics that are clearly preserved in the fossil record. Moreover, Sage and Cowling (1999) hypothesized that evolutionary innovations to enhance CO2 uptake during glacial periods may have produced selection pressures that could limit the ability of plants to benefit from rising [CO2] in modern and future atmospheres.
SPECIES INTERACTIONS AND THE EVOLUTION OF PLANT PHYSIOLOGY IN RESPONSE TO CLIMATE CHANGE
Plant species evolve in complex environments with networks of interacting species. Climate change will affect plant physiology and evolution indirectly by altering interactions with mutualists, antagonists, and competitors (Fig. 1; Gilman et al., 2010; Kiers et al., 2010; Lau et al., 2014). Interacting species are potent agents of selection that can drive the evolution of plant physiology through direct effects on physiological processes (e.g. effects of mycorrhizal fungi on plant carbon and nutrient dynamics) or through shifts in physiological tradeoffs (e.g. investment in defensive compounds versus growth). The ecoevolutionary consequences of altered species interactions with climate change may, in some cases, be as or even more important than the direct effects of climate change on plant physiology (Alexander et al., 2015). Below, we use plant-herbivore, plant-pollinator, and mycorrhizal associations as case studies to illustrate several mechanisms by which climate change is altering species interactions and, thereby, influencing plant evolutionary and physiological responses to complex environmental changes.
Plant-Herbivore Interactions
Plants have evolved elaborate defenses against diverse and abundant herbivore assemblages (Núñez-Farfán et al., 2007). By altering plant physiology, climate change could disrupt the production of secondary metabolites that provide antiherbivore defense (Alnsour and Ludwig-Muller, 2015), alter the strength of physiological tradeoffs between herbivore defense and plant growth, and reduce the nutritional value of plant tissues (Robinson et al., 2012). For example, meta-analysis reveals that elevated [CO2] reduces plant nutritional quality for many herbivore species by increasing leaf carbon-nitrogen ratios (Robinson et al., 2012). Consequently, herbivores will need to consume more plant tissue to meet their nutritional demands (DeLucia et al., 2008; Robinson et al., 2012), which may alter selection for plant defensive and tolerance traits.
Direct climate change effects on herbivore physiology and population dynamics also can generate ecoevolutionary feedbacks that impact selection on plant traits. First, higher temperatures may accelerate insect population growth rates, potentially increasing the frequency and severity of plant damage (Liu et al., 2011; Mitton and Ferrenberg, 2012). Indeed, foliar damage from insect herbivores increased dramatically with mean annual temperature across millions of years in the fossil record (Currano et al., 2010). Warmer winter temperatures also may reduce overwinter mortality among herbivores (Bale et al., 2002) and increase foraging opportunities during prolonged growing seasons (Brodie et al., 2012). Second, climate change may increase herbivory by disrupting herbivore-predator interactions. If predators can no longer forage during certain periods of the day because temperatures exceed their thermal tolerances, then herbivores may inflict greater damage on plants (Barton et al., 2009). Third, owing to their fast generation times and high mobility, insect herbivores may have a greater capacity than plants to adapt to ongoing climate change or to migrate to more suitable locations. For example, the rapid migration of natural enemies into previously inhospitable habitats could expose naive plant populations to increased levels of damage (Kurz et al., 2008), thereby imposing novel selection on these populations.
Increased rates of herbivory with climate change could alter plant physiology, reduce plant fitness and population growth rates, deplete genetic diversity, and diminish adaptive potential (Maron and Crone, 2006). It remains to be seen whether plants can counter the rapid responses of herbivores to changing climates. Preexisting genetic diversity in plant defense (Rasmann and Agrawal, 2011) and gene flow among populations could facilitate adaptation to novel herbivore communities. Additionally, plant populations that have historically experienced spatiotemporal variation in herbivore damage may have evolved multiple defense strategies (Carmona and Fornoni, 2013) that may decrease susceptibility to altered herbivore assemblages, especially if projected increases in climate variability translate into greater temporal variation in herbivory. Finally, simultaneous changes in both [CO2] and climate will likely mediate plant and herbivore responses in surprising ways (Copolovici et al., 2014), resulting in novel ecoevolutionary dynamics.
Plant-Pollinator Interactions
Pollinators influence the evolution of plant traits and the diversification of flowering plant lineages (Cardinal and Danforth, 2013). Climate change may alter pollination mutualisms via effects on plant physiology and physiological tradeoffs. For example, many pollinators prefer larger flowers, although increased frequency or severity of drought may impose selection for smaller flowers that reduce water loss (Galen, 2000). Elevated [CO2] alters the nutritional quality of nectar rewards through direct effects on photosynthesis and sugar production (Watanabe et al., 2014). Increases in [CO2] over the past 170 years also reduced pollen protein concentration in Solidago canadensis (Ziska et al., 2016). Such changes in either nectar or pollen rewards could adversely affect pollinators and the strength of pollinator-mediated selection on plant traits. Over longer periods of time, climate change effects on water stress and sugar production in plants could restrict evolutionary shifts in pollination syndromes if changes in nectar traits alter pollinator selection.
