Breast shielding impaired coronary CT angiography image quality but had no significant effect on γ-H2AX foci levels among blood samples exposed to ex vivo radiation under breast shields or in vivo radiation of circulating lymphocytes.
Abstract
Purpose
To examine the effect of breast shielding on blood lymphocyte deoxyribonucleic acid (DNA) double-strand-break levels resulting from in vivo radiation and ex vivo radiation at breast-tissue level, and the effect of breast shielding on image quality.
Materials and Methods
The study was approved by institutional review and commpliant with HIPAA guidelines. Adult women who underwent 64-section coronary computed tomographic (CT) angiography and who provided informed consent were prospectively randomized to the use (n = 50) or absence (n = 51) of bismuth breast shields. Peripheral blood samples were obtained before and 30 minutes after in vivo radiation during CT angiography to compare DNA double-strand-break levels by γ-H2AX immunofluorescence in blood lymphocytes. To estimate DNA double-strand-break induction at breast-tissue level, a blood sample was taped to the sternum for ex vivo radiation with or without shielding. Data were analyzed by linear regression and independent sample t tests.
Results
Breast shielding had no effect on DNA double-strand-break levels from ex vivo radiation of blood samples under shields at breast-tissue level (unadjusted regression: β = .08; P = .43 versus no shielding), or in vivo radiation of circulating lymphocytes (β = −.07; P = .50). Predictors of increased DNA double-strand-break levels included total radiation dose, increasing tube potential, and tube current (P < .05). With current radiation exposures (median, 3.4 mSv), breast shielding yielded a 33% increase in image noise and 19% decrease in the rate of excellent quality ratings.
Conclusion
Among women who underwent coronary CT angiography, breast shielding had no effect on DNA double-strand-break levels in blood lymphocytes exposed to in vivo radiation, or ex vivo radiation at breast-tissue level. At present relatively low radiation exposures, breast shielding contributed to an increase in image noise and a decline in image quality. The findings support efforts to minimize radiation by primarily optimizing CT settings.
© RSNA, 2016
Clinical trial registration no. NCT02617888
Introduction
Cardiovascular computed tomography (CT) is an established technique with numerous applications for the evaluation of patients suspected of having cardiovascular diseases (1). With expanded indications for cardiovascular CT and increased use of diagnostic imaging, both patients and clinicians note concerns regarding the potential genotoxic effects of ionizing radiation (2–4). In response, imaging providers led numerous efforts to minimize radiation exposure with the development of appropriate use criteria (1), and guidelines to optimize radiation exposure as low as reasonably achievable (5). As a result, modern cardiovascular CT techniques significantly reduced radiation exposures (6,7). By example, the median radiation dose from cardiovascular CT decreased by more than 30% without image quality degradation in a 3-year period between 2008 and 2011 across 15 imaging centers in the Advanced Cardiovascular Imaging Consortium (7).
Despite this progress, many patients are not eligible for low-dose cardiovascular CT protocols because of a variety of factors (eg, high body mass index, high heart rate despite rate control medications, or arrhythmias). Moreover, provider experience and access to modern CT technology varies, and particular concern remains in the exposure of young women and pediatric patients to ionizing radiation. In these patients, efforts to limit radiation exposure expanded to include shielding of radiosensitive organs during CT. Although previous studies (8–10) reported an ability of shielding to lower CT radiation exposure by 30%–60% at the breast-tissue level, available data remains limited to phantom models and biodosimeters. To our knowledge, no studies examined the direct biologic effects of breast shielding. Additionally, previous studies demonstrated (5,11,12) that breast shielding may impair CT image quality by inducing streak artifacts and increasing image noise. Consequently, guidelines for the performance of coronary CT angiography recommend that until more conclusive data are available, shielding is not considered a routine tool to lower radiation exposure (11).
To examine the effect of radiation on cellular injury, immunofluorescence of the histone γ-H2AX was used to quantify deoxyribonucleic acid (DNA) double-strand-break levels with a linear dose response to radiation exposure (13). As a sensitive marker of DNA damage, enumeration of γ-H2AX foci was applied to in vivo and ex vivo human tissues to monitor DNA damage produced by low-level radiation from diagnostic imaging (14–16). In this study of women who underwent coronary CT angiography, we aimed to examine the effect of breast shielding on blood lymphocyte DNA double-strand-break levels resulting from in vivo radiation and ex vivo radiation at breast-tissue level, and the effect of breast shielding on image quality.
