The physiological and proteomic profiles induced during iron limitation indicate a trade-off between growth and susceptibility to oxidative stress in a marine diatom.
Abstract
Diatoms are single-celled, photosynthetic, bloom-forming algae that are responsible for at least 20% of global primary production. Nevertheless, more than 30% of the oceans are considered “ocean deserts” due to iron limitation. We used the diatom Phaeodactylum tricornutum as a model system to explore diatom’s response to iron limitation and its interplay with susceptibility to oxidative stress. By analyzing physiological parameters and proteome profiling, we defined two distinct phases: short-term (<3 d, phase I) and chronic (>5 d, phase II) iron limitation. While at phase I no significant changes in physiological parameters were observed, molecular markers for iron starvation, such as Iron Starvation Induced Protein and flavodoxin, were highly up-regulated. At phase II, down-regulation of numerous iron-containing proteins was detected in parallel to reduction in growth rate, chlorophyll content, photosynthetic activity, respiration rate, and antioxidant capacity. Intriguingly, while application of oxidative stress to phase I and II iron-limited cells similarly oxidized the reduced glutathione (GSH) pool, phase II iron limitation exhibited transient resistance to oxidative stress, despite the down regulation of many antioxidant proteins. By comparing proteomic profiles of P. tricornutum under iron limitation and metatranscriptomic data of an iron enrichment experiment conducted in the Pacific Ocean, we propose that iron-limited cells in the natural environment resemble the phase II metabolic state. These results provide insights into the trade-off between optimal growth rate and susceptibility to oxidative stress in the response of diatoms to iron quota in the marine environment.
Phytoplankton are single-celled photosynthetic microorganisms that live in the upper, illuminated layer of the oceans. Like plants in terrestrial ecosystems, phytoplankton are the basis of marine food webs. High turnover, estimated to be weekly based, due to high growth and death rates mediated mainly by availability of nutrients, grazing, and viral infections, make phytoplankton very responsive to climate change (Falkowski et al., 1998; Bidle and Falkowski, 2004; Chavez et al., 2011; Behrenfeld and Boss, 2014; Bidle, 2015). Diatoms dominate phytoplankton communities and are responsible for at least 20% of global primary production (Nelson and Brzezinski, 1997; Armbrust, 2009; Arrigo et al., 2012). They are abundant in well-mixed coastal, upwelling regions, and near the sea-ice edge, where they form massive blooms (Arrigo et al., 2012). Diatoms are thus central to the biogeochemical cycling of important nutrients such as carbon, nitrogen, iron, and silica (Field et al., 1998; Morel and Price, 2003).
Algal growth is highly dependent on iron (Fe) bioavailability (Behrenfeld and Kolber, 1999), due to its involvement in fundamental enzymatic and metabolic reactions such as photosynthesis (PSI, ferredoxin, cytochrome b6f), TCA cycle (aconitase, fumarase), and nitrate assimilation (nitrate and nitrite reductases; Milligan and Harrison, 2000; Behrenfeld and Milligan, 2013). Iron-limited diatoms have reduced chlorophyll content, photosynthetic efficiency (Greene et al., 1991; Marchetti and Harrison, 2007), and nitrate assimilation rate (Milligan and Harrison, 2000), all of which lead to limited growth rate. More than a decade of mesoscale open ocean experiments revealed that iron is limiting primary productivity in about 30% of the ocean ecosystems (Behrenfeld and Milligan, 2013; Moore et al., 2013). These observations support “the iron hypothesis,” which claims that iron is the limiting factor for phytoplankton blooms in high-nitrate, low-chlorophyll ocean areas (Behrenfeld et al., 1996, 2006); therefore, artificial addition of iron to these areas will induce blooms that would potentially activate the biological pump and reduce atmospheric CO2 (Martin, 1990). Natural and artificial iron enrichments lead to rapid increase in phytoplankton biomass, where addition of dissolved iron to a body of water with <0.05 nM iron led to diatom-dominated blooms and enhanced carbon export to the deep sea (Boyd et al., 2007; Marchetti et al., 2012; Smetacek et al., 2012; Xiu et al., 2014).
Diatoms developed diverse strategies to cope with low and fluctuating iron quota, such as high-affinity iron uptake systems, iron storage capacity, substitution of iron-containing proteins with nonferrous functional equivalents, and mechanisms to mitigate the risk of damage from reactive oxygen species (ROS) produced in the presence of this redox-active metal (Allen et al., 2008; Lommer et al., 2012; Marchetti et al., 2012; Morrissey and Bowler, 2012). Iron uptake systems in diatoms include a high-affinity reductive system, containing ferric reductase that dissociates Fe3+ from organic ligands, a multicopper oxidase that oxidizes the released Fe2+ to Fe3+, and an iron permease that receives Fe3+ for translocation across the cell membrane (Maldonado and Price, 2001; Maldonado et al., 2006; Kustka et al., 2007). Recently, a copper-independent, nonreductive mechanism was reported to directly bind and concentrate Fe3+ by Iron Starvation Induced Protein 2a (ISIP2a; Morrissey et al., 2014). After acquiring the iron, intracellular concentrations must be tightly regulated to avoid oxidative damage. Ferritin, an iron-storage protein used by plants, animals, and microorganisms to concentrate and store iron (Theil, 2004), is present in all diatom classes but is probably used to buffer iron concentrations and not for long-term storage (Marchetti et al., 2009; Groussman et al., 2015; Pfaffen et al., 2015). During iron limitation, diatoms can substitute the common iron-sulfur redox protein ferredoxin with flavodoxin, a functionally equivalent, non-iron-containing protein (La Roche et al., 1993; McKay et al., 1997, 1999; Erdner et al., 1999). Induction of carotenoids biosynthesis, tocopherols, dehydroascorbate, and alternative oxidase may all compensate for down-regulation of iron-containing antioxidants such as heme peroxidase, while proteorhodopsins may partially compensate for impaired iron-rich PSI (Allen et al., 2008; Marchetti et al., 2015).
Despite the advantage of iron-based metabolism, iron might be cytotoxic for living cells, as the production of harmful ROS such as hydroxyl radical can be triggered by free iron (Fe+2) in the Fenton/Haber-Weiss reaction (Kolthoff and Medalia, 1949). Therefore, iron uptake sequestration is tightly controlled to avoid the accumulation of potentially dangerous free iron in the cell. In contrast, iron limitation can lead to oxidative stress as a result of inefficient activity of the photosynthetic electron transport chain that uses iron as an essential cofactor and reduction in iron-containing antioxidants (Niyogi, 1999). In the centric diatom Thalassiosira pseudonana, iron limitation led to oxidative stress and further induction of hallmarks of programmed cell death (PCD; Thamatrakoln et al., 2012; Luo et al., 2014). In plants, long-term iron limitation can lead to H2O2 accumulation, indicating iron deficiency-induced oxidative stress (Le et al., 2016). Due to their toxic effects, ROS levels are tightly controlled by the cellular antioxidant network (Mittler et al., 2004). Iron is an important cofactor in numerous ROS-degrading enzymes (Fe-superoxide dismutase, superoxide reductase, and catalases; Ravet and Pilon, 2013), suggesting an important interplay between ROS and iron metabolism. Iron-based metabolism can make the cells more susceptible to oxidative stress, as high ROS level can damage Fe-S-containing proteins, impairing their biochemical activity and leading to the release of Fe+2, which can trigger the Fenton reaction. The aim of this study was to examine the effect of iron limitation on the response to oxidative stress in the model diatom Phaeodactylum tricornutum. We measured physiological parameters and conducted proteome profiling during different phases of iron limitation in which the susceptibility to oxidative stress changed markedly.
RESULTS
Physiological Response to Iron Starvation
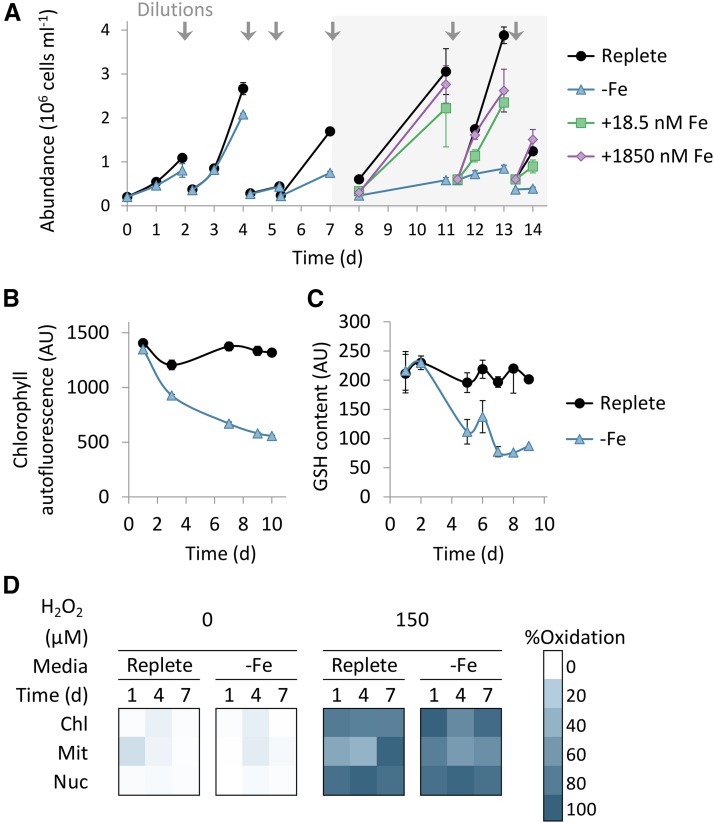
To investigate the effect of iron limitation on cell physiology and growth, we monitored P. tricornutum growth under iron limitation in the presence of the iron chelator desferrioxamine B (DFB) as described in the methods (Hutchins et al., 1999). Iron starvation reduced growth rate and chlorophyll autofluorescence per cell (Fig. 1, A and B), together with decrease in PSII photosynthetic efficiency, measured by variable fluorescence (Fv/Fm) and oxygen production rate (Supplemental Fig. S1, A and B). Respiration rates as measured by oxygen consumption in the dark were stable during the early stages, and significant reduction was found only on day 7 of iron limitation (Supplemental Fig. S1C). To examine how iron limitation affects antioxidant capacity, we monitored the cellular reduced glutathione (GSH) pool of the same cultures during iron limitation by the florescent stain Monochlorobimane, which stains reduced thiols of small molecules, and detected a decrease in the GSH pool (Fig. 1C). At day 7 of iron limitation, growth rates, chlorophyll autofluorescence per cell, GSH content, Fv/Fm, oxygen production, and respiration were 38%, 49%, 43%, 18%, 25%, and 3% of replete cultures, respectively (Fig. 1, A–C, Supplemental Fig. S1, A–C). Upon resupply of 1,850 nM (as in replete) or 18.5 nM Fe to iron-limited cultures at day 7, growth rates recovered within 4 d (Fig. 1A). Ten days following resupply of 1,850 nM or 18.5 nM Fe (day 17), GSH levels were 120% and 72% of cells under Fe-replete conditions, respectively, while under iron depletion, the GSH pool was 51% of replete (Supplemental Fig. S2). We further used P. tricornutum cells expressing the redox-sensitive GFP (roGFP) targeted to the chloroplast, mitochondria, or nucleus (Rosenwasser et al., 2014; Graff van Creveld et al., 2015) to examine the effect of iron limitation on the GSH redox potential. H2O2-dependent cell death was similar to wild-type cell death in the roGFP transformant lines (Supplemental Fig. S3). No remarkable changes in roGFP oxidation were detected in iron-limited cultures compared to control cells (<10% oxidation, P values between replete and iron limitation >0.09; Fig. 1D). A decrease in the GSH pool without oxidation of the roGFP probe suggests that the glutathione pool was smaller in size but was retained in a highly reduced state during 7 d of iron limitation.