Climate change effects on plant and pollinator physiology also may result in mismatches between flowering time and pollinator activity (Forrest, 2015). Many plant species are emerging and reproducing earlier in the year due to increasing temperature and [CO2] (Amano et al., 2010; Ward et al., 2012; CaraDonna et al., 2014), while some species are delaying phenological events or are unresponsive to climate change (Sherry et al., 2007; Cook et al., 2012). The timing of these life history transitions depends on complex environmental cues that affect plant physiology (Forrest and Miller-Rushing, 2010). Climate change may alter such cues, resulting in dramatic shifts in flowering time (Springer et al., 2008; Wahl et al., 2013). If plants and their pollinators differ in their environmental sensitivities, then climate change could induce asynchronous phenologies, which could modify patterns of gene flow (Elzinga et al., 2007), alter coevolutionary dynamics between pollinators and plants (Gilman et al., 2012), reduce seed production (Forrest, 2015), and limit resource availability for pollinators (Memmott et al., 2007; but see Forrest and Thomson, 2011). Predicting the extent of temporal asynchrony under future climates will require physiological studies that determine the specific environmental cues that elicit life history transitions in plants and pollinators.
Asynchronous migration of (specialist) plant or pollinator mutualists with climate change could limit the pace of migration for the partner species, reduce the fitness of both interacting species, and alter ecoevolutionary dynamics within pollination mutualisms (Gilman et al., 2010). For example, bee diversity in alpine ecosystems in Colorado has increased with the influx of lower elevation bee species over the past 40 years (Miller-Struttmann and Galen, 2014). Additionally, some alpine bee species evolved significantly shorter tongues, which allow these bees to forage on a wider variety of plant species (Miller-Struttmann et al., 2015). These changes in the pollinator community have led to a functional mismatch between alpine plants and their pollinators, since average flower size in this system has not changed with warming temperatures (Miller-Struttmann et al., 2015). In this case, climate-induced shifts in the pollination network may have cascading effects on the evolution of plant traits. It remains unclear how changes in pollinator-mediated selection will interact with the physiological constraints of increasing temperature and drought to drive plant physiological and evolutionary responses.
Mycorrhizal Associations
Mycorrhizal associations are widespread symbioses involving plants and root-colonizing fungi (Smith and Read, 2008). Physiological mechanisms that control carbon and nutrient acquisition are tightly linked in mycorrhizal plant species; thus, climate change effects on plant physiology can alter the functioning of these ancient and ubiquitous interactions (Kiers et al., 2010; Mohan et al., 2014). Since plants supply mycorrhizal fungi with sugars, genetic and environmental factors that limit photosynthesis can reduce the amount of carbohydrates available to support fungal symbionts (Johnson et al., 2015). For example, C3 plants are generally more carbon limited than C4 plants, especially in dry environments. This physiological constraint may explain the higher responsiveness of C4 plants to mycorrhizal fungi (Reinhart et al., 2012). In exchange for carbohydrates, mycorrhizal fungi supply their hosts with soil nutrients (Smith and Read, 2008); some fungi also enhance plant drought tolerance (Lehto and Zwiazek, 2011), pathogen resistance (Powell et al., 2009), and herbivore defense (Johnson and Gilbert, 2015).
Mutually beneficial mycorrhizal associations are hypothesized to occur in nutrient-limited ecosystems where plants can effectively trade surplus carbohydrates for soil nutrients (Johnson et al., 2015). However, climate change may shift the relative resource limitations within host plants, thereby altering mycorrhizal dynamics and plant investment in these mutualisms (Kiers et al., 2010; Mohan et al., 2014). For example, increased photosynthesis under elevated [CO2] reduces the relative cost of supporting mycorrhizal fungi, but plants require more nutrients to maintain high rates of photosynthesis and growth. To meet their nutrient demands, plants generally allocate more resources to fungal symbionts under elevated [CO2], resulting in increased fungal growth and more beneficial partnerships (Compant et al., 2010). In some cases, mycorrhizal responses to elevated [CO2] decrease over time, possibly due to the progressive nitrogen limitation of photosynthesis and competition between plants and fungi for this critical resource (Alberton et al., 2007). Climate conditions that limit photosynthesis (e.g. drought) also could reduce net mycorrhizal benefits and potentially cause growth depressions within host plants (Correa et al., 2006; Johnson et al., 2015). Delineating the independent and synergistic effects of increasing [CO2], temperature, and drought will provide novel insights into environmental and physiological drivers of mycorrhizal dynamics.
Functional diversity and rapid evolution in fungal populations can mediate plant physiological responses to climate change and the evolution of plant traits within complex environments. For example, increasing herbivory or pathogen load with climate change may strengthen the importance of mycorrhizal fungi to plant defenses (Pineda et al., 2013). Some mycorrhizal functions, such as pathogen protection, are phylogenetically conserved within fungal lineages (Powell et al., 2009). Thus, variation in fungal community composition within and among plant communities could generate selection mosaics that alter plant adaptation to novel environmental stressors. Furthermore, plants can preferentially allocate carbohydrates to more beneficial mycorrhizal fungi (Bever, 2015), which could enable plants to maintain beneficial partnerships across variable environments and strengthen ecoevolutionary feedbacks within these symbioses.