Materials and Methods
The study was approved by institutional review, compliant with Health Insurance Portability and Accountability Act guidelines, and registered at ClinicalTrials.gov (NCT02617888). All included patients provided written informed consent. Funding support was provided by the American Medical Association Foundation, National Institute of Allergy and Infectious Diseases Radiation/Nuclear Countermeasures Program, and the Intramural Research Program of the Center for Cancer Research (National Cancer Institute, Bethesda, Maryland). The authors are solely responsible for study design and conduct, all study analyses, the drafting and editing of the manuscript, and the final contents.
Patient Population
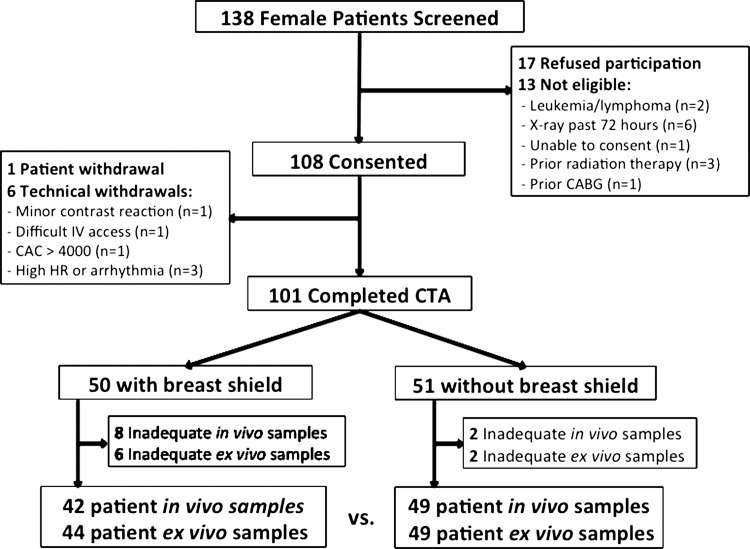
Female patients 18 years or older who were clinically referred to undergo coronary CT angiography between August 2012 and July 2014 at Walter Reed National Military Medical Center (Bethesda, Md) were eligible for enrollment. We excluded patients who had an inability to consent, were pregnant, had prior leukemia or lymphoma, had previous radiation therapy, underwent radiography or scintigraphy within 72 hours, or underwent previous coronary revascularization (Fig 1). Eligible patients who provided informed consent were randomized in a one-to-one ratio to the use or absence of breast shielding by a random allocation sequence (encoded by M.K.C. in Excel [Microsoft, Redmond, Wash]). Informed consent was obtained and randomization was performed at enrollment (P.D., A.S.K., M.K.C., and J.B., with 2, 6, 8, and 15 years of experience in clinical medicine and research). Patients were not blinded to the randomized intervention. Providers were blinded to shielding status in CT angiography planning, scan interpretation, and blood sample processing. Baseline characteristics were prospectively collected, including a history of current smoking, defined as cigarette or cigar use within 30 days. Chest circumference was recorded by a single observer (J.B.) with measurement tape at the nipple level.
Figure 1:
Schematic of patient enrollment. CABG = coronary artery bypass graft, CAC = coronary artery calcium score, CTA = coronary CT angiography, HR = heart rate, IV = intravenous.
Sample Collection and Processing
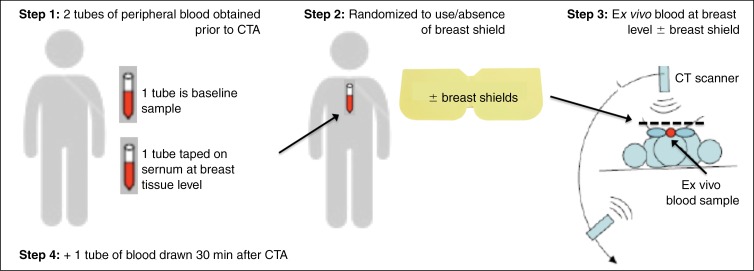
Peripheral blood samples were obtained in a fasting state before and 30 minutes after the patient underwent CT angiography (Fig 2), consistent with previous research (17,18) that demonstrated peak γ-H2AX foci levels 30 minutes after undergoing CT. Samples were collected by using 6-mL blood tubes with heparin. One tube of blood was taped on the sternum for ex vivo radiation at the breast-tissue level, under shielding in patients randomized to their use. To monitor conditions for double-strand break formation, blood sample temperatures were recorded with a calibrated, noncontact infrared thermometer (Model RY230; Santa Medical, Tustin, Calif) at baseline and 30 minutes after the patient underwent CT angiography in a subset of patients (60.4%; 61 of 101 patients). After collection, blood samples were placed on ice until further processing by an independent laboratory with 20 years of expertise in γ-H2AX analysis (WMB Laboratory; National Cancer Institute, Bethesda, Md) (14,18).
