Laticifer- and latex-deficient mutants identify multiple loci regulating laticifer differentiation, growth, and metabolic activity.
Abstract
Laticifer cells are specialized plant cells that synthesize and accumulate latex. Studies on laticifers have lagged behind in recent years, and data regarding the functional role of laticifers and their fitness benefit still remain elusive. Laticifer differentiation and its impact on plant growth and development also remain to be investigated. Here, cellular, molecular, and genetic tools were developed to examine the distribution, differentiation, ontogeny, and other characteristic features, as well as the potential developmental role of laticifer cells in the latex-bearing plant Euphorbia lathyris. The organization of the laticiferous system within the E. lathyris plant body is reported, emerging as a single elongated and branched coenocytic cell, constituting the largest cell type existing in plants. We also report the ontogeny and organization of laticifer cells in the embryo and the identification of a laticifer-associated gene expression pattern. Moreover, the identification of laticifer- and latex-deficient mutants (pil mutants) allowed for the identification of distinct loci regulating laticifer differentiation, growth, and metabolic activity. Additionally, pil mutants revealed that laticifer cells appear nonessential for plant growth and development, thus pointing toward their importance, instead, for specific ecophysiological adaptations of latex-bearing plants in natural environments.
In vascular plants, two prominent tubing systems, the tracheal-appearing xylem and the phloem, have been widely studied. Laticifer cells form an additional tubing system based on living cell(s). They occur throughout the Plantae, yet not as extensively as the xylem and phloem. Laticifers are specialized cells (or row of cells) that synthesize and accumulate latex (Fahn, 1990). The latex produced is highly variable in its chemical composition, not necessarily of a dense milky appearance but frequently white, and contains suspended colloids and carries a variety of dissolved solutes and macromolecules (Konno, 2011). According to Kekwick (2002), latex is produced in approximately 12,500 plant species, representing approximately 10% of all flowering plants (angiosperms), which belong to 900 genera of approximately 20 plant families that grow in a variety of ecological settings (Metcalfe, 1967; Lewinsohn, 1991; Agrawal and Konno, 2009). Thus, laticifers appear to be polyphyletic in origin. Moreover, their absence in primitive angiosperms suggests that these cells developed more recently than most other cell types. Despite their widespread presence in the plant kingdom, studies on laticifers have been lagging behind in recent years. Aspects that have been more deeply studied are those related to the physiology and role of laticifers in the production of latex in rubber tree (Hevea brasiliensis) or opium poppy (Papaver somniferum) as a source of rubber and opium, respectively. Also, the relevance of laticifers for insect defense and their involvement in the transport pathways of natural products has been pointed out (Hagel et al., 2008). However, there remains a paucity of information regarding the mechanisms of laticifer cell differentiation, the precise ontogenic origin, and the organization of the laticifer system within the plant body. In fact, to find comprehensive reviews about laticifer cells it is necessary to visit the early contributions provided by K. Esau (1965) and her coetaneous authors (Fahn, 1990; Mahlberg, 1993).
Early anatomical and morphological observations of laticifers were mostly provided by plant anatomists during the 19th century who focused on the large cosmopolitan family Euphorbiaceae (De Bary, 1884). As a result, two laticifer cell types, nonarticulated and articulated, were identified with distinct modes of cellular organization and different ontogenic origins (Fahn, 1990). Nonarticulated laticifers are single elongated cells that develop and grow intrusively between other cells via tip growth. This process requires a partial disassembly of the cell wall components and a disruption of cell wall connections with surrounding mesophyll cells (Mahlberg, 1959, 1963). Nonarticulated laticifers develop from cells that are present in the embryo (i.e. laticifer initials; Mahlberg, 1961; Mahlberg and Sabharwal, 1968). As the embryo grows into a mature plant, the laticifer initials elongate and undergo karyokinesis without forming cell plates. Thus, nonarticulated laticifers become large (e.g. tens of centimeters long), and constitute the largest cell type described in plants to date. Articulated laticifers, as found in dandelion (Taraxacum spp.) or rubber tree, arise from a series of initials that derive from meristematic regions (i.e. apical meristem and cambium) that originate at different phases of plant growth. Within this region, adjacent cell walls undergo partial or complete perforation (due to the gradual removal of wall materials) to form a series of somewhat elongated cells that are connected through highly perforated cell walls (Nessler and Mahlberg, 1979, 1981).
No specific function has yet been ascribed to laticifers (Pickard, 2008). It has been observed that nonarticulated laticifers do not contain chloroplast (Sacchetti et al., 1999), and they have no plasmodesmatal connections with their neighbors. Thus, these cells presumably obtain energy inputs from the apoplast. Laticifer loading has also been attributed to the symplastic transport of nutrients from phloem to parenchymal cells adjacent to the laticifers followed by entry into the apoplast, from which nutrients are taken up by laticifers (Bouteau et al., 1991; St-Pierre et al., 1999; Santana et al., 2002) and converted into latex, the most obvious characteristic that distinguishes laticifer from other cell types. This latex is enriched in different isoprenoid molecules following species-specific patterns, and it oozes copiously whenever a laticifer is punctured. It is hypothesized that unpunctured laticifers are turgid as a result of osmotic water uptake. From an ecological perspective, laticifers have been touted as a defense against insect herbivory for more than a century (Dussourd and Eisner, 1987) where the pressurized flow of latex may function as a form of physical defense, in addition to the potential antibiotic effects of the secondary metabolites stored in the latex (Agrawal and Konno, 2009; Huber et al., 2016).
In this study, cellular, molecular, and genetic tools were developed to examine the distribution, differentiation, and ontogeny of laticifer cells in the latex-bearing plant Euphorbia lathyris. We describe the distribution of the nonarticulated laticifer network within entire organs and approach the ontogeny of laticifer initials in the embryo. Furthermore, through the identification of laticifer mutants, we show that laticifers and latex production appear not to be essential for plant growth and development, and instead probably have importance for the ecophysiological adaptation of plants in natural environments.
RESULTS
Organization of the Laticiferous System within E. lathyris Plant Body
Knowledge of laticifer cells in latex-bearing plants has been derived from microscopy studies of cryostat or paraffin sections of plant tissues and conventional staining techniques. However, this approach does not allow for the three-dimensional distribution of laticifer cells within a plant organ. Moreover, laticifer cells frequently adopt a sinuous elongation pattern of cellular growth, moving in and out of plane. Thus, tissue sections only provide information for short distances along a piece of plant tissue and not along a longitudinal axis of a complex tissue as a whole. To simplify the identification of laticifer cells and the characterization of the three-dimensional relationships between them and their surrounding tissues in an intact seedling or plant organ, a whole-mount histochemical staining procedure employing Sudan Black B staining was developed. Since this technique renders other tissues translucent or transparent while staining the laticifer cells, it permits mapping of entire laticifer supply patterns of organs.
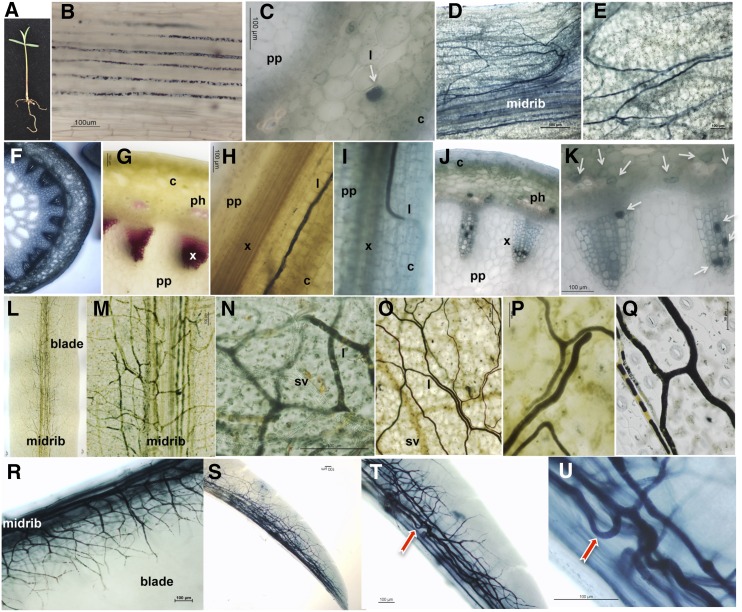
Whole-mount staining of intact E. lathyris seedlings revealed the longitudinal growth of laticifer cells along the hypocotyl axis (Fig. 1, A and B). On average, 18 to 21 laticifer cells were detected in the hypocotyl, and these cells ran parallel to each other from the base of the hypocotyl toward the shoot apical meristem (SAM), reaching an average of at least 9 cm in length, which is the length of the hypocotyl under our growing conditions. Therefore, laticifer cells constitute the largest plant cell type, if not the largest cell in nature. The laticifer cell asset of the hypocotyl was concentric to the central vascular cylinder and embedded among the mesophyll cells of the cortex (Fig. 1C). Supplemental Figure S1 presents a scheme of laticifer organization in the hypocotyl.