Species Interactions: Unanswered Questions and Future Directions
Our understanding of climate change effects on species interactions has grown considerably in recent years (Kiers et al., 2010; Robinson et al., 2012; Forrest, 2015); however, the potential for climate change to affect plant physiological evolution through species interactions is not well understood. Given the complexity of plant-species interactions and their potential to drive evolution, studies that simulate climate change under realistic natural conditions with a full complement of interacting species could reveal plant physiological and evolutionary responses to direct and indirect effects of novel climates (Barton et al., 2009). Studies that take advantage of genetic mutants or natural variation in plant traits, such as herbivore defenses, can provide further insights into the genetic basis of traits under selection by interacting species. Pairing these mechanistic experiments with phylogenetic analyses of the evolution of plant traits and species interactions following historic climate change events could provide a framework for predicting how species interactions will shape plant physiological and evolutionary responses to climate change in the future.
CONCLUSION
In the introduction, we define a series of questions that are critical to the field of evolutionary physiology, and we provide examples of how these questions are being addressed in highly innovative ways (see “Outstanding Questions”). Moreover, the field of evolutionary physiology can inform us about the future trajectory of plant responses to climate change as well as provide insights into how evolutionary history has shaped the current responses of plants to their environment. This is a field that has provided a foundation for our understanding of the resilience (or lack thereof) of plants to survive rapid climate change. Moreover, continued work in this area as well as the application of new knowledge is critical for our own adaptive potential to climate change, since food and water security and ecosystem services are highly dependent on the evolutionary and physiological responses of plants to future conditions. We argue that the integration of plant physiological studies coupled with evolutionary approaches will enhance our understanding of past and future plant communities and the roles they play in driving ecosystem functioning through time.
OUTSTANDING QUESTIONS
To what degree will phenotypic plasticity or gene flow enhance or impede adaptive evolution in plant physiological traits?
What microevolutionary and ecological mechanisms contribute to altered species diversification rates following environmental change?
How have plant responses to past climate conditions influenced physiological and evolutionary responses to rapid climate change during contemporary time periods?
What are the relative influences of direct effects of climate change versus indirect effects via shifts in biotic interactions on the evolution of plant physiological traits?
How will potential physiological constraints interact with evolutionary history and species interactions to mediate plant responses to future changes in multiple environmental factors?
To what degree can we predict the resiliency of plants to survive rapid climate change?
Acknowledgments
We thank Robert Teisberg, president of Ancientwood, Ltd., for his generous contribution of glacial and modern Agathis spp. specimens for research purposes and John Southon at the University of California-Irvine W.M. Keck Carbon Cycle Accelerator Mass Spectrometry Laboratory for radiocarbon dating of ancient specimens.
Glossary
- WUE
water-use efficiency
- CCM
CO2-concentrating mechanism
- CAM
Crassulacean acid metabolism
Footnotes
This work was supported by the National Science Foundation (grant no. DEB 1553408 to J.T.A. and S.M.W., grant no. IOS 1457236 to K.M.B and J.K.W., and grant no. DEB 1455894 to L.M.G.), by the National Institutes of Health (grant no. 5F32GM110988-03 to C.A.W.), and by a Research Investment Council grant from the University of Kansas to K.M.B. and J.K.W.
Articles can be viewed without a subscription.
References
- Ackerly DD, Dudley SA, Sultan SE, Schmitt J, Coleman JS, Linder CR, Sandquist DR, Geber MA, Evans AS, Dawson TE, et al. (2000) The evolution of plant ecophysiological traits: recent advances and future directions. Bioscience 50: 979–995 [Google Scholar]
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Aitken SN, Whitlock MC (2013) Assisted gene flow to facilitate local adaptation to climate change. Annu Rev Ecol Evol Syst 44: 367–388 [Google Scholar]
- Alberto FJ, Aitken SN, Alía R, González-Martínez SC, Hänninen H, Kremer A, Lefèvre F, Lenormand T, Yeaman S, Whetten R, et al. (2013) Potential for evolutionary responses to climate change: evidence from tree populations. Glob Change Biol 19: 1645–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberton O, Kuyper TW, Gorissen A (2007) Competition for nitrogen between Pinus sylvestris and ectomycorrhizal fungi generates potential for negative feedback under elevated CO2. Plant Soil 296: 159–172 [Google Scholar]
- Alexander JM, Diez JM, Levine JM (2015) Novel competitors shape species’ responses to climate change. Nature 525: 515–518 [DOI] [PubMed] [Google Scholar]