Figure 1.
Physiological response to iron limitation in the model diatom P. tricornutum. A, Time course of growth of batch cultures incubated in replete Fe conditions (black circles, f/2 media, 1,850 nM), or in −Fe (blue triangles, f/2 without iron, with 1 µM DFB). After 7 d, Fe-limited cultures were supplemented with either18.5 nM (green squares) and 1,850 nM (purple diamonds). Arrows indicate culture dilutions into fresh media as described in the method section. Gray background represents iron resupply at day 7. B, Chlorophyll autofluorescence per cell measured by flow cytometry (ex: 488 nm, em: 665 nm) during iron limitation. C, Quantification of the reduced GSH pool per cell during iron limitation measured as monochlorobimane fluorescence using flow cytometry (ex: 405 nm, em: 455 nm). D, roGFP oxidation in the chloroplast (Chl), mitochondria (Mit), and nucleus (Nuc), during iron limitation and 30 min after addition of 0 and 150 µM H2O2. Oxidation was measured by flow cytometry (ex: 405 nm or 488 nm, em: 525 nm). In all experiments, flow cytometry analysis is based on fluorescent measurements of at least 5,000 cells per sample. Error bars represent SE of biological triplicates.
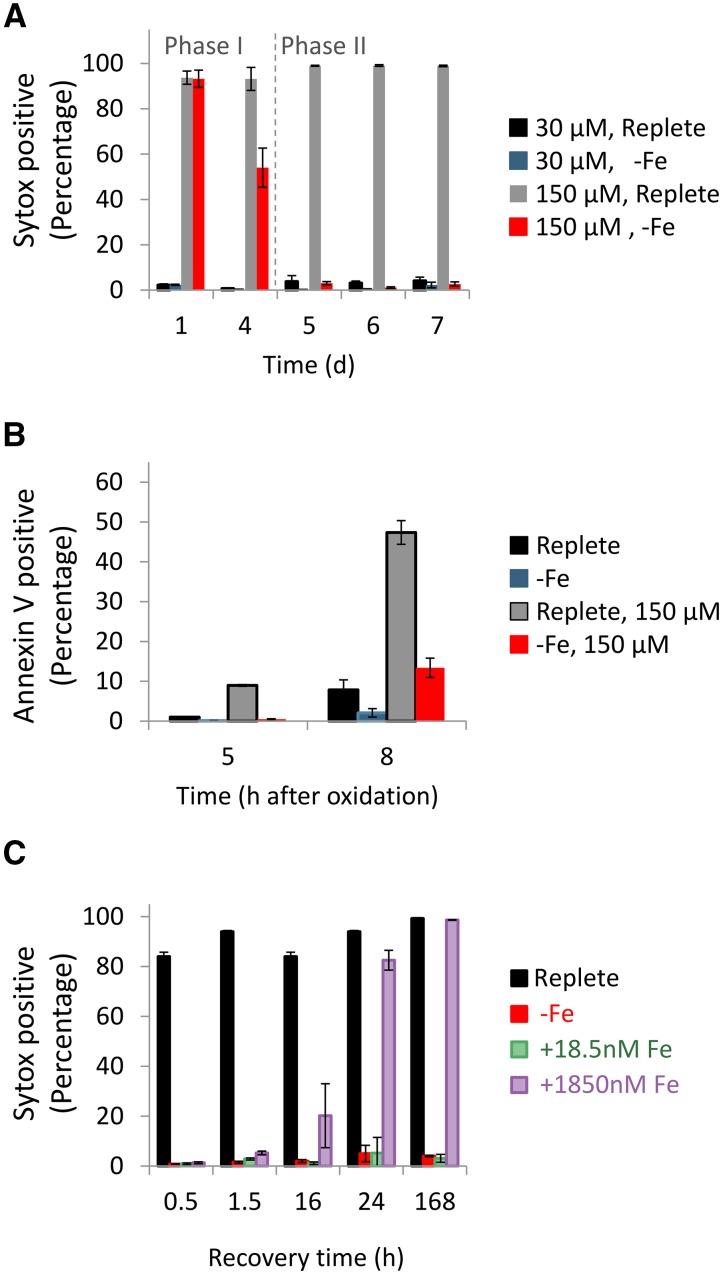
To examine how the observed decrease in the GSH pool may remodel the susceptibility to oxidative stress during iron limitation, we treated the cells with 30 or 150 µM H2O2 and measured cell death after 24 h by using Sytox Green DNA-binding fluorescent stain, which is used to assess membrane integrity and phosphatidyl-Ser externalization (Annexin V), both considered as a good proxy for induction of cell death (Graff van Creveld et al., 2015). No cell death was detected in response to 30 µM H2O2 in replete or in iron-limited cells, while application of 150 µM H2O2 induced cell death in both iron levels as measured by fluorescence markers for membrane permeability and phosphatidyl-Ser externalization in replete cultures (Fig. 2, A and B). Quantification of phosphatidyl-Ser externalization at day 6 of iron limitation exhibited 47% positive cells at 8 h post 150 µM H2O2 treatment (Fig. 2B); at this time point, there were <12% Sytox positive cells (Supplemental Fig. S4). Intriguingly, whereas the iron-limited cultures showed similar sensitivity to oxidative stress during the first 4 d of limitation, they exhibited a profound resistance to H2O2 from day 5 of iron limitation (Fig. 2A). This resistance to H2O2 treatment was transient, with 4% Sytox positive cells at 24-h posttreatment, but 92% Sytox positive cells within 48 h, as in the replete cells (Supplemental Fig. S5A). The same H202 treatment at day 6 of iron limitation led to 2% Sytox positive cells 24 h posttreatment, preceded to 37% and 97% Sytox positive cells at 48 and 102 h, respectively (Supplemental Fig. S5B). The observed delay in cell death suggests that the chronically iron-starved cells were in a metabolic and physiological state that enabled them to survive oxidative stress.
Figure 2.
Chronic iron limitation confers transient resistance to oxidative stress in the P. tricornutum. A, Cell death assessed by Sytox staining measured 24 h after treatment with 30 or 150 µM H2O2. The time (days) in the x axis represents the duration of iron limitation, similar to the time depicted in Figure 1. B, Phosphatidyl-Ser externalization measured by Annexin V stain at day 6 of iron limitation. Cells treated with 0 and 150 µM H2O2 were stained with Annexin V at 5 and 8 h posttreatment. C, Cell death assessed by Sytox positive cells, measured 24 h after treatment with 150 µM H2O2. Application of H2O2 was done 0.5, 1.5, 16, 24, and 168 h following iron resupply at day 7 of iron limitation. Flow cytometry analysis is based on fluorescent measurements of at least 5,000 cells per sample. Error bars represent SE of biological triplicates.
Accordingly, we defined two physiological phases in iron-limited cultures: “phase I” in which H2O2 sensitivity was similar to iron-replete cells (days 1–3 of iron limitation) and “phase II” in which chronically iron-limited cells (>5 d of iron limitation) acquired resistance to H2O2. Oxidation of organelle-specific roGFP in response to 150 µM H2O2 was comparable under replete Fe conditions and during phase I (day 1) and phase II (day 7) of iron limitation (Fig. 1D), indicating that H2O2 permeability and GSH oxidation in response to H2O2 in the specific organelles was not modified under iron limitation. To examine the reversibility and dependence of H2O2 susceptibility on iron availability, iron-limited cells (at day 7) were resupplied with 18.5 nM (1% of replete) or 1,850 nM (100% of replete) FeCl3 (Fig. 2C). Growth was resumed after 4 d of resupply at either 18.5 or 1,850 nM (Fig. 1A). We subsequently examined susceptibility to oxidative stress by treating the cells with H2O2 (150 µM) at 0.5, 1.5, 16, 24, and 168 h after iron resupply, and cell death was monitored 24 h posttreatment. Resupply of 1,850 nM Fe reversed the H2O2 susceptibility within 24 h, while following resupply of 18.5 nM Fe, cells remained H2O2 resistant for 168 h (Fig. 2C). In summary, resistance to oxidative stress in diatom cells was closely associated with low iron quota and availability.