Figure 1.
Distribution pattern of laticifer cells in E. lathyris intact plant structures as revealed by whole-mount staining with Sudan Black B. A, Seedling, showing the first pair of true leaves, employed to identify laticifer cells as in B to E. B, A sector of the hypocotyl showing rows of laticifers running in parallel along the hypocotyl. C, Cross section of a hypocotyl region with laticifer cells (marked by a white arrow) specifically stained for isoprenoids. D and E, Longitudinal sector of a whole-mount stained cotyledon, along the lamina, in a region proximal to the node (D), where longitudinal laticifers cells concentrate along the midrib and in a distal region from the node and midrib (E), where laticifers appear scattered. F and G, Cross section of the stem stained with toluidine blue (F) and phloroglucinol (G), where the lignified xylem poles become specifically stained in red. H and I, Close-up of a whole-mount preparation of the stem showing an ascending laticifer running parallel to one of the poles of the vascular cylinder (H) or curving toward the vasculature from the cortex in search of a xylem pole (I). J and K, Close-up of cross sections of the stem showing some laticifer cells occupying the internal cavity of the hollow cylinder of different tracheary elements. L, Sector of a blade from a whole-mount stained fully expanded leaf. M, Magnification of a sector showing the midrib of the leaf blade and the abundant presence of laticifer cells. N and O, Magnification of leaf blade sectors showing the different dispositions and distribution patterns of laticifer cells. P and Q, Magnification of leaf blade laticifer cells showing characteristic Y- and H-bifurcation patterns. R to U, Whole-mount preparation of an emerging leaf close to the apical meristem, with predominant distribution of laticifer cells close to the midrib (R) and details of a sector close to the leaf tip at different magnifications (S–U). c, Cortex; l, laticifer; ph, phloem; pp, pith parenchyma; sv, secondary vasculature; x, xylem.
Laticifer cells were not identified in the roots of E. lathyris seedlings. Conversely, laticifer cells in the cotyledonary region were observed to group together to form rows of longitudinal cells that were entangled along the midrib of the petiole (Fig. 1D). This tier of longitudinal- and vascular-associated laticifer cells expanded up to the tip of the cotyledon. From this central disposition along the midrib, laticifers were profusely bifurcated and elongated, and they encompassed the entire blade of the cotyledon (Fig. 1E). Consequently, a complex labyrinth of laticifer cells formed, and no regular pattern of cellular distribution could be inferred.
Along the stem, which is typically composed of 24 xylem/phloem poles (Fig. 1, F and G), laticifer cells were observed to elongate toward the apical meristem and paralleled to secondary vascular strands. Due to the complexity of the tissues at this developmental stage, the exact number of laticifers could not be accurately determined. However, laticifer cells were predominant in the cortex and in an area proximal to the vascular bundle (Fig. 1H; Supplemental Fig. S2). Scanning electron microscopy (SEM) across transverse sections of the stem confirmed the distribution of laticifers observed in whole-mount preparations. Laticifer cells proximal to the vascular bundle had a larger internal diameter than those in the external cortex (Supplemental Fig. S3). A frequent and peculiar observation was the angling of the laticifer cells toward a nearby secondary xylem pole (Fig. 1I) and their entry into the lumen of hollowed tracheary elements to occupy the empty cavity (Fig. 1, J and K). This phenomenon represents to our knowledge a previously unknown cellular strategy by which a laticiferous system can enhance its invasive growth style.
In the leaves, laticifers appeared distributed parallel and along the midrib of all leaves (Fig. 1, L and M; Supplemental Fig. S4). Frequent bifurcations of the central laticifer cells occurred at right or almost right angles, and these cells subsequently continued to elongate and bifurcate to expand along every direction of the leaf blade. Thus, a myriad of laticifer cell-based crossroads appeared along the leaf lamina (Fig. 1, N and O). The density of these cells progressively decreased at the leaf margins, recalling a circulatory system like that of animals, exhibiting an unpredictable pattern of distribution with occasional aggregations of laticifers assembled in parallel to one another and with Y- and H-type bifurcations (Fig. 1, P and Q). However, these laticifers did not undergo anastomosis. Along the leaf blade, laticifers were distributed among the mesophyll cells, primarily on the abaxial side of the leaf lamina. Direct contact with the epidermal cell layer was not observed, although their proximity to the epidermis resulted in frequent dragging of the laticifer tubular structures with epidermal manual peels (Fig. 1Q). Whole-mount staining of intact true leaves emerging from the apical meristem, which are undergoing leaf expansion, provided a three-dimensional visualization of the interconnected tubular-like organization of the laticiferous system, exhibiting both sinuous and wave growth patterns with frequent loops and curls that altered the direction of the elongating laticifer growth. In addition, repeated bifurcations occurred and progressed toward the leaf margins as the leaf expanded (Fig. 1, R–U).
Characterization of Isolated Laticifer Cells
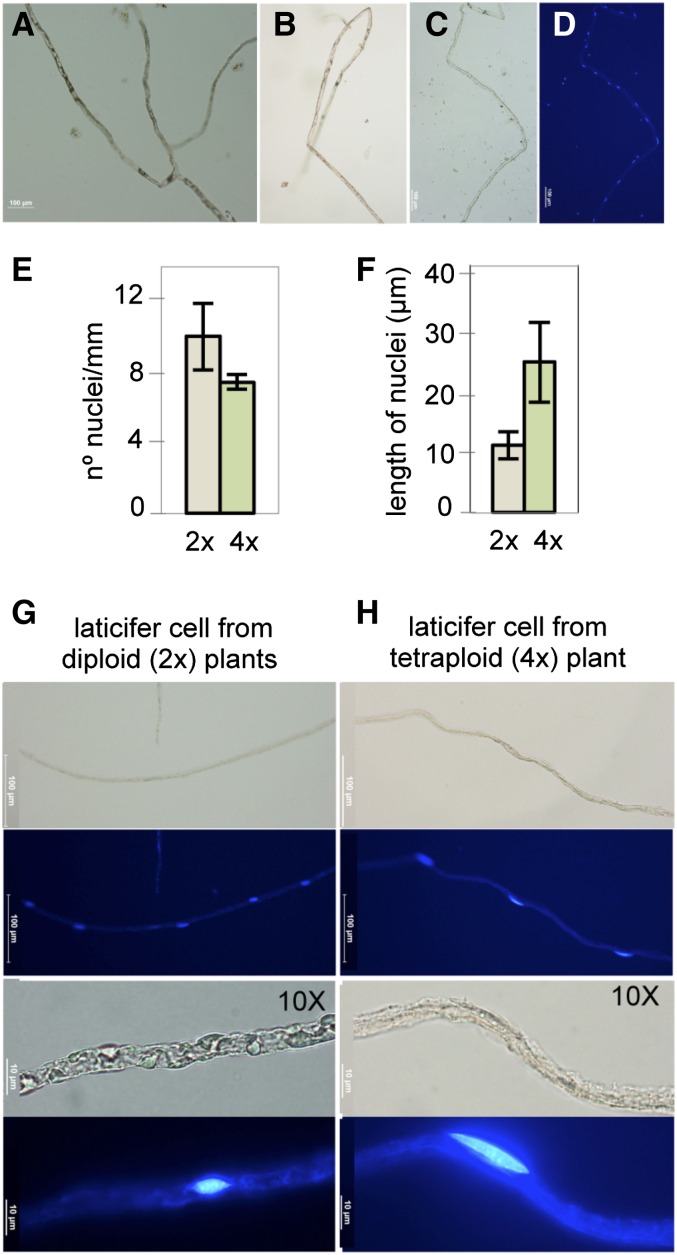
Nonarticulated laticifer cells have been documented as coenocytic cells that undergo karyokinesis without forming a cell plate (Mahlberg and Sabharwal, 1966). To obtain a detailed characterization of E. lathyris laticifer cells, laticifer protoplasts, corresponding to large fragments of individual laticifer cells (up to 1 cm), were isolated, and the distribution and spacing of nuclei were studied upon 4′,6-diamino-phenylindole (DAPI) staining (Fig. 2, A–D). The coenocytic nature of laticifer cells was confirmed with nuclei fitting and occupying the internal lumen of the tubular laticifer cells. Nuclei appear flattened at the poles, adopting an oblate shape with an averaged length of 10 μm and with a lineal and apparent constant spacing of 10 nuclei per mm of laticifer cell length (Fig. 2, E–G). When individual laticifer protoplasts were derived from stable tetraploid plants (Supplemental Fig. S5), the size of the nuclei doubled (Fig. 2–H) and the nuclear spacing was retained along the coenocyte (Fig. 2E). Therefore, the lack of a cell wall septum and consistent nuclei spacing, giving the appearance of beads in a rosary, help define the longitudinal coenocytic organization of a laticifer cell.
