Herbs display a wide range of embolism resistance and do not show pronounced embolism formation throughout the growing season.
Abstract
The water transport pipeline in herbs is assumed to be more vulnerable to drought than in trees due to the formation of frequent embolisms (gas bubbles), which could be removed by the occurrence of root pressure, especially in grasses. Here, we studied hydraulic failure in herbaceous angiosperms by measuring the pressure inducing 50% loss of hydraulic conductance (P50) in stems of 26 species, mainly European grasses (Poaceae). Our measurements show a large range in P50 from −0.5 to −7.5 MPa, which overlaps with 94% of the woody angiosperm species in a worldwide, published data set and which strongly correlates with an aridity index. Moreover, the P50 values obtained were substantially more negative than the midday water potentials for five grass species monitored throughout the entire growing season, suggesting that embolism formation and repair are not routine and mainly occur under water deficits. These results show that both herbs and trees share the ability to withstand very negative water potentials without considerable embolism formation in their xylem conduits during drought stress. In addition, structure-function trade-offs in grass stems reveal that more resistant species are more lignified, which was confirmed for herbaceous and closely related woody species of the daisy group (Asteraceae). Our findings could imply that herbs with more lignified stems will become more abundant in future grasslands under more frequent and severe droughts, potentially resulting in lower forage digestibility.
Terrestrial biomes provide numerous ecosystem services to humans, such as biodiversity refuges, forage supply, carbon sequestration, and associated atmospheric feedback (Bonan, 2008). Drought frequency and severity are predicted to increase across various ecosystems (Dai, 2013), and its impact on the fate of terrestrial biomes has aroused great concern for stakeholders over the past decade. For instance, worldwide forest declines have been associated with drought events (Allen et al., 2010), and the sustainability of grasslands, one of the most important agro-ecosystems representing 26% of the world land area, is threatened due to increasing aridity in the light of climate change (Tubiello et al., 2007; Brookshire and Weaver, 2015). Since the maintenance of grasslands is of prime importance for livestock, and several of the most valuable crops are grasses, herbaceous species deserve more attention from a hydraulic point of view to understand how they will cope with shifts in precipitation and temperature patterns.
During water deficit, hydraulic failure in trees has been put forward as one of the primary causes of forest decline (Anderegg et al., 2015, 2016). Drought exacerbates the negative pressure inside the water conducting cells, making the liquid xylem sap more metastable, and thus more vulnerable, to air entry (i.e. gas embolism; Lens et al., 2013a). Extensive levels of embolisms may lead to desiccation, leaf mortality, branch sacrifice, and ultimately plant death (Barigah et al., 2013; Urli et al., 2013). Plant resistance to embolism is therefore assumed to represent a key parameter in determining the drought tolerance of trees and is estimated using so-called vulnerability curves (VCs), from which the P50, i.e. the sap pressure inducing 50% loss of hydraulic conductivity, can be estimated (Cochard et al., 2013). P50 values are therefore good proxies for drought stress tolerance in woody plants and have been published for hundreds of angiosperm and gymnosperm tree species (Delzon et al., 2010; Choat et al., 2012), illustrating a wide range from −0.5 to −19 MPa (Larter et al., 2015).
Studies focusing on P50 values of herbs are limited to stems of ∼14 angiosperm species (see Supplemental Table S1 and references cited therein). Half of the herbaceous angiosperms studied so far (Supplemental Table S1) have a stem P50 between 0 and −2 MPa, indicating that many herbs are highly vulnerable to embolism. Moreover, positive root pressure has been reported in various herbs, including many grasses (Poaceae) with hydathodes in their leaves (Evert, 2006), and root pressure is hypothesized to refill embolized conduits overnight when transpiration is low (Miller, 1985; Neufeld et al., 1992; Cochard et al., 1994; Macduff and Bakken, 2003; Saha et al., 2009; Cao et al., 2012). This could suggest that embolism formation and repair follow a daily cycle in herbs. In other words, the midday water potential that herbs experience in the field may often be more negative than P50, which would result in an extremely vulnerable hydraulic pipeline characterized by a negative hydraulic safety margin (expressed as the minimum midday water potential minus P50). In contrast to herbs, most trees operate at a slightly positive hydraulic safety margin (Choat et al., 2012), and woody plants are often too tall to allow refilling by positive root and/or stem pressure in the upper stems (Ewers et al., 1997; Fisher et al., 1997). Therefore, it could be postulated that herbaceous species possess a hydraulic system that is more vulnerable to embolism than that of woody species. In this study, we want to underpin possible differences in embolism resistance between stems of herbaceous and woody angiosperms.
The scarcity of P50 measures in herbaceous angiosperms, including grasses and herbaceous eudicots, is mainly due to their fragile stems and low hydraulic conductivity, making VCs technically more challenging. Using minor adaptations to existing centrifuge techniques (Supplemental Text S1), we obtained a P50 stem data set of 26 herbaceous angiosperm species (mainly grasses) from various collection sites in France and Switzerland. In addition, we compared our data set with published data from woody (gymnosperm and angiosperm) species, confronted some of our herbaceous eudicot measurements with original P50 data from derived, woody relatives, and performed anatomical observations in grasses to investigate a possible link between stem anatomical characters and differences in P50 among the species studied. Three main research questions are central in our article: (1) Are stems of herbaceous angiosperms more vulnerable to embolism than those of woody angiosperms? (2) Do grasses operate with highly vulnerable, negative hydraulic safety margins? (3) Do grasses show structure-function trade-offs in their stems with respect to embolism resistance?