Figure 2a:
Schematic shows the study design. (a) Peripheral blood sample obtained before the patient underwent coronary CT angiography (CTA) provides baseline for comparison to repeat sample drawn 30 minutes after in vivo radiation exposure. A second tube of blood obtained prior to CT angiography is taped to the patient’s sternum for ex vivo radiation at the breast-tissue level with or without breast shielding (top right). (b) γ-H2AX foci induced by CT angiography, comparing baseline blood sample versus blood sample obtained 30 minutes after CT angiography. Green, γ-H2AX foci; red, DNA stained with propidium iodide.
Blood lymphocyte DNA double-strand-break levels were analyzed by two independent observers (A.S.B. and C.E.R., with 3 and 15 years of experience, respectively, in γ-H2AX analysis) blinded to clinical variables and shielding status by using previously described methods (Appendix E1 [online]) (18,19).
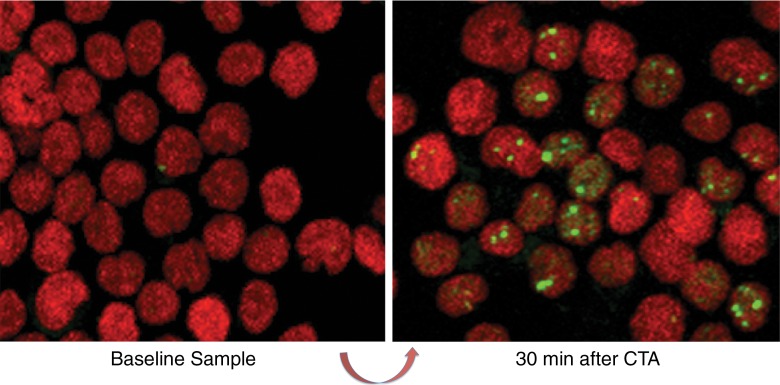
Images were obtained by using a laser-scanning confocal microscope (C.E.R., A.S.B.) (PCM-2000; Nikon, Augusta, Ga). Optical sections (0.5 μm) through the thickness of the sample were imaged and combined in a maximum projection with software (Simple32; Compix, Cranberry, Pa) so that all of the visible foci in a nucleus were recorded (Fig 2b). Manual counting of γ-H2AX foci in approximately 1000 cells per sample was performed by an experienced reader (C.E.R.) and compared with a software enumeration technique (A.S.B.). For this, projected images that used a tagged image file were processed by using publicly available software (ImageJ; National Institutes of Health, Bethesda, Md) with a customized macro to obtain the number of γ-H2AX foci (Appendix E2 [online]) (20). Excess γ-H2AX foci per cell was determined by subtracting postexposure levels from nonirradiated baseline controls, and represented the amount of radiation-induced foci.
Figure 2b:
Schematic shows the study design. (a) Peripheral blood sample obtained before the patient underwent coronary CT angiography (CTA) provides baseline for comparison to repeat sample drawn 30 minutes after in vivo radiation exposure. A second tube of blood obtained prior to CT angiography is taped to the patient’s sternum for ex vivo radiation at the breast-tissue level with or without breast shielding (top right). (b) γ-H2AX foci induced by CT angiography, comparing baseline blood sample versus blood sample obtained 30 minutes after CT angiography. Green, γ-H2AX foci; red, DNA stained with propidium iodide.