Figure 2.
Isolation of coenocytic laticifer E. lathyris protoplasts and distribution of nuclei. A to D, Fragments of different long individual laticifer protoplast, either with bifurcations or lineal, viewed under the optical microscope (A–C) or by fluorescence microscopy upon staining with DAPI (D). E, Nuclear density along the longitudinal laticifer protoplasts from a diploid (2x) and a tetraploid (4x) plant. F, Length of nuclei from 2x and 4x plants. Bars represent mean ± sd. For nuclear density, 40 individual laticifer protoplasts of different lengths were analyzed. For nuclear length determination, 100 different nuclei from 10 different laticifer cells were measured. G and H, Magnification details of isolated laticifer protoplasts from 2x and 4x plants.
Ontogeny and Organization of Laticifer Cells in the E. lathyris Embryo
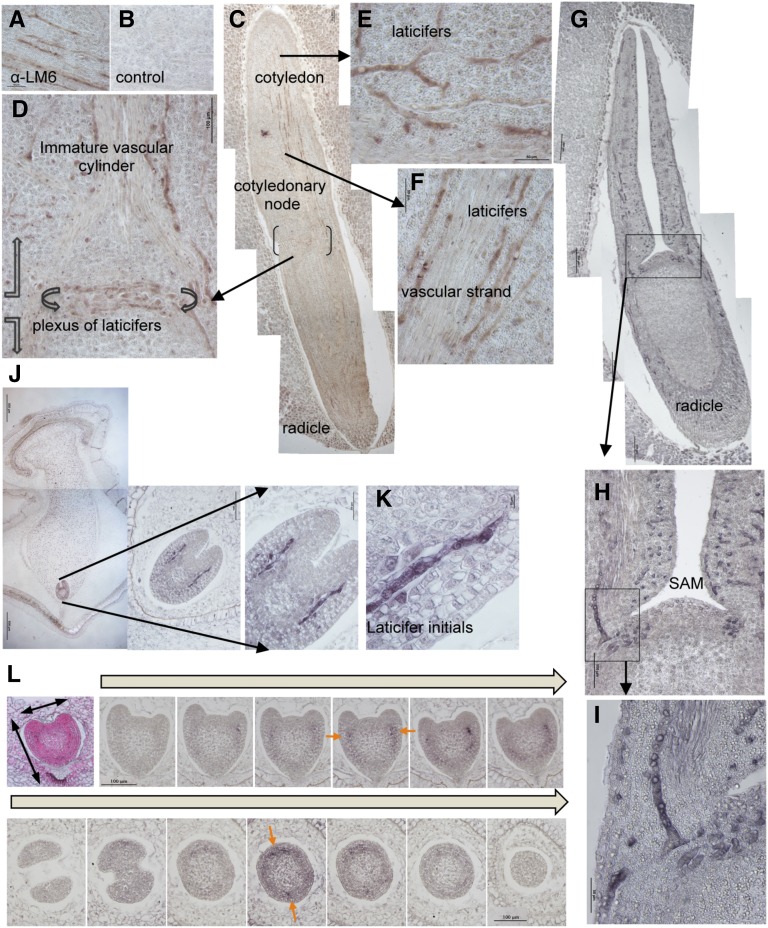
Early anatomical studies of laticifer cells in embryos from different plant species were conducted by Schmalhausen (1877) and by Chauveaud (1891). These observations, along with more recent studies by Mahlberg (1961, 1993) and Mahlberg and Sabharwal (1968), have indicated that nonarticulated laticiferous systems arise from a series of embryonal initials and their intrusively growing branches. Since whole-mount staining in the embryo is impeded by the presence of the seed coat, an immunohistochemical approach using fixed sections was applied to study laticifer organization in the embryo of E. lathyris and its ontogeny. Intrusive growth requires both disassembly and synthesis processes of different cell wall constituents. Consequently, differences in the wall composition of laticifer cells were expected. From a panel of antibodies raised against various cell wall components, we identified the LM6 antibody to specifically immunodecorate laticifer cells in the mature embryo (Fig. 3, A and B). LM6 specifically recognizes (1-5)-α-l-arabinan epitopes (Willats et al., 1998). When the LM6 antibody was used to study the complexity of laticifer organization in the embryo, a ring of interwoven laticifer cells, here designed as a “plexus,” was observed (Fig. 3D). These cells were located at the cotyledonary node, from which branches of laticifers extended upwards into the cotyledons parallel to the immature vascular strand (Fig. 3F). These laticifers subsequently branched at right angles and extended laterally into the swollen cotyledonary tissues (Fig. 3E). A frontal view of a mature embryo (Fig. 3G) showed the laticifer branches of the plexus that ascended into the cotyledons along the course of the immature vascular strand from a different angle (Fig. 3, H and I). The SAM appeared to be devoid of laticifer strands (Fig. 3H). In addition, branches that extended from the plexus downward into the radicle were much less abundant than those extending toward the cotyledons (Fig. 3, C and G).
Figure 3.
Organization and ontogeny of the laticiferous system in the E. lathyris embryo. A and B, Comparative immunohistochemical staining of E. lathyris embryo sections with LM6 (A) and a nonspecific (B) antibody revealed the specificity of LM6 to immunodecorate laticifer cells in the embryo. C, Sagittal section along the longitudinal axis of the mature embryo revealed with LM6 allowed identifying laticifer cellular structures along the embryo axis. D, Magnification of the sector shown in C showing the ring of interwoven laticifer cells (plexus) in the cotyledonary node and the ascending row of laticifers parallel to the immature vascular strand in the cotyledon. E and F, Details of branches of laticifer cell structures extending laterally (E) in the cotyledon tissues or extending upwards and parallel to the immature vascular strand (F). G–I, Frontal view of a mature embryo section immunodecorated with LM6 (G; magnification details (H and I) allowed identification of laticifer branches ascending into the cotyledons. Observed that the sam appears devoid of laticifer strands. J, Detection of elongated laticifer initials in immature embryos (i.e. heart stage) as embedded in the seed. The laticifers appeared immunodecorated at the base of the emerging cotyledons. K, Detail of a laticifer initial terminating as a narrow tip with acuminate ends. L, Detection of earliest laticifer appearance detected using LM6 antibodies at late globular stages of embryo development. Serial longitudinal sectioning (top) and cross-sectioning (bottom) of embryos revealed the presence of a single pair of nonelongated initials, marked with orange arrows and discernible at the time primordia of cotyledons start to form in the immature embryo.
We subsequently wondered when and where laticifer initials originate during embryo development. Symmetric pairs of elongated laticifer initials in the early stages of laticifer cellular differentiation (e.g. carrying eight to 12 nuclei) were detected at the heart stage of embryo development, at the base of emerging cotyledons, and in the region where future procambial tissue eventually differentiate (Fig. 3J). These laticifer initials appear to elongate bidirectionally, with the lower end penetrating downward toward the root apex of the immature embryo and the upper end penetrating toward the base of the developing cotyledons. Initially, each end of the laticifer intrusively thrusted its way between neighboring cells, with the initials decreasing in diameter until they terminated as a narrow tip with acuminate ends that wedged between two adjacent cells (Fig. 3K). The growing tips then followed the path of the middle lamella and did not fuse or penetrate adjacent cells. During their intrusive growth, the laticifers established contacts with new cells, while neighboring cells were forced apart from their original point of contacts. Thus, intrusive growth appears established in the very early stages of laticifer differentiation and is maintained throughout the life span of the laticifer. To monitor the abundance of the laticifer initials, both transversal and radial serial sectioning were performed along the entire embryo at the heart stage of development. In this serial sectioning approach, up to 26 to 28 initials were found to be present at this stage of embryo development (Supplemental Fig. S6A). Furthermore, these laticifers were radially distributed and exhibited different degrees of differentiation, as if the differentiation process was not completely simultaneous for all the initials. From that point, the laticifer initials began to branch and elongate until they conformed to the laticifer complexity that characterizes the mature embryo as described above. The earliest laticifer initials were detected when the embryo leaves the globular stage (e.g. when no initials were detected; Supplemental Fig. S6B) and enters the early heart stage. At this point, the embryo is approximately 180 μm in length (Fig. 3L). At the time the primordia of the cotyledons start to form in the embryo, we identified a single pair of nonelongated and symmetrically positioned laticifer initials that occupied a median position relative to the cotyledonary primordia. Both, serial longitudinal sectioning (Fig. 3L, top) and cross-sectioning (Fig. 3L, bottom) of embryos confirmed the presence of these two initials. These observations may be taken as indicative that the laticiferous system is initiated with the early differentiation of a single initial on either side of the bilateral axis of an immature embryo. Soon after, further cellular differentiation events occur concurrent with the embryo entering the heart stage of development. Supplemental Figure S7 summarizes this process of differentiation and growth in the developing embryo for a single laticifer initial.