RESULTS AND DISCUSSION
Comparable P50 Range in Herbs Compared to Woody Species
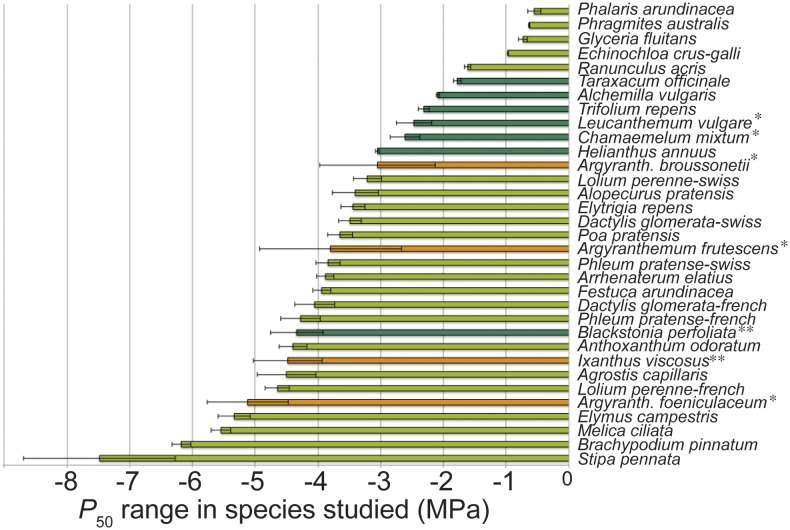
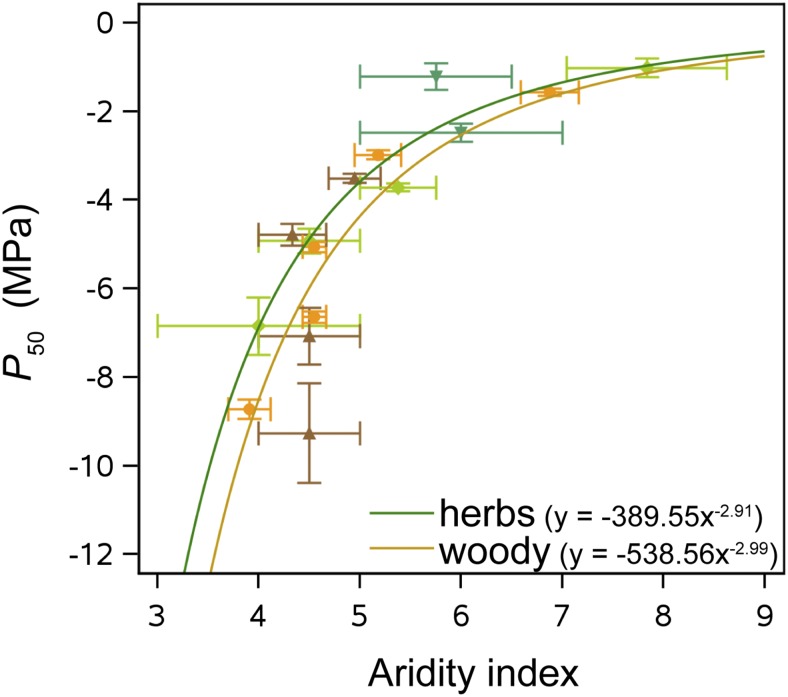
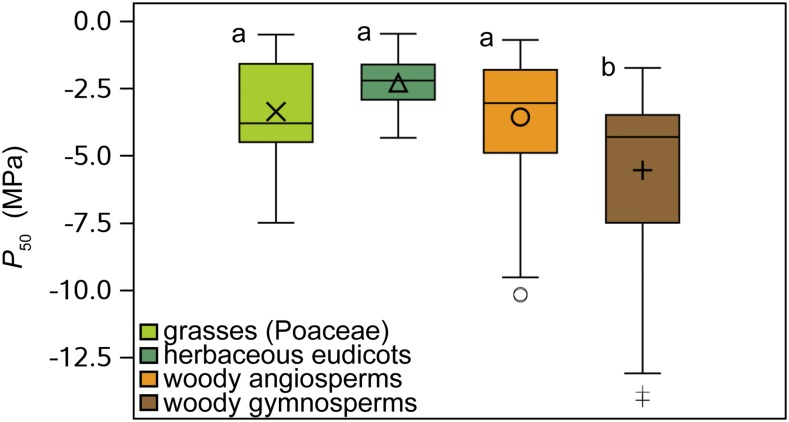
Our herbaceous data set including 26 angiosperm species reveals a broad range in P50 from −0.5 to −7.5 MPa (Fig. 1). If we compare the overlap between the range of this herbaceous data set and the range observed in a large, published woody data set (including P50 values of 404 woody angiosperm and gymnosperm species; see “Materials and Methods”; Supplemental Table S2), 89% of the woody species fall within this 0.5 to 7.5 MPa range. This P50 overlap further increases to 94% when only the woody angiosperms are taken into account (301 species). Since herbaceous species (n = 28, Spearman’s r = 0.6003, P = 0.0007) as well as woody species (n = 124, Spearman’s r = 0.6006, P < 0.0001) with a more negative P50 grow in drier environments (lower aridity index; Fig. 2), we expect that further sampling of herbs from (semi)desert-like environments will further increase the P50 range toward more negative extremes. This would generate an even stronger overlap in P50 between herbaceous and woody plants. Generally, we find that herbaceous angiosperms (mean P50 = −2.93 MPa, coefficient of variation [CV] = 57%) are significantly more vulnerable to embolism than woody species, including angiosperms and gymnosperms (mean P50 = −4.07 MPa, CV = 62%; F1,441 = 7.64, P = 0.0059; Supplemental Fig. S1). However, when splitting up the data set into grasses (Poaceae, mean P50 = −3.37 MPa, CV = 57%), herbaceous eudicots (mean P50 = −2.3 MPa, CV = 43%), woody angiosperms (mean P50 = −3.57 MPa, CV = 59%), and woody gymnosperms (mean P50 = −5.55 MPa, CV = 55%), only the woody gymnosperms are different from the rest (Fig. 3; Supplemental Tables S1 and S2), while the differences between grasses, herbaceous eudicots, and woody angiosperms are not significant (Supplemental Table S3), especially the similarity in stem P50 between grasses and woody angiosperms is remarkable (least squares means differences P = 0.98; Supplemental Table S3). These results emphasize that both herbaceous and woody angiosperms share the ability to withstand low water potentials without experiencing considerable embolism formation in their xylem conduits during water deficit (Fig. 3).