Breast Shielding
In patients randomized to shielding, four-ply bismuth breast shields (model 532708 AttenuRad; Cone Instruments, Caledonia, Mich) were used to cover the breasts. The shields consisted of a 1-mm-thick bismuth-impregnated synthetic rubber with a 6.35-mm foam offset, and were 0.06-mm lead equivalent. The foam offset was placed directly over the patient’s gown, covering the breasts and ex vivo blood samples taped to the sternum at breast-tissue level. The bismuth-impregnated synthetic rubber surface was directed anteriorly.
CT Angiographic Imaging
Coronary CT angiography was performed by using a 64-section CT scanner (Discovery CT 750 HD; GE Healthcare, Waukesha, Wis). Unless contraindicated, patients received before-scan heart rate control and sublingual nitroglycerin according to guidelines (11). By design, initial nonenhanced CT images were obtained in a majority of patients (96%) by using prospective electrocardiogram triggering with a constant 120-kV tube potential and fixed tube current. In patients randomized to their use, breast shields were placed after the initial topogram images and before the coronary artery calcium scan. CT angiography variables were determined by the imaging provider blinded to shielding and consistent with guidelines for radiation dose optimization (Fig E1 [online]) (5,11). Contrast agent–enhanced CT angiographic images were obtained by prospective electrocardiogram triggering (n = 100) or retrospective electrocardiogram gating with dose modulation after a timing bolus (n = 1). In all patients, the scan range covered the heart from just below the carina to the diaphragm.
For each patient, images were reconstructed by using Digital Imaging and Communications in Imaging data with a limited field of view to exclude breast shielding. All axial CT angiography images were reconstructed with a section thickness of 0.625 mm in overlapping 0.4-mm increments by using 50% adaptive statistical iterative reconstruction. CT angiography scans were interpreted jointly (by consensus) by a level-3–certified cardiologist and radiologist with best phase images blinded to shielding (T.C.V. and B.N., each with 15 years of experience in cardiovascular CT). By using an 18-segment model, each coronary segment larger than 1.5-mm diameter was assessed for coronary artery disease severity by visual grading as follows: normal (no plaque and no stenosis), nonobstructive (1%–49% stenosis), obstructive (≥50%), or uninterpretable (one or more uninterpretable segment). Image signal and noise were measured in the left main coronary ostium (12), with visual grading of overall image quality on a four-point Likert scale as follows: 1, uninterpretable; 2, poor but adequate for stenosis; 3, good with minor artifacts; and 4, excellent or no artifacts.
Radiation dose was recorded as dose-length product (in milligray centimeters) and effective dose (in millisieverts). Effective dose was calculated as the product of dose-length product and a conversion factor for the chest (κ = 0.014 mSv/mGy · cm) (21).
Statistical Analysis
Continuous variables with normal distributions are expressed as mean ± standard deviation and compared by Student t test for independent groups and one-way analysis of variance between groups. Continuous variables with nonnormal distributions are expressed as median ± interquartile range, and compared with Wilcoxon rank-sum test. Categorical variables are expressed as frequencies (in percent) and compared by Pearson χ2 test. Pearson correlation was performed to compare manual and software enumeration of γ-H2AX foci, with agreement by Bland-Altman analysis. Linear regression was used to demonstrate associations between excess γ-H2AX foci, patient characteristics, sample processing variables, CT angiography parameters, and radiation exposure. Unadjusted regression coefficient (β) is reported, and a two-tailed P value of less than .05 was considered to indicate statistical significance. To examine factors that decreased the likelihood of a CT angiography quality rating of excellent, we performed unadjusted and multivariable regression analysis, reporting odds ratio and 95% confidence intervals. All analyses were performed by using statistical software (Stata version 13.1; Statacorp, College Station, Tex) (M.K.C. and T.C.V.).
The study had a sufficient sample size with 80% power and significance level α of .01 to detect a difference of 0.15 Gy · cm in absorbed radiation dose between samples exposed to radiation with versus without shielding. To determine sample size, radiation dose was used as a proxy for excess γ-H2AX foci per cell on the basis of evidence that γ-H2AX foci formation exhibits a strong linear relationship with radiation exposure (R2 = 0.96) (17), and that a significant increase in γ-H2AX foci can be detected with a dose-length product as low as 0.15 Gy · cm (17).