- Alnsour M, Ludwig-Muller J (2015) Potential effects of climate change on plant primary and secondary metabolism and its influence on plant ecological interactions. Endocytobiosis Cell Res 26: 90–99 [Google Scholar]
- Amano T, Smithers RJ, Sparks TH, Sutherland WJ (2010) A 250-year index of first flowering dates and its response to temperature changes. Proc Biol Sci 277: 2451–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JT. (2016) Plant fitness in a rapidly changing world. New Phytol 210: 81–87 [DOI] [PubMed] [Google Scholar]
- Anderson JT, Inouye DW, McKinney AM, Colautti RI, Mitchell-Olds T (2012) Phenotypic plasticity and adaptive evolution contribute to advancing flowering phenology in response to climate change. Proc Biol Sci 279: 3843–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki M, Christin PA, Nyffeler R, Lendel A, Eggli U, Ogburn RM, Spriggs E, Moore MJ, Edwards EJ (2011) Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc Natl Acad Sci USA 108: 8379–8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badyaev AV. (2005) Stress-induced variation in evolution: from behavioural plasticity to genetic assimilation. Proc Biol Sci 272: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK, Butterfield J, Buse A, Coulson JC, Farrar J, et al. (2002) Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Change Biol 8: 1–16 [Google Scholar]
- Barton BT, Beckerman AP, Schmitz OJ (2009) Climate warming strengthens indirect interactions in an old-field food web. Ecology 90: 2346–2351 [DOI] [PubMed] [Google Scholar]
- Battipaglia G, Saurer M, Cherubini P, Calfapietra C, McCarthy HR, Norby RJ, Francesca Cotrufo M (2013) Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol 197: 544–554 [DOI] [PubMed] [Google Scholar]
- Becklin KM, Medeiros JS, Sale KR, Ward JK (2014) Evolutionary history underlies plant physiological responses to global change since the last glacial maximum. Ecol Lett 17: 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ. (2005) Evolutionary responses of land plants to atmospheric CO2. In Ehleringer JR, Cerling TE, Dearing MD, eds, A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Springer, New York, pp 114–132 [Google Scholar]
- Beerling DJ, Chaloner WG, Huntley B, Pearson JA, Tooley MJ (1993) Stomatal density responds to the glacial cycle of environmental change. Proc Biol Sci 251: 133–138 [Google Scholar]
- Berner R. (2006) GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim Cosmochim Acta 70: 5653–5664 [Google Scholar]
- Besnard G, Muasya AM, Russier F, Roalson EH, Salamin N, Christin PA (2009) Phylogenomics of C4 photosynthesis in sedges (Cyperaceae): multiple appearances and genetic convergence. Mol Biol Evol 26: 1909–1919 [DOI] [PubMed] [Google Scholar]
- Bever JD. (2015) Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytol 205: 1503–1514 [DOI] [PubMed] [Google Scholar]
- Billington HL, Pelham J (1991) Genetic variation in the date of budburst in Scottish birch populations: implications for climate change. Funct Ecol 5: 403–409 [Google Scholar]
- Bone RE, Smith JA, Arrigo N, Buerki S (2015) A macro-ecological perspective on Crassulacean acid metabolism (CAM) photosynthesis evolution in Afro-Madagascan drylands: Eulophiinae orchids as a case study. New Phytol 208: 469–481 [DOI] [PubMed] [Google Scholar]
- Bouchenak‐Khelladi Y, Muasya AM, Linder HP (2014) A revised evolutionary history of Poales: origins and diversification. Bot J Linn Soc 175: 4–16 [Google Scholar]
- Bridle JR, Vines TH (2007) Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol Evol 22: 140–147 [DOI] [PubMed] [Google Scholar]
- Brodie J, Post E, Watson F, Berger J (2012) Climate change intensification of herbivore impacts on tree recruitment. Proc Biol Sci 279: 1366–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaraDonna PJ, Iler AM, Inouye D (2014) Shifts in flowering phenology reshape a subalpine plant community. Proc Natl Acad Sci USA 111: 4916–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal S, Danforth BN (2013) Bees diversified in the age of eudicots. Proc R Soc B 280: 20122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona D, Fornoni J (2013) Herbivores can select for mixed defensive strategies in plants. New Phytol 197: 576–585 [DOI] [PubMed] [Google Scholar]
- Chevin LM, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol 8: e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Boxall SF, Gregory R, Edwards EJ, Hartwell J, Osborne CP (2013a) Parallel recruitment of multiple genes into C4 photosynthesis. Genome Biol Evol 5: 2174–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Edwards EJ, Besnard G, Boxall SF, Gregory R, Kellogg EA, Hartwell J, Osborne CP (2012) Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Curr Biol 22: 445–449 [DOI] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Chatelet DS, Columbus JT, Besnard G, Hodkinson TR, Garrison LM, Vorontsova MS, Edwards EJ (2013b) Anatomical enablers and the evolution of C4 photosynthesis in grasses. Proc Natl Acad Sci USA 110: 1381–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin PA, Osborne CP, Sage RF, Arakaki M, Edwards EJ (2011) C4 eudicots are not younger than C4 monocots. J Exp Bot 62: 3171–3181 [DOI] [PubMed] [Google Scholar]