Figure 1.
P50 values of species measured. The range in P50 among the 26 herbaceous and 4 woody species studied varies from −0.5 up to −7.5 MPa. Light-green bars indicate grasses (Poaceae), dark-green bars represent herbaceous eudicots, and the orange ones are woody eudicot shrubs that have evolved from some of the herbaceous relatives studied (*daisy lineage; **gentian lineage). Each bar represents the average value for three specimens of the same species, and error bars show se.
Figure 2.
P50 versus aridity index in herbs and woody species. Herbaceous and woody species that are more resistant to embolism formation (more negative P50) grow in drier environments (lower aridity index; Julve, 1998). P50 values were averaged for each plant group every 2 MPa (light-green diamonds, grasses; dark-green triangles, herbaceous eudicots; orange circles, woody angiosperms; brown triangles, woody gymnosperms). Error bars show se.
Figure 3.
Box plots showing P50 range among different plant groups. There is a striking similarity in P50 between grasses, herbaceous eudicots, and woody angiosperms. On the other hand, woody gymnosperms have a statistically more negative P50 than each of the angiosperm groups. Mean values are shown with either a cross (grasses), triangle (herbaceous eudicots), circle (woody angiosperms), or plus sign (woody gymnosperms); “a” and “b” indicate statistical differences (Supplemental Table S3).
Hydraulic Safety Margins in Stems of Grasses Are Positive
We assessed the range of native embolism in five grass species with a P50 between −3 and −4.5 MPa from the Swiss field sites (Table I). Therefore, we measured the midday leaf water potential throughout the entire growing season from April to October and related these values with their VCs in order to estimate native embolism over the operating range of water potential. Interestingly, midday leaf water potentials in spring were substantially less negative than P50, suggesting very low levels of native embolism (<16% loss of hydraulic conductance; Table I; Supplemental Table S4). This contradicts the general assumption that grasses undergo daily or short-term embolism/repair cycles during mild conditions. Furthermore, the most negative leaf water potential (Psi min), experienced by the plants during the driest period of the year (July), corresponded to low levels of native embolism in the stems, ranging from 10 to 22% loss of hydraulic conductance, which is far below 50% as defined by P50 (Table I). Consequently, midday leaf water potential data in the five grass species studied show evidence for positive hydraulic safety margins varying from 1.40 to 2.19 MPa (Table I).
Table I. Embolism is not pronounced in grasses.
Summary of hydraulic parameters for grasses from the Swiss collections, including mean leaf water potential during three time points in spring time (mean Psimidday during spring time), its corresponding native levels of embolism (PLCmidday, %), the minimum leaf water potential measured throughout the growing season (=Psimin), and its corresponding PLC. Values are means ±1 se for n = 6. More detailed information throughout the growing season is provided in Supplemental Table S4.
| Species | P50 (MPa) | Mean Psimidday in Spring Time (MPa) | Mean PLCmidday in Spring Time (%) | Psimin (MPa) | PLC at Psimin (%) |
|---|---|---|---|---|---|
| Dactylis glomerata | −3.49 | −1.47 ± 0.06 | 14.56 ± 0.67 | −2.06 ± 0.14 | 22.30 ± 2.22 |
| Lolium perenne | −3.21 | −1.37 ± 0.03 | 15.80 ± 0.35 | −1.81 ± 0.05 | 21.75 ± 0.73 |
| Phleum pratense | −3.84 | −1.24 ± 0.12 | 5.51 ± 0.86 | −1.90 ± 0.10 | 10.49 ± 1.05 |
| Poa pratensis | −3.65 | Species not yet growing | Species not yet growing | −2.06 ± 0.15 | 11.06 ± 2.18 |
| Agrostis capillaris | −4.50 | −2.05 ± 0.15 | 8.98 ± 1.20 | −2.31 ± 0.14 | 11.06 ± 1.20 |
In summary, our data suggest that daily embolism/repair cycles in grasses are not the rule throughout the growing season, at least not in stems, despite ample evidence for positive root pressure in grasses (Miller, 1985; Neufeld et al., 1992; Cochard et al., 1994; Saha et al., 2009; Cao et al., 2012). The broad range in embolism resistance of the grasses studied, in combination with these low levels of native embolism in the moderately resistant grasses studied suggest that embolism refilling may play a less significant role for grasses than previously thought (Cao et al., 2012). In other words, our findings suggest that frequent cycles of xylem embolism and repair are not pronounced in grasses, which is in agreement with observations in woody plants (Wheeler et al., 2013; Sperry, 2013; Delzon and Cochard, 2014). If the Psi min monitoring in our five grass species studied could be confirmed in a broader sampling of herbaceous species, this would raise questions about the generally accepted role of root pressure in repairing embolized conduits. Root pressure may simply be a by-product of nutrient absorption by roots, allowing water transport via a leaky hydraulic pipeline with hydathodes. Evidently, root pressure needs to be quantified in relation to P50 and midday leaf/stem water potentials across a broad sampling of herbaceous species to better understand this enigmatic phenomenon. Moreover, we should know more about the specific climatic conditions under which root pressure development is physically possible, since drought will decrease the soil water content (Supplemental Table S4), making root pressure more challenging.