Results
Study characteristics conform to the Consolidated Standards of Reporting Trials, or CONSORT, guidelines for reporting randomized clinical trials (22). Baseline characteristics are displayed in Table 1, with no significant differences between patients randomized to the use or absence of breast shielding. Overall, patients had a mean age of 52 years ± 14 (standard deviation) and were predominantly overweight, and 50% were white. Blood sample processing was similar between groups, with follow-up in vivo blood samples obtained at 36.5 minutes (interquartile range, 31–42 minutes) after CT angiography. CT angiography results are shown in Table 2, with no significant difference in CT angiography settings between groups. Total radiation exposure in all patients was relatively low (median radiation dose, 3.4 mSv [interquartile range, 2.5–4.2 mSv]).
Table 1.
Baseline Characteristics and Sample Processing

Note.—Data are mean ± standard deviation unless otherwise noted.
*Data in parentheses are percentages.
†Data are medians and data in parentheses are interquartile ratio.
‡Temperature immediately before placement of sample on ice, as recorded in a subset of patients.
Table 2.
CT Angiography Settings and Results
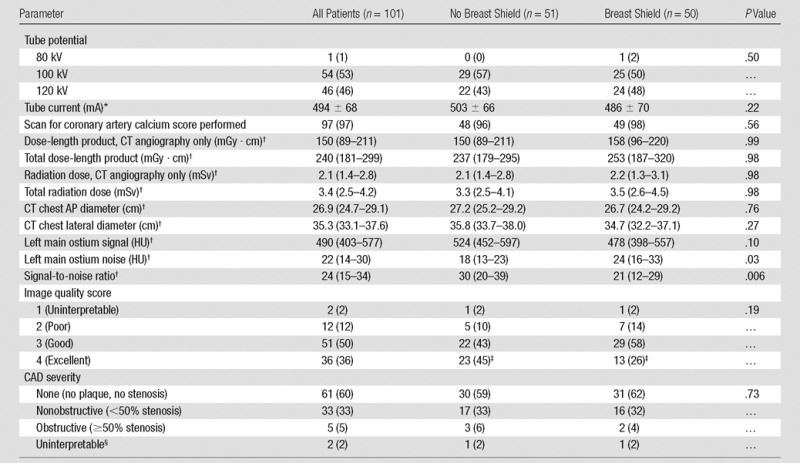
Note.—Data are numerators with percentages in parentheses unless otherwise indicated. AP = anterior-posterior, CAD = coronary artery disease.
*Data are mean ± standard deviation.
†Data are median; data in parentheses are interquartile range.
‡P value is .045 by comparing the frequency of image quality ratings of excellent between groups.
§Uninterpretable study defined as ≥1 uninterpretable coronary segment.
DNA Damage: Enumeration of γ-H2AX Foci
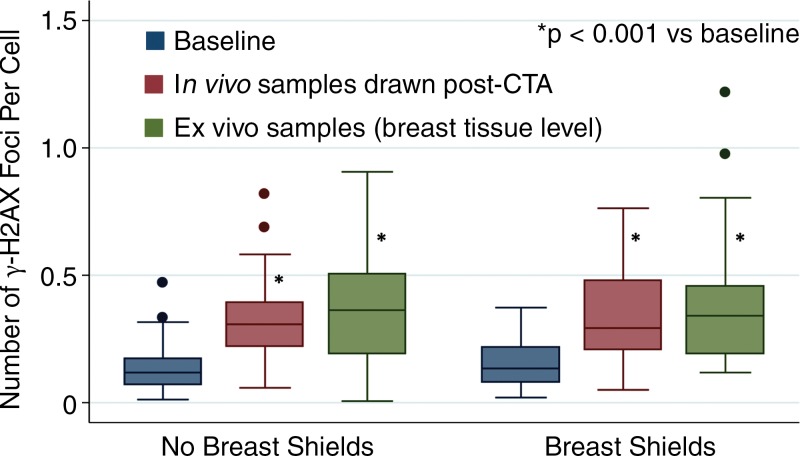