- Compant S, van der Heijden MG, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol Ecol 73: 197–214 [DOI] [PubMed] [Google Scholar]
- Conner JK, Karoly K, Stewart C, Koelling VA, Sahli HF, Shaw FH (2011) Rapid independent trait evolution despite a strong pleiotropic genetic correlation. Am Nat 178: 429–441 [DOI] [PubMed] [Google Scholar]
- Cook BI, Wolkovich EM, Parmesan C (2012) Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci USA 109: 9000–9005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolovici L, Kannaste A, Remmel T, Niinemets U (2014) Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environ Exp Bot 100: 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Strasser RJ, Martins-Loucao MA (2006) Are mycorrhiza always beneficial? Plant Soil 279: 65–73 [Google Scholar]
- Currano ED, Labandeira CC, Wilf P (2010) Fossil insect folivory tracks paleotemperature for six million years. Ecol Monogr 80: 547–567 [Google Scholar]
- Dawson T, Mambelli S, Plamboeck A, Templer P, Tu K (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33: 507–559 [Google Scholar]
- D’Costa DM, Palmer J, Hogg A, Turney C, Fifield LK, Ogen J (2008) Stratigraphy, pollen, and 14C dating of Johnston’s Gum Hole, a late Quaternary fossil kauri (Agathis australis) site, Northland, New Zealand. J Quaternary Sci 24: 47–59 [Google Scholar]
- DeLucia EH, Casteel CL, Nabity PD, O’Neill BF (2008) Insects take a bigger bite out of plants in a warmer, higher carbon dioxide world. Proc Natl Acad Sci USA 105: 1781–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13: 77–81 [DOI] [PubMed] [Google Scholar]
- Dippery JK, Tissue DT, Thomas RB, Strain BR (1995) Effects of low and elevated CO2 on C3 and C4 annuals. 1. Growth and biomass allocation. Oecologia 101: 13–20 [DOI] [PubMed] [Google Scholar]
- Donohue K. (2003) Setting the stage: phenotypic plasticity as habitat selection. Int J Plant Sci 164: S79–S92 [Google Scholar]
- Easlon HM, Carlisle E, McKay JK, Bloom AJ (2015) Does low stomatal conductance or photosynthetic capacity enhance growth at elevated CO2 in Arabidopsis? Plant Physiol 167: 793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. Int J Plant Sci 173: 724–733 [Google Scholar]
- Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, Bond WJ, Christin PA, Cousins AB, Duvall MR, Fox DL, Freckleton RP, et al. (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA (2010) Phylogenetic analyses reveal the shady history of C4 grasses. Proc Natl Acad Sci USA 107: 2532–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE (1995) Atmospheric CO2 and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol 15: 105–111 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112: 285–299 [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Monson RK (1993) Evolutionary and ecological aspects of photosynthetic pathway variation. Annu Rev Ecol Syst 24: 411–439 [Google Scholar]
- Eller ASD, McGuire KL, Sparks JP (2011) Responses of sugar maple and hemlock seedlings to elevated carbon dioxide under altered above- and belowground nitrogen sources. Tree Physiol 31: 391–401 [DOI] [PubMed] [Google Scholar]
- Elliot M, Neall V, Wallace C (2005) A Late Quaternary pollen record from Lake Tongonge, far northern New Zealand. Rev Palaeobot Palynol 136: 143–158 [Google Scholar]
- Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22: 432–439 [DOI] [PubMed] [Google Scholar]
- Etterson JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 294: 151–154 [DOI] [PubMed] [Google Scholar]
- Falconer D, Mackay T (1996) Introduction to Quantitative Genetics, Ed 4 Addison Wesley Longman, Harlow, UK [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Flint-Garcia SA, Thornsberry JM, Buckler ES IV (2003) Structure of linkage disequilibrium in plants. Annu Rev Plant Biol 54: 357–374 [DOI] [PubMed] [Google Scholar]
- Forrest J, Miller-Rushing AJ (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc Lond B Biol Sci 365: 3101–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest J, Thomson JD (2011) An examination of synchrony between insect emergence and flowering in Rocky Mountain meadows. Ecol Monogr 81: 469–491 [Google Scholar]
- Forrest JRK. (2015) Plant-pollinator interactions and phenological change: what can we learn about climate impacts from experiments and observations? Oikos 124: 4–13 [Google Scholar]
- Frankham R. (2005) Genetics and extinction. Biol Conserv 126: 131–140 [Google Scholar]
- Franks PJ, Beerling DJ (2009) CO2-forced evolution of plant gas exchange capacity and water-use efficiency over the Phanerozoic. Geobiology 7: 227–236 [DOI] [PubMed] [Google Scholar]
- Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA 104: 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN (2014) Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl 7: 123–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C. (2000) High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae). Am Nat 156: 72–83 [DOI] [PubMed] [Google Scholar]
- Galloway LF, Etterson JR (2007) Transgenerational plasticity is adaptive in the wild. Science 318: 1134–1136 [DOI] [PubMed] [Google Scholar]
- Geber M, Griffen LR (2003) Inheritance and natural selection on functional traits. Int J Plant Sci 164: S21–S42 [Google Scholar]
- Gerhart LM, Harris JM, Nippert JB, Sandquist DR, Ward JK (2012) Glacial trees from the La Brea tar pits show physiological constraints of low CO₂. New Phytol 194: 63–69 [DOI] [PubMed] [Google Scholar]
- Gerhart LM, Ward JK (2010) Plant responses to low [CO2] of the past. New Phytol 188: 674–695 [DOI] [PubMed] [Google Scholar]
- Gilman RT, Fabina NS, Abbott KC, Rafferty NE (2012) Evolution of plant-pollinator mutualisms in response to climate change. Evol Appl 5: 2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25: 325–331 [DOI] [PubMed] [Google Scholar]
- Givnish TJ. (1987) Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytol 106: 131–160 [Google Scholar]
- Givnish TJ, Spalink D, Ames M, Lyon SP, Hunter SJ, Zuluaga A, Iles WJ, Clements MA, Arroyo MT, Leebens-Mack J, et al. (2015) Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proc R Soc B 282: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbranson E, Ryberg P (2013) Paleobotanical and geochemical approaches to studying fossil tree rings: quantitative interpretations of paleoenvironment and ecophysiology. Palaios 28: doi/10.1098/rspb.2015.1553 [Google Scholar]
- Gunderson CA, O’Hara KH, Campion CM, Walker AV, Edwards NT (2010) Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. Glob Change Biol 16: 2272–2286 [Google Scholar]
- Hatch MD. (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106 [Google Scholar]
- Hausmann NJ, Juenger TE, Sen S, Stowe KA, Dawson TE, Simms EL (2005) Quantitative trait loci affecting delta13C and response to differential water availability in Arabidopsis thaliana. Evolution 59: 81–96 [PubMed] [Google Scholar]
- Heard SB, Hauser DL (1995) Key evolutionary innovations and their ecological mechanisms. Hist Biol 10: 151–173 [Google Scholar]
- Heschel MS, Sultan S, Glover S, Sloan D (2004) Population differentiation and plastic responses to drought stress in the generalist annual Polygonum persicaria. Int J Plant Sci 165: 817–824 [Google Scholar]
- Horn JW, Xi Z, Riina R, Peirson JA, Yang Y, Dorsey BL, Berry PE, Davis CC, Wurdack KJ (2014) Evolutionary bursts in Euphorbia (Euphorbiaceae) are linked with photosynthetic pathway. Evolution 68: 3485–3504 [DOI] [PubMed] [Google Scholar]
- Horrocks M, Nichol SL, Augustinus PC, Barber IG (2007) Late Quaternary environments, vegetation and agriculture in northern New Zealand. J Quaternary Sci 22: 267–279 [Google Scholar]
- IPCC (2013) Climate Change 2013: The Physical Science Basis. Intergovernmental Panel on Climate Change, Cambridge, UK [Google Scholar]
- Johnson D, Gilbert L (2015) Interplant signalling through hyphal networks. New Phytol 205: 1448–1453 [DOI] [PubMed] [Google Scholar]
- Johnson MTJ, Agrawal AA, Maron JL, Salminen JP (2009) Heritability, covariation and natural selection on 24 traits of common evening primrose (Oenothera biennis) from a field experiment. J Evol Biol 22: 1295–1307 [DOI] [PubMed] [Google Scholar]
- Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205: 1473–1484 [DOI] [PubMed] [Google Scholar]
- Jump AS, Peñuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8: 1010–1020 [DOI] [PubMed] [Google Scholar]
- Keuskamp DH, Sasidharan R, Pierik R (2010) Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signal Behav 5: 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL (2010) Mutualisms in a changing world: an evolutionary perspective. Ecol Lett 13: 1459–1474 [DOI] [PubMed] [Google Scholar]
- Kimball S, Gremer JR, Angert AL, Huxman TE, Venable DL (2012) Fitness and physiology in a variable environment. Oecologia 169: 319–329 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150: 1–23 [DOI] [PubMed] [Google Scholar]
- Kremer A, Potts BM, Delzon S (2014) Genetic divergence in forest trees: understanding the consequences of climate change. Funct Ecol 28: 22–36 [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452: 987–990 [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37: 1210–1226 [DOI] [PubMed] [Google Scholar]
- Lau JA, Lennon JT (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proc Natl Acad Sci USA 109: 14058–14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JA, Shaw RG, Reich PB, Tiffin P (2014) Indirect effects drive evolutionary responses to global change. New Phytol 201: 335–343 [DOI] [PubMed] [Google Scholar]
- Lehto T, Zwiazek JJ (2011) Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21: 71–90 [DOI] [PubMed] [Google Scholar]
- Leimu R, Mutikainen PIA, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94: 942–952 [Google Scholar]
- Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320: 1768–1771 [DOI] [PubMed] [Google Scholar]
- Lenormand T. (2002) Gene flow and the limits to natural selection. Trends Ecol Evol 17: 183–189 [Google Scholar]
- Liancourt P, Boldgiv B, Song DS, Spence LA, Helliker BR, Petraitis PS, Casper BB (2015) Leaf-trait plasticity and species vulnerability to climate change in a Mongolian steppe. Glob Change Biol 21: 3489–3498 [DOI] [PubMed] [Google Scholar]
- Liu Y, Reich PB, Li G, Sun S (2011) Shifting phenology and abundance under experimental warming alters trophic relationships and plant reproductive capacity. Ecology 92: 1201–1207 [DOI] [PubMed] [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD (2009) The velocity of climate change. Nature 462: 1052–1055 [DOI] [PubMed] [Google Scholar]
- Maddison WP, Midford PE, Otto SP (2007) Estimating a binary character’s effect on speciation and extinction. Syst Biol 56: 701–710 [DOI] [PubMed] [Google Scholar]
- Marchin RM, Sage EL, Ward JK(2008) Population-level variation of Fraxinus americana L. (white ash) is influenced by precipitation differences across the native range. Tree Physiol 28: 151–159 [DOI] [PubMed] [Google Scholar]
- Maron JL, Crone E (2006) Herbivory: effects on plant abundance, distribution and population growth. Proc Biol Sci 273: 2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayewski PA, Rohling EE, Stager JC, Karlén W, Maasch KA, Meeker LD, Meyerson EA, Gasse F, van Kreveld S, Holmgren K, et al. (2004) Holocene climate variability. Quat Res 62: 243–255 [Google Scholar]
- Medeiros JS, Ward JK (2013) Increasing atmospheric [CO2] from glacial to future concentrations affects drought tolerance via impacts on leaves, xylem and their integrated function. New Phytol 199: 738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvigy D, Beaulieu C (2012) Trends in daily solar radiation and precipitation coefficients of variation since 1984. J Clim 25: 1330–1339 [Google Scholar]
- Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecol Lett 10: 710–717 [DOI] [PubMed] [Google Scholar]
- Merilä J. (2012) Evolution in response to climate change: in pursuit of the missing evidence. BioEssays 34: 811–818 [DOI] [PubMed] [Google Scholar]
- Miller-Struttmann NE, Galen C (2014) High-altitude multi-taskers: bumble bee food plant use broadens along an altitudinal productivity gradient. Oecologia 176: 1033–1045 [DOI] [PubMed] [Google Scholar]
- Miller-Struttmann NE, Geib JC, Franklin JD, Kevan PG, Holdo RM, Ebert-May D, Lynn AM, Kettenbach JA, Hedrick E, Galen C (2015) Functional mismatch in a bumble bee pollination mutualism under climate change. Science 349: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T. (1996) Pleiotropy causes long-term genetic constraints on life-history evolution in Brassica rapa. Evolution 50: 1849–1858 [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Willis JH, Goldstein DB (2007) Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat Rev Genet 8: 845–856 [DOI] [PubMed] [Google Scholar]
- Mitton JB, Ferrenberg SM (2012) Mountain pine beetle develops an unprecedented summer generation in response to climate warming. Am Nat 179: E163–E171 [DOI] [PubMed] [Google Scholar]
- Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, et al. (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol 10: 3–19 [Google Scholar]
- Monson RK, Littlejohn Jr RO, Williams GJ III (1983) Photosynthetic adaptation to temperature in four species from the Colorado shortgrass steppe: a physiological model for coexistence. Oecologia 58: 43–51 [DOI] [PubMed] [Google Scholar]
- Muir CD. (2015) Making pore choices: repeated regime shifts in stomatal ratio. Proc Biol Sci 282: 20151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F, et al. (2010) Plant phenotypic plasticity in a changing climate. Trends Plant Sci 15: 684–692 [DOI] [PubMed] [Google Scholar]
- Norby RJZ, Donald R (2011) Ecological lessons from free-air CO2 enrichment (FACE) experiments. Annu Rev Ecol Evol Syst 42: 181–203 [Google Scholar]
- Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38: 541–566 [Google Scholar]
- O’Hara NB, Rest JS, Franks SJ (2016) Increased susceptibility to fungal disease accompanies adaptation to drought in Brassica rapa. Evolution 70: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. (1994) Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc Biol Sci 255: 37–45 [Google Scholar]
- PAGES 2k Consortium (2013) Continental-scale temperature variability during the past two millennia. Nat Geosci 6: 339–346 [Google Scholar]
- Perry AL, Low PJ, Ellis JR, Reynolds JD (2005) Climate change and distribution shifts in marine fishes. Science 308: 1912–1915 [DOI] [PubMed] [Google Scholar]
- Pineda A, Dicke M, Pieterse CMJ, Pozo MJ (2013) Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct Ecol 27: 574–586 [Google Scholar]
- Polley HW, Johnson HB, Marino BD, Mayeux HS (1993) Increase in C3 plant water-use efficiency and biomass over glacial to present CO2 concentrations. Nature 361: 61–64 [Google Scholar]
- Powell JR, Parrent JL, Hart MM, Klironomos JN, Rillig MC, Maherali H (2009) Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc Biol Sci 276: 4237–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabosky DL. (2014) Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9: e89543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, Agrawal AA (2011) Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity and induction following herbivory. Ecol Lett 14: 476–483 [DOI] [PubMed] [Google Scholar]
- Reinhart KO, Wilson GWT, Rinella MJ (2012) Predicting plant responses to mycorrhizae: integrating evolutionary history and plant traits. Ecol Lett 15: 689–695 [DOI] [PubMed] [Google Scholar]
- Robinson EA, Ryan GD, Newman JA (2012) A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol 194: 321–336 [DOI] [PubMed] [Google Scholar]