Despite the observed conservative nature of embolism/refilling cycles in the grass stems studied, Holloway-Phillips and Brodribb (2011) showed that Lolium perenne, one of our Swiss species studied, operates very close to its hydraulic limits based on whole leaf hydraulic data, suggesting a hydraulic decoupling between stem and leaves. While the stem P50 reaches −3.21 MPa in the individuals we studied (Supplemental Table S1), the authors found a vulnerable whole leaf P50 (leaf P50: −1 MPa; leaf P95: −2.2 MPa), and complete stomatal closure happened very late at −2.35 MPa. In other words, while our stem observations for L. perenne indicate no or low levels of native embolisms throughout the growing season in combination with a positive safety margin, leaf hydraulic measures suggest much narrower or even negative hydraulic safety margins. This contradicting result could be explained by recent articles on leaf hydraulics, showing that the observed decrease in hydraulic conductance in needles and leaves is not due to xylem embolism but rather to a conductivity drop in the extra-xylary pathway (Bouche et al., 2016; C. Scoffoni, personal communication). This suggests that there are no robust assessments of leaf vulnerability to embolism so far, but it is expected that the new optical technique developed by Brodribb et al. (2016) will shed new light on a better understanding of the hydraulic connection between stems and leaves.
Embolism Resistance in Herbs Comes at a Lignification Cost
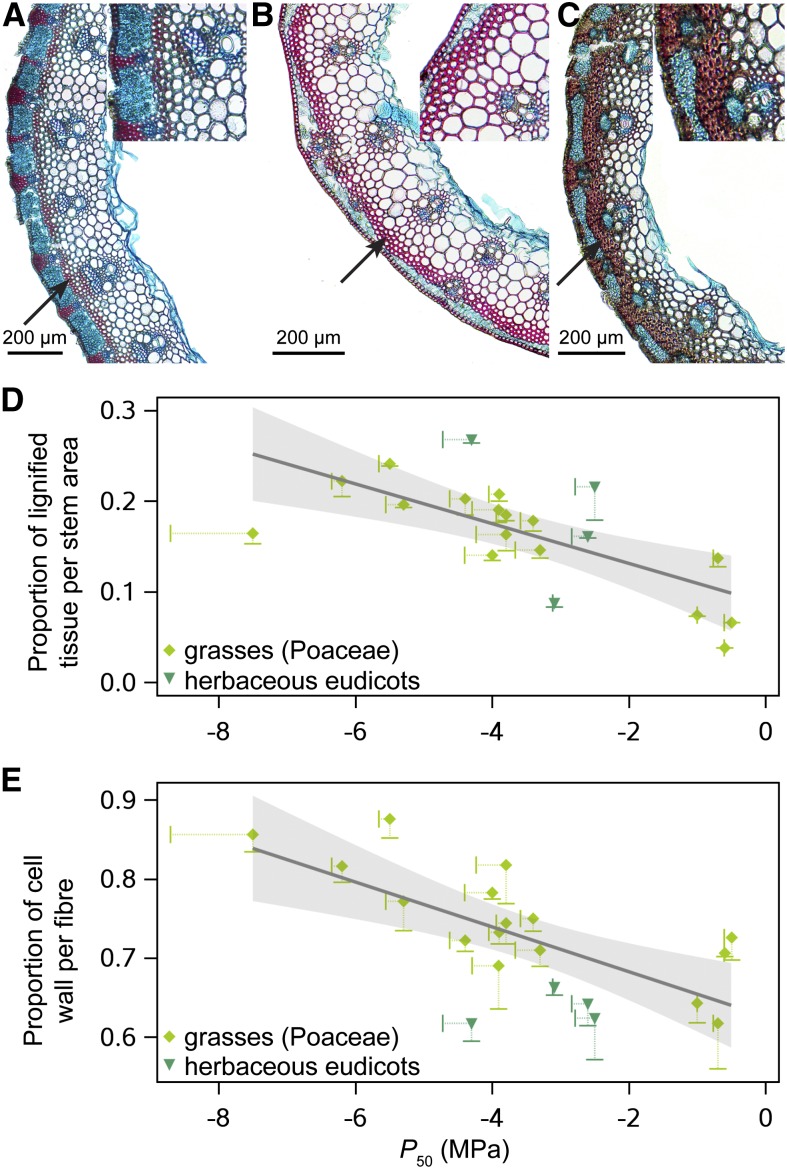
Based on our 20 herbaceous species for which we have anatomical observations (mainly based on internode cross sections of grasses; Supplemental Tables S1, S5, and S6), Figure 4 shows that the more resistant herbs have a higher proportion of lignified tissue in their stems (P = 0.0066, partial R2 = 0.40; Fig. 4, A–D) and develop thicker cell walls in the fibers of this lignified zone (P = 0.0005, partial R2 = 0.57; Fig. 4, A–C and E). When only the grass data set is analyzed, the relative proportion of lignified tissue becomes marginally significant (P = 0.0457, partial R2 = 0.32), while the relative proportion of cell wall per lignified fiber remains highly significant (P = 0.0014, partial R2 = 0.62; Supplemental Table S6). Therefore, we argue that developing embolism resistant stems in herbs requires up-regulation of the energy-consuming lignin pathway, which is a costly process. The relative size of the pith and the hydraulically weighted (metaxylem) vessel diameter did not significantly contribute to variation in P50. Likewise, there was no trade-off between P50 and the intervessel pit membrane thickness between adjacent metaxylem vessels in vascular bundles of six selected grass species, which ranged from on average 131 nm in L. perenne to 313 nm in Elytrigia repens (F1,4 = 0.03, P = 0.87). This is unexpected considering the strong evidence for functional relevance of intervessel pit membrane thickness among woody angiosperms (Jansen et al., 2009; Lens et al., 2011, 2013a; Li et al., 2016).