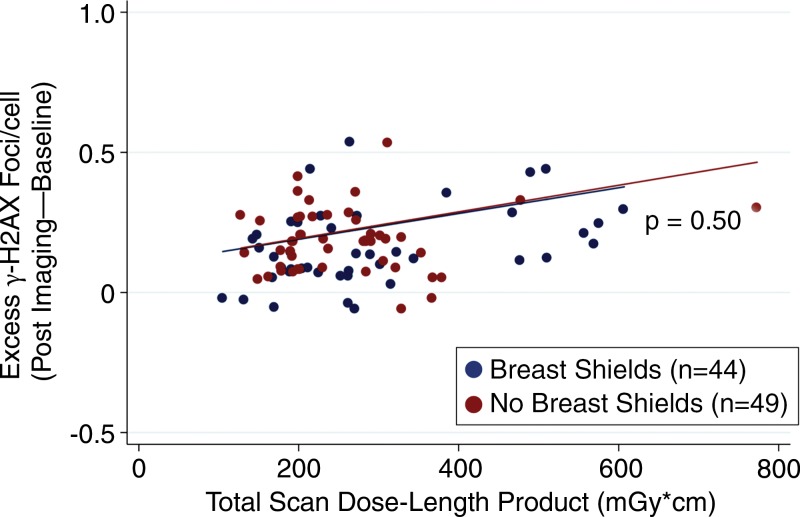
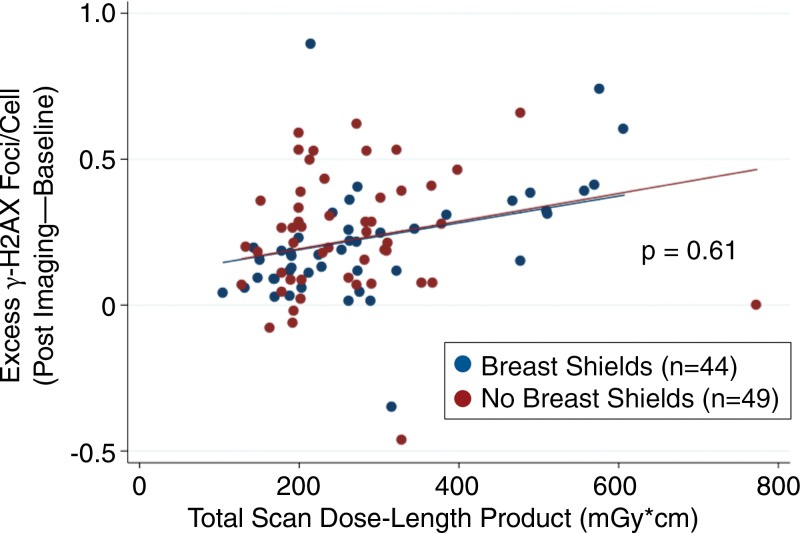
As expected, there was a statistically significant increase in γ-H2AX foci among in vivo and ex vivo blood samples after undergoing CT angiography compared with baseline (Fig 3). In Figure 3, note the higher median γ-H2AX foci levels of ex vivo samples compared with in vivo samples. We attribute this difference primarily to tissue attenuation decreasing radiation exposure to circulating in vivo samples, and the static nature of ex vivo samples (as with breast tissue) increasing radiation exposure at this level. Excess γ-H2AX foci per cell was determined by subtracting after-exposure levels from baseline controls, and it represents the amount of radiation-induced foci. By linear regression, breast shielding had no effect on excess γ-H2AX foci among in vivo blood samples (P = .50 vs no shielding; Fig 4). Additionally, breast shielding had no statistically significant effect on excess γ-H2AX foci in blood samples exposed to ex vivo radiation at breast-tissue level (P = .61 vs no shielding; Fig 5). Also noted in Figures 5 and 6 is the dose-dependent increase in excess γ-H2AX with increasing radiation exposures.
Figure 3:
Absolute number of γ-H2AX foci per cell at baseline versus after CT angiography (CTA). Findings demonstrate a significant increase in all samples after CT angiography compared with baseline.
Figure 4:
Scatterplot shows blood samples of excess γ-H2AX foci that resulted from in vivo radiation during coronary CT angiography. Excess DNA double-strand-break levels measured by γ-H2AX immunofluorescence before and after coronary CT angiography demonstrated no difference in DNA damage between patients randomized to the use versus absence of breast shielding (P = .50 between groups).