Identification of Laticifer-Associated Gene Expression Pattern
Oozing latex carries the cytosolic constituents of laticifers. Therefore, to identify genes related to laticifer cell activity, we isolated latex from leaves, isolated its RNA, and performed massive 454 sequencing. Whole leaves were similarly processed and sequenced. The nucleotide sequences obtained were compared with the entries in databases to search for homologous sequences. A subset of genes (Supplemental Table S1) was further selected for expression analysis by quantitative reverse transcription PCR (RT-qPCR). As shown in Supplemental Figure S8, mRNAs encoding conserved ACAT or HMGCoAR enzymes, key for the activity of the cytosolic mevalonate pathway (MVA), were abundant in the latex as well as in the leaves, stem, and roots. In contrast, genes encoding DXS and DXR were found to be poorly expressed in laticifer cells compared to other plant organs (Supplemental Fig. S8). The latter are markers of the methylerythritol phosphate (MEP) pathway of the chloroplast. Both the MVA and MEP pathways contribute to the synthesis of the common five carbons in isopentenyl pyrophosphate precursor required for isoprenoid biosynthesis in photosynthetic tissues (Vranová et al., 2013). Thus, isopentenyl pyrophosphate precursors in laticifer cells may be primarily provided by the cytosolic MVA pathway. This possibility is further supported by the observation that laticifer cells are devoid of chloroplasts (Mahlberg, 1993), which indeed contain the components of the MEP pathway (Vranová et al., 2013).
Genes encoding cardinal enzymatic constituents of the triterpenoid pathway, including SQS, SQE, CAS, and SMT1, are also expressed both in laticifers and in the different plant organs. Other genes highly expressed in laticifer cells, however, are expressed at much lower levels in samples representing whole plant organs. These genes may represent potential markers of laticifer cells, such as PE and EG (Supplemental Fig. S8). These later genes encode proteins involved in cell wall remodeling that might contribute to the disassembly of the cell walls of surrounding cells during the intrusive growth of laticifers. Other laticifer-specific markers included MLP, or EH, of unknown function, and DHDDS, involved in membrane metabolism. Conversely, two genes were found strongly expressed in various plant organs but strongly repressed in laticifer cells (i.e. PEI or PL). These latter proteins are hypothesized to provide a counterbalancing effect of the mesophyll cells to the cell wall remodeling enzymes secreted by laticifers. A subset of these genes was subsequently employed as markers for the presence of laticifers.
Dynamics of Laticifer Cells in the Plant
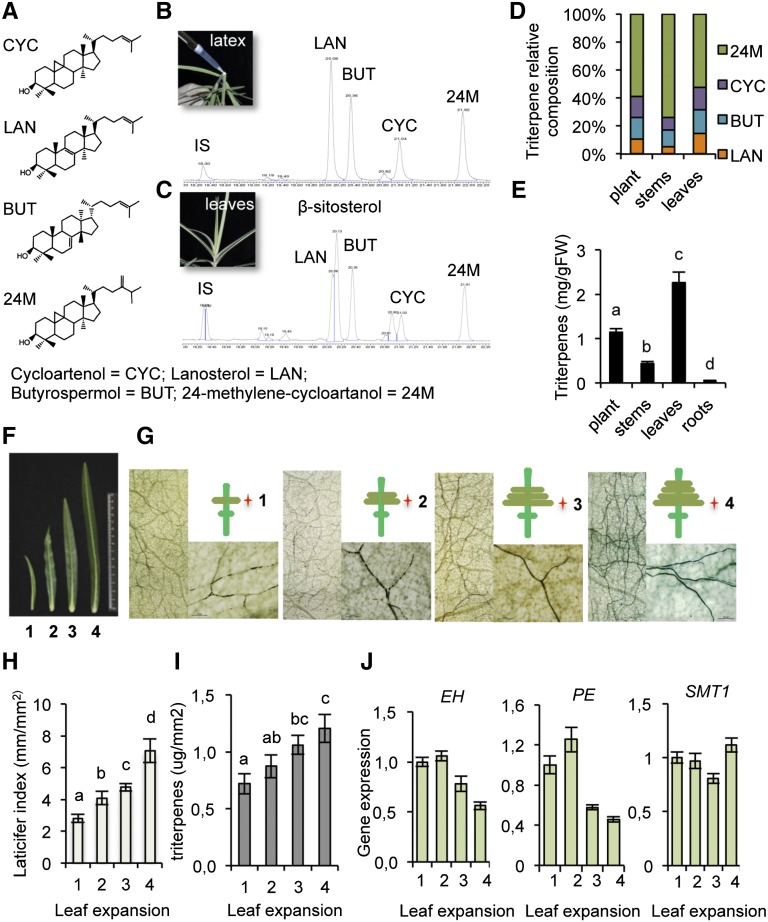
To study laticifer metabolic activity, the latex components were analyzed by gas chromatography-mass spectrometry (GC-MS). Four major isoprenoid species were found: cycloartenol (CYC), lanosterol (LAN), butyrospermol (BUT), and 24-methylene cycloartanol (24M), with distinctive retention times (Fig. 4, A and B). In whole-leaf extracts, all four latex-associated isoprenoids, along with β-sitosterol, which was absent in latex, where detected (Fig. 4C). Thus, the CYC, LAN, BUT, and 24M isoprenoid profile represents a metabolic fingerprint for E. lathyris laticifer cells. The relative content of each of these four isoprenoids varies between leaves and stems, with 24M accumulating at higher levels in stems, apparently at the expense of the three other isoprenoids (Fig. 4D). Thus, organ-specific adjustments in the isoprenoid pathway may be made to allow common squalene intermediate to be transformed into any of the four major isoprenoids that accumulate in laticifer cells (Supplemental Fig. S8). A comparative analysis of isoprenoid content in stems, leaves, and roots was also conducted with respect to the net accumulation of isoprenoids in the whole plant. A 3-fold increase in isoprenoid content was detected in the leaves compared to the stems (Fig. 4E), while they were barely detectable in roots. Taken together, these results reconcile with findings deduced from whole-mount staining (Fig. 1).
Figure 4.
Dynamics of E. lathyris laticifer differentiation and activity in the whole plant and during leaf development. A, Major isoprenoids present in latex: CYC, cycloartenol; LAN, lanosterol; BUT, butyrospermol; 24M, 24-methylene-cycloartanol. B and C, GC-MS analysis of latex extracts (B) and whole-leaf extracts (C). D, Relative content of each of the four major latex isoprenoids in extracts derived from whole plants, stems, and leaves. E, Comparative analysis of triterpenoid content in stems, leaves, and roots with respect to its net accumulation in the whole developed plant. Bars represent mean ± sd, n = 9 independent plants. An ANOVA was conducted to assess significant differences in isoprenoid content, with a priori P < 0.05 level of significance; the letters above the bars indicate different homogeneous groups with statistically significant differences. F, Image of E. lathyris leaves at the four stages (1–4) of leaf expansion. G, Whole-mount staining of leaves and close-up of a sector of the leaf blade, showing the presence of laticifer cells at the four stages of leaf expansion shown in F. The insert cartoons serve to indicate the relative position of the selected leaves in the stem. H, LI recording for each stage of leaf expansion. I, Triterpenoid content in leaves at different stages of leaf expansion. Bars represent mean ± sd, n = 9 independent plants. J, Expression of the EH, PE, and SMT1 genes at different stages of leaf expansion. Relative expression was assayed by RT-qPCR on total RNA from leaves at the indicated stages. Data represent means ± sd (n = 3 biological replicates). Expression was normalized to the constitutive Histone H3 gene, then to expression attained at stage 1 of leaf expansion.
The abundance of laticifer cells was estimated using a “laticifer index” (LI) calculated based on the total length (in mm) of the tubular laticifer cells that occupied a microscopic field area (in mm2) of the leaf lamina upon whole-mount staining. Laticifer abundance was found to positively correlate with the extent of leaf expansion at a fixed position in the stem (Fig. 4, F–H); leaf expansion was arbitrarily numbered from 1 to 4 (Fig. 4, F and G). Moreover, LI increase was observed in parallel with greater isoprenoid accumulation as the leaf expands (Fig. 4I). RT-qPCR analysis expression of SMT1, serving as a marker for isoprenoid biosynthesis, remained stable during leaf expansion (Fig. 4J). Conversely, the expression of laticifer marker genes (e.g. PE and EH) declined in the late stages of leaf expansion, indicative that laticifers slow down their growth when leaf expansion decays. Thus, isoprenoid metabolism within laticifers remains fully active even in the absence of laticifer growth.