Figure 4.
Lignification and P50. A to C, Cross sections of hollow stems through the internodes of the grasses Phalaris arundinacea (A; P50 = −0.5 MPa), L. perenne (B; P50 = −4.6 MPa), and Brachypodium pinnatum (C; P50 = −6.2 MPa), showing more lignification in the outer zones of the stems (arrows) and thicker-walled fibers (insets) with increasing P50. D and E, Grasses and herbaceous eudicots that are more resistant to embolism have a higher proportion of lignified tissues in their stems (D) and thicker-walled fibers (E). Error bars show se (only lower limits are presented for clarity purposes, and each point represents the average value for three specimens of the same species). Marked zones apply to the 95% confidence limit of the regression. See Supplemental Table S6 for multiple regression analysis of P50 and anatomical features as predictive variables.
The distribution pattern of lignified tissues between grasses and herbaceous eudicots is completely different. In grasses, lignification is mainly confined to the outer parts of the stems along the entire axis (Fig. 4, A–C) and is related to provide mechanical strength and perhaps also to avoid water loss during periods of drought. Lignification in the herbaceous eudicots, however, is concentrated in the narrow wood cylinder at the base of the stem (Lens et al., 2012a, 2012b; Kidner et al., 2016; Supplemental Fig. S2, A and B). Our anatomical data set, including mainly grass species, shows that lignification scales positively with embolism resistance. The link between increased embolism resistance and increased lignification has also been experimentally demonstrated in the herbaceous eudicot Arabidopsis (Arabidopsis thaliana; Lens et al., 2013a; Tixier et al., 2013), in several transgenic poplars modified for lignin metabolism (Awad et al., 2012), and is further corroborated in this study by comparing the vulnerable, herbaceous daisies Chamaemelum (P50 −2.6 MPa) and Leucanthemum (P50 −2.5 MPa) with closely related members of the derived, more embolism resistant, woody genus Argyranthemum (P50 between −3 and −5.1 MPa; Supplemental Fig. S2, A and C). Based on these observations, it seems that plants invest more energy resources to develop a mechanically stronger, embolism resistant stem (Lens et al., 2013a), which is in agreement with previous studies linking embolism resistance with higher wood densities and thickness-to-span ratios of water conducting cells (Hacke et al., 2001) and thicker interconduit pit membranes (Jansen et al., 2009; Lens et al., 2011, 2013a; Li et al., 2016). Likewise, intervessel pit membranes of the embolism resistant, woody Argyranthemum species are thicker than in the more vulnerable, herbaceous Leucanthemum and Chamaemelum (between on average 370 to 485 nm and 290 to 350 nm, respectively).
However, more lignification/wood formation is not per definition needed to obtain a higher level of embolism resistance across flowering plants: the Gentianaceae sister pair Blackstonia perfoliata (herbaceous) and Ixanthus viscosus (woody) shows a similar P50 value (−4.5 MPa), despite the marked difference in wood formation (Supplemental Fig. S2, B and D). Likewise, some other woody eudicot lineages that have evolved from herbaceous relatives grow in extremely wet environments, such as Cyrtandra (Cronk et al., 2005) or Begonia (Kidner et al., 2016). Also in ferns, where a thick ring of sclerenchyma fibers is located just below the epidermis of the leaf rachis, comparable to the situation in grass stems, no structural investment trade-offs in vulnerability to embolism were found (Watkins et al., 2010; Pittermann et al., 2011).
In conclusion, there is a remarkable range in P50 among 26 herbaceous species, overlapping with 94% of woody angiosperm species in a published data set. The large variation in P50 in herbs and trees scales tightly with climatic conditions. Despite the potential refilling capacity by root pressure, embolism formation in grasses does not seem to be common throughout the growing season. This suggests that herbs and woody plants are more similar in their ability to avoid drought-induced embolism than previously expected, especially within the angiosperms. We also found that embolism resistance generally comes at a lignification cost in herbs. This could lead to selection for species with more lignified stems in future grasslands that have to cope with more frequent and intensive droughts, potentially resulting in a lower forage digestibility.
MATERIALS AND METHODS
Sampling Strategy
In total, 26 herbaceous angiosperm species, including 18 grass species (family Poaceae) and eight eudicots, and four woody angiosperm species were investigated. Details about species and sampling sites are given in Supplemental Text S1 and Supplemental Table S1. Canary Island species were collected in order to compare stem anatomy and P50 values of some of the herbaceous eudicots with closely related, woody descendants. Examples are Argyranthemum species that have evolved within the largely herbaceous daisy group, including among others Chamaemelum and Leucanthemum (Fig. 1). Likewise, we studied Ixanthus viscosus, a woody Canary Island species that is derived from the herbaceous Blackstonia native to continental Europe (Lens et al., 2013b; Fig. 1; Supplemental Table S1). To expand the wood data set, we used an updated version of the Xylem Functional Traits Database (Choat et al., 2012; Supplemental Table S2, and references cited therein), in which we removed the angiosperms with long vessels and high P50 values (>−1 MPa) to account for the vessel length artifact (Cochard et al., 2013), and adopted the P50 values with those published by Brendel and Cochard (2011) for 18 species that showed more than 40% intraspecific variation compared to other studies (mainly because of vessel length issues). In addition, we updated the wood data set with more recent references and with four Canary Island species measured in this study (Supplemental Table S2).