Figure 5:
Scatterplot shows γ-H2AX foci with and without breast shielding during coronary CT angiography and ex vivo blood sample radiation at breast-tissue level. Excess DNA double-strand-break levels measured by γ-H2AX immunofluorescence before and after coronary CT angiography provide an estimation of radiation-induced DNA damage. Findings demonstrate no difference in DNA double-strand-break levels in blood samples exposed to ex vivo radiation at the breast-tissue level among women randomized to the use or absence of breast shielding (P = .61 between groups).
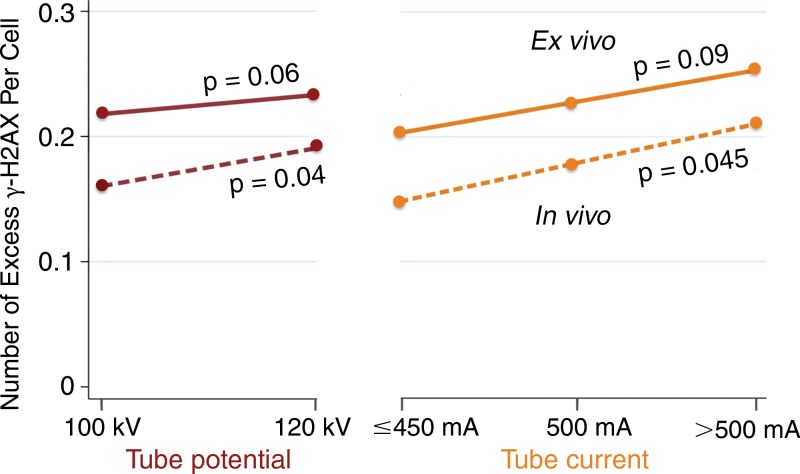
Figure 6:
Graph shows effect of CT angiography settings on excess γ-H2AX foci. Demonstrates increase in excess γ-H2AX foci per cell with increasing tube potential and range of tube current. Dot values represent an average of excess foci per cell among ex vivo (solid line) and in vivo (dashed line) samples. P values are unadjusted, between group comparisons by Student t test (tube potential) and one-way analysis of variance for multiple groups (tube current).
Among baseline clinical variables and CT angiography settings, only effective radiation dose demonstrated a statistically significant prediction for excess DNA double-strand-break levels by univariate regression (Table 3). Contributing to this effect, we observed a statistically significant increase in excess γ-H2AX foci levels across an increasing range of tube potential and tube current among in vivo blood samples, with a similar trend among ex vivo samples (Fig 6).
Table 3.
Unadjusted Regression Analysis to Examine Predictors of Excess γ-H2AX Foci
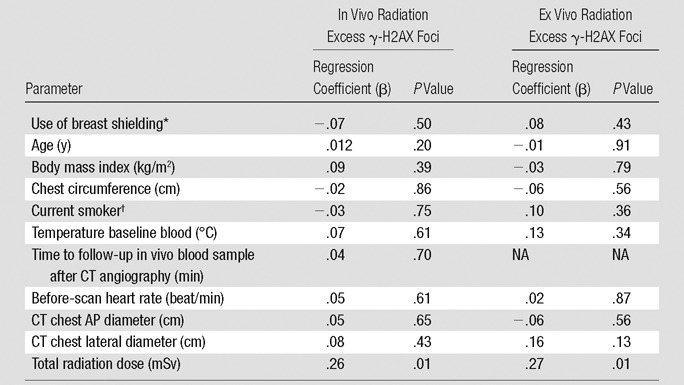
Note.—Excess γ-H2AX foci defined as the number of γ-H2AX foci 30 minutes after CT angiography minus the number of γ-H2AX foci at baseline. The regression coefficients (β) were obtained by unadjusted linear regression of excess DNA double-strand-break levels. AP = anterior-posterior, NA = not applicable.
*Compared with patients without breast shielding.
†Compared with nonsmokers.
CT Image Quality
Compared with no shielding, patients with breast shields demonstrated a 33% increase in image noise and a 30% decrease in signal-to-noise ratio (Table 2). While there was no difference between groups in the low rate of uninterpretable or poor-quality scans or uninterpretable segments, the rate of CT angiography studies graded as excellent decreased by 19% with shielding (P = .045 between groups; Table 2, Fig E2 [online]). By univariate analysis, predictors of decreased image quality included increasing body mass index, chest circumference, before-scan heart rate, and shielding (Table 4). By multivariate analysