Plant physiological and growth characteristics drive linear and nonlinear shifts in the functioning of mycorrhizal associations across a 180 to 1,000 µL L−1 [CO2] gradient.
Abstract
Rising atmospheric carbon dioxide concentration ([CO2]) may modulate the functioning of mycorrhizal associations by altering the relative degree of nutrient and carbohydrate limitations in plants. To test this, we grew Taraxacum ceratophorum and Taraxacum officinale (native and exotic dandelions) with and without mycorrhizal fungi across a broad [CO2] gradient (180–1,000 µL L−1). Differential plant growth rates and vegetative plasticity were hypothesized to drive species-specific responses to [CO2] and arbuscular mycorrhizal fungi. To evaluate [CO2] effects on mycorrhizal functioning, we calculated response ratios based on the relative biomass of mycorrhizal (MBio) and nonmycorrhizal (NMBio) plants (RBio = [MBio − NMBio]/NMBio). We then assessed linkages between RBio and host physiology, fungal growth, and biomass allocation using structural equation modeling. For T. officinale, RBio increased with rising [CO2], shifting from negative to positive values at 700 µL L−1. [CO2] and mycorrhizal effects on photosynthesis and leaf growth rates drove shifts in RBio in this species. For T. ceratophorum, RBio increased from 180 to 390 µL L−1 and further increases in [CO2] caused RBio to shift from positive to negative values. [CO2] and fungal effects on plant growth and carbon sink strength were correlated with shifts in RBio in this species. Overall, we show that rising [CO2] significantly altered the functioning of mycorrhizal associations. These symbioses became more beneficial with rising [CO2], but nonlinear effects may limit plant responses to mycorrhizal fungi under future [CO2]. The magnitude and mechanisms driving mycorrhizal-CO2 responses reflected species-specific differences in growth rate and vegetative plasticity, indicating that these traits may provide a framework for predicting mycorrhizal responses to global change.
Atmospheric carbon dioxide concentration ([CO2]) has more than doubled over the past 20,000 years, rising from a minimum value of approximately 180 µL L−1 during the Last Glacial Maximum (LGM; Augustin et al., 2004) to a current value of 401 µL L−1. Due to ongoing fossil fuel emissions, [CO2] is expected to reach 700 to 1,000 µL L−1 by the end of this century (IPCC, 2013). Rising [CO2] has greatly impacted plant physiology since the LGM (Sage and Coleman, 2001; Ainsworth and Rogers, 2007; Gerhart and Ward, 2010), likely altering interactions between plants and their microbial symbionts over geologic and contemporary time scales.
Mycorrhizal associations are ancient plant-fungal symbioses (Remy et al., 1994) where host plants and their fungal partners exchange photosynthetically derived carbohydrates for soil nutrients (Smith and Read, 2008). These associations play a critical role in modern ecosystems via their effects on plant physiology, species coexistence, carbon and nutrient cycling, and net primary productivity (Hodge and Fitter, 2010; Clemmensen et al., 2015; Lin et al., 2015). Temporal changes in [CO2] since the LGM have likely influenced the functioning of mycorrhizal associations along a continuum from mutualism to parasitism, hereafter referred to as the M-P continuum (Johnson et al., 1997), by altering plant carbohydrate production and nutrient demand. Previous mycorrhizal-CO2 studies have focused mainly on the effects of modern versus future conditions (Alberton et al., 2005; Mohan et al., 2014), and little is known about mycorrhizal responses to low [CO2] of the past (Treseder et al., 2003; Procter et al., 2014). Assessing mycorrhizal responses to a broad, temporal [CO2] gradient is critical to establish a baseline for how these symbioses functioned prior to anthropogenic forcing, which will provide insight into potential constraints on mycorrhizal responses to future conditions (Ogle et al., 2015). In addition, rising [CO2] is known to cause nonlinear shifts in plant physiology and growth (Gerhart and Ward, 2010); therefore, nonlinear shifts in mycorrhizal functioning also are likely to occur. Characterizing such responses is critical to accurately predict how these symbioses will impact plant physiology and growth in the future and requires experimentation that manipulates mycorrhizal associations at both low and elevated [CO2]. Furthermore, to fully understand the physiological mechanisms driving plant responses to mycorrhizal fungi, linkages between host plant physiology and mycorrhizal functioning across the full M-P continuum need to be assessed. A broad [CO2] gradient will likely generate both mutualistic and parasitic symbioses and provide insight into physiological traits that vary with mycorrhizal functioning.
In this study, we examined arbuscular mycorrhizal (AM) associations in two closely related C3 plant species, Taraxacum ceratophorum and Taraxacum officinale (native and exotic common dandelions; Asteraceae), across a glacial through future [CO2] gradient. AM fungi are the predominant, ancestral type of mycorrhizal fungi (Smith and Read, 2008), and approximately 80% of modern plant species form AM associations (Brundrett, 2009). These fungi are primarily associated with increased phosphorus (P) uptake (Johnson, 2010), and past studies show that as much as 90% of plant P is acquired via fungal symbionts (Pearson and Jakobsen, 1993; Smith et al., 2009). AM fungi also can increase nitrogen (N) uptake, especially NH4+, although the fungal contribution to plant N uptake is challenging to measure and highly variable among studies (e.g. 0%–74% of total plant N; Hodge and Storer, 2015). In exchange for these nutrients, plants allocate an estimated 5% to 10% of their carbohydrates to AM fungi (Bryla and Eissenstat, 2005).
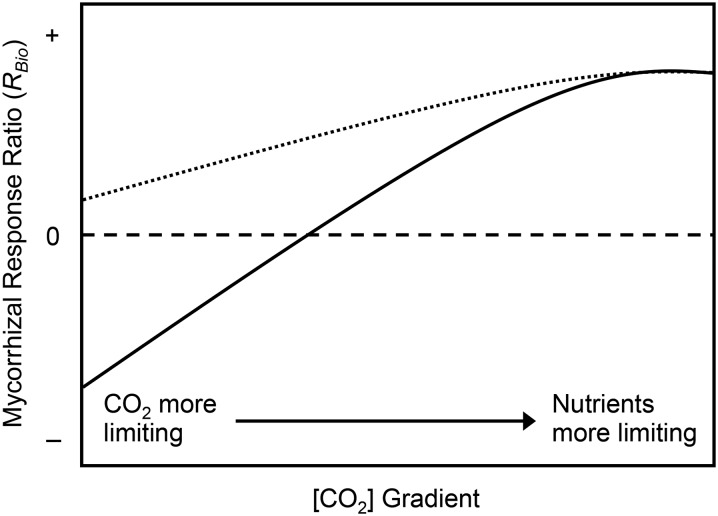
The net effect of AM fungi on plants is often measured in terms of plant growth, with some associations promoting faster growth rates and larger, more competitive plants (mutualism), while other associations restrict plant growth, resulting in smaller, less competitive plants (parasitism; Johnson et al., 1997). Where an interaction falls along the M-P continuum is highly dependent on physiological tradeoffs and resource limitations in the host. In general, nutrient availability is thought to be the primary driver of mycorrhizal functioning, with AM fungi increasing plant growth when nutrients, especially P, are more limiting than carbohydrates (Johnson et al., 2015). Changes in [CO2] will likely modulate nutrient effects on AM associations by altering the relative degree of nutrient and carbohydrate limitations in plants (Fig. 1; Johnson, 2010). More specifically, there is strong evidence that low [CO2] during the LGM produced major carbon (C) limitations within C3 plants, and modern plants grown at glacial [CO2] generally exhibit greater than 50% reductions in growth and photosynthetic rates relative to modern [CO2] (Polley et al., 1993; Tissue et al., 1995; Sage and Coleman, 2001; Beerling, 2005; Gerhart and Ward, 2010; Gerhart et al., 2012). Furthermore, the majority of C3 plants show some level of increased photosynthetic rates, leaf carbohydrate levels, and biomass when grown at future [CO2] (Ainsworth and Rogers, 2007; Prior et al., 2011). However, plants may require more nutrients in order to maintain high rates of photosynthesis and growth at elevated [CO2] (Campbell and Sage, 2006; Lewis et al., 2010). Thus, rising [CO2] may cause mycorrhizal associations to shift along the M-P continuum by reducing plant carbohydrate limitation while simultaneously increasing plant nutrient limitation.
Figure 1.
[CO2] is predicted to mediate nutrient effects on mycorrhizal associations by altering relative resource limitation in host plants. Specifically, the mycorrhizal response ratio (RBio) is predicted to increase with rising [CO2] due to simultaneous increases in plant carbohydrate production and plant nutrient limitation. RBio is calculated from the biomass of mycorrhizal (MBio) and nonmycorrhizal (NMBio) plants (RBio = [MBio − NMBio]/NMBio). The dashed line represents a neutral association (no difference in plant size). RBio may be negative (solid line) or marginally positive (dotted line) at low [CO2], depending on mycorrhizal effects on plant physiological responses to a CO2-limiting environment.
Most mycorrhizal-CO2 studies compared the effects of current (340–400 µL L−1) and future (540–750 µL L−1) [CO2] (Alberton et al., 2005; Mohan et al., 2014), but very few studies included more than two [CO2] treatments, preventing the assessment of potential nonlinear patterns. These studies indicate that AM fungi generally increase plant growth at elevated [CO2] and that rising [CO2] promotes stronger mutualism (Mohan et al., 2014). However, the functioning of mycorrhizal associations at low [CO2] of the past remains unclear. One possibility is that AM fungi reduce plant growth at low [CO2] because these symbionts are a major sink for carbohydrates that are expensive to produce when CO2 is limiting (Gerhart and Ward, 2010; Gerhart et al., 2012). Studies that show reduced AM fungal abundance in soils exposed to preindustrial [CO2] provide tentative support for this hypothesis (Treseder et al., 2003; Procter et al., 2014). Alternatively, some plants may partially compensate for low [CO2] by increasing their investment in the photosynthetic machinery, including the enzyme Rubisco (Sage and Coleman, 2001; Becklin et al., 2014). This strategy enhances CO2 uptake but comes at the cost of greater demand for N (Sage and Coleman, 2001). P limitation also has been shown to restrict the rate of ribulose bisphosphate regeneration, with subsequent effects on photosynthesis under preindustrial [CO2] (Campbell and Sage, 2006). Thus, by alleviating nutrient limitations on plant physiology, AM fungi may promote plant growth even under low [CO2].
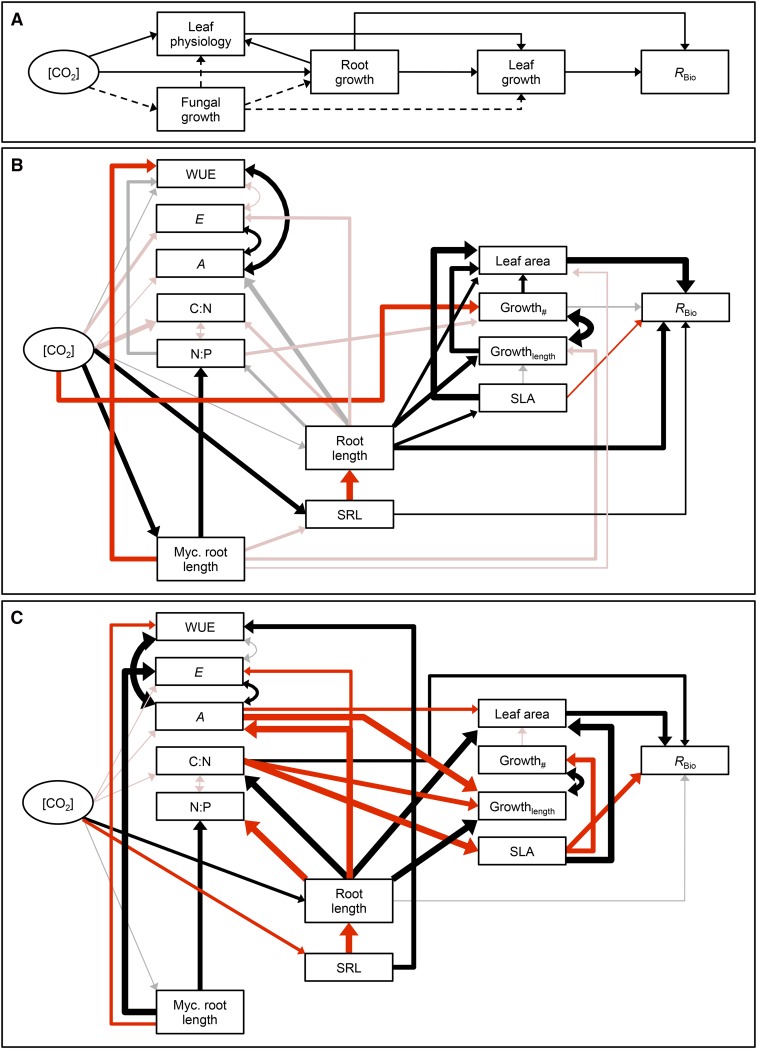
Characterizing CO2 effects on AM associations is critical for understanding the unique properties of these symbioses as well as for predicting how these interactions respond to both past and future environments. Here, we examined mycorrhizal responses across a glacial through future [CO2] gradient in a controlled-environment experiment. We predicted that mycorrhizal associations would become more beneficial (i.e. have a larger positive effect on plant growth) with rising [CO2] (Fig. 1). We further hypothesized two possible outcomes for mycorrhizal functioning under low [CO2] of the past: (1) AM fungi will exacerbate carbohydrate constraints and restrict plant growth under low [CO2], resulting in parasitic mycorrhizal associations when CO2 is limiting (Fig. 1, solid line); and (2) fungal effects on nutrient uptake will alleviate nutrient constraints on plant physiological responses to low [CO2], resulting in mutualistic mycorrhizal associations under glacial conditions (Fig. 1, dotted line). Additionally, studies indicate that many C3 plants respond nonlinearly to rising [CO2], with stronger responses to changes in [CO2] below the modern value compared with above (Gerhart and Ward, 2010). These nonlinear shifts in plant physiology and growth will likely alter plant resource limitations and mycorrhizal functioning across a broad [CO2] gradient. Thus, we further predicted that plants will be most responsive to increases from glacial to modern [CO2], resulting in nonlinear shifts in plant physiology, plant growth, and mycorrhizal functioning across the full [CO2] gradient. To better understand the mechanisms that alter plant responses to mycorrhizal fungi and overall patterns of plant productivity with rising [CO2], we conducted detailed studies of plant physiology, intraradical fungal growth, and plant biomass allocation in mycorrhizal plants. We then tested for causal relationships among these traits and overall plant responses to mycorrhizal fungi using structural equation modeling (Fig. 2).
Figure 2.
A, Piecewise structural equation models describing [CO2] effects on RBio via changes in plant and fungal traits. B, Model results for T. ceratophorum indicate that both direct and indirect effects of [CO2] on plant traits contributed to shifts in RBio. C, Model results for T. officinale indicate that primarily direct effects of [CO2] on plant traits contributed to shifts in RBio. In B and C, boxes represent measured traits and solid arrows indicate significant pathways in the model (P < 0.05). Black and red arrows indicate positive and negative correlations, respectively. The thickness of solid arrows was scaled to reflect the magnitude of the standardized regression coefficients. r2 values for each component model and standardized pathway coefficients are listed in Supplemental Table S4.
RESULTS
Our study focuses on plant physiological mechanisms driving shifts in mycorrhizal functioning across a broad [CO2] gradient representing glacial through future conditions. Native (T. ceratophorum) and invasive (T. officinale) host species responded differently to AM fungi, with T. ceratophorum exhibiting stronger, but more variable, mycorrhizal responses than T. officinale. [CO2] altered the functioning of mycorrhizal associations along the M-P continuum, although the pattern and magnitude of these shifts depended on the host species. For T. officinale, mycorrhizal associations shifted from parasitism at low [CO2] to mutualism at elevated [CO2]. In contrast, AM fungi increased the growth of T. ceratophorum at glacial to modern [CO2]; this effect was reversed in T. ceratophorum plants grown at future [CO2]. Mechanisms contributing to shifts in mycorrhizal functioning with [CO2] also differed between host species. [CO2] and fungal effects on plant traits related to C sink strength were highly correlated with shifts in mycorrhizal functioning in T. ceratophorum. In contrast, [CO2] effects on photosynthetic rates and vegetative plasticity drove shifts in mycorrhizal functioning in T. officinale. Overall, our results indicate that mycorrhizal associations functioned differently at the low [CO2] of the past and that nonlinear effects could limit mycorrhizal responses to future [CO2] in some plant species.
Leaf-Level Physiology
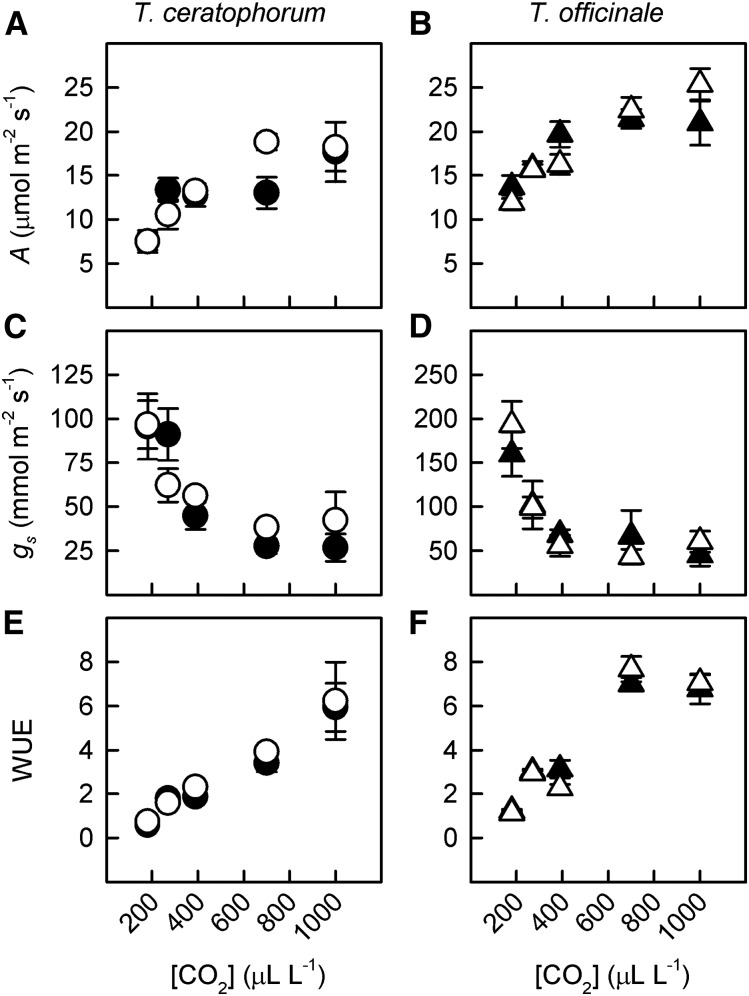
[CO2] similarly affected leaf physiology in the Taraxacum spp. hosts, while the effect of AM fungi on plant-CO2 responses depended on the trait and species (Supplemental Tables S1 and S2). For T. ceratophorum, instantaneous photosynthetic rate (A) increased nonlinearly with [CO2] (Fig. 3A; CO2 main effect, PC = 0.0001), and this effect was similar between mycorrhizal and control plants (CO2 by mycorrhizal interaction, PCM = 0.07). A also increased with [CO2] in T. officinale (Fig. 3B; PC = 0.0001), although the magnitude of this response was reduced in mycorrhizal plants grown at elevated [CO2] (PCM = 0.05). Stomatal conductance (gs) decreased with [CO2] in both host species, and the magnitude of this effect was greater at lower [CO2] (Fig. 3, C and D; PC = 0.0001 and 0.002 for T. ceratophorum and T. officinale, respectively). Mycorrhizal treatment did not affect gs in T. ceratophorum (mycorrhizal main effect, PM > 0.6). For T. officinale, gs tended to decrease more rapidly with [CO2] in control plants compared with mycorrhizal plants (PCM = 0.09). Instantaneous water use efficiency (WUE) was affected significantly by [CO2] but not by mycorrhizal treatment. Additionally, the shape of the relationship between WUE and [CO2] differed between Taraxacum spp., in that WUE increased linearly for T. ceratophorum and nonlinearly for T. officinale across the [CO2] gradient (Fig. 3, E and F; PC = 0.0001 for both species).
Figure 3.
[CO2] and mycorrhizal effects on leaf gas-exchange rates were similar between T. ceratophorum and T. officinale. Graphs show mean photosynthetic rate (A and B), gs (C and D), and WUE (E and F) for mycorrhizal (black symbols) and control (white symbols) plants.
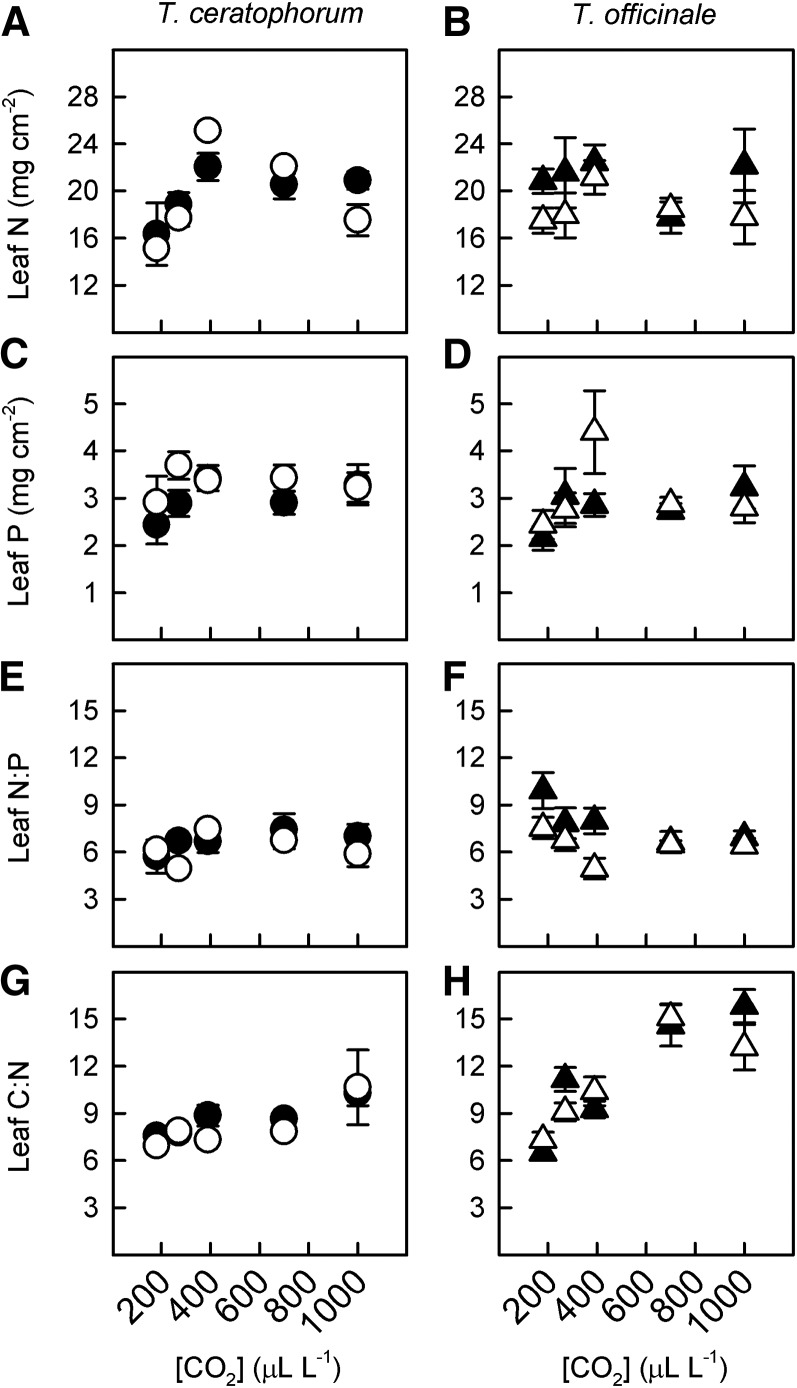
[CO2] and mycorrhizal treatment also impacted leaf nutrient content and stoichiometry, although these effects differed between host species (Supplemental Tables S1 and S2). In T. ceratophorum, leaf N content increased from 180 to 390 µL L−1 [CO2] and then decreased in plants grown at elevated [CO2] (Fig. 4A; PC = 0.0001). Neither [CO2] nor mycorrhizal treatment significantly affected leaf P content or leaf nitrogen-phosphorus (N-P) ratio in T. ceratophorum (Fig. 4, C and E; P > 0.1 for both traits). However, leaf carbon-nitrogen (C-N) ratio increased across the [CO2] gradient in this host (Fig. 4G; PC = 0.0001). [CO2] had no effect on leaf N or P content in T. officinale (Fig. 4, B and D; P > 0.3), but leaf N content was significantly higher in mycorrhizal plants versus control plants (PM = 0.048). Both [CO2] and mycorrhizal treatment affected leaf N-P ratio in T. officinale (Fig. 4F). Specifically, leaf N-P ratio decreased across the [CO2] gradient, with the greatest change occurring from 180 to 390 µL L−1 [CO2] (PC = 0.02). Leaf N-P ratio also was higher in mycorrhizal T. officinale plants compared with control plants (PM = 0.03), although this effect was driven largely by differences in the 180 to 390 µL L−1 treatments. Additionally, leaf C-N ratio increased in T. officinale across the [CO2] gradient, and changes in this ratio were greatest at lower [CO2] (Fig. 4H; PC = 0.002).
Figure 4.
[CO2] and mycorrhizal effects on leaf stoichiometry differed between T. ceratophorum and T. officinale. Graphs show mean N content (A and B), P content (C and D), N-P ratio (E and F), and C-N ratio (G and H) for mycorrhizal (black symbols) and control (white symbols) plants.
Plant Growth and Biomass Allocation
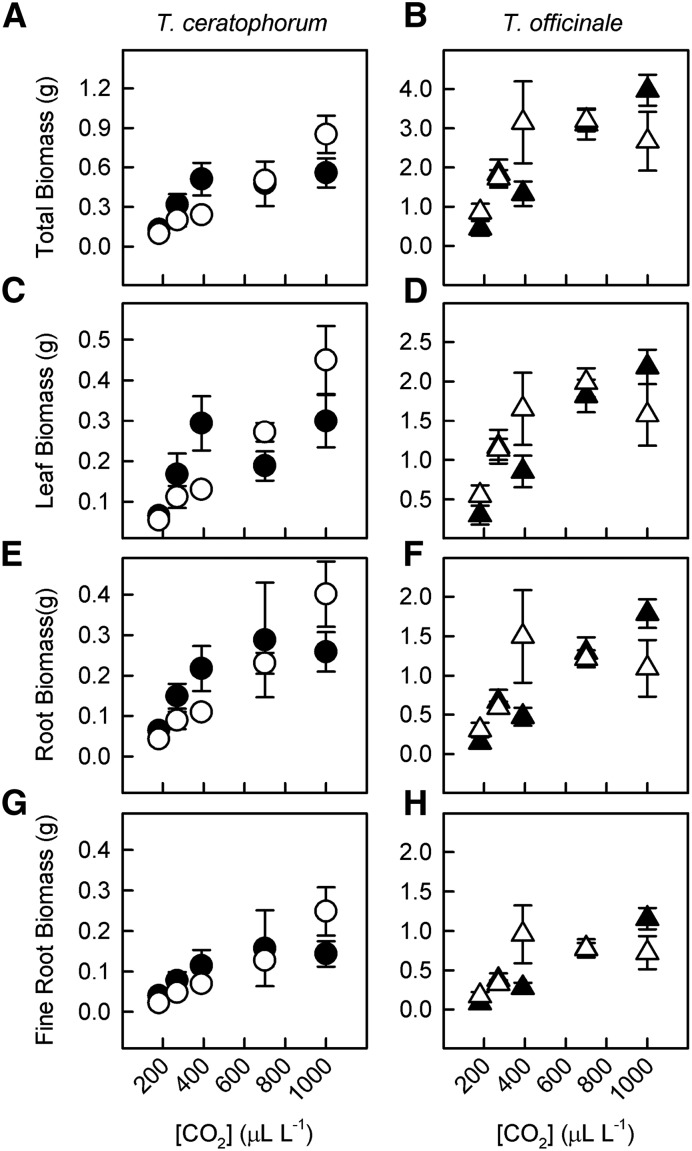
[CO2] and mycorrhizal effects on plant growth and allocation depended on the host species (Supplemental Tables S1 and S2). For T. ceratophorum, rising [CO2] significantly increased leaf, root, and total plant biomass (Fig. 5; PC = 0.0001 for all traits). [CO2] effects on total plant biomass differed between mycorrhizal and control plants (Fig. 5A; nonlinear CO2 by mycorrhizal interaction, PCCM = 0.05). In the control treatment, total plant biomass increased linearly with [CO2] (P = 0.0001). In the mycorrhizal treatment, total plant biomass increased from 180 to 390 µL L−1 [CO2], but then remained relatively constant with further increases in [CO2] (P = 0.01). Consequently, control plants were larger than mycorrhizal plants at elevated [CO2] (P = 0.02). Although the mycorrhizal effect was only significant at the whole-plant level, similar patterns were observed for leaf and total root biomass (Fig. 5, C and E; PCCM = 0.08 and 0.06, respectively). Mycorrhizal treatment did not significantly affect fine root biomass in T. ceratophorum (PM = 0.3 and PCCM = 0.2). Neither [CO2] nor mycorrhizal treatment affected the proportion of total biomass allocated to leaves and roots in this host (P > 0.6).
Figure 5.
The relative strength of [CO2] and mycorrhizal effects on plant biomass differed between T. ceratophorum and T. officinale. Graphs show total plant biomass (A and B), leaf biomass (C and D), total root biomass (E and F), and fine root biomass (G and H) for mycorrhizal (black symbols) and control (white symbols) plants. These data were used to calculate the mycorrhizal response ratios shown in Figure 8.
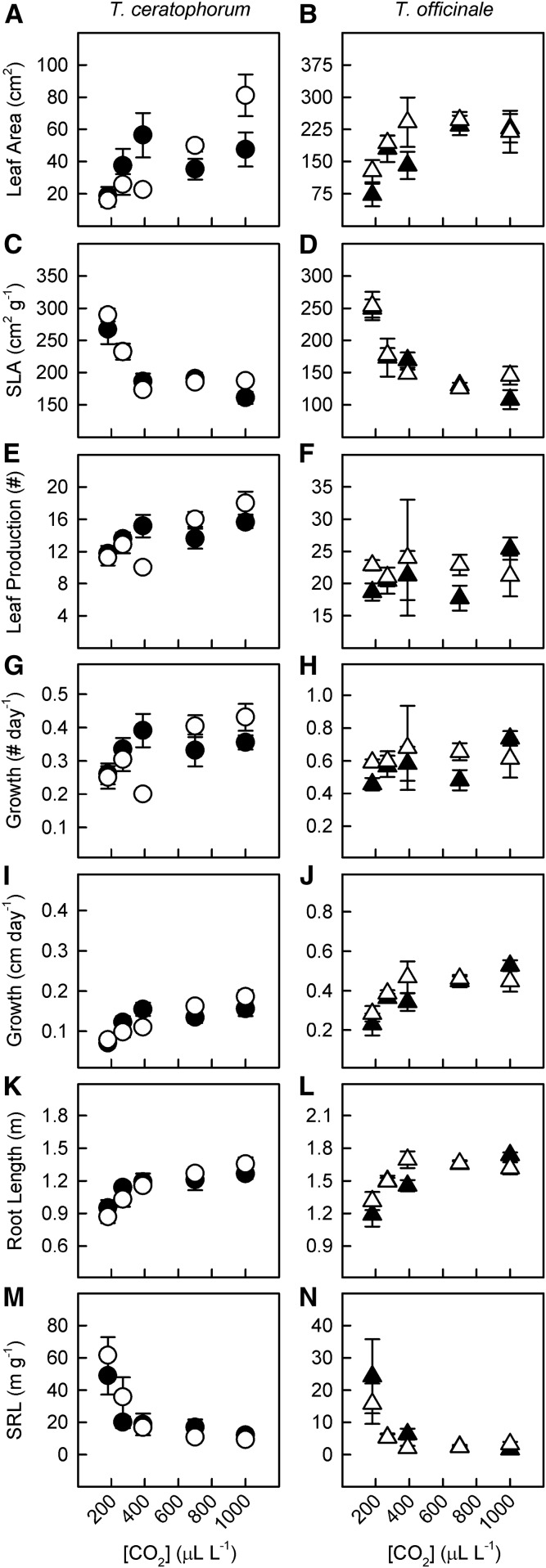
The number of leaves produced by T. ceratophorum, total leaf area, and total root length increased with [CO2] (Fig. 6; PC = 0.0001 for all traits). As with total biomass, the slope of these relationships differed between mycorrhizal treatments, with control plants producing more leaves, leaf area, and root length than mycorrhizal plants at elevated [CO2] (PCM < 0.05 for all traits). Differences in leaf production by T. ceratophorum were reflected in aboveground growth rates. Specifically, the rate of change in leaf production and leaf length increased with [CO2], but this effect was reduced in mycorrhizal plants (Fig. 6, G and I; PCM < 0.05 for both rates). Therefore, mycorrhizal T. ceratophorum plants grew at a slower rate than control plants at elevated [CO2]. Specific leaf area (SLA) and specific root length (SRL) decreased nonlinearly with [CO2] in this host (Fig. 6, C and M; PC = 0.0002 and 0.003, respectively). SRL also was generally reduced in mycorrhizal plants compared with control plants at low [CO2] (PCM = 0.07).
Figure 6.
The relative strength of [CO2] and mycorrhizal effects on leaf and root traits differed between T. ceratophorum and T. officinale. Graphs show mean leaf area (A and B), specific leaf area (C and D), leaf number (E and F), change in leaf number per day (G and H), change in leaf length per day (I and J), total root length (K and L), and specific root length (M and N) for mycorrhizal (black symbols) and control (white symbols) plants.
For T. officinale, [CO2] had a stronger effect on plant growth than the mycorrhizal treatment. Rising [CO2] significantly increased leaf, root, and total plant biomass (Fig. 5; PC < 0.05 for all traits). With the exception of fine roots, changes in plant biomass were greater at low [CO2] (significant nonlinear response). [CO2] effects on total plant biomass and leaf biomass tended to differ between mycorrhizal and control T. officinale plants (Fig. 5, B and D; PCCM = 0.06 for both traits). Specifically, total plant biomass and leaf biomass increased linearly with [CO2] in the mycorrhizal treatment and nonlinearly with [CO2] in the control treatment (P = 0.0001 for all analyses). [CO2] and mycorrhizal treatment significantly affected biomass allocation to T. officinale leaves and fine roots (PCM = 0.048). In the mycorrhizal treatment, plants allocated relatively more biomass to leaves compared with fine roots when grown at 180 to 390 µL L−1 [CO2]. Relative biomass allocation to fine roots increased in mycorrhizal plants grown at elevated [CO2]. In the control treatment, [CO2] had no effect on relative biomass allocation to aboveground and belowground tissues. The effects of [CO2] and mycorrhizal treatment on biomass allocation in T. officinale were reduced when comparing leaf biomass with total root biomass (PCM = 0.07).
Total leaf area and total root length increased nonlinearly with [CO2] in T. officinale (Fig. 6, B and L; PC = 0.01 and 0.0006, respectively), and these responses were similar between mycorrhizal treatments (PM > 0.3 for both traits). Leaf growth rate (change in leaf length per day) also increased nonlinearly across the [CO2] gradient (Fig. 6J; PC = 0.03). Neither [CO2] nor mycorrhizal treatment significantly affected the number of leaves or the rate of leaf production in T. officinale (Fig. 6, F and H; P > 0.1 for both traits). SLA and SRL decreased nonlinearly with [CO2] (Fig. 6, D and N; PC = 0.0002 and 0.003, respectively). As with T. ceratophorum, these responses were similar between mycorrhizal treatments (PCM > 0.1 and 0.7, respectively).
Fungal Growth
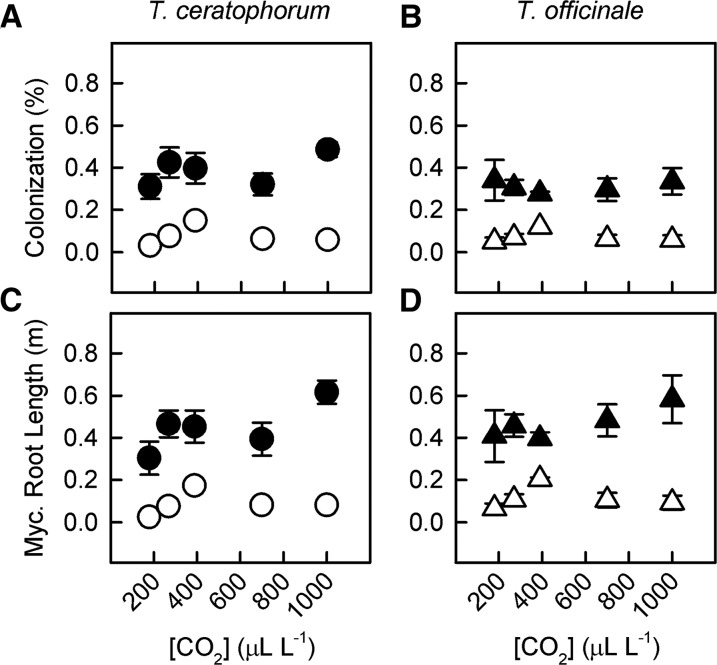
Fungal colonization rates in the mycorrhizal treatment ranged from 16% to 72% (average = 39%) of T. ceratophorum root length and 16% to 60% (average = 31%) of T. officinale root length. [CO2] had a minimal effect on fungal growth within Taraxacum spp. roots (Supplemental Tables S1 and S2). Mycorrhizal root length increased linearly with [CO2] in T. ceratophorum (Fig. 7C; PC = 0.058), but this effect was not significant until the shift from 700 to 1,000 µL L−1 [CO2]. Neither mycorrhizal colonization rate nor mycorrhizal root length varied with [CO2] in T. officinale (Fig. 7, B and D; PC = 0.9 and 0.3, respectively).
Figure 7.
[CO2] had minimal effects on fungal growth in T. ceratophorum and T. officinale roots. Graphs show mean AM fungal colonization (A and B) and mycorrhizal root length (C and D) for mycorrhizal (black symbols) and control (white symbols) plants.
Mycorrhizal Response Ratio
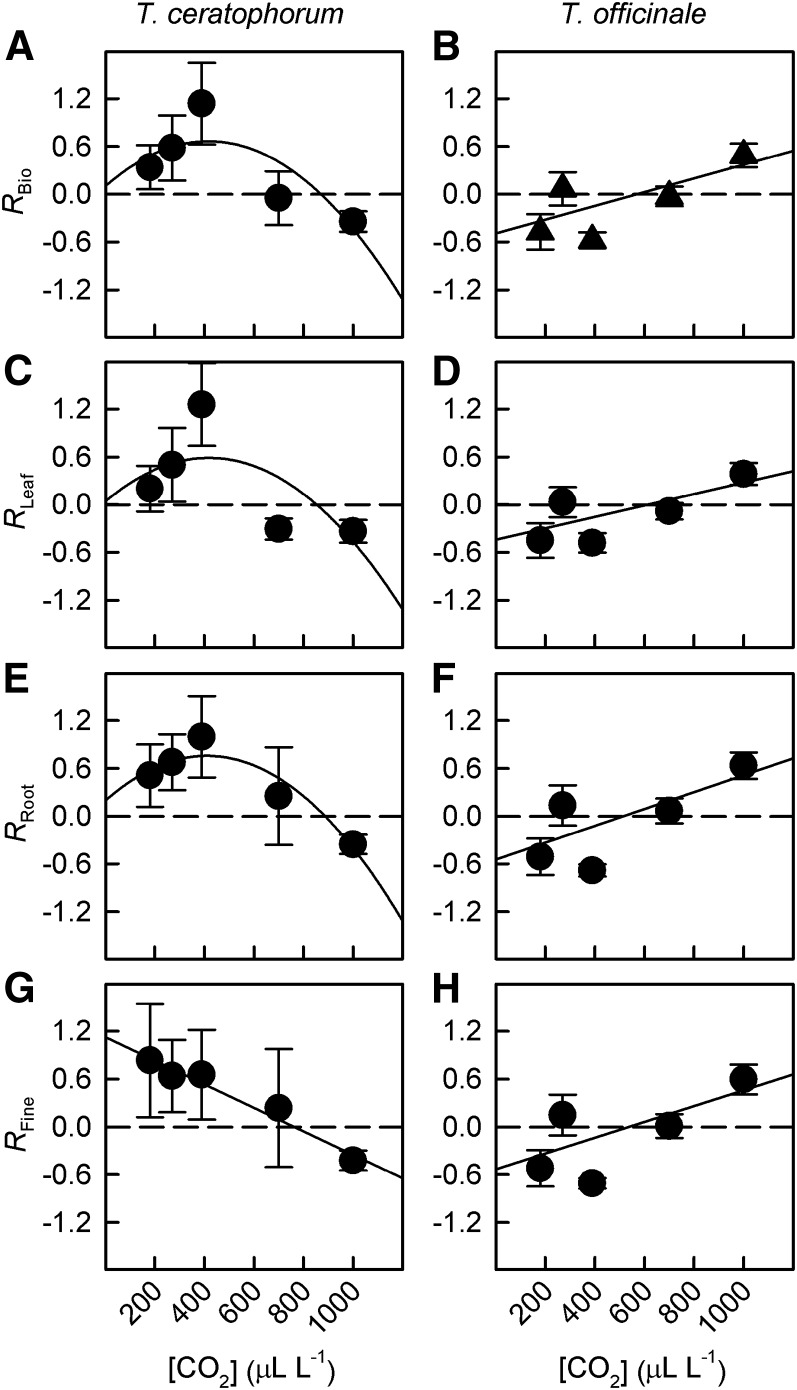
[CO2] differentially affected the mycorrhizal response ratios based on total plant biomass of T. ceratophorum and T. officinale hosts (RBio). For T. officinale, RBio increased linearly across the [CO2] gradient, and 700 µL L−1 [CO2] represented a critical point above which RBio shifted from negative to positive (Fig. 8B; PC = 0.005). Similar patterns were observed for mycorrhizal response ratios based on leaf and root biomass (Fig. 8). RBio also increased with [CO2] in T. ceratophorum plants grown at 180 to 390 µL L−1 [CO2]. However, further increases in [CO2] caused RBio to decrease and eventually shift from positive to negative values at elevated [CO2] (Fig. 8A; PC = 0.03). Mycorrhizal response ratios based on leaf and total root biomass also shifted nonlinearly with [CO2] (Fig. 8, C and E). In contrast, the response ratio based on fine root biomass was more variable, and decreased linearly with rising [CO2] (Fig. 8G).
Figure 8.
[CO2] differentially affected the mycorrhizal response ratios of T. ceratophorum and T. officinale. Graphs show response ratios calculated based on total biomass (RBio; A and B), leaf biomass (RLeaf; C and D), total root biomass (RRoot; E and F), and fine root biomass (RFine; G and H).
Plant physiological mechanisms contributing to the observed shifts in RBio depended on the host (Fig. 2). In general, models that included both [CO2] and fungal effects on plant traits performed better than models that excluded one of these factors (Supplemental Table S3). For T. ceratophorum, the primary traits associated with shifts in RBio were total leaf area, total root length, SRL, and SLA (Fig. 2B; Supplemental Table S4). Aboveground growth metrics also were strongly correlated with root-based response ratios in this host. [CO2] effects on fungal growth were weakly correlated with decreased mycorrhizal effects on aboveground growth rates and leaf area. Variation in fungal growth also was correlated with increased leaf N-P ratio and decreased WUE in mycorrhizal hosts. The main traits associated with shifts in RBio in T. officinale plants were total leaf area, SLA, and leaf C-N ratio (Fig. 2C; Supplemental Table S4). In this case, [CO2] influenced RBio primarily through positive effects on root growth, which translated into increased total leaf area. Response ratios based on photosynthetic rate also were negatively correlated with mycorrhizal effects on total leaf area. Variation in fungal growth influenced response ratios based on leaf physiological traits, including gs, leaf N-P ratio, and WUE.
DISCUSSION
Long-term changes in atmospheric [CO2] are expected to alter mycorrhizal functioning and the role of these symbioses in terrestrial ecosystems due to integrated shifts in plant and fungal physiology. Developments in mycorrhizal theory (Johnson, 2010; Johnson et al., 2015) have provided a framework for predicting how these symbioses will function as plant carbohydrate and nutrient limitations shift with rising [CO2]. Yet, our understanding of mycorrhizal-CO2 responses is primarily limited to studies comparing modern and future [CO2] (Alberton et al., 2005; Mohan et al., 2014). The lack of information about mycorrhizal responses to low [CO2] during the recent geologic past limits the characterization of physiological mechanisms that drive shifts in mycorrhizal functioning prior to and after anthropogenic influence. By examining host plant physiology and mycorrhizal functioning across glacial through future [CO2], we show that plant responses to mycorrhizal fungi were weaker under the low [CO2] of the past, although in T. ceratophorum, fungal symbionts still promoted plant growth under CO2-limiting conditions. Our results also support the hypothesis that mycorrhizal associations will become more beneficial with rising [CO2] (Mohan et al., 2014); however, nonlinear responses may limit mycorrhizal benefits to some plants at the elevated [CO2] of the future (Alberton et al., 2007). Furthermore, physiological mechanisms driving shifts in mycorrhizal associations were linked to host-specific differences in plant growth rate (Koziol and Bever, 2015) and vegetative plasticity as well as potential constraints on plant physiology across a long-term [CO2] gradient. Overall, this work addresses key gaps in our understanding of [CO2] effects on plant physiology and mycorrhizal associations during the recent geologic past and into the future.
Host-Specific Differences in Mycorrhizal Functioning
The overall strength of plant responses to AM fungi differed for T. ceratophorum and T. officinale, highlighting the potential for mycorrhizal associations to vary even between closely related plant species. On average, T. ceratophorum responded more positively to AM fungi than T. officinale under low to modern [CO2], and this difference corresponds to the observed variation in mycorrhizal associations in modern Taraxacum spp. populations. In particular, previous work indicates that T. ceratophorum is more heavily colonized by AM fungi than T. officinale in the field (Becklin and Galen, 2009) and that colonization rate more strongly predicts plant growth benefits in the native host (Becklin, 2010). Together, these data suggest overall stronger mycorrhizal associations in the native host. Yet, T. ceratophorum also exhibited more variation than T. officinale in mycorrhizal functioning across the full [CO2] gradient. Changes in other variables, such as light and water availability, also have been shown to influence mycorrhizal functioning to a greater degree in T. ceratophorum compared with T. officinale (Becklin, 2010), indicating that mycorrhizal associations in the native host may be more sensitive to environmental conditions. Differences in mycorrhizal functioning between T. ceratophorum and T. officinale may be amplified in field populations, where colonization rates are generally higher and more variable than what we observed in this study.
Species-specific differences in mycorrhizal responsiveness may reflect variable growth strategies and native/invasive status. For example, Koziol and Bever (2015) recently showed that mycorrhizal responsiveness was inversely related to plant growth rate in an artificial community of 30 prairie plant species. In our study, T. ceratophorum had a slower growth rate than T. officinale (Fig. 6, G and I) and tended to invest proportionally more biomass belowground. These growth strategies may facilitate more beneficial mycorrhizal associations in T. ceratophorum, especially when [CO2] limits plant growth. A recent meta-analysis further shows that mycorrhizal responses are often dampened in invasive plant species (Bunn et al., 2015). Furthermore, the positive relationship between fungal colonization and plant size that is often observed for native hosts is generally absent in invasive hosts. The evolution of reduced dependence on mycorrhizal fungi (Seifert et al., 2009) may allow invasive plants to spread more easily into novel environments, since they are less likely to be restricted by the absence of fungal partners. Mismatches between invasive plants and species of mycorrhizal fungi in the novel range also could explain dampened mycorrhizal responses (Klironomos, 2003). Differences in fungal species composition between cooccurring T. ceratophorum and T. officinale plants in the field (Becklin et al., 2012) suggest that mismatches could play a role during the initial invasion. Finally, many invasive plants have fast growth rates and high degrees of plasticity that allow them to spread rapidly into novel habitats; these growth strategies also could contribute to lower mycorrhizal responsiveness in T. officinale.
Shifts in Mycorrhizal Functioning with [CO2]
Plant growth responses to AM fungi increased with rising [CO2], supporting our original hypothesis that these associations will become more beneficial due to reduced plant carbohydrate limitations and increased plant nutrient limitations with rising [CO2] (Johnson, 2010; Mohan et al., 2014). This pattern was most evident in T. officinale, which showed linear increases in RBio across the full [CO2] gradient. In contrast, RBio increased in T. ceratophorum only from glacial to modern [CO2]. Results for T. officinale also supported the hypothesis that AM fungi would reduce plant growth (function as parasites) under low [CO2] due to severe carbon limitations on plant physiology. Negative plant responses to AM fungi, such as what we saw for T. officinale, may have generated negative feedbacks that reduced the frequency or abundance of these associations during low [CO2] periods in the past. Indeed, studies that examined AM fungi under preindustrial [CO2] noted reduced fungal growth in the soil environment (Treseder et al., 2003; Procter et al., 2014). Unlike what we saw for T. officinale, T. ceratophorum responded positively to AM fungi even at low [CO2]. This result supports the hypothesis that mycorrhizal fungi could promote CO2 assimilation under low [CO2] by increasing the uptake of nutrients that are necessary to maximize photosynthetic capacity in a C-limited environment. Thus, the ability to form mycorrhizal associations and the strength of these partnerships may represent an important adaptation that enabled some plant species to persist during low [CO2] periods in the past (Becklin et al., 2014).
Elevated [CO2] greatly altered mycorrhizal functioning in these cooccurring plant species. Specifically, AM fungi reduced the growth of T. ceratophorum plants but increased the growth of T. officinale plants in the 1,000 µL L−1 treatment. For both plant species, 700 µL L−1 represented a critical point along the [CO2] gradient where RBio shifted from negative to positive values (or vice versa). This indicates that future anthropogenic changes in [CO2] could drastically alter the nature of mycorrhizal associations as well as their role in plant physiological acclimation and adaptation to global change. Characterizing physiological mechanisms that underlie these differential shifts in mycorrhizal functioning at elevated [CO2] (see below) will be critical for predicting mycorrhizal feedbacks on plant physiology in future environments.
Both Taraxacum spp. hosts showed evidence of nonlinear responses to rising [CO2] that could only be assessed across a large [CO2] gradient. As expected, [CO2] caused significant nonlinear shifts in leaf gas-exchange rates, leaf stoichiometry, and plant growth, with generally greater shifts in these traits at the lower end of the [CO2] gradient. These nonlinear shifts often are attributed to strong carbon limitations imposed on plant physiology at low [CO2] and the emergence of other limiting factors (e.g. nutrient and water availability) at elevated [CO2] (Gerhart and Ward, 2010; Gerhart et al., 2012). Here, we show that mycorrhizal fungi can mediate some of these nonlinear plant responses across glacial through future [CO2]. Indeed, shifts in RBio and trait-specific response ratios often reflected differences in the shape of [CO2] responses in mycorrhizal and control plants. For example, the biomass of T. ceratophorum plants increased linearly with [CO2] in the control treatment but nonlinearly in the mycorrhizal treatment. The reduction in mycorrhizal plant growth (relative to control plants) resulted in negative RBio under elevated [CO2]. Characterizing these types of nonlinear responses to [CO2] is critical to accurately predict how long-term global change will impact mycorrhizal associations as well as the potential constraints imposed by mycorrhizal fungi on plant physiology. For example, mycorrhizal fungi may alleviate increased plant nutrient limitations at elevated [CO2]. However, if fungal growth also becomes increasingly nutrient limited, then competition for nutrients between the host plant and its fungal partners could constrain plant physiology, resulting in photosynthetic down-regulation or even growth depressions at elevated [CO2] (Alberton et al., 2007; Johnson, 2010). Modeling these physiological feedbacks requires knowledge of potential threshold responses that identify points along the [CO2] gradient that cause critical shifts in mycorrhizal functioning within individual hosts as well as shifts in mycorrhizal dynamics within the larger plant community.
In our system, shifts in the functioning of mycorrhizal associations with rising [CO2] could alter plant-soil feedbacks (PSF; Mangan et al., 2010; Bever et al., 2012) that influence invasive plant dynamics (Johnson et al., 2013). PSF can facilitate the spread of invasive plants if the invader disrupts positive PSF in the native community (Callaway et al., 2008) or if the invader experiences positive PSF itself (Zhang et al., 2010; Hayward et al., 2015). Our results indicate that positive PSF are likely stronger in T. ceratophorum than in T. officinale under current conditions. Furthermore, previous work indicates that native AM fungi partially restrict the distribution of T. officinale at our field site (Becklin, 2010). However, if rising [CO2] enhances plant responses to AM fungi in T. officinale, thereby strengthening PSF in this host, then mycorrhizal associations could potentially facilitate the spread of T. officinale in the future.
Physiological Mechanisms Driving Shifts in Mycorrhizal Functioning
The strong correlation between aboveground and belowground response ratios suggests that C sink strength drove shifts in RBio in T. ceratophorum. That is, T. ceratophorum plants responded more positively to AM fungi in [CO2] treatments where fungal colonization stimulated root growth. Indeed, response ratios based on fine root biomass were highest in the [CO2] treatments that caused the greatest reductions in root growth (180–390 µL L−1; Figs. 5G and 8G). Thus, AM fungi had a proportionally larger influence on root growth and overall plant size in these low [CO2] treatments.
At elevated [CO2], mycorrhizal root length increased and RBio shifted to negative values in T. ceratophorum. This pattern may reflect resource competition between host plants and their fungal symbionts, which is predicted to increase under elevated [CO2] (Alberton et al., 2007; Johnson, 2010). This type of plant-fungal competition could influence photosynthetic down-regulation in the host as well as constrain plant growth responses to rising [CO2] in the future. Alternatively, if nutrients were not limiting to either partner, then increased fungal growth could enhance the carbohydrate sink strength within host plants without providing additional nutrient benefits, resulting in plant growth depressions (Johnson, 2010). If this were the case, then we would expect increases in fungal growth to be strongly correlated with decreases in RBio as well as greater growth depressions under low [CO2] when carbon availability limited plant physiology and growth. Since that was not the case, it is unlikely that increased fungal C demand in T. ceratophorum roots explains the shift from positive to negative response values at elevated [CO2]. Instead, we propose that plant-fungal competition for nutrients is a more likely scenario.
Mycorrhizal response ratios in T. officinale were associated primarily with fungal effects on leaf growth across the [CO2] gradient. This species is known to adjust allocation to leaf growth based on environmental conditions (Brock et al., 2005); thus, it is not surprising that mycorrhizal effects appear to be mediated through vegetative plasticity in this host. Interestingly, mycorrhizal effects on photosynthetic rate were negatively correlated with shifts in leaf growth. In other words, AM fungi promoted leaf growth even when photosynthetic rate was reduced in mycorrhizal plants (relative to control plants; Figs. 3B and 8D). This suggests that AM fungi may have increased the C use efficiency of T. officinale plants, at least under elevated [CO2], where a relative reduction in photosynthesis between mycorrhizal and control plants occurred. Although [CO2] did not significantly affect fungal growth in T. officinale plants, variation in mycorrhizal root length was correlated with variation in leaf-level physiology (e.g. gs and WUE). These physiological effects of AM fungi could become more important when considering the interactive effects of rising [CO2] and climate change (Mohan et al., 2014). In particular, the negative correlation between mycorrhizal root length and WUE suggests that AM fungi may decrease the ability of T. officinale to tolerate drought.
CONCLUSION
A glacial through future [CO2] gradient caused differential shifts in the functioning of mycorrhizal associations within two closely related dandelion species. In both cases, studying mycorrhizal responses to a broad, temporal [CO2] gradient provided support for contrasting hypotheses regarding mycorrhizal responses to low [CO2]. Our results suggest that these critical symbioses were generally weaker under low [CO2] representing glacial and preindustrial conditions. Exposing host plants to the full range of [CO2] experienced over recent geologic history also resulted in mycorrhizal associations that spanned the M-P continuum. Since nutrient availability was kept constant, this experiment isolated [CO2] as the driver of these shifts in mycorrhizal functioning and identified a critical [CO2] (700 µL L−1) that caused mycorrhizal response ratios to shift between positive and negative values. Differential responses of the two plant species across the [CO2] gradient were linked to variation in plant physiology and growth strategies, and our results suggest that key plant traits, such as growth rate, may provide a framework for predicting mycorrhizal functioning. Here, we focused primarily on host plant responses; however, significant [CO2] effects on fungal species composition have been noted in other studies (Treseder et al., 2003; Cotton et al., 2015). Such changes could alter the functional pathway by which mycorrhizal fungi impact plant physiology and growth (Sikes et al., 2010) and should be investigated further for CO2 responses. Finally, [CO2] is an important driver of evolutionary adaptation in plants (Ward and Strain, 1999; Sage and Coleman, 2001; Ward and Kelly, 2004; Beerling and Berner, 2005; Grossman and Rice, 2014), and our findings suggest that mycorrhizal fungi may contribute to these responses. Thus, integrating both physiological and evolutionary perspectives into mycorrhizal-CO2 studies will be critical for predicting the long-term consequences of global change on ecosystems.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Taraxacum ceratophorum, the native alpine dandelion, and Taraxacum officinale, the exotic dandelion, are close congeners that cooccur at the treeline in the Rocky Mountains (Brock, 2004; Becklin and Galen, 2009). These C3 plants differ in several ecological and physiological traits that could influence their responses to [CO2] and AM fungi. Specifically, T. officinale has a faster growth rate and greater plasticity in biomass allocation to aboveground and belowground tissues (Brock and Galen, 2005; Brock et al., 2005). In contrast, T. ceratophorum is a smaller plant with a slower growth rate, higher WUE (CO2 uptake per water molecule lost), and greater allocation belowground. Additionally, T. ceratophorum is more heavily colonized and exhibits a larger growth response to AM fungi than T. officinale under field conditions (Becklin and Galen, 2009; Becklin, 2010). In a field survey, AM fungi colonized 45% to 100% (average = 78%) of T. ceratophorum roots and 0% to 97% (average = 72%) of T. officinale roots (Becklin and Galen, 2009). AM fungal community composition also varies between cooccurring T. ceratophorum and T. officinale hosts (Becklin et al., 2012). Given these physiological and ecological distinctions, rising [CO2] is expected to differentially affect plant physiology, plant growth, and mycorrhizal functioning in these Taraxacum spp.
To test this hypothesis, T. ceratophorum and T. officinale seeds and field soil (the source of fungal inoculum) were collected from well-studied populations on Pennsylvania Mountain (approximately 3,623 m above sea level; Park County, Colorado). Both species were grown with and without AM fungi in a full factorial design with five [CO2] treatments representing glacial (180 µL L−1), preindustrial (270 µL L−1), modern (390 µL L−1), and future (700 and 1,000 µL L−1) conditions. Each [CO2] treatment was replicated in two separate Conviron BDR16 growth chambers. Chamber conditions were maintained at 22°C/14°C and 65%/90% relative humidity (day/night). Daytime light intensity was maintained at approximately 950 μmol m−2 s−1 for a 14-h photoperiod. Plants were watered once daily to saturation and fertilized with 25 mL of one-half-strength Hoagland solution (modified to one-quarter-strength P concentration) during a 30-d growth period.
All seeds were surface sterilized and germinated in petri dishes. Immediately upon germination, seedlings were transplanted into 2-L microcosms containing a sterilized growth medium (2:1:1 sand:gravel:turface). Half of the microcosms were inoculated with AM fungi by adding 20 g of homogenized living field soil. Control microcosms were inoculated with 20 g of sterilized field soil. To minimize nontarget microbial differences between inoculated and control treatments, all microcosms received 20 mL of a soil filtrate containing nonmycorrhizal soil microbes (Johnson et al., 2010). We assessed the AM fungal colonization of mycorrhizal and control plants after harvest. A subsample of the fine roots from each plant in the experiment was stained with Trypan Blue (Phillips and Hayman, 1970) and analyzed for the presence of arbuscules using the gridline intersection method (McGonigle et al., 1990). Root colonization rate was calculated as the percentage of root length containing arbuscules. Mycorrhizal root length was estimated by multiplying total root length and colonization rate. Some control plants had very low levels of AM fungi (range = 0%–15% of root length); in these cases, the intensity of colonization within the root also was very low compared with plants in the mycorrhizal treatment and random across [CO2] treatments (P > 0.1). These low levels of colonization may actually represent a more realistic control, since true nonmycorrhizal Taraxacum spp. plants are unlikely to occur in nature (AM fungi colonized all but one Taraxacum spp. plant examined in a field survey; Becklin and Galen, 2009). Additionally, control plants with low colonization levels were not statistically different in size from plants with no AM fungi (P > 0.1); thus, it is unlikely that the low colonization of control plants affected our results.
Leaf-Level Physiology
Leaf gas-exchange measurements were made using an LI-6400XT device (Li-Cor Biosciences) between 9 am and 12 noon on the day of harvest using a fully expanded green leaf that was exposed to full light. Cuvette conditions were set to match growth conditions. Leaves were allowed to acclimate to cuvette conditions until the instantaneous A and gs were stable. Instantaneous WUE was calculated as the ratio of photosynthetic rate to transpiration rate. Plants were harvested immediately following gas-exchange measurements.
For most plants in the experiment, mass-based leaf N and P contents were measured following acid digestion of 250 mg of dry leaf biomass using a Technicon AAII auto analyzer (Agronomy Soil Testing Laboratory, Kansas State University). For samples with less than 250 mg, leaf P content was analyzed following microwave digestion of 50 to 100 mg of dry leaf biomass using a Spectro Genesis inductively coupled plasma optical emission spectrometer (North Dakota State University). Mass-based leaf C content was analyzed on 3 to 4 mg of dry leaf biomass using a Costech 4010 elemental analyzer (Keck Paleoenvironmental and Environmental Stable Isotope Laboratory, University of Kansas). For samples with less than 250 mg, leaf N content was analyzed in conjunction with leaf C content. Replicated samples were analyzed using all approaches to correct for potential biases in the N and P results produced using different methods. N and P results were converted from mass-based percentages to nutrient content per leaf area using the ratio of total leaf biomass and total leaf area. Leaf N-P ratio and C-N ratio were calculated from mass-based percentages. In a few cases, plant biomass was too small to measure leaf N, P, and C contents on the same sample; thus, we were not able to calculate N-P ratio and C-N ratio for all plants in the experiment.
Plant Growth and Biomass Allocation
Plant growth was monitored weekly by counting the total number of leaves and the length of the longest leaf on each plant. Leaf length is correlated with leaf area in Taraxacum spp. (P < 0.0001, r2 > 0.75 for both species); thus, this measure reflects changes in photosynthetic area. Growth rates were calculated as the change in leaf number or leaf length per day.
After 30 d, we analyzed plant size and allocation to leaf and root fractions. Root tissue was further divided into taproot and fine root fractions. The biomass of each fraction was determined after drying the plants at 60°C for 72 h. Total fresh leaf area was measured using an LI-3100 leaf area meter (Li-Cor Biosciences). SLA was calculated as fresh leaf area per dry leaf biomass. To estimate total root length, we collected a random subsample of fresh fine roots from each plant in the experiment. Root length was determined from a scanned image of these roots using ImageJ. We then dried the imaged roots and used regression analysis to determine the coefficient relating root length to root biomass for each species and [CO2] treatment (mycorrhizal treatment did not affect this relationship). This coefficient was used to estimate total root length from measures of fine root biomass as well as SRL (fresh root length per dry root biomass).
Mycorrhizal Response Ratio
Shifts in mycorrhizal functioning along the M-P continuum were characterized using mycorrhizal response ratios (RBio). RBio represents the overall effect of AM fungi on plant growth and is generally calculated by comparing the total biomass of mycorrhizal (MBio) and nonmycorrhizal (NMBio) plants of the same species and age grown under similar conditions (RBio = [MBio − NMBio]/NMBio). Thus, RBio integrates the growth benefits of fungus-mediated nutrient uptake and the growth costs of carbohydrate allocation to fungal symbionts. A positive RBio indicates that AM fungi increase plant growth (mutualism), while a negative RBio indicates that AM fungi reduce plant growth (parasitism). Critical shifts in RBio include (1) shifts from positive to negative values, (2) nonlinear shifts in RBio, and (3) changes in the direction that RBio shifts across a resource gradient (e.g. increasing versus decreasing trends). For this study, we calculated RBio using the total biomass of mycorrhizal plants and the average total biomass of control plants for each host species and [CO2] treatment. We calculated similar response ratios using leaf gas-exchange rates, leaf carbon and nutrient contents, aboveground growth rates, total leaf area and SLA, leaf and root biomass, and total root length and SRL.
Statistical Analysis
All plant and fungal variables were analyzed using generalized linear models with mycorrhizal treatment as a categorical variable and [CO2] as a continuous variable. To assess the shape of plant responses to [CO2], we conducted both linear and quadratic models and tested for interactions between [CO2] and mycorrhizal treatment (C×M and C×C×M interaction terms in Supplemental Tables S1 and S2). A significant C×M interaction indicates that the presence of AM fungi mediated plant responses to [CO2]. Similarly, a significant C×C×M interaction indicates that the shape (linear or nonlinear) of the response differed between control and mycorrhizal treatments. Models were compared using the Akaike information criterion corrected for sample size (AICc). We also conducted allometric analyses of biomass allocation to aboveground and belowground plant structures via regression with [CO2] as a covariate and mycorrhizal treatment as a categorical variable. All data were analyzed separately for T. ceratophorum and T. officinale.
[CO2] effects on RBio were analyzed using regression with [CO2] as a continuous variable. Again, we compared linear and quadratic models using AICc. To assess the mechanisms that drive shifts in RBio, we calculated response ratios for each plant trait measured in this study. Because these response ratios are relative to nonmycorrhizal controls grown at the same [CO2], they minimize variation due to inherent plant-CO2 responses and allow us to evaluate traits that contributed to size differences between mycorrhizal and control plants across the [CO2] gradient. At the leaf level, we focused on response ratios calculated from instantaneous gas-exchange rates that determine carbohydrate production and are sensitive to [CO2] in C3 plants (Ainsworth and Rogers, 2007; Gerhart and Ward, 2010). We also examined ratios based on leaf N-P ratio and C-N ratio, because these traits reflect relative resource limitations in leaves and may be more relevant for predicting plant responses to mycorrhizal fungi than absolute leaf nutrient content (Johnson, 2010). Relationships among physiological traits, plant growth, and overall RBio were analyzed using piecewise structural equation modeling (Lefcheck, 2016). Specifically, we compared the following models: (1) [CO2] affected response ratios indirectly via changes in fungal growth (no direct [CO2] pathways); (2) [CO2] affected response ratios directly (no fungus-mediated pathways); and (3) both [CO2] and fungal growth affected response ratios in Taraxacum spp. (Fig. 2). Conditional independence within each model was evaluated using Shipley’s test of direction separation (Shipley, 2009), and overall model fit was evaluated using AICc. Modeling analyses were conducted separately for T. ceratophorum and T. officinale.
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. [CO2] and mycorrhizal effects on fungal growth, plant physiology, and plant growth in T. ceratophorum.
Supplemental Table S2. [CO2] and mycorrhizal effects on fungal growth, plant physiology, and plant growth in T. officinale.
Supplemental Table S3. Comparison of structural equation models for T. ceratophorum and T. officinale.
Supplemental Table S4. Standardized pathway coefficients and submodel r2 values for the T. ceratophorum and T. officinale models with the lowest AICc values.
Supplementary Material
Acknowledgments
We thank J. Medeiros and M. Walker for help with plant care; J. Stern and W. Wang for sample preparation; and D. Jacobs for analysis of leaf nutrients.
Glossary
- LGM
Last Glacial Maximum
- M-P
mutualism-to-parasitism
- AM
arbuscular mycorrhizal
- P
phosphorus
- N
nitrogen
- C
carbon
- WUE
water use efficiency
- N-P
nitrogen-phosphorus
- C-N
carbon-nitrogen
- SLA
specific leaf area
- SRL
specific root length
- PSF
plant-soil feedbacks
- AICc
Akaike information criterion corrected for sample size
Footnotes
This work was supported by the National Institutes of Health (Institutional Research and Academic Career Development Award fellowship to K.M.B.), the National Science Foundation (grant no. IOS 1457236 to J.K.W.), and a Research Investment Council grant from the University of Kansas.
Articles can be viewed without a subscription.
References
- Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30: 258–270 [DOI] [PubMed] [Google Scholar]
- Alberton O, Kuyper TW, Gorissen A (2005) Taking mycocentrism seriously: mycorrhizal fungal and plant responses to elevated CO2. New Phytol 167: 859–868 [DOI] [PubMed] [Google Scholar]
- Alberton O, Kuyper TW, Gorissen A (2007) Competition for nitrogen between Pinus sylvestris and ectomycorrhizal fungi generates potential for negative feedback under elevated CO2. Plant Soil 296: 159–172 [Google Scholar]
- Augustin L, Barbante C, Barnes PR, Barnola JM, Bigler M, Castellano E, Cattani O, Chappellaz J, Dahl-Jensen D, Delmonte B, et al. (2004) Eight glacial cycles from an Antarctic ice core. Nature 429: 623–628 [DOI] [PubMed] [Google Scholar]
- Becklin KM (2010) Friends in high places: ecology of mycorrhizal associations in alpine plant communities. PhD thesis. University of Missouri, Columbia [Google Scholar]
- Becklin KM, Galen C (2009) Intra- and interspecific variation in mycorrhizal associations across a heterogeneous habitat gradient in alpine plant communities. Arct Antarct Alp Res 41: 183–190 [Google Scholar]
- Becklin KM, Hertweck KL, Jumpponen A (2012) Host identity impacts rhizosphere fungal communities associated with three alpine plant species. Microb Ecol 63: 682–693 [DOI] [PubMed] [Google Scholar]
- Becklin KM, Medeiros JS, Sale KR, Ward JK (2014) Evolutionary history underlies plant physiological responses to global change since the last glacial maximum. Ecol Lett 17: 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling DJ. (2005) Evolutionary responses of land plants to atmospheric CO2. In Ehleringer JR, Cerling TE, Dearing MD, eds, A History of Atmospheric CO2 and Its Effects on Plants, Animals and Ecosystems. Springer, New York, pp 114–132 [Google Scholar]
- Beerling DJ, Berner RA (2005) Feedbacks and the coevolution of plants and atmospheric CO2. Proc Natl Acad Sci USA 102: 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever JD, Platt TG, Morton ER (2012) Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu Rev Microbiol 66: 265–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock MT. (2004) The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. Am J Bot 91: 656–663 [DOI] [PubMed] [Google Scholar]
- Brock MT, Galen C (2005) Drought tolerance in the alpine dandelion, Taraxacum ceratophorum (Asteraceae), its exotic congener T. officinale, and interspecific hybrids under natural and experimental conditions. Am J Bot 92: 1311–1321 [DOI] [PubMed] [Google Scholar]
- Brock MT, Weinig C, Galen C (2005) A comparison of phenotypic plasticity in the native dandelion Taraxacum ceratophorum and its invasive congener T. officinale. New Phytol 166: 173–183 [DOI] [PubMed] [Google Scholar]
- Brundrett MC. (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37–77 [Google Scholar]
- Bryla DR, Eissenstat DM (2005) Respiratory costs of mycorrhizal associations. In Lambers H, Ribas-Carbo M, eds, Plant Respiration. Springer, Dordrecht, The Netherlands, pp 207–224 [Google Scholar]
- Bunn RA, Ramsey PW, Lekberg Y (2015) Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J Ecol 103: 1547–1556 [Google Scholar]
- Callaway RM, Cipollini D, Barto K, Thelen GC, Hallett SG, Prati D, Stinson K, Klironomos J (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89: 1043–1055 [DOI] [PubMed] [Google Scholar]
- Campbell CD, Sage RE (2006) Interactions between the effects of atmospheric CO2 content and P nutrition on photosynthesis in white lupin (Lupinus albus L.). Plant Cell Environ 29: 844–853 [DOI] [PubMed] [Google Scholar]
- Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD (2015) Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol 205: 1525–1536 [DOI] [PubMed] [Google Scholar]
- Cotton TEA, Fitter AH, Miller RM, Dumbrell AJ, Helgason T (2015) Fungi in the future: interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol 205: 1598–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart LM, Harris JM, Nippert JB, Sandquist DR, Ward JK (2012) Glacial trees from the La Brea tar pits show physiological constraints of low CO₂. New Phytol 194: 63–69 [DOI] [PubMed] [Google Scholar]
- Gerhart LM, Ward JK (2010) Plant responses to low [CO2] of the past. New Phytol 188: 674–695 [DOI] [PubMed] [Google Scholar]
- Grossman JD, Rice KJ (2014) Contemporary evolution of an invasive grass in response to elevated atmospheric CO2 at a Mojave Desert FACE site. Ecol Lett 17: 710–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J, Horton TR, Pauchard A, Nuñnez MA (2015) A single ectomycorrhizal fungal species can enable a Pinus invasion. Ecology 96: 1438–1444 [DOI] [PubMed] [Google Scholar]
- Hodge A, Fitter AH (2010) Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA 107: 13754–13759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386: 1–19 [Google Scholar]
- IPCC (2013) Climate Change 2013: The Physical Science Basis. IPCC, New York [Google Scholar]
- Johnson NC. (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185: 631–647 [DOI] [PubMed] [Google Scholar]
- Johnson NC, Angelard C, Sanders IR, Kiers ET (2013) Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol Lett (Suppl 1) 16: 140–153 [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135: 575–586 [Google Scholar]
- Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci USA 107: 2093–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, Wilson GWT, Wilson JA, Miller RM, Bowker MA (2015) Mycorrhizal phenotypes and the law of the minimum. New Phytol 205: 1473–1484 [DOI] [PubMed] [Google Scholar]
- Klironomos JN. (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84: 2292–2301 [Google Scholar]
- Koziol L, Bever JD (2015) Mycorrhizal response trades off with plant growth rate and increases with plant successional status. Ecology 96: 1768–1774 [DOI] [PubMed] [Google Scholar]
- Lefcheck JS. (2015) piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods Ecol Evol 7: 573–579 [Google Scholar]
- Lewis JD, Ward JK, Tissue DT (2010) Phosphorus supply drives nonlinear responses of cottonwood (Populus deltoides) to increases in CO2 concentration from glacial to future concentrations. New Phytol 187: 438–448 [DOI] [PubMed] [Google Scholar]
- Lin G, McCormack ML, Guo D (2015) Arbuscular mycorrhizal fungal effects on plant competition and community structure. J Ecol 103: 1224–1232 [Google Scholar]
- Mangan SA, Herre EA, Bever JD (2010) Specificity between Neotropical tree seedlings and their fungal mutualists leads to plant-soil feedback. Ecology 91: 2594–2603 [DOI] [PubMed] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115: 495–501 [DOI] [PubMed] [Google Scholar]
- Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, et al. (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol 10: 3–19 [Google Scholar]
- Ogle K, Barber JJ, Barron-Gafford GA, Bentley LP, Young JM, Huxman TE, Loik ME, Tissue DT (2015) Quantifying ecological memory in plant and ecosystem processes. Ecol Lett 18: 221–235 [DOI] [PubMed] [Google Scholar]
- Pearson JN, Jakobsen I (1993) The relative contribution of hyphae and roots to phosphorus uptake by arbuscular mycorrhizal plants, measured by dual labelling with 32P and 33P. New Phytol 124: 489–494 [Google Scholar]
- Phillips JM, Hayman JD (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhiza fungi for rapid assessment of infection. Trans Br Mycol Soc 55: 158–161 [Google Scholar]
- Polley HW, Johnson HB, Marino BD, Mayeux HS (1993) Increase in C3 plant water-use efficiency and biomass over glacial to present CO2 concentrations. Nature 361: 61–64 [Google Scholar]
- Prior SA, Runion GB, Marble SC, Rogers HH, Gilliam CH, Torbert HA (2011) A review of elevated atmospheric CO2 effects on plant growth and water relations: implications for horticulture. HortScience 46: 158–162 [Google Scholar]
- Procter AC, Ellis JC, Fay PA, Polley HW, Jackson RB (2014) Fungal community responses to past and future atmospheric CO2 differ by soil type. Appl Environ Microbiol 80: 7364–7377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy W, Taylor TN, Hass H, Kerp H (1994) Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA 91: 11841–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage RF, Coleman JR (2001) Effects of low atmospheric CO2 on plants: more than a thing of the past. Trends Plant Sci 6: 18–24 [DOI] [PubMed] [Google Scholar]
- Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90: 1055–1062 [DOI] [PubMed] [Google Scholar]
- Shipley B. (2009) Confirmatory path analysis in a generalized multilevel context. Ecology 90: 363–368 [DOI] [PubMed] [Google Scholar]
- Sikes BA, Powell JR, Rillig MC (2010) Deciphering the relative contributions of multiple functions within plant-microbe symbioses. Ecology 91: 1591–1597 [DOI] [PubMed] [Google Scholar]
- Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182: 347–358 [DOI] [PubMed] [Google Scholar]
- Smith SE, Read D (2008) Mycorrhizal Symbiosis. Academic Press, London [Google Scholar]
- Tissue DT, Griffin KL, Thomas RB, Strain BR (1995) Effects of low and elevated CO2 on C3 and C4 annuals. II. Photosynthesis and leaf biochemistry. Oecologia 101: 21–28 [DOI] [PubMed] [Google Scholar]
- Treseder KK, Egerton-Warburton ML, Allen FM, Cheng Y, Oechel CW (2003) Alteration of soil carbon pools and communities of mycorrhizal fungi in chaparral exposed to elevated carbon dioxide. Ecosystems (N Y) 6: 786–796 [Google Scholar]
- Ward JK, Kelly JK (2004) Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecol Lett 7: 427–440 [Google Scholar]
- Ward JK, Strain BR (1999) Elevated CO2 studies: past, present and future. Tree Physiol 19: 211–220 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang R, Tang J, Yang H, Hu S, Chen X (2010) Positive feedback between mycorrhizal fungi and plants influences plant invasion success and resistance to invasion. PLoS ONE 5: e12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.