The variation in habitat among the herbaceous species and the adjusted data set of Choat et al. (2012) was captured by the Julve index, an aridity index characterizing the edaphic humidity environment that was specifically designed for the French flora (Julve, 1998; http://perso.wanadoo.fr/philippe.julve/catminat.htm, download “French Flora Database (Baseflor),” column AD “Humidité_édaphique” corresponding to edaphic humidity). Baseflor is a floristic database indexing about 11,000 taxa from the French vascular flora. For each taxon, the database includes phytosociological characteristics and chorological, ecological, and biological descriptions. In the Baseflor database, the Ellenberg’s “F”-values are modified to take into account the French ecological context of each taxon, describing xerophytic to aquatic species (from small to high values). The Julve index was documented for 28 herbaceous species and 124 woody species present in our data sets (Supplemental Table S2).
Embolism Resistance Measurements
All the species were measured using the centrifuge technique. The static centrifuge technique (Alder et al., 1997) was applied when the conductance was too low (most of the grass species from France), while the cavitron (in situ flow centrifuge) technique (Cochard et al., 2005) was used for the other species because the hydraulic conductivity was high enough (Supplemental Table S1). Both centrifuge techniques are explained in Supplemental Text S1, and S-shaped VCs were fitted according to a sigmoid function (Pammenter and Vander Willigen, 1998).
Leaf Water Potential Measures
For the species of the Swiss collection, midday leaf water potential was determined using a Scholander pressure chamber (SKPM; Skye Instruments) along the entire growing season of 2015 (from April to October) between 11 am and 1 pm on sunny days and every 2 weeks. Then, the minimum midday leaf water potential value experienced in the field for each species was used as minimum water potential (Psi min), which in all cases corresponded to the driest period of the year, i.e. in July.
Anatomical Observations
For all the French (n = 20) and Canary Island (n = 4) species, cross sections of three individuals per species were made at the level of the internodes according to resin embedding (Hamann et al., 2011) or standard wood sectioning (Lens et al., 2005), respectively, observed with the light microscope, photographed with a digital camera, and measured with ImageJ (Supplemental Table S5). Details are given in the Supplemental Text S1. We also investigated intervessel pit membrane thickness based on transmission electron microscope observations for six selected grass species from the French site with a P50 range between −0.5 and −6.2 MPa (Anthoxanthum odoratum, Brachypodium pinnatum, Elymus campestris, Elytrigia repens, Lolium perenne, and Phalaris arundinacea; stored in −20°C freezer before fixation, with transverse sections through the nodes) and all the eight eudicot species belonging to the daisy and Gentianaceae lineage. After hydraulic measures, we immediately submerged the stems in Karnovsky fixative (Karnovsky, 1965) and followed the protocol explained in the Supplemental Text S1.
Statistics
The correlation between P50 and the aridity index (Fig. 2) was tested using Spearman correlation for herbaceous species (n = 28) and woody species (n = 124 species) separately (PROC CORR, in SAS Software, SAS University Edition). To assess differences between embolism resistance across plant groups (Fig. 3), we compared P50 variability (1) among angiosperms (including grasses, herbaceous eudicots, and woody angiosperms) and gymnosperms and (2) between herbaceous species and woody species using General Linear models (PROC GLM). For the first type of analysis (1), we used posthoc least squares means using the Tukey-Kramer approximation adapted for multiple comparisons with unbalanced sample sizes (Supplemental Table S3).
We used multiple regression analyses (PROC REG) to test the contribution of anatomical features (independent variables) to P50 variability (dependent variable). Several of the anatomical features measured were correlated because many of them were merged to calculate additional traits. To select predictive factors, we screened for multicollinearity by calculating variance inflation factors in multiple regression analyses (VIF option in PROC REG). This resulted in four predictive characters in our model: proportion of lignified tissues compared to entire stem diameter, proportion of pith compared to entire stem area, proportion of cell wall per fiber, and hydraulically weighted (metaxylem) vessel diameter. The VIFs for the predictor variables in our regression model were <2, which indicates that multicollinearity did not cause a loss of precision. This multiple regression model was applied independently to the 16 grasses and 20 herbaceous species for which we measured anatomical features (Supplemental Tables S1, S5, and S6). Finally, we tested the relationship between P50 and intervessel pit membrane thickness between metaxylem vessels in six grass species using a simple linear regression.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Global P50 comparison between herbs and woody species.
Supplemental Figure S2. Differences in anatomy between herbs and related woody species.
Supplemental Table S1. P50 data set of herbaceous species from our study and published papers.
Supplemental Table S2. Entire P50 and Julve data set of woody and herbaceous species from our study and published papers.
Supplemental Table S3. Posthoc comparisons of P50 LS means across species groups (see Fig. 3).
Supplemental Table S4. Hydraulic measures throughout the growing season for the five Swiss grass species.
Supplemental Table S5. List of the anatomical measurements carried out for the species in this study (three replicates per species).