Decoupling between Early Oxidation and Subsequent Induction of Cell Death under Chronic Iron Limitation
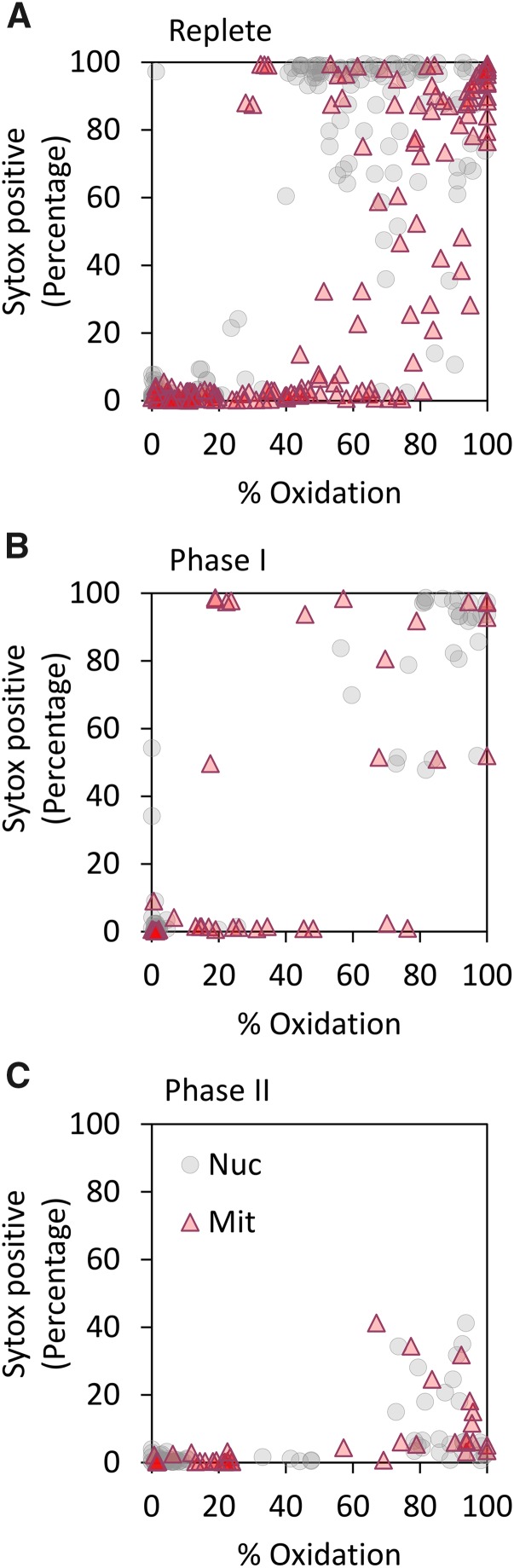
We previously demonstrated that early oxidation of mitochondrial roGFP, which represents the mitochondrial GSH redox potential, is an early perturbation event in the subsequent induction of a cell death biochemical cascade (Graff van Creveld et al., 2015). In line with these findings, we detected threshold levels in oxidation in the mitochondria (70%) or nucleus (40%) within 3 h of H2O2 treatment under iron-replete conditions. These thresholds were indicative of subsequent induction of PCD-like process 24 h posttreatment in replete cells (Fig. 3A). Iron-limited cells in phase I (days 1–3) displayed a similar trend (Fig. 3B). In contrast, no correlation between mitochondrial or nuclear roGFP oxidation and subsequent induction of cell death was detected in phase II (day 5 onward) iron-limited cells (Fig. 3C; Supplemental Table S1). Cells that were oxidized above threshold did not undergo cell death within 24 h, pointing toward a decoupling between early roGFP oxidation and subsequent induction of cell death. This indicates that cells under chronic iron limitation (phase II) exhibit a distinct metabolic state, characterized by transient resistance to H2O2.
Figure 3.
Decoupling between early oxidation and subsequent induction of cell death under chronic iron limitation. Scatter plots of degree roGFP oxidation in P. tricornutum cells targeted to the nucleus (3 h post H2O2 treatment, circles) and mitochondria (0.5 h post H2O2 treatment, triangles) in response to 0 to 150 µM H2O2, and level of cell death measured by Sytox positive cells 24 h posttreatment. A, Replete. B, Phase I (days 1–4 of iron limitation). C, Phase II (since day 5 of iron limitation). Each graph presents at least 130 samples.
Global Proteomic Profiling during Different Phases of Iron Limitation
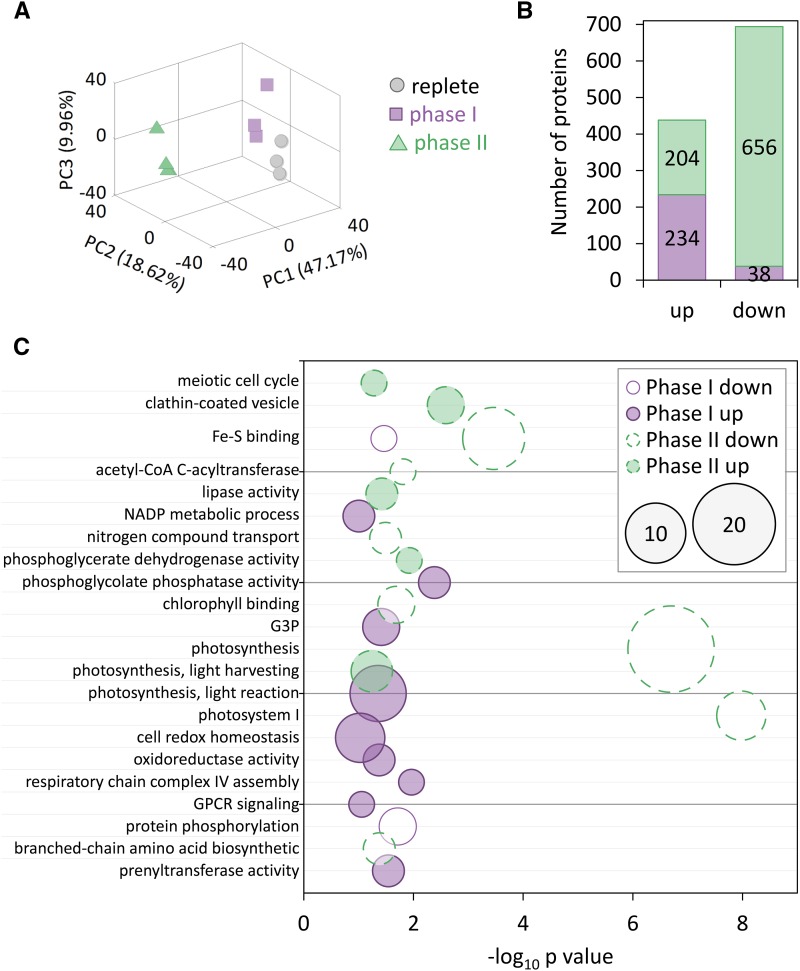
To better characterize the metabolic states of replete (day 3 of replete), phase I (day 3 of iron limitation), and phase II (day 5 of iron limitation) cells, we conducted a comparative liquid chromatography-mass spectrometry-based proteomic experiment, which provides protein expression profiles of cells in different iron limitation phases. Principle component analysis clearly shows that phase II (resistant to oxidative stress) is clustered distinctively from phase I and replete cultures (Fig. 4A). Of 2191 identified proteins, approximately 10% were up-regulated (fold change >1.5, P value < 0.05) in phase I (day 3 of iron limitation compared to the replete culture) or in phase II (day 5 compared to day 3 of iron limitation), while 2% and 30% of the identified proteins were down-regulated (fold change < −1.5, P value < 0.05) in phase I and phase II, respectively (Fig. 4B).
Figure 4.
Global proteomic profiling in P. tricornutum response to iron limitation. A, Principal component analysis of the proteomic data derived from Fe replete (replete, day 3, gray circles), phase I (day 3 of Fe limitation, purple squares), and phase II (day 5 of Fe- limitation, green triangles) cultures. B, Number of differentially expressed proteins (fold change < −1.5 or >1.5, P < 0.05) in phase I (purple, Fe-limited cells on day 3 compared to cells in Fe-replete on day 3) and phase II (green, Fe-limited cells on day 5 compared Fe-limited cells on day 3) cultures. C, Significantly enriched GO terms (hypergeometric test, P < 0.05, down-regulated < −1.5-fold change, up-regulated >1.5-fold change) related to each phase for up- or down-regulated proteins. x Axis represents −log10 of P value, size represents number of genes in each GO term.
Functional analysis of enriched biological terms was performed for each treatment using Gene Ontology (GO) analysis. In phase I (day 3 of iron limitation compared to replete culture), GO terms related to cell redox homeostasis, oxidoreductase activity, and NADP metabolic process (GO: 0045454, 0016668, 0006739) were significantly enriched (fold enrichments: 1.76, 3.59, and 2.61, respectively) in up-regulated proteins, while Fe-S binding and protein phosphorylation (GO: 0051537, 0006468) were enriched (fold enrichments: 6.69, 3.79) in down-regulated proteins (Fig. 4C; Supplemental Table S2). In phase II (day 5 compared to day 3 of iron limitation), proteins related to meiosis and vesicle trafficking (GO: 0051321, 0030136) were significantly enriched in up-regulated proteins. While photosynthesis, PSI, and Fe-S binding proteins (GO: 0015979, 0009522, 0051536) were significantly enriched in down-regulated proteins (Fig. 4C; Supplemental Table S2), light harvesting proteins (GO: 0009765) such as fucoxanthin chlorophyll binding protein (protein ID 219110471, 219129382) were up-regulated (Supplemental Table S4), which may mediate tolerance to photo-oxidative stress under iron-limited conditions (Lommer et al., 2012).
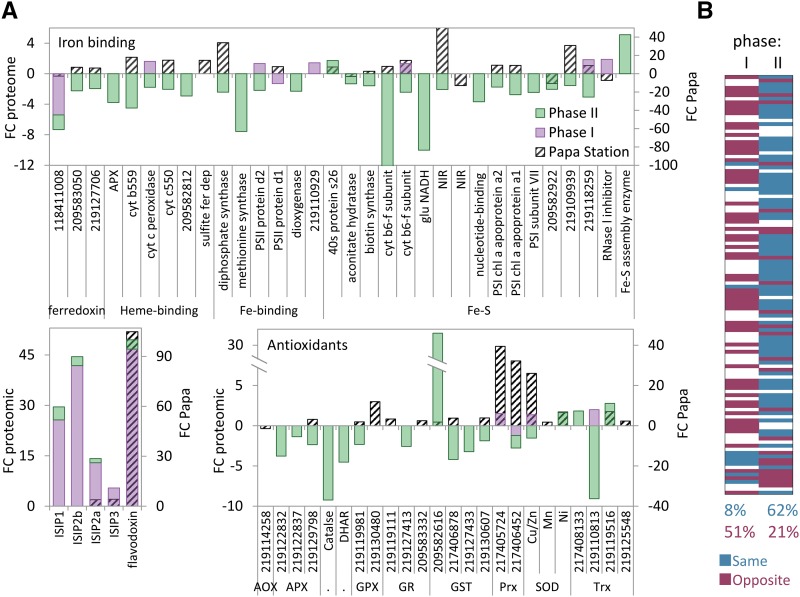
The ISIPs and the canonical iron starvation protein flavodoxin, which substitutes the iron-containing ferredoxin, were induced during iron limitation (Fig. 5A). These proteins were highly up-regulated in phase I and further induced in phase II, corroborating our experimental setup, indicating existence of iron-limitation already in phase I. Ferredoxins, heme binding, Fe binding, and Fe-S binding proteins (defined by GO terms) were down-regulated mainly in phase II (Fig. 5A; Supplemental Table S3).
Figure 5.
Comparison between P. tricornutum proteomic profiling under iron limitation and metatranscriptome study at Ocean Station Papa. A, Fold change of iron-responsive and antioxidant proteins in phase I (purple) and phase II (green). At 98 h following iron enrichment in Ocean Station Papa (striped black) is presented on the secondary y axis (Marchetti et al., 2012). Gene numbers represent P. tricornutum GI numbers. B, Gene-to-gene comparison of proteins present in both P. tricornutum phase I or phase II proteomic data (P value < 0.05), and Papa Station, total of 116 genes. Genes with the same trend (induced in iron limitation proteomics in P. tricornutum and reduced in iron fertilization metatranscriptome or the opposite) are marked in blue. Genes with the opposite trend (induced in iron limitation proteomics in P. tricornutum and in iron fertilization metatranscriptome or the opposite) are marked in red. Genes with fold change between −1.5 to 1.5 are marked in white.
ROS Metabolism under Iron Limitation
Since we could phenotypically categorize the different phases during iron-limitation based on susceptibility to oxidative stress, we were motivated to explore how proteins related to ROS metabolism were modulated during iron limitation in the two phases. Antioxidant proteins were generally unchanged or slightly up-regulated in phase I and down-regulated in phase II (Fig. 5A). While peroxiredoxin (Prx), Cu/Zn superoxide dismutase (Cu/Zn-SOD), and thioredoxin (Trx) were slightly up-regulated and 17 antioxidants were unchanged in phase I, 12 antioxidant enzymes were significantly down-regulated in phase II, with the exception of one glutathione-S-transferase (GST) and 2 Trxs (Fig. 5A; Supplemental Table S3). Interestingly, bacterial-like Ni-SOD was up-regulated in phase II (Fig. 5A; Supplemental Table S3). The general reduction in antioxidant capacity during phase II (Fig. 5A) and the major decrease in GSH content (Fig. 1C) emphasize the paradox in the observed resistance to oxidative stress, displayed by the cells in this phase of iron limitation.
To conclude, we detected two phases of response to iron limitation. In the first phase, we observed induction of cellular strategies to cope with iron deficiency (as ISIPs and flavodoxin) with no decrease in growth rate or photosynthetic efficiency, while in chronic iron limitation (phase II), we observed a decrease in growth rate, chlorophyll content, photosynthetic efficiency, and glutathione content and a large proteomic shift (about 40% of the proteome) that were coupled with resistance to oxidative stress.
Iron-Limited Cells in the Ocean Resemble Phase II Metabolic State
Based on various physiological parameters, response to oxidative stress, and proteome profiling, we defined two distinct cellular states representing early (phase I) and late (phase II) cellular responses to iron limitation. We were encouraged to investigate whether any of the phases is reflected in natural diatom populations in the ocean. We compared the P. tricornutum proteome with metatranscriptomic data from Ocean Station Papa in the Pacific Ocean (50°N, 145°W), where iron enrichment (addition of 4 nM to oceanic water with <0.05 nM Fe) led to induction of phytoplankton growth dominated by diatoms of the genera Pseudo-nitzschia and Fragilariopsis (Marchetti et al., 2012). A recent study described a coordinated transcriptome and proteome patterns in diatom response to phosphorus limitation (Dyhrman et al., 2012). Nevertheless, since quantitative proteomics and transcriptomics data are not necessarily comparable (Bertrand et al., 2012), we examined the trend similarities between the proteome and the metatranscriptome studies by comparing between lists of differentially expressed proteins and transcripts (up- or down-regulation). This qualitative comparative approach can provide a straightforward way to determine the similarity in expression profiles between datasets retrieved using different methodologies. Diatom-specific gene expression profiles were retrieved from Marchetti and co-workers (Marchetti et al., 2012) and compared to the P. tricornutum proteome using best hit blast. A total of 1,190 proteins were detected in both datasets. For example, Fe-containing ferredoxins, which were down-regulated in the iron-limited proteome, were induced in iron resupply metatranscriptome as expected; however, Fe-free flavodoxin, which is known to replace ferredoxin under low-iron conditions, was further induced upon iron resupply (Fig. 5A). Interestingly, 98 h upon iron resupply, diatoms rely on Fe-free flavodoxin and plastocyanin and not on Fe-containing ferredoxin and cytochrome C6, despite the existence of the genes encoding for Fe-containing enzymes in the genome of the associated diatoms species (Marchetti et al., 2012). These data raise fundamental questions about diatoms’ response to variation in iron quota and suggest possible benefits of the iron-free metabolic state. Interestingly, ISIP2a, ISIP3, and flavodoxin were also induced upon iron enrichment (Fig. 5A). A general induction of antioxidant proteins was detected as well, for example, ascorbate peroxidase, glutathione peroxidase, glutathione reductase, GST, Prx, and SOD were induced in the environmental iron enrichment (Fig. 5A), indicating the importance of antioxidants enzymes in iron-rich environment.
Venn diagrams showing iron limitation-induced proteins (defined as proteins with fold change >1.5 in phase I or phase II, or genes with fold change < −1.5 in Papa Station), and iron limitation down-regulated proteins (defined as proteins with fold change < −1.5 in phase I or phase II, or genes with fold change >1.5 in Papa Station) suggest that iron limitation in the ocean is more similar to phase II than to phase I in culture experiments (Supplemental Fig. S6). Of 1,192 proteins down-regulated in phase I, phase II, or Papa Station, 8 were common between phases I and II, 11 between phase I and Papa Station, and 284 between phase II and Papa Station (Supplemental Fig. S6). For example, ascorbate peroxidase, glutathione peroxidase, GST, and Cu/Zn-SOD are down-regulated in response to iron limitation in phase II and Papa Station, but not in phase I (Fig. 5A). Gene-to-gene comparison of 116 genes that were significantly altered (P value < 0.05, fold change either >1.5 or < −1.5) in phase I and phase II and had homologous genes in Papa Station metatranscriptome revealed that in phase I, 8% of the genes respond in the same trend as in Papa Station (either induced in phase I and down-regulated in Papa Station or vice versa), while 62% of the genes exhibited the same trend in phase II and Papa Station (Fig. 5B). Of 51 iron-binding encoded proteins identified in the P. tricornutum proteome, 31 were identified in the metatranscriptome as well, 5 were down-regulated, and 26 were up-regulated upon iron resupply, indicating that chronic iron limitation conditions led to major remodeling of diatoms’ metabolism under low-iron quota that is similar to phase II in our laboratory experiments.
DISCUSSION
The increase in abundance of iron-containing proteins during evolution, despite the decrease in iron bioavailability due to ocean oxygenation, points at an advantage of iron-based metabolism in promoting cellular growth (David and Alm, 2011). Iron is an ideal transition metal for redox reactions in biological systems during photosynthesis, respiration, and diverse enzymatic reactions involved in nitrogen assimilation, pigment synthesis, fatty acid saturation, and DNA synthesis. Nevertheless, a recent metatranscriptomic experiment suggests a possible benefit of low-iron metabolism, where a fraction of the metatranscriptome-encoded proteins remained iron free, although iron was added to the seawater samples (Marchetti et al., 2012). By examining sensitivity to oxidative stress, during iron limitation in parallel to protein expression profiles, we detected two distinct metabolic phases in the response of diatoms to iron limitation. While phase I is characterize by up-regulation of several antioxidant proteins and sensitivity to oxidative stress, phase II cells exhibited resistance to oxidative stress along with a decrease in the cellular GSH pool, antioxidant proteins, iron-containing proteins, photosynthetic efficiency, respiration rate, and growth rate. Importantly, our data indicate that the differential sensitivity to oxidative stress between phase I and phase II was due to neither differential H2O2 permeability nor differential oxidization of the GSH pool, which was highly comparable between the two phases (Fig. 1D). These results suggest a tradeoff between high growth rates, achieved by the iron-based metabolism, and resistance to environmental stress conditions (i.e. high light, nitrogen limitation, and infochemicals). These conditions can induce organelle specific oxidative stress that is comparable to external application of H2O2 (Rosenwasser et al., 2014; Graff van Creveld et al., 2015). Furthermore, early oxidation of mitochondrial roGFP was found to predict subsequent cell death in cells under natural environmental stress conditions and in response to H2O2 (Fig. 3, A and B; Graff van Creveld et al., 2015). The only exception we detected so far is in the response of chronically iron-limited (phase II) cells to H2O2 (Fig. 3C). This implicates that diatom survival in response to external stress is defined by their internal iron quota.
The ability to donate and accept electrons makes iron not only a crucial cofactor in enzymes that catalyze redox reactions, but also a producer of oxidative damage, especially when free Fe+2 react with H2O2 in the Fenton reaction. Consequently, oxidative stress and iron metabolism are tightly connected, and regulated cell death from iron-dependent oxidative injury was described as “ferroptosis” in mammalian cells (Dixon et al., 2012; Reed and Pellecchia, 2012). While iron chelators can directly prevent cell death in cancer and neurodegenerative diseases, the direct iron-containing, peroxide-sensitive target is still unknown (Antunes et al., 2001; Seiler et al., 2008; Weinreb et al., 2010; Hamacher-Brady et al., 2011; Dixon and Stockwell, 2014). To the best of our knowledge, ferroptosis was never described in photosynthetic organisms; nevertheless, free Fe2+ have a crucial role in plant PCD (Swidzinski et al., 2004). In contrast to mammalian cells, many diatoms are adapted to low-iron environments and have large internal storage of iron (Sutak et al., 2012). In this light, it is not surprising that diatoms resistant to oxidative stress are observed only in long-term, chronic iron limitation and following application of a strong chelator, when the internal storage of iron is completely depleted.
Due to its high reactivity, iron homeostasis has to be tightly controlled, restricting iron availability only to specific targeted proteins (Finney and O’Halloran, 2003). To avoid the toxicity of free iron, ferritin is used in all kingdoms to safely store iron (Andrews et al., 1992). In plants, ferritin is crucial to utilize iron while avoiding iron-induced oxidative damage and toxicity (Ravet and Pilon, 2013). In the absence of iron, the ferritin mutant plants do not exhibit any macroscopic phenotype but have elevated ROS levels and display induction of antioxidants. However, under high iron conditions, wild-type plants induced growth, while the ferritin mutant is overwhelmed by oxidative damage (Ravet et al., 2009). Ferritin was found in all diatom classes (Groussman et al., 2015), and it is important in maintaining high growth rates in sporadic availability of iron (Marchetti et al., 2009; Pfaffen et al., 2013). Intriguingly, the role of the diatom ferritin is probably to buffer the iron quota rather than to store it, as this ferritin is not optimized for iron mineralization, enabling rapid release of iron when it is required (Pfaffen et al., 2015).
Upon iron limitation, algae (red and green linages) and cyanobacteria replace the iron-containing ferredoxin with the iron-free flavodoxin, which is absent in genomes of land plants (Tognetti et al., 2006). Flavodoxin and especially the ratio between flavodoxin and ferredoxin were suggested as in situ markers for iron limitation in the marine environment, due to induction of flavodoxin in iron limitation and its down-regulation upon iron resupply (La Roche et al., 1995, 1996; Erdner et al., 1999; Chappell et al., 2015). Ectopic expression of flavodoxin in land plants not only improves fitness during iron starvation but also confers multiple stress-tolerance, including tolerance to oxidative stress (Tognetti et al., 2006, 2007; Lodeyro et al., 2012). Part of the effect may be due to replacement of ferredoxin functions, as ferredoxin is down-regulated under diverse stress conditions. Nevertheless, overexpression of ferredoxin did not induce stress tolerance as flavodoxin did (Ceccoli et al., 2011). The induction in flavodoxin expression during iron limitation cannot by itself explain the H2O2 resistance that was detected in phase II, as flavodoxin was highly induced already during phase I, and the decrease in ferredoxin was mainly in phase I (Fig. 5A). Remarkably, in the metatranscriptomic experiment, 4 d after iron enrichment there was some induction of ferredoxin and 10-fold higher induction in flavodoxin (Fig. 5A). High flavodoxin levels may contribute to general stress tolerance or as cellular preparation for fluctuating iron availability in the ocean. Another possible explanation is that 4 d following iron enrichment (4 nM Fe), iron is mainly consumed by nitrogen assimilation pathway and photosynthesis proteins, therefore hindering induction of ferredoxin (Marchetti et al., 2012). This the strength of the proteomic approach in increasing the detection resolution of the cellular response to varying iron concentrations which enabled better characterization of the two phases of iron limitation.
In addition to the regulation of iron homeostasis in the cells, the cellular antioxidant network that is composed of antioxidant enzymes and small molecules serves in detoxifying harmful ROS and prevents their interaction with free iron. Indeed, in phase II of iron limitation, we detected a general decrease in antioxidant proteins such as ascorbate peroxidase, catalase, dehydroascorbate reductase, glutathione peroxidase, glutathione reductase, and Prx (Fig. 5A), suggesting the role of these antioxidants in iron-replete environments. Up-regulation of antioxidant proteins, such as glutathione peroxidase, Prx, Trx, and SODs, was detected following iron enrichment in Ocean Station Papa (Fig. 5A). In contrast, few antioxidants were up-regulated in chronic iron limitation, including two Trxs, one of four GSTs, and Ni-SOD (Fig. 5A). Although two Trxs are induced in phase II, Trx reductase, which is essential to reduce Trx, is induced in phase I and down-regulated in phase II (fold changes 2.1, −7.4 respectively; P values < 0.05; Supplemental Table S4). The specific induction of one GST may induce glutathionylation and protection of specific target proteins that are crucial for the resistance to oxidative stress. However, as there is no complete growth arrest in phase II, there is still a demand for antioxidant enzymes to detoxify normal byproducts of photosynthesis and respiration.
Diatoms under iron limitation were shown to substitute Fe-SOD with other Fe-free isoforms to minimize oxidative stress under low iron availability (Allen et al., 2008). In our proteome, we identified three SODs: Cu/Zn-SOD, Ni-SOD, and Mn/Fe-SOD (Supplemental Table S3). Cu/Zn-SOD exhibited down-regulation in phase II (fold change −1.53, P value 0.002), while the Ni-SOD was induced in phase II (fold change 1.63, P value 0.045). Ni-SOD is prevalent in open ocean species, where iron concentrations are low, and is considered to be acquired via horizontal gene transfer, concomitant to the loss of Fe-SOD (Dupont et al., 2008, 2010).
Comparison of P. tricornutum proteome to a metatranscriptome of a naturally iron-limited population (Marchetti et al., 2012) suggested that natural populations of diatoms exhibit a metabolic state that is more similar to phase II than to phase I of iron limitation (Fig. 5B). This suggests that diatom cells in natural low-iron conditions may benefit from low-iron metabolism by resistance to oxidative stress as in phase II of iron limitation (Fig. 2A). Remarkably, following resupply of a low amount of iron (18.5 nM), chronically iron-starved cells (phase II) can maintain high growth rates while still displaying resistance to oxidative stress (Figs. 1A and 2C), suggesting the existence of a metabolic state in which cells benefit from iron-based metabolism in parallel to resistance to stress conditions. In such conditions, when only low concentrations of iron are available, prioritizing the iron distribution between different metabolic pathways would be critical. Indeed, based on metatranscriptomics analysis during iron enrichment in Ocean Station Papa, pennate diatoms (i.e. Pseudo-nitzschia and Fragilariopsis) probably shunt the iron to the nitrogen assimilation pathway, while other metabolic pathways were kept partially iron free (Marchetti et al., 2012). Notably, in P. tricornutum, up-regulation of ferritin was detected (Supplemental Table S3), enabling safe iron storage. In agreement, upon iron resupply to the ocean, the blooming species are usually pennate diatoms that encode for ferritin (Marchetti et al., 2012; Morrissey and Bowler, 2012; Groussman et al., 2015). As ferritin is crucial for iron utilization and avoiding iron toxicity, it is important to note that some pennate, and many centric diatoms, do not have the gene encoding for ferritin (Groussman et al., 2015). These diatoms may be subjected to high oxidative damage in response to natural or artificial iron enrichments. Taken together, these metabolic and physiological consequences should be considered when suggesting to artificially fertilize the ocean with iron in order to enhance biological productivity and remove atmospheric CO2. Here, we revealed a possible cost of iron-based metabolism as high sensitivity to oxidative stress. While the scale of the iron-induced blooms and the export to the deep ocean are widely investigated, our data suggest that physiological tradeoff and metabolic remodeling should be further investigated in higher resolution. Defining the physiological and metabolic states under iron limitation, based on hundreds of coexpressed proteins, provides a sensitive systems approach to reveal a gradient in phenotypic strategies employed by marine algae in response to stress in the marine environment.
MATERIALS AND METHODS
Culture Growth
Phaeodactylum tricornutum, accession Pt1 8.6 (CCMP2561 in the Provasoli-Guillard National Center for Culture of Marine Phytoplankton) cells were batch grown in f/2 medium at 18°C under a 16-h-light:8-h-dark cycle and light intensity of 80 μmol photons·m−2·s−1 supplied by cool-white LED lights. Exponentially growing cells (1·106 cells mL−1) were harvested by centrifugation (4000 g, 10 min), washed three times by resuspension in 0.2 µm filtered and autoclaved seawater and centrifugation, and used to inoculate triplicate cultures of either replete f/2 medium (replete) or f/2 medium without added iron and with the efficient iron chelator, 1 µM DFB (Fe-limited, or −Fe) at a cell concentration of 2·105 cells·mL−1. DFB may compete with cell surface binding sites for available Fe2+ (Shaked et al., 2005). To keep iron as the only limiting factor, we diluted the cultures every few days. Dilutions were done into fresh media with the same properties (replete or −Fe media), cells were counted prior to dilution to dilute replete and −Fe cells to the same cell density. Seawater used for media formulations was collected from coastal Israel Mediterranean Sea, and iron concentration was <0.005 mg·l−1.
Iron resupply: cultures were centrifuged (4000 g, 10 min), washed three times in filtered and autoclaved seawater, and “−Fe” cultures were divided into three: one part was resuspended in replete f/2 media (+Fe, 1,850 nM), a second was resuspended in f/2 media with 18.5 nM iron (+Fe, 18.5 nM), and a third part was kept without iron, with DFB (−Fe). “Replete” cultures were resuspended in replete f/2 media.
Samples were removed daily and monitored for cell abundance, photosynthetic efficiency, respiration, chlorophyll autofluorescent per cell, GSH contents, roGFP oxidation, and sensitivity to oxidation stress.
Monitoring Physiological Response to Iron Limitation
Cell abundance was determined using the Multisizer 4 Coulter Counter (Beckman Coulter, Fullerton, CA).
Oxygen evolution and respiration was measured using a Clark-type oxygen electrode at 20°C (Oxy-Lab, Hansatech Instruments, King’s Lynn, UK). For each measurement, cells were concentrated by 10-min centrifugation at 4,000 g to a final concentration of 107 cells·mL−1 and dark-adapted for 20 min. Respiration was measured in the dark andO2 evolution was measured at 1,000 μmol photon·m−2·s −1 provided by light-emitting diodes with an emission maximum around 650 nm.
Photosynthetic efficiency was measured using an imaging PAM system (Heinz Walz). Cells were concentrated by 10-min centrifugation at 4,000 g to a final concentration of 107 cells·mL−1 and were dark-adapted for 20 min. Photosynthetic efficiency was determined as Fv/Fm, calculated according to Maxwell and Johnson, 2000, where Fm is the maximum fluorescence emission level in the dark measured with a saturating pulse of light (emission peak at 450 nm, 2,700 μmol photons·m−2·s−1, 800 ms) and Fv = Fm − F0. GSH content was measured by the fluorescence dye Monochlorobimane (Sigma), solubilized in dimethyl sulfoxide (DMSO) at final concentration of 1 µM. Samples were incubated at room temperature in the dark for 40 min before analysis by flow cytometry (ex: 405 nm, em: 455/50 nm). Leakage of chlorophyll autofluorescence to the blue channel was subtracted by measurement of unstained samples.
Degree of oxidation was measured using transformant lines expressing roGFP in the chloroplast, mitochondria, or nucleus as described in Rosenwasser et al. (2014) and Graff van Creveld et al. (2015). All the experiments were preformed simultaneously in wild-type and roGFP expressing lines, and all the examined physiological parameters such as growth rates, chlorophyll auto fluorescence per cell, and H2O2 resistance (e.g. Sytox green stain for cell death assay) were similar between the wild-type and roGFP expressing transformants. Basically, roGFP was measured by flow cytometry in the green channel (525/50 nm) following excitation at 405 nm (oxidized) and 488 nm (reduced), and leakage of autofluorescence to the green channel was subtracted by measurement of wild-type cells in parallel to the roGFP transformants. RoGFP degree of oxidation was calculated based on fluorescent ratios (ex: 405/488, em:525) calibrated against fully oxidized (H2O2) and fully reduced (DTT) roGFP samples as described in Graff van Creveld et al. (2015).
Membrane permeability was determined by Sytox Green (Invitrogen) at a final concentration of 1 µM. Samples were incubated at room temperature in the dark for 30 min prior to measurement. Phosphatidyl-Ser externalization was estimated using Annexin-V-Alexa Fluor 488 (Invitrogen). Then 106 cells were harvested via centrifugation, washed in cold PBS, resuspended in 150 µL of Annexin binding buffer (10 mm HEPES, 140 mm NaCl, 2.5 mm CaCl2, pH 7.4), and stained with 7.5 µL of Annexin V for 15 min at room temperature in the dark, after which 600 µL of Annexin binding buffer was added. After staining, cells were pelleted via centrifugation, washed once with PBS, and measured. Sytox Green and Annexin V staining were measured by flow cytometry in the green channel (ex: 488 nm, em: 525 nm).
All flow cytometry measurements (chlorophyll autofluorescent per cell, GSH contents, Sytox, Annexin V stains, and roGFP oxidation) were obtained using the Eclipse iCyt flow cytometer (Sony Biotechnology Inc., Champaign, IL), equipped with 405-nm and 488-nm solid-state, air-cooled lasers, both with 25 mW on the flow cell and with standard filter set-up. Each experiment was repeated at least three times and 5,000 cells were measured per sample.
Protein Extraction
Proteins were extracted from P. tricornutum cells at day 3 of replete and day 3 and 5 of iron limitation, using 10% TCA-acetone. For protein precipitation, cells were collected by centrifugation of 250-mL cultures (10 min, 4,000 g, 4°C) and resuspended in 500 µL of 10% TCA-acetone. Then, cells were sonicated gently and kept at –20°C for 1 h. After centrifugation (10 min, 10,000 g), the supernatant was discarded and the pellet was washed twice with 5% TCA-acetone and stored at –20°C for 1 h. Precipitated proteins were collected by centrifugation (10 min, 10,000 g, 4°C) and the pellet was dried under nitrogen flow. Pellets were solubilized using 8 m urea (Sigma). Samples were then diluted to 1.6 m urea and proteins reduced using 5 mm DTT (Sigma) for 30 min in 56°C. This was followed by alkylation using 10 mM iodoacetamide for 30 min in the dark at room temperature. Trypsin was then added at a ratio of 50:1 (protein:trypsin) at 37°C overnight. Digestion was stopped using 1% formic acid. Samples were desalted using Oasis HLB (Waters). Samples were frozen in –80°C until mass spectrometry analysis.
Liquid Chromatography
Ultra performance liquid chromatography/mass spectrometry (UPLC/MS) grade solvents were used for all chromatographic steps. Each sample was loaded using splitless nano-Ultra Performance Liquid Chromatography (10 kpsi nanoAcquity; Waters, Milford, MA) in high-pH/low-pH reversed phase 2-dimensional liquid chromatography mode. Then 15 μg of digested protein from each sample was loaded onto a C18 column (XBridge, 0.3 × 50 mm, 5-μm particles, Waters). The following two buffers were combined: (A) 20 mm ammonium formate, pH 10, and (B) acetonitrile. Peptides were released from the column using a step gradient: 6.9% B, 10.4% B, 12.1% B, 13.5% B, 14.7% B, 15.9% B, 17.3% B, 18.8% B, 20.9% B, and 65% B. Each fraction flowed directly to the second dimension of chromatography. The buffers used in the low-pH reversed phase were: (A) H2O + 0.1% formic acid and (B) acetonitrile + 0.1% formic acid. Desalting of samples was performed online using a reverse-phase C18 trapping column (180-µm i.d., 20-mm length, 5-µm particle size, Waters). Then the peptides were separated using a C18 HSS T3 nano-column (75-µm i.d., 150-mm length, 1.8-µm particle size, Waters) run at 0.4 µL·min−1. Finally, peptides were eluted from the column and loaded onto the mass spectrometer using the following protocol: 3% to 30% B over 60 min, 30% to 95% B over 5 min, 95% maintained for 7 min (and then back to initial conditions).
Mass Spectrometry
The nanoLC was coupled online through a nanoESI emitter (7-cm length, 10-mm tip; New Objective, Woburn, MA) to a quadrupole ion mobility time-of-flight mass spectrometer (Synapt G2 HDMS, Waters) tuned to 20,000 mass resolution (full width at one-half height). Data were acquired using Masslynx version 4.1 in HDMSE positive ion mode in which the quadrupole was set to transfer all ions. The ions were separated in the T-Wave ion mobility chamber and transferred into the collision cell. Collision energy was alternated from low to high throughout the acquisition time. In low-energy (MS1) scans, the collision energy was set to 5 eV, and this was ramped from 27 to 50 eV for high-energy scans. For both scans, the mass range was set to 50 to 2,000 D with a scan time set to 1 s. A reference compound (Glu-Fibrinopeptide B; Sigma) was infused continuously for external calibration using a LockSpray and scanned every 30 s.
Data Processing, Searching, and Analysis
Quantification of protein expression was conducted using MS1 intensity based label-free quantification as described in Levin et al. (2011). Raw data were imported into Rosetta Elucidator System version 3.3 (Rosetta Biosoftware, Seattle, WA). Elucidator was used for retention time alignment and extraction of MS1 feature intensities. In parallel, database searching was performed using ProteinLynx Global Server (IdentityE) version 2.5. Database searching was carried out using the Ion Accounting algorithm described by Li et al., 2009. Trypsin was set as the protease, one missed cleavage was allowed, and fixed modification was set to carbamidomethylation of cysteines. Variable modifications included oxidation of Met.
Data were searched against a P. tricornutum protein database that was built by combining three publically available protein databases: The National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/protein/?term=tricornutum), Doe Joint Genome Institute (http://genome.jgi.doe.gov/Phatr2/Phatr2.download.html), and TrEMBL (http://www.ebi.ac.uk/uniprot/database/download.html) as in Rosenwasser et al., 2014. A target-decoy strategy was performed using reversed sequences. The criteria for protein identification were set to minimum of three fragments per peptide, five fragments per protein, and minimum peptide score of 6.7, which corresponds to the false identification rate of 1%. The approach for setting the minimum identification score is based on reports by Keller et al. and termed Peptide Prophet (Keller et al., 2002; Nesvizhskii et al., 2003). Identifications were imported automatically into Elucidator for annotation of features, applying a “match between runs” approach of propagating identifications between samples. Protein quantification inference was conducted using the Hi-3 method (Silva et al., 2006). A Student’s t-test was used for statistical evaluation after logarithmic transformation of protein intensities. Fold changes were determined by dividing the arithmetic mean of the three biological replicates in each group. When a protein was detected in only one of the conditions, a fold change value of ±1,000 was assigned. All mass spectrometry data, including raw data, processed spectra, and identifications, have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner (Vizcaíno et al., 2013) with the dataset identifier PXD004694.
Protein Functional Annotation and Enrichment Analysis
GO enrichment was assessed utilizing the Ontologizer application (http://compbio.charite.de/contao/index.php/ontologizer2.html; Bauer et al., 2008).
Comparison to Metatranscriptomic Data
Diatom-derived expression datasets from Papa Station metatranscriptomes (Marchetti et al., 2012) were obtained from Adrian Marchetti to compare with the P. tricornutum proteome. Diatom sequences were retrieved using Uniport databases (http://www.uniprot.org/). To obtain orthologous protein pairs, these proteins were blasted against the P. tricornutum databases (Rosenwasser et al., 2014) using BlastP matched based on best hit best hit (e-value < 0.05).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Photosynthetic efficiency and respiration during iron limitation.
Supplemental Figure S2. GSH content during iron limitation and resupply.
Supplemental Figure S3. Cell death 24 h after H2O2 treatment as measured on roGFP transformant lines.
Supplemental Figure S4. Cell death assessed by Sytox staining, measured 5 and 8 h after treatment with 0 and 150 µM H2O2 at day 6 of iron limitation.
Supplemental Figure S5. Cell death following H2O2 treatment.
Supplemental Figure S6. Venn diagrams comparing between P. tricornutum proteomic profiles and Ocean Station Papa metatranscriptome during iron limitation and enrichment.
Supplemental Table S1. Number of samples in each cluster presented in Figure 3; “reduced and live,” “reduced and dead,” “oxidized and live,” or “oxidized and dead” in replete, phase I, and phase II.
Supplemental Table S2. Functional analysis of enriched biological terms was performed for phase I (day 3 of iron limitation compared to replete) and phase II (day 5 compared to day 3 of iron limitation) using GO. These data are visualized in Figure 4C.
Supplemental Table S3. Fold changes and P values in phase I, phase II, and 98 h following iron enrichment in Ocean Station Papa (Marchetti et al., 2012).
Supplemental Table S4. Fold changes and P values in phase I, Phase II, and 98 h following iron enrichment in Ocean Station Papa (Marchetti et al., 2012).
Supplementary Material
Acknowledgments
We are grateful to Adrian Marchetti and Ginger Armbrust for assistance with metatranscriptome datasets from Ocean Station Papa. We thank Prof. Ziv Reich and Dr. Anat Shperberg from the Department of Biomolecular Sciences at the Weizmann Institute of Science for their kind help with measuring respiration and oxygen evolution rates in our experimental setup. We thank Daniella Schatz for critical comments on the manuscript.
Glossary
- Cu/Zn-SOD
Cu/Zn superoxide dismutase
- DFB
desferrioxamine B
- GO
Gene Ontology
- GSH
reduced glutathione
- GST
glutathione-S-transferase
- PCD
programmed cell death
- Prx
peroxiredoxin
- roGFP
redox-sensitive GFP
- ROS
reactive oxygen species
- Trx
thioredoxin
References
- Allen AE, Laroche J, Maheswari U, Lommer M, Schauer N, Lopez PJ, Finazzi G, Fernie AR, Bowler C (2008) Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc Natl Acad Sci USA 105: 10438–10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SC, Arosio P, Bottke W, Briat JF, von Darl M, Harrison PM, Laulhère JP, Levi S, Lobreaux S, Yewdall SJ (1992) Structure, function, and evolution of ferritins. J Inorg Biochem 47: 161–174 [DOI] [PubMed] [Google Scholar]
- Antunes F, Cadenas E, Brunk UT (2001) Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem J 356: 549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbrust EV. (2009) The life of diatoms in the world’s oceans. Nature 459: 185–192 [DOI] [PubMed] [Google Scholar]
- Arrigo KR, Perovich DK, Pickart RS, Brown ZW, van Dijken GL, Lowry KE, Mills MM, Palmer MA, Balch WM, Bahr F, et al. (2012) Massive phytoplankton blooms under Arctic sea ice. Science 336: 1408. [DOI] [PubMed] [Google Scholar]
- Bauer S, Grossmann S, Vingron M, Robinson PN (2008) Ontologizer 2.0--a multifunctional tool for GO term enrichment analysis and data exploration. Bioinformatics 24: 1650–1651 [DOI] [PubMed] [Google Scholar]
- Behrenfeld MJ, Bale AJ, Kolber ZS, Aiken J, Falkowski PG (1996) Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature 383: 508–511 [Google Scholar]
- Behrenfeld MJ, Boss ES (2014) Resurrecting the ecological underpinnings of ocean plankton blooms. Annu Rev Mar Sci 6: 167–194 [DOI] [PubMed] [Google Scholar]
- Behrenfeld MJ, Kolber ZS (1999) Widespread iron limitation of phytoplankton in the south pacific ocean. Science 283: 840–843 [DOI] [PubMed] [Google Scholar]
- Behrenfeld MJ, Milligan AJ (2013) Photophysiological expressions of iron stress in phytoplankton. Annu Rev Mar Sci 5: 217–246 [DOI] [PubMed] [Google Scholar]
- Behrenfeld MJ, Worthington K, Sherrell RM, Chavez FP, Strutton P, McPhaden M, Shea DM (2006) Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature 442: 1025–1028 [DOI] [PubMed] [Google Scholar]
- Bertrand EM, Allen AE, Dupont CL, Norden-Krichmar TM, Bai J, Valas RE, Saito MA (2012) Influence of cobalamin scarcity on diatom molecular physiology and identification of a cobalamin acquisition protein. Proc Natl Acad Sci USA 109: E1762–E1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidle KD. (2015) The molecular ecophysiology of programmed cell death in marine phytoplankton. Ann Rev Mar Sci 7: 341–375 [DOI] [PubMed] [Google Scholar]
- Bidle KD, Falkowski PG (2004) Cell death in planktonic, photosynthetic microorganisms. Nat Rev Microbiol 2: 643–655 [DOI] [PubMed] [Google Scholar]
- Boyd PW, Jickells T, Law CS, Blain S, Boyle EA, Buesseler KO, Coale KH, Cullen JJ, de Baar HJW, Follows M, et al. (2007) Mesoscale iron enrichment experiments 1993-2005: synthesis and future directions. Science 315: 612–617 [DOI] [PubMed] [Google Scholar]
- Ceccoli RD, Blanco NE, Medina M, Carrillo N (2011) Stress response of transgenic tobacco plants expressing a cyanobacterial ferredoxin in chloroplasts. Plant Mol Biol 76: 535–544 [DOI] [PubMed] [Google Scholar]
- Chappell PD, Whitney LP, Wallace JR, Darer AI, Jean-Charles S, Jenkins BD (2015) Genetic indicators of iron limitation in wild populations of Thalassiosira oceanica from the northeast Pacific Ocean. ISME J 9: 592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez FP, Messié M, Pennington JT (2011) Marine primary production in relation to climate variability and change. Annu Rev Mar Sci 3: 227–260 [DOI] [PubMed] [Google Scholar]
- David LA, Alm EJ (2011) Rapid evolutionary innovation during an Archaean genetic expansion. Nature 469: 93–96 [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, et al. (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Stockwell BR (2014) The role of iron and reactive oxygen species in cell death. Nat Chem Biol 10: 9–17 [DOI] [PubMed] [Google Scholar]
- Dupont CL, Buck KN, Palenik B, Barbeau K (2010) Nickel utilization in phytoplankton assemblages from contrasting oceanic regimes. Deep Res Part I-Oceanographic Res Pap 57: 553–566 [Google Scholar]
- Dupont CL, Neupane K, Shearer J, Palenik B (2008) Diversity, function and evolution of genes coding for putative Ni-containing superoxide dismutases. Environ Microbiol 10: 1831–1843 [DOI] [PubMed] [Google Scholar]
- Dyhrman ST, Jenkins BD, Rynearson TA, Saito MA, Mercier ML, Alexander H, Whitney LP, Drzewianowski A, Bulygin VV, et al. (2012) The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS One 7: e33768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdner DL, Price NM, Doucette GJ, Peleato ML, Anderson DM (1999) Characterization of ferredoxin and flavodoxin as markers of iron limitation in marine phytoplankton. Mar Ecol Prog Ser 184: 43–53 [Google Scholar]
- Falkowski PG, Barber RT, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281: 200–207 [DOI] [PubMed] [Google Scholar]
- Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281: 237–240 [DOI] [PubMed] [Google Scholar]
- Finney LA, O’Halloran TV (2003) Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300: 931–936 [DOI] [PubMed] [Google Scholar]
- Graff van Creveld S, Rosenwasser S, Schatz D, Koren I, Vardi A (2015) Early perturbation in mitochondria redox homeostasis in response to environmental stress predicts cell fate in diatoms. ISME J 9: 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RM, Geider RJ, Falkowski PG (1991) Effect of iron limitation on photosynthesis in a marine diatom. Limnol Oceanogr 36: 1772–1782 [Google Scholar]
- Groussman RD, Parker MS, Armbrust EV (2015) Diversity and evolutionary history of iron metabolism genes in diatoms. PLoS One 10: e0129081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR (2011) Artesunate activates mitochondrial apoptosis in breast cancer cells via iron-catalyzed lysosomal reactive oxygen species production. J Biol Chem 286: 6587–6601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins D, Franck V, Brzezinski M, Bruland K (1999) Inducing phytoplankton iron limitation in iron-replete coastal waters with a strong chelating ligand. Limnol Oceanogr 44: 1009–1018 [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392 [DOI] [PubMed] [Google Scholar]
- Kolthoff IM, Medalia AI (1949) The reaction between ferrous iron and peroxides. I. Reaction with hydrogen peroxide in the absence of oxygen. J Am Chem Soc 71: 3777–3783 [Google Scholar]
- Kustka AB, Allen AE, Morel FMM (2007) Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatom. J Phycol 43: 715–729 [Google Scholar]
- Le CTT, Brumbarova T, Ivanov R, Stoof C, Weber E, Mohrbacher J, Fink-Straube C, Bauer P (2016) ZINC FINGER OF ARABIDOPSIS THALIANA12 (ZAT12) Interacts with FER-LIKE IRON DEFICIENCY-INDUCED TRANSCRIPTION FACTOR (FIT) Linking Iron Deficiency and Oxidative Stress Responses. Plant Physiol 170: 540–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin Y, Hradetzky E, Bahn S (2011) Quantification of proteins using data-independent analysis (MSE) in simple andcomplex samples: a systematic evaluation. Proteomics 11: 3273–3287 [DOI] [PubMed] [Google Scholar]
- Li GZ, Vissers JPC, Silva JC, Golick D, Gorenstein MV, Geromanos SJ (2009) Database searching and accounting of multiplexed precursor and product ion spectra from the data independent analysis of simple and complex peptide mixtures. Proteomics 9: 1696–1719 [DOI] [PubMed] [Google Scholar]
- Lodeyro AF, Ceccoli RD, Pierella Karlusich JJ, Carrillo N (2012) The importance of flavodoxin for environmental stress tolerance in photosynthetic microorganisms and transgenic plants. Mechanism, evolution and biotechnological potential. FEBS Lett 586: 2917–2924 [DOI] [PubMed] [Google Scholar]
- Lommer M, Specht M, Roy AS, Kraemer L, Andreson R, Gutowska MA, Wolf J, Bergner SV, Schilhabel MB, Klostermeier UC, et al. (2012) Genome and low-iron response of an oceanic diatom adapted to chronic iron limitation. Genome Biol 13: R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo CS, Liang JR, Lin Q, Li C, Bowler C, Anderson DM, Wang P, Wang XW, Gao YH (2014) Cellular responses associated with ROS production and cell fate decision in early stress response to iron limitation in the diatom Thalassiosira pseudonana. J Proteome Res 13: 5510–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MT, Allen AE, Chong JS, Lin K, Leus D, Karpenko N, Harris SL (2006) Copper-dependent iron transport in coastal and oceanic diatoms. Limnol Oceanogr 51: 1729–1743 [Google Scholar]
- Maldonado MT, Price NM (2001) Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae). J Phycol 37: 298–309 [Google Scholar]
- Marchetti A, Catlett D, Hopkinson BM, Ellis K, Cassar N (2015) Marine diatom proteorhodopsins and their potential role in coping with low iron availability. ISME J 9: 2745–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A, Harrison PJ (2007) Coupled changes in the cell morphology and elemental (C, N, and Si) composition of the pennate diatom Pseudo-nitzschia due to iron deficiency. Limnol Oceanogr 52: 2270–2284 [Google Scholar]
- Marchetti A, Parker MS, Moccia LP, Lin EO, Arrieta AL, Ribalet F, Murphy MEP, Maldonado MT, Armbrust EV (2009) Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 457: 467–470 [DOI] [PubMed] [Google Scholar]
- Marchetti A, Schruth DM, Durkin CA, Parker MS, Kodner RB, Berthiaume CT, Morales R, Allen AE, Armbrust EV (2012) Comparative metatranscriptomics identifies molecular bases for the physiological responses of phytoplankton to varying iron availability. Proc Natl Acad Sci USA 109: E317–E325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. (1990) Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography 5: 1–13 [Google Scholar]
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence--a practical guide. J Exp Bot 51: 659–668 [DOI] [PubMed] [Google Scholar]
- McKay RML, Geider RJ, LaRoche J (1997) Physiological and biochemical response of the photosynthetic apparatus of two marine diatoms to fe stress. Plant Physiol 114: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RML, La Roche J, Yakunin AF, Durnford DG, Geider RJ (1999) Accumulation of ferredoxin and flavodoxin in a marine diatom in response to Fe. J Phycol 35: 510–519 [Google Scholar]
- Milligan AJ, Harrison PJ (2000) Effects of non-steady-state iron limitation on nitrogen assimilatory enzymes in the marine diatom Thalassiosira weissflogii (bacillariophyceae). J Phycol 36: 78–86 [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, Boyd PW, Galbraith ED, Geider RJ, et al. (2013) Processes and patterns of oceanic nutrient limitation. Nat Geosci 6: 701–710 [Google Scholar]
- Morel FMM, Price NM (2003) The biogeochemical cycles of trace metals in the oceans. Science 300: 944–947 [DOI] [PubMed] [Google Scholar]
- Morrissey J, Bowler C (2012) Iron utilization in marine cyanobacteria and eukaryotic algae. Front Microbiol 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J, Sutak R, Paz-Yepes J, Tanaka A, Moustafa A, Veluchamy A, Thomas Y, Botebol H, Bouget FY, McQuaid JB, et al. (2015) A novel protein, ubiquitous in marine phytoplankton, concentrates iron at the cell surface and facilitates uptake. Curr Biol 25: 364–371 [DOI] [PubMed] [Google Scholar]
- Nelson D, Brzezinski M (1997) Diatom growth and productivity in an oligotrophic midocean gyre: A 3-yr record from the Sargasso Sea near Bermuda. Limnol Oceanogr 42: 473–486 [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658 [DOI] [PubMed] [Google Scholar]
- Niyogi KK. (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Pfaffen S, Abdulqadir R, Le Brun NE, Murphy MEP (2013) Mechanism of ferrous iron binding and oxidation by ferritin from a pennate diatom. J Biol Chem 288: 14917–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffen S, Bradley JM, Abdulqadir R, Firme MR, Moore GR, Le Brun NE, Murphy MEP (2015) A diatom ferritin optimized for iron oxidation but not iron storage. J Biol Chem 290: 28416–28427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravet K, Pilon M (2013) Copper and iron homeostasis in plants: the challenges of oxidative stress. Antioxid Redox Signal 19: 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57: 400–412 [DOI] [PubMed] [Google Scholar]
- Reed JC, Pellecchia M (2012) Ironing out cell death mechanisms. Cell 149: 963–965 [DOI] [PubMed] [Google Scholar]
- La Roche J, Boyd PW, McKay RML, Geider RJ (1996) Flavodoxin as an in situ marker for iron stress in phytoplankton. Nature 382: 802–805 [Google Scholar]
- La Roche J, Geider RJ, Graziano LM, Murray H, Lewis K (1993) Induction of specific proteins in eukaryotic algae grown under iron-deficient, phosphorus-deficient, or nitrogen-deficient conditions. J Phycol 29: 767–777 [Google Scholar]
- La Roche J, Murray H, Orellana M, Newton J (1995) Flavodoxin expression as an indicator of iron limitation in marine diatoms. J Phycol 31: 520–530 [Google Scholar]
- Rosenwasser S, Graff van Creveld S, Schatz D, Malitsky S, Tzfadia O, Aharoni A, Levin Y, Gabashvili A, Feldmesser E, Vardi A (2014) Mapping the diatom redox-sensitive proteome provides insight into response to nitrogen stress in the marine environment. Proc Natl Acad Sci USA 111: 2740–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, et al. (2008) Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 8: 237–248 [DOI] [PubMed] [Google Scholar]
- Shaked Y, Kustka AB, Morel FMM (2005) A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol Oceanogr 50: 872–882 [Google Scholar]
- Silva JC, Gorenstein MV, Li GZ, Vissers JP, Geromanos SJ (2006) Absolute quantification of proteins by LCMSE: a virtue of parallel MS acquisition. Mol Cell Proteomics 5: 144–156 [DOI] [PubMed] [Google Scholar]
- Smetacek V, Klaas C, Strass VH, Assmy P, Montresor M, Cisewski B, Savoye N, Webb A, d’Ovidio F, Arrieta JM, et al. (2012) Deep carbon export from a Southern Ocean iron-fertilized diatom bloom. Nature 487: 313–319 [DOI] [PubMed] [Google Scholar]
- Sutak R, Botebol H, Blaiseau PL, Léger T, Bouget FY, Camadro JM, Lesuisse E (2012) A comparative study of iron uptake mechanisms in marine microalgae: iron binding at the cell surface is a critical step. Plant Physiol 160: 2271–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidzinski JA, Leaver CJ, Sweetlove LJ (2004) A proteomic analysis of plant programmed cell death. Phytochemistry 65: 1829–1838 [DOI] [PubMed] [Google Scholar]
- Thamatrakoln K, Korenovska O, Niheu AK, Bidle KD (2012) Whole-genome expression analysis reveals a role for death-related genes in stress acclimation of the diatom Thalassiosira pseudonana. Environ Microbiol 14: 67–81 [DOI] [PubMed] [Google Scholar]
- Theil EC. (2004) Iron, ferritin, and nutrition. Annu Rev Nutr 24: 327–343 [DOI] [PubMed] [Google Scholar]
- Tognetti VB, Palatnik JF, Fillat MF, Melzer M, Hajirezaei MR, Valle EM, Carrillo N (2006) Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell 18: 2035–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognetti VB, Zurbriggen MD, Morandi EN, Fillat MF, Valle EM, Hajirezaei M-R, Carrillo N (2007) Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc Natl Acad Sci USA 104: 11495–11500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno JA, Côté RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, et al. (2013) The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res 41: D1063–D1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Mandel S, Kupershmidt L, Youdim MBH (2010) Neuroprotective multifunctional iron chelators: from redox-sensitive process to novel therapeutic opportunities. Antioxid Redox Signal 13: 919–949 [DOI] [PubMed] [Google Scholar]
- Xiu P, Thomas AC, Chai F (2014) Satellite bio-optical and altimeter comparisons of phytoplankton blooms induced by natural and artificial iron addition in the Gulf of Alaska. Remote Sens Environ 145: 38–46 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.