Influence of the SAM on Laticifer Growth in the Cotyledon
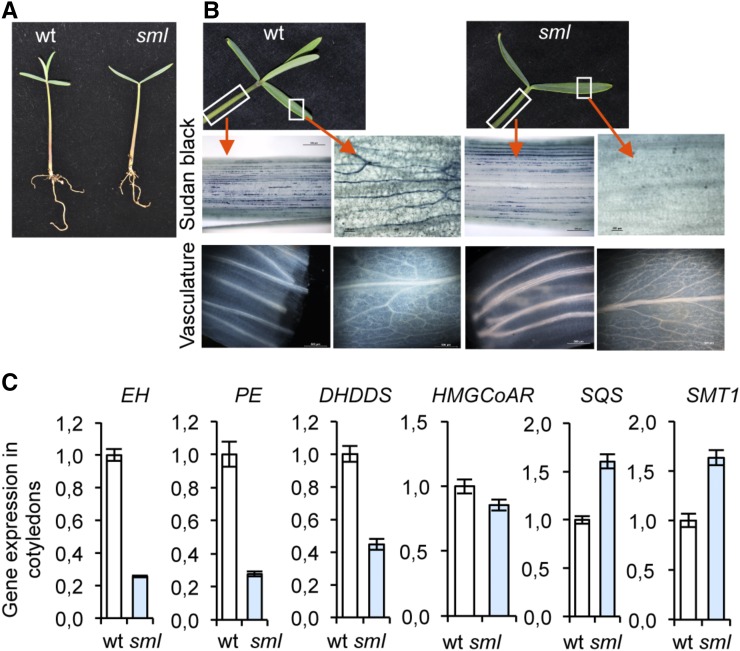
Early reports proposed that a relationship between laticifer growth and the meristematic activity in the apex might exist (Schaffstein, 1932). It was thus contended that the SAM was the source of a factor(s) influencing laticifer differentiation and growth. To determine the importance of the SAM on the laticiferous system, we searched for a mutant defective in shoot apical meristem. To achieve this goal, we mutagenized 9000 seeds of E. lathyris, which were allowed to grow and selfed. M2 seeds were screened for mutants defective in SAM. One mutant was identified. When it was assayed in homozygosis, it lacked shoot apical growth due to the lack of a distinguishable SAM and thus did not develop true leaves (Fig. 5A). This mutant was named shoot meristemless (sml).
Figure 5.
Influence of the SAM on laticifer growth in the cotyledon of E. lathyris. A, Developing seedlings from wild type and the sml mutant. Observe the lack of true leaf formation in the mutant. B, Close-up of the cotyledons and hypocotyls from wild type and sml mutant. Below is shown the presence and disposition of laticifers (top) and the vascular strands and venation pattern (bottom) in each genetic background and for both organs, and as revealed by whole-mount staining with Sudan Black B or upon acetone clarification, respectively, and visualization under the light microscope. C, Expression of the EH, PE, DHDDS, HMGCoAR, SQS, and SMT1 genes in cotyledons from wild-type and sml plants. Relative expression was assayed by RT-qPCR on total RNA from leaves at the indicated stages. Data represent means ± sd (n = 3 biological replicates). Expression was normalized to the constitutive Histone H3 gene, then to expression attained in wild-type plants.
In the early stages of seedling development, whole-mount staining of sml seedlings and wild-type seedlings showed that the arrangement and growth of the laticifer cells in the hypocotyl were similar, as it was also the case for the disposition of vascular strands (Fig. 5B). Conversely, laticifer cell establishment was found to be severely compromised in the cotyledons of the sml mutant, where laticifer structures were only sporadically observed and showed no branching (Fig. 5B), while the development of the vasculature suffers no variation with respect to the parental line. RT-qPCR analysis of the mRNAs extracted from cotyledons showed that expression of laticifer markers (e.g. EH, PE, and DHDDS) was severely repressed in the sml mutant (Fig. 5C), consistent with the diminution of laticifer cells in this organ. In contrast, expression of triterpenoid biosynthesis-related genes (e.g. HMGCoAR, SQS, or SMT1) remained nearly invariant in the sml mutant. Therefore, the sml mutant confirmed that SAM plays an important role in the organization of the laticiferous system in cotyledons, reminiscent of a laticifer-specific chemotropic response.
Identification of E. lathyris Mutants Defective in Laticifer Organization and Activity
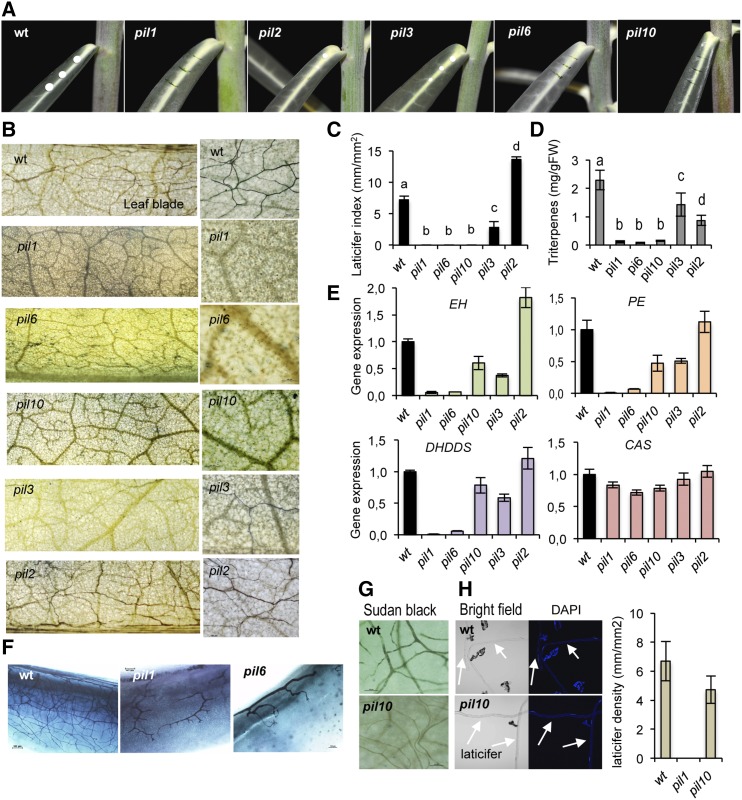
We next approached the identification of mutants defective in laticifer differentiation and latex production to unravel the importance of laticifer cells and latex production for plant growth and development. We screened for mutants devoid of latex production based on the rationale that these mutants would reflect defects in laticifer differentiation and growth or, alternatively, have defects in latex production. A total of 3000 M2 plants were screened by pricking leaves and observing whether latex oozed, and candidate mutants were selfed. Five mutants were reconfirmed in the M3 and M4 generations. These mutants, which showed reduced or no bleeding of latex upon severe injury, were coined poor in latex (pil). Three of them (i.e. pil1, pil6, and pil10) produced no latex in either the leaves or stems. The pil2 and pil3 mutants produced some latex, albeit much less copiously than the parental plants (Fig. 6A). Backcrossing with parental plants and segregation analysis of the F2 progenies revealed that the mutants manifested as Mendelian recessive genes (Supplemental Table S2), except for pil3, which behaved as dominant. As a complementation test, reciprocal crosses of the five pil mutants with each other and characterization of F1 plants revealed a wild-type phenotype in all cases, thereby indicating that none of the pil mutants were allelic.
Figure 6.
Characterization of E. lathyris pil mutants. A, Comparative oozing of latex upon pricking of leaves from wild-type, pil1, pil2, pil3, pil6, and pil10 plants. B, Whole-mount Sudan Black B staining of leaves and close-up of a sector of the leaf blade, showing the relative abundance of laticifer cells in the indicated genetic backgrounds. C, Comparison of LI as recorded in leaves located at the same position in plants of the indicated genetic backgrounds. D, Triterpenoid content in leaves from wild-type plants and the pil mutants. Bars represent mean ± sd, n = 9 independent plants. E, Expression of the EH, PE, DHDDS, and CAS genes in leaves from wild-type plants and pil mutants. Relative expression was assayed by RT-qPCR on total RNA from leaves of the indicated genotypes. Data represent means ± sd (n = 3 biological replicates). Expression was normalized to the constitutive Histone H3 gene, then to expression attained in wild-type plants. F, Whole-mount preparation of emerging leaves close to the apical meristem showing defective laticifer elongation in pil1 and pil6 mutants. G, Presence of laticifer cells in leaves of pil10 plants, with a density similar to that observed in wild-type plants, as revealed under the optical microscope upon Sudan Black B staining, intense clarification with ethanol, and illumination with white bright light. H, Long individual laticifer protoplast fragments released from wild-type plants and pil10 plants view under bright field in the optical microscope (left) or by fluorescence microscopy upon staining with DAPI (right). An estimation of laticifer density (mm of longitudinal protoplast released to the medium per optical surface area recorded) from independent protoplast preparations (n = 4) from wild-type and pil10 leaves is shown at the right. The pil1 mutant was used as a negative control revealing the lack of laticifer protoplast released to the medium in this mutant.
Characterization of the pil1, pil6, and pil10 Mutants
The five PIL loci were found to affect distinct aspects of laticifer activity and organization. In pil1, pil6, and pil10 mutants, no identifiable laticifer cells were observed along the leaf lamina of full expanded leaves (Fig. 6B). Thus, a zero value LI was calculated for each mutant (Fig. 6C). The lack of detectable laticifers was also consistent with the depletion of CYC, LAN, BUT, and 24M triterpenes in the crude leaf extracts obtained from each of the three mutants (Fig. 6D; Supplemental Fig. S9). Moreover, laticifer identity genes (e.g. EH, PE, and DHDDS) were severely repressed in pil1 and pil6 mutants and much less acute in the pil10 mutant (Fig. 6E). This posed a distinction of pil10 with respect to pil1 and pil6.
A detailed inspection of whole-mount preparations of the intact primary meristematic leaves from each of the five mutants revealed that the primary leaves from pil1 and pil6 exhibited only rudimentary versions of laticifer cells (Fig. 6F), which were only proximal to the central vein and were highly branched but did not elongate. The characteristic network of laticifers engulfing the entire leaf lamina in wild-type plants was absent in these two mutants. Therefore, it was hypothesized that the pil1 and pil16 mutants carry defects in genes required for the elongation, but not for branching, of laticifers. Remarkably, careful observation of whole-mount preparations of primary leaves in pil10 plants revealed the mutant indeed contained laticifer cells, albeit they did not become stained with the colorant; pil10 laticifers appeared translucent and could awkwardly be visualized upon illuminating the cleared whole-mount preparations with intense bright light (Fig. 6G). Thus, while the pil10 plants maintained a normal laticifer network, they lacked production of latex. When longitudinal arrays of laticifer protoplasts were retrieved from wild-type and pil10 fully expanded leaves (Fig. 6H), with pil1 plants serving as a negative control, DAPI staining further showed that pil10 laticifer protoplasts retained the characteristic coenocytic organization observed in the wild type (Fig. 6H). Thus, laticifer differentiation, growth, and organization can occur in the absence of latex and isoprenoid accumulation.
In marked contrast with the adult mutant plants, immunohistochemical staining with an LM6 antibody showed that the growth and organization of laticifer cells in these three mutant embryos were not severely compromised. However, distribution and shape of the laticifer cells in pil1 and pil6 were partially distorted (Supplemental Fig. S10). These observations indicate that the defective laticiferous system observed in planta is most likely due to a postembryonary cell elongation defect, presumably in response to growth signals from vegetative tissues. This would reconcile with a chemiotropic model of laticifer growth in planta. Moreover, complementation of the mutant phenotypes, upon generating chimeric E. lathyris plants obtained by grafting where the rootstock and scion were reciprocally interchanged between wild-type plants and the pil1 and pil6 plants, was not achieved (Supplemental Fig. S11), thus indicating cell autonomy of PIL1 and PIL6 genes during laticifer cell growth.
With the exception of only a minor reduction in height of pil1 plants, which, however, recovered at later development stages, the pil plants grew and developed similarly to the wild-type plants (Supplemental Fig. S12). Therefore, laticifer cells and latex production are not essential for plant development.
Characterization of the pil2 and pil3 Mutants
In the pil3 plants, laticifer cell differentiation and growth occurred, yet it was characterized by a marked reduction in the LI value (Fig. 6, B and C). Despite this reduction, laticifer cells in the pil13 mutant elongate along the leaf lamina to an extent similar to that achieved in wild-type plants. Expression of laticifer marker genes (Fig. 6E) and triterpene content (Fig. 6D; Supplemental Fig. S9) also decline in pil3 plants, consistent with the observed reduced LI value. Therefore, the PIL3 protein might be controlling a process related to branching of laticifers but not to elongation, indicative that the two cellular processes distinctly regulate laticifer growth and density.
The pil2 plants exhibited an inverse laticifer cellular phenotype compared to that of the pil3 plants. Despite having reduced latex production, pil2 plants had enhanced LI value (Fig. 6, B and C) and concurring increases in the expression of laticifer marker genes (Fig. 6E). However, pil2 laticifers stained less intensively with Sudan Black B compared with wild-type ones. Consequently, we hypothesize that the higher complexity of the laticiferous system observed in pil2 plants might be due to the enhanced branching activity of the laticifer cells. However, despite the enhanced LI value in pil2, lower levels of triterpenoid accumulation were observed compared to the wild-type plants (Fig. 6D). Thus, laticifer growth via branching and certain aspects of triterpenoid metabolism may be negatively interlinked through the PIL2 locus.
Taken together, these findings suggest that PIL2 and PIL3 may function as negative and positive regulators, respectively, of laticifer growth via tip branching. Instead, PIL1 and PIL6 appear to be pivotal for laticifer growth by tip elongation, and PIL10 appears to be critical for coupling laticifer differentiation to latex production.
DISCUSSION
Terpenoids characterize the cytoplasmic content of laticifers (Hagel et al., 2008), and these specialized elongated cells or vessel-like series of cells, first described by de Bary in 1884 (De Bary, 1884), represent the lengthiest eukaryotic cell identified to date. Hagel et al. (2008) have performed a clade diagram representing the evolutionary relationship of the various taxa that possess laticifers that suggests that laticifers have arisen independently more than once during evolution. This points toward the importance of laticifers for adaptation of plants to specific natural environments. Previously, knowledge of the presence of laticifers was based on observations made on classical microscopy methods for tissue sections. In the current study, a whole-mount histochemical staining procedure allowed us to identify and map the entire laticifer supply within entire plant organs and seedlings. Consequently, the polarized growth of rows of laticifers along the longitudinal axis of the hypocotyl and toward the SAM could be observed. In contrast, an intricate and complex distribution pattern of laticifers along the cotyledon and leaf lamina was observed: a few major laticifer cells run parallel to the primary vasculature of the leaf and rendered, by repeated bifurcations, lateral laticifers that subsequently elongated via intrusive growth. Subsequent repetitions of this dual process, branching and elongation, led to the formation of a laticifer network that encompassed the entire leaf organ. The pattern of this network of laticifers resembled the distribution of blood vessels in the circulatory system of animal organs. Establishment of this laticifer network in the leaf correlates with the expansion and growth of this organ, indicative that both processes are coordinated. In fact, early studies on laticifer organization support the hypothesis that laticifer growth and meristematic activity are related and that the shoot meristem is the source of a factor that influences laticifer differentiation and growth (Schaffstein, 1932; Mahlberg, 1993). The identification of the sml mutant, compromised in the maintaining and organizing the meristematic activity, in a manner similar to that described for the Arabidopsis (Arabidopsis thaliana) STM gene (Endrizzi et al., 1996), revealed that for a correct organization and growth of the laticiferous system, SAM intactness is required and evokes a laticifer-specific chemotropic response for a signal synthesized in the meristem.
In the embryo, the pattern of laticifer cell distribution mirrors what will be later seen in an adult plant, with particular enrichment in cotyledons and absence in roots, suggesting an early pre-establishment of the laticiferous system. Moreover, a plexus of laticifers in the mature embryo, at a certain distance below the meristem, from which tangential rows of laticifers emanated to elongate and reach distal tissues of the embryo, appears to function as a supply of laticifers for the establishment of the laticifer network. These observations are consistent with early interpretations by Mahlberg (1961) in his search for the organization of laticifer cells in Nerium oleander embryos and thus indicates that this characteristic organization of laticifer organization is evolutionarily conserved.
Our immunohistochemical studies also revealed that the primary events of laticifer differentiation in E. lathyris, when the first single pair of laticifer initials was identified, occurred during the transition from the globular to the early heart stage of embryo development. The initials were symmetrically positioned, according to the bilateral axis, at a median position relative to the cotyledonary primordia. This first differentiation event was then followed by the rapid appearance of up to 26 to 28 additional laticifer initials, which were distributed concentric to the perimeter of the maturing embryo. Once the ring of differentiated laticifer initials was set, they started elongating via intrusive growth along the longitudinal axis of the embryo. This elongation was accompanied by sequential karyokinesis events and did not include the formation of cell plates. As a result, a characteristic coenocytic-type appearance was observed for the incipient laticifers. When elongation of the laticifer initials was initiated (e.g. when a row of six to eight nuclei was identified within the laticifer initials), the laticifers began branching at oblique angles and started forming the complex laticifer network observed in the mature embryos.
Our understanding of the role of laticifers in plant growth and development has remained very limited. Consequently, the identification the pil mutants provided valuable insight onto how laticifer cells grow and become organized within the plant body. The PIL1 and PIL6 loci appear to function as positive regulators of laticifer elongation, but not branching. Conversely, The PIL2 and PIL3 loci appear to function as negative and positive regulators, respectively, of laticifer branching without affecting cell elongation. Therefore, the concerted action of these PIL determinants on elongation and branching might be on the basis for the final conformation of the laticifer network in the plant. On the other hand, the identification of the PIL10 locus allowed us to conclude that laticifer differentiation and growth is independent of latex production. Therefore, PIL10 may represent a metabolic switcher for latex formation in fully differentiated laticifer cells. Moreover, pil plants did not appear to have any noticeable phenotypic effect on normal growth and development, this being indicative of laticifer cells representing a specialized cellular adaptation to fulfill a specific role in latex-bearing plants in their natural environments.
In sum, our results provide valuable insight into the paradigmatic mechanisms involving cellular differentiation, morphogenesis, and growth characteristics of specialized laticifer cells that have long been recognized yet have remained poorly understood. The ecophysiological tradeoffs and fitness effects of pil mutants in natural environments, as well as the identification of the different PILs, is our next challenge for the future.
MATERIALS AND METHODS
Plants Growth Conditions and Latex Isolation
Euphorbia lathyris plants were grown in a growth chamber (19°C–23°C, 85% relative humidity, 100 μE m−2 s−1 fluorescent illumination) on a 16 h:8 h light:dark photoperiod. For latex isolation, leaves were pricked at the central vein, and the latex oozing stored at 280°C.
Leaf Enzymatic Digestion, Laticifer Isolation, and DAPI Staining
Whole plants were vacuum infiltrated in ethanol-acetic acid-formaldehyde (50:5:10) for 20 min and fixed during 16 h at 4°C. Leaves were excised, washed, and immersed in an enzymatic solution containing 1% (w/v) Driselase (Sigma-Aldrich) in MP 0.6 medium (MS mineral solution [Murashige and Skoog, 1962], 2.5 mm MES, 100 mm Suc, 400 mm mannitol, 100 mm Gly, 14 mm CaCl2, pH 5.7) during 16 h at 28°C in the dark. The digested tissue was stained with DAPI.
Generation of Tetraploid Plants
Hypocotyl explants were cultured in basal medium (MS solution, B5 Gamborg vitamins [Gamborg et al., 1968], 2.5 mm MES, 87 mm Suc, pH 5.7) supplemented with 0.009 mm 6-benzylaminopurine and 0.0014 mm oryzalin. After 3 d, explants were transferred to fresh medium without oryzalin. After 3 weeks, developed shoots were excised from the explant and transferred to a root induction medium (basal medium supplemented with 0.068 mm indole-3-acetic acid). Ploidy level was evaluated by flow cytometry (Smulders et al., 1994) with the CyFlow Ploidy Analyzer (Partec). Over 5000 nuclei were measured per sample. Tetraploid (2n = 4x), diploid (2n = 2x) or mixoploid (with 2x and 4x nuclei) plants could be identified.
Seed Mutagenesis
Seeds were mutagenized by gamma ray at 300 Gy at the International Atomic Energy Agency Laboratories in Seibersdorf (Vienna, Austria). The irradiation dosage was chosen based on the observation that a dose of 300 Gy resulted in approximately 97% survival of M1 plants.
Genetic Analysis
Upon backcrossing with the parental line, segregation of phenotypes in the F2 generation was analyzed with the χ2 test for goodness of fit. For complementation analysis of the different pil mutants, each pil mutant was crossed with each of the other mutants and appearance of the pil phenotype in F1 plants recorded.
RNA Extraction, RT, and qPCR
RNA extraction and reverse transcription was performed as described (López et al., 2011). qPCRs were performed using an ABI PRISM 7000 sequence detection system and SYBR-Green (Perkin-Elmer Applied Biosystems). Histone H3 was chosen as the reference gene. Primers for amplicons covering each of the genes studied are listed in Supplemental Table S3.
454 Sequencing
A 1 µg aliquot of mRNA was used as the template for first-strand cDNA synthesis using a MINT-2 cDNA synthesis kit (Biocat). cDNA normalization was performed with a Trimmer-2 cDNA normalization kit (Biocat). cDNA was digested with GsuI (Fermentas) and purified using QIAquick columns (Qiagen) to eliminate oligo(dT). The cDNA quality was verified with an Agilent 2100 Bioanalyzer (Agilent). A 1 µg aliquot of each cDNA or noncoding DNA sample was nebulized to produce fragments of a mean size of between 400 and 800 bp. Preparation of cDNA fragment libraries and emulsion PCR conditions were as described in the Roche GS FLX manual. Pyrosequencing was performed on a Roche Genome Sequencer FLX instrument (454LifeScience-Roche Diagnostics). The quality of the reads was assessed with PERL scripts developed at Lifesequencing S.L. for trimming and validation of high-quality sequences. Adaptor sequences used for library preparation were entered in an adaptor-trimming database to the PERL Program. New SFF output files were generated with the sfftools (454 Life Science/Roche). Trimmed reads were assembled with NEWBLER version 2.3 (454 Life Science/Roche) with default parameters.
SEM
Tissue samples were processed for SEM as described (Dobón et al., 2015). Pictures were taken with a JSM-5410 scanning electron microscope (JEOL).
Isoprenoids Extraction and GC-MS Analysis
Isoprenoids were extracted with heptane in a Soxhlet and saponified (Koops et al., 1991). Triterpenols were derivatized to trimethylsilylether derivatives with Sylon HTP (Sigma-Aldrich) and analyzed by GC-MS on an Agilent 6890N gas chromatograph attached to a low-resolution quadrupolar mass spectrometer Agilent 5973 with a HP-5MS UI (30 m, 0.25 mm inner diameter, 0.25 µm) column. Mass spectra were taken over the m/z 30 to 500 range with an ionizing voltage of 70 eV. The individual compounds were identified by matching the acquired mass spectra with those stored in the reference libraries (National Institute of Standards and Technology), and lanosterol and cycloartenol were compared with samples of pure compounds (Sigma-Aldrich). A calibration curve was performed with lanosterol samples using 5-α-cholestan-3-one (Sigma-Aldrich) as internal standard.
Whole-Mount Staining and Laticifer Index
Entire plants were immersed in fixative (formaldehyde-acetic acid-ethanol, 3.5:10:50) overnight at 4°C. Plant sectors were washed with 70% ethanol and stained with Sudan Black B (0.1% [w/v] in 70% ethanol [Jensen, 1962]) for 3 to 4 h at room temperature, washed with 70% ethanol then with water, and placed in 2.5 m NaOH until the leaves were cleared (Ruzin, 1999). Tissues were observed under an EclipseE600 (Nikon) light microscope. LI was used to estimate the profusion of laticifer cells in a tissue and was calculated by measuring, with the ImageJ software, the total length (in mm) of the Sudan Black B-stained laticifer cells in a microscopic field area (in mm2).
Immunohistochemical Identification of Laticifers in Embryos
Embryos were fixed overnight with 4% paraformaldehyde and embedded in paraffin. Samples were sectioned on a HM330 microtome at 8 μm and were blocked and incubated with the monoclonal antibody LM6 [binding to a pectic polysaccharide, anti-(1-5)-α-l-arabinan; PlantProbes] diluted 1:20 in PBS containing 0.1% (w/v) BSA and 0.05% (v/v) Tween 20. Control slides were treated similarly with a nonspecific monoclonal diluted 1:20. An anti-rat IgG conjugated with alkaline phosphatase (Sigma), diluted 1:2000, was used as a secondary antibody, and sections were revealed with nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate.
Accession Numbers
Nucleotide sequence data for the genes described in this article are available from the GenBank database under the following accession numbers: ACAT (JQ434427), HMGCoAR (JQ694150), DXS (KT003670), DXR (JQ694151), SQS (JQ694152), SQE (JQ694153), CAS (JQ694154), SMT1 (JQ694155), EH (JQ694156), DHDDS (JQ694157), MLP (JQ694158), EG (JQ694159), PE (JQ694160), PEI (JQ694161), PL (JQ694162), and H3 (JQ966276).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Scheme summarizing the disposition and abundance of laticifer cells along the hypocotyl axis of E. lathyris seedlings.
Supplemental Figure S2. Scheme summarizing the disposition and abundance of laticifer cells along the stem and in leaves of an E. lathyris plant.
Supplemental Figure S3. SEM across the stem of a developing E. lathyris plant.
Supplemental Figure S4. SEM across the lamina of a developing E. lathyris leaf.
Supplemental Figure S5. Generation of E. lathyris tetraploid plants.
Supplemental Figure S6. Ontogeny and early distribution of the laticiferous system in the embryo of E. lathyris.
Supplemental Figure S7. Scheme summarizing the appearance and abundance of laticifer initials at different stages of embryo development in E. lathyris.
Supplemental Figure S8. RT-qPCR analysis of genes in latex and in intact plant organs in E. lathyris.
Supplemental Figure S9. Comparative triterpenoid GC-MS analysis of parental E. lathyris plants and pil mutants.
Supplemental Figure S10. Organization of the laticiferous system in E. lathyris in mature embryos of pil mutants in comparison to wild-type plants.
Supplemental Figure S11. Chimeric E. lathyris plants generated by grafting between wild-type and pil1 or pil6 plants.
Supplemental Figure S12. Appearance of pil mutants of E. lathyris grown under glasshouse conditions.
Supplemental Table S1. Marker genes selected for transcriptomic analysis in E. lathyris.
Supplemental Table S2. Segregation analysis in pil mutants of E. lathyris.
Supplemental Table S3. Primer sequences used in this study for RT-qPCR analyses in E. lathyris.
Supplementary Material
Acknowledgments
We thank Javier Paz-Ares and Antonio Leyva for critical reading of the manuscript, Carlos Alonso for assistance during seed mutagenesis, Vicente Ramírez for helpful discussions, and Maria Dolores Arocas and Marina Moliner for taking care of the plants.
Glossary
- SAM
shoot apical meristem
- DAPI
4′,6-diamino-phenylindole
- GC-MS
gas chromatography-mass spectrometry
- LI
laticifer index
Footnotes
This work was supported by the Spanish MINECO (BFU2015-68199-R to P.V.) and Generalitat Valenciana (Prometeo 2014/024 to P.V.).
References
- Agrawal AA, Konno K (2009) Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu Rev Ecol Evol Syst 40: 311–331 [Google Scholar]
- Bouteau F, Lacrotte RL, Cornel D, Monestiez M, Bousquet U, Pennarun AM, Rona JP (1991) Electrogenic active proton pumps in Hevea brasiliensis laticiferous cells: its role in activating sucrose/H+ and glucose/H+ symports at the plasma membrane. Bioelectrochem Bioenerg 26: 223–236 [Google Scholar]
- Chauveaud LG. (1891) Recherches Embryogéniques sur l’Appareil Laticifère des Euphorbiacées, Urticacées, Apocynées et Asclépiadées. Ann Sci Nat Bot 14: 1–160 [Google Scholar]
- De Bary A. (1884) Comparative Anatomy of the Vegetative Organs of the Phanerogams and Ferns. Clarendon Press, Oxford, UK [Google Scholar]
- Dobón A, Canet JV, García-Andrade J, Angulo C, Neumetzler L, Persson S, Vera P (2015) Novel disease susceptibility factors for fungal necrotrophic pathogens in Arabidopsis. PLoS Pathog 11: e1004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussourd DE, Eisner T (1987) Vein-cutting behavior: insect counterploy to the latex defense of plants. Science 237: 898–901 [DOI] [PubMed] [Google Scholar]
- Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T (1996) The SHOOT MERISTEMLESS gene is required for maintenance of undifferentiated cells in Arabidopsis shoot and floral meristems and acts at a different regulatory level than the meristem genes WUSCHEL and ZWILLE. Plant J 10: 967–979 [DOI] [PubMed] [Google Scholar]
- Esau K. (1965) Plant Anatomy. Wiley, New York [Google Scholar]
- Fahn A. (1990) Plant Anatomy, Ed 4 Pergamon Press, Oxford, UK [Google Scholar]
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Hagel JM, Yeung EC, Facchini PJ (2008) Got milk? The secret life of laticifers. Trends Plant Sci 13: 631–639 [DOI] [PubMed] [Google Scholar]
- Huber M, Epping J, Schulze Gronover C, Fricke J, Aziz Z, Brillatz T, Swyers M, Köllner TG, Vogel H, Hammerbacher A, et al. (2016) A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol 14: e1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen WA. (1962) Botanical Histochemistry: Principles and Practice. W.H. Freeman and Company, San Francisco [Google Scholar]
- Kekwick RG. (2002) Latex and laticifers. In eLS. John Wiley & Sons, Chichester, UK, www.els.net, doi/0.1038/npg.els.0000913 [Google Scholar]
- Konno K. (2011) Plant latex and other exudates as plant defense systems: roles of various defense chemicals and proteins contained therein. Phytochemistry 72: 1510–1530 [DOI] [PubMed] [Google Scholar]
- Koops AJ, Baas WJ, Groeneveld HW (1991) The composition of phytoesterols, latex triterpenols and wax triterpenoids in the seedling of E. lathyris L. Plant Sci 74: 185–191 [Google Scholar]
- Lewinsohn TM. (1991) The geographical distribution of plant latex. Chemoecology 2: 64–68 [Google Scholar]
- López A, Ramírez V, García-Andrade J, Flors V, Vera P (2011) The RNA silencing enzyme RNA polymerase v is required for plant immunity. PLoS Genet 7: e1002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlberg PG. (1959) Karyokinesis in the non-articulated laticifers of Nerium oleander L. Phytomorphology 9: 110–118 [Google Scholar]
- Mahlberg PG. (1961) Embryogeny and histogenesis in Nerium oleander L. II. Origin and development of the non-articulated laticifers. Am J Bot 48: 90–99 [Google Scholar]
- Mahlberg PG. (1963) Development of non-articulated laticifer in seedling axis of Nerium oleander L. Bot Gaz 124: 224–231 [Google Scholar]
- Mahlberg PG. (1993) Laticifers: an historical perspective. Bot Rev 59: 1–23 [Google Scholar]
- Mahlberg P, Sabharwal P (1966) Mitotic waves in laticifers of Euphorbia marginata. Science 152: 518–519 [DOI] [PubMed] [Google Scholar]
- Mahlberg PG, Sabharwal PS (1968) Origin and early development of non-articulated laticifers in embryos of Euphorbia marginata. Am J Bot 55: 375–381 [Google Scholar]
- Metcalfe CR. (1967) Distribution of latex in the plant kingdom. Econ Bot 21: 115–127 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nessler CL, Mahlberg PG (1979) Ultrastructure of laticifers in redifferentiated organs on callus from Papaver somniferum (Papaveraceae). Can J Bot 57: 675–685 [Google Scholar]
- Nessler CL, Mahlberg PG (1981) Cytochemical localization of cellulase activity in articulated anastomosing laticifers of Papaver somniferum L. (Papaveraceae). Am J Bot 68: 730–732 [Google Scholar]
- Pickard WF. (2008) Laticifers and secretory ducts: two other tube systems in plants. New Phytol 177: 877–888 [DOI] [PubMed] [Google Scholar]
- Ruzin SE. (1999) Plant Microtechnique and Microscopy. Oxford University Press, New York [Google Scholar]
- Sacchetti G, Ballero M, Serafini M, Romagnoli C, Bruni A, Poli F (1999) Laticifer tissue distribution and alkaloid location in Vinca sardoa (Stearn) Pign. (Apocynaceae), an endemic plant of Sardinia (Italy). Phyton 39: 265–275 [Google Scholar]
- Santana MA, Vásquez V, Matehus J, Aldao RR (2002) Linamarase expression in cassava cultivars with roots of low- and high-cyanide content. Plant Physiol 129: 1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffstein G. (1932) Untersuchungen an ungegliederten Milchröhren. Botanisches Centralblatt 49: 197–220 [Google Scholar]
- Schmalhausen J. 1877. Beiträge zur Kenntnis der Milchsaftbehälter. Mémoiries de l’Académie Impériale des Sciences de St. Pétersbourg 7: 1–27 [Google Scholar]
- Smulders MJM, Rus-Kortekaas W, Gillissen LJW (1994) Development of polysomaty during differentiation in diploid and tetraploid tomato (Solanum lycopersicon) plants. Plant Sci 97: 53–60 [Google Scholar]
- St-Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11: 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranová E, Coman D, Gruissem W (2013) Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu Rev Plant Biol 64: 665–700 [DOI] [PubMed] [Google Scholar]
- Willats WGT, Marcus SE, Knox JP (1998) Generation of monoclonal antibody specific to (1-->5)-α-L-arabinan. Carbohydr Res 308: 149–152 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.