Supplemental Table S6. Multiple regression model of anatomical features as explaining factors of P50 variability in herbaceous species and grass species.
Supplemental Text S1. More detailed “Materials and Methods” descriptions about sampling strategy, embolism resistance measurements, and anatomical observations.
Acknowledgments
We thank MSc student Jérémy Rivière for his measurements on 16 grasses from the French sites and the two reviewers for their valuable feedback. This article is supported by COST Action FP1106 STReESS.
Glossary
- VC
vulnerability curve
Footnotes
Articles can be viewed without a subscription.
This project was funded by the Climagie Project within the Metaprogramme Adaptation of Agriculture and Forests to Climate Change (AAFCC) of the French National Institute for Agriculture Research (INRA) and by the program “Investments for the Future” (ANR-10-EQPX-16 and XYLOFOREST) from the French National Agency for Research. The Swiss contribution was part of the project GrassAlt, funded by the Swiss National Science Foundation (SNF CR31I3_156282/1). F.L. received support from the Alberta Mennega Foundation.
References
- Alder NN, Pockman WT, Sperry JS, Nuismer S (1997) Use of centrifugal force in the study of xylem cavitation. J Exp Bot 48: 665–674 [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage 259: 660–684 [Google Scholar]
- Anderegg WRL, Hicke JA, Fisher RA, Allen CD, Aukema J, Bentz B, Hood S, Lichstein JW, Macalady AK, McDowell N, et al. (2015) Tree mortality predicted from drought-induced vascular damage. Nat Geosci 8: 367–371 [Google Scholar]
- Anderegg WRL, Klein T, Bartlett M, Sack L, Pellegrini AFA, Choat B, Jansen S (2016) Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc Natl Acad Sci USA 113: 5024–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad H, Herbette S, Brunel N, Tixier A, Pilate G, Cochard H, Badel E (2012) No trade-off between hydraulic and mechanical properties in several transgenic poplars modified for lignin metabolism. Environ Exp Bot 77: 185–195 [Google Scholar]
- Barigah TS, Charrier O, Douris M, Bonhomme M, Herbette S, Améglio T, Fichot R, Brignolas F, Cochard H (2013) Water stress-induced xylem hydraulic failure is a causal factor of tree mortality in beech and poplar. Ann Bot (Lond) 112: 1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonan GB. (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320: 1444–1449 [DOI] [PubMed] [Google Scholar]
- Bouche PS, Delzon S, Choat B, Badel E, Brodribb TJ, Burlett R, Cochard H, Charra-Vaskou K, Lavigne B, Li S, et al. (2016) Are needles of Pinus pinaster more vulnerable to xylem embolism than branches? New insights from X-ray computed tomography. Plant Cell Environ 39: 860–870 [DOI] [PubMed] [Google Scholar]
- Brendel O, Cochard H (2011) How plant species cope with water stress. In Birot Y, Gracia C, Palahi M, eds, Water for Forest and People in the Mediterranean: A Challenging Balance. European Forest Institute, Joensuu, Finland, pp 76–80 [Google Scholar]
- Brodribb TJ, Skelton RP, McAdam SAM, Bienaimé D, Lucani CJ, Marmottant P (2016) Visual quantification of embolism reveals leaf vulnerability to hydraulic failure. New Phytol 209: 1403–1409 [DOI] [PubMed] [Google Scholar]
- Brookshire ENJ, Weaver T (2015) Long-term decline in grassland productivity driven by increasing dryness. Nat Commun 6: 7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KF, Yang S-J, Zhang Y-J, Brodribb TJ (2012) The maximum height of grasses is determined by roots. Ecol Lett 15: 666–672 [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–755 [DOI] [PubMed] [Google Scholar]
- Cochard H, Damour G, Bodet C, Tharwat I, Poirier M, Améglio T (2005) Evaluation of a new centrifuge technique for rapid generation of xylem vulnerability curves. Physiol Plant 124: 410–418 [Google Scholar]
- Cochard H, Badel E, Herbette S, Delzon S, Choat B, Jansen S (2013) Methods for measuring plant vulnerability to cavitation: a critical review. J Exp Bot 64: 4779–4791 [DOI] [PubMed] [Google Scholar]
- Cochard H, Ewers FW, Tyree MT (1994) Water relations of a tropical vine-like bamboo (Rhipidocladum racemiflorum): root pressures, vulnerability to cavitation and seasonal changes in embolism. J Exp Bot 45: 1085–1089 [Google Scholar]
- Cronk QCB, Kiehn M, Wagner WL, Smith JF (2005) Evolution of Cyrtandra (Gesneriaceae) in the Pacific Ocean: the origin of a supertramp clade. Am J Bot 92: 1017–1024 [DOI] [PubMed] [Google Scholar]
- Dai AG. (2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3: 52–58 [Google Scholar]
- Delzon S, Cochard H (2014) Recent advances in tree hydraulics highlight the ecological significance of the hydraulic safety margin. New Phytol 203: 355–358 [DOI] [PubMed] [Google Scholar]
- Delzon S, Douthe C, Sala A, Cochard H (2010) Mechanism of water-stress induced cavitation in conifers: bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant Cell Environ 33: 2101–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert RF. (2006) Esau’s Plant Anatomy, Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, Ed 3. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- Ewers FW, Cochard H, Tyree MT (1997) A survey of root pressures in vines of a tropical lowland forest. Oecologia 110: 191–196 [DOI] [PubMed] [Google Scholar]
- Fisher JB, Angeles GA, Ewers FW, Lopez-Portillo J (1997) Survey of root pressure in tropical vines and woody species. Int J Plant Sci 158: 44–50 [Google Scholar]
- Hacke UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461 [DOI] [PubMed] [Google Scholar]
- Hamann TD, Smets E, Lens F (2011) A comparison of paraffin and resin-based techniques used in bark anatomy. Taxon 60: 841–851 [Google Scholar]
- Holloway-Phillips MM, Brodribb TJ (2011) Minimum hydraulic safety leads to maximum water-use efficiency in a forage grass. Plant Cell Environ 34: 302–313 [DOI] [PubMed] [Google Scholar]
- Jansen S, Choat B, Pletsers A (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96: 409–419 [DOI] [PubMed] [Google Scholar]
- Julve P. (1998) Baseflor. Index botanique, écologique et chorologique de la flore de France, Version Julve 2005-2014. http://perso.wanadoo.fr/philippe.julve/catminat.htm
- Karnovsky MJ. (1965) A formaldehyde-glutaraldehyde fixative of light osmolality for use in electron microscopy. J Cell Biol 27: 137A–138A [Google Scholar]
- Kidner C, Groover A, Thomas D, Emelianova K, Soliz-Gamboa C, Lens F (2016) First steps in studying the origins of secondary woodiness in Begonia (Begoniaceae): combining anatomy, phylogenetics, and stem transcriptomics. Biol J Linn Soc Lond 117: 121–138 [Google Scholar]
- Larter M, Brodribb TJ, Pfautsch S, Burlett R, Cochard H, Delzon S (2015) Extreme aridity pushes trees to their physical limits. Plant Physiol 168: 804–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Davin N, Smets E, Del Arco M (2013b) Insular woodiness on the Canary Islands: remarkable case of convergent evolution. Int J Plant Sci 174: 992–1013 [Google Scholar]
- Lens F, Dressler S, Jansen S, van Evelghem L, Smets E (2005) Relationships within balsaminoid Ericales: a wood anatomical approach. Am J Bot 92: 941–953 [DOI] [PubMed] [Google Scholar]
- Lens F, Eeckhout S, Zwartjes R, Smets E, Janssens SB (2012a) The multiple fuzzy origins of woodiness within Balsaminaceae using an integrated approach. Where do we draw the line? Ann Bot (Lond) 109: 783–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens F, Smets E, Melzer S (2012b) Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytol 193: 12–17 [DOI] [PubMed] [Google Scholar]
- Lens F, Sperry JS, Christman MA, Choat B, Rabaey D, Jansen S (2011) Testing hypotheses that link wood anatomy to cavitation resistance and hydraulic conductivity in the genus Acer. New Phytol 190: 709–723 [DOI] [PubMed] [Google Scholar]
- Lens F, Tixier A, Cochard H, Sperry JS, Jansen S, Herbette S (2013a) Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr Opin Plant Biol 16: 287–292 [DOI] [PubMed] [Google Scholar]
- Li S, Lens F, Espino S, Karimi Z, Klepsch M, Schenk HJ, Schmitt M, Schuldt B, Jansen S (2016) Intervessel pit membrane thickness as a key determinant of embolism resistance in angiosperm xylem. IAWA J 37: 152–171 [Google Scholar]
- Macduff JH, Bakken AK (2003) Diurnal variation in uptake and xylem contents of inorganic and assimilated N under continuous and interrupted N supply to Phleum pratense and Festuca pratensis. J Exp Bot 54: 431–444 [DOI] [PubMed] [Google Scholar]
- Miller DM. (1985) Studies of root function in Zea mays: III. Xylem sap composition at maximum root pressure provides evidence of active transport into the xylem and a measurement of the reflection coefficient of the root. Plant Physiol 77: 162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld HS, Grantz DA, Meinzer FC, Goldstein G, Crisosto GM, Crisosto C (1992) Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiol 100: 1020–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter NW, Vander Willigen C (1998) A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiol 18: 589–593 [DOI] [PubMed] [Google Scholar]
- Pittermann J, Limm E, Rico C, Christman MA (2011) Structure-function constraints of tracheid-based xylem: a comparison of conifers and ferns. New Phytol 192: 449–461 [DOI] [PubMed] [Google Scholar]
- Saha S, Holbrook NM, Montti L, Goldstein G, Cardinot GK (2009) Water relations of Chusquea ramosissima and Merostachys claussenii in Iguazu National Park, Argentina. Plant Physiol 149: 1992–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry J. (2013) Cutting-edge research or cutting-edge artefact? An overdue control experiment complicates the xylem refilling story. Plant Cell Environ 36: 1916–1918 [DOI] [PubMed] [Google Scholar]
- Tixier A, Cochard H, Badel E, Dusotoit-Coucaud A, Jansen S, Herbette S (2013) Arabidopsis thaliana as a model species for xylem hydraulics: does size matter? J Exp Bot 64: 2295–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubiello FN, Soussana JF, Howden SM (2007) Crop and pasture response to climate change. Proc Natl Acad Sci USA 104: 19686–19690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013) Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol 33: 672–683 [DOI] [PubMed] [Google Scholar]
- Watkins JE Jr, Holbrook NM, Zwieniecki MA (2010) Hydraulic properties of fern sporophytes: Consequences for ecological and evolutionary diversification. Am J Bot 97: 2007–2019 [DOI] [PubMed] [Google Scholar]
- Wheeler JK, Huggett BA, Tofte AN, Rockwell FE, Holbrook NM (2013) Cutting xylem under tension or supersaturated with gas can generate PLC and the appearance of rapid recovery from embolism. Plant Cell Environ 36: 1938–1949 [DOI] [PubMed] [Google Scholar]