- Rothwell GW, Wyatt SE, Tomescu AMF (2014) Plant evolution at the interface of paleontology and developmental biology: an organism-centered paradigm. Am J Bot 101: 899–913 [DOI] [PubMed] [Google Scholar]
- Royer DL. (2001) Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev Palaeobot Palynol 114: 1–28 [DOI] [PubMed] [Google Scholar]
- Sage RF, Christin P-A, Edwards EJ (2011) The C4 plant lineages of planet Earth. J Exp Bot 62: 3155–3169 [DOI] [PubMed] [Google Scholar]
- Sage RF, Coleman JR (2001) Effects of low atmospheric CO2 on plants: more than a thing of the past. Trends Plant Sci 6: 18–24 [DOI] [PubMed] [Google Scholar]
- Sage RF, Cowling SA (1999) Implications of stress in low CO2 atmospheres of the past: are today’s plants too conservative for a high CO2 world? In Luo Y, Mooney HA, eds, Carbon Dioxide and Environmental Stress. Academic Press, New York, pp 289–304 [Google Scholar]
- Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Sandquist DR, Ehleringer JR (2003) Carbon isotope discrimination differences within and between contrasting populations of Encelia farinosa raised under common-environment conditions. Oecologia 134: 463–470 [DOI] [PubMed] [Google Scholar]
- Schluter D, Price T, Mooers AØ, Ludwig D (1997) Likelihood of ancestor states in adaptive radiation. Evolution 51: 1699–1711 [DOI] [PubMed] [Google Scholar]
- Schmitt J, Wulff RD (1993) Light spectral quality, phytochrome and plant competition. Trends Ecol Evol 8: 47–51 [DOI] [PubMed] [Google Scholar]
- Shaw RG, Etterson JR (2012) Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol 195: 752–765 [DOI] [PubMed] [Google Scholar]
- Shefferson RP, Salguero-Gómez R (2015) Eco-evolutionary dynamics in plants: interactive processes at overlapping time-scales and their implications. J Ecol 103: 789–797 [Google Scholar]
- Sherry RA, Zhou X, Gu S, Arnone JA III, Schimel DS, Verburg PS, Wallace LL, Luo Y (2007) Divergence of reproductive phenology under climate warming. Proc Natl Acad Sci USA 104: 198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D, Zizka G, Schulte K (2014) Disentangling the effects of key innovations on the diversification of Bromelioideae (Bromeliaceae). Evolution 68: 163–175 [DOI] [PubMed] [Google Scholar]
- Simpson GG. (1953) Major Features of Evolution. Columbia University Press, New York [Google Scholar]
- Smith SE, Read D (2008) Mycorrhizal Symbiosis, Ed 3. Academic Press, London [Google Scholar]
- Spriggs EL, Christin PA, Edwards EJ (2014) C4 photosynthesis promoted species diversification during the Miocene grassland expansion. PLoS ONE 9: e97722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer CJ, Orozco RA, Kelly JK, Ward JK (2008) Elevated CO2 influences the expression of floral-initiation genes in Arabidopsis thaliana. New Phytol 178: 63–67 [DOI] [PubMed] [Google Scholar]
- Tripati AK, Roberts CD, Eagle RA (2009) Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 326: 1394–1397 [DOI] [PubMed] [Google Scholar]
- Voelker SL, Brooks JR, Meinzer FC, Anderson R, Bader MKF, Battipaglia G, Becklin KM, Beerling D, Bert D, Betancourt JL, et al. (2016) A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2: evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob Change Biol 22: 889–902 [DOI] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wang T, O’Neill GA, Aitken SN (2010) Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol Appl 20: 153–163 [DOI] [PubMed] [Google Scholar]
- Ward JK. (2005) Evolution and growth of plants in a low CO2 world. In Ehleringer J, Cerling T, Dearing D, eds, A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Springer-Verlag, New York, pp 232–257 [Google Scholar]
- Ward JK, Antonovics J, Thomas RB, Strain BR (2000) Is atmospheric CO2 a selective agent on model C3 annuals? Oecologia 123: 330–341 [DOI] [PubMed] [Google Scholar]
- Ward JK, Kelly J (2004) Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol Lett 7: 427–440 [Google Scholar]
- Ward JK, Samanta Roy D, Chatterjee I, Bone CR, Springer CJ, Kelly JK (2012) Identification of a major QTL that alters flowering time at elevated [CO2] in Arabidopsis thaliana. PLoS ONE 7: e49028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe CK, Sato S, Yanagisawa S, Uesono Y, Terashima I, Noguchi K (2014) Effects of elevated CO2 on levels of primary metabolites and transcripts of genes encoding respiratory enzymes and their diurnal patterns in Arabidopsis thaliana: possible relationships with respiratory rates. Plant Cell Physiol 55: 341–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilczek AM, Cooper MD, Korves TM, Schmitt J (2014) Lagging adaptation to warming climate in Arabidopsis thaliana. Proc Natl Acad Sci USA 111: 7906–7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Smith JAC (1996) An introduction to Crassulacean acid metabolism: biochemical principles and ecological diversity. In Winter K and Smith JAC, eds, Crassulacean Acid Metabolism. Springer, Berlin Heidelberg, pp 1–13 [Google Scholar]
- Ziska LH, Pettis JS, Edwards J, Hancock JE, Tomecek MB, Clark A, Dukes JS, Loladze I, Polley HW (2016) Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc R Soc B 283: doi/10.1098/rspb.2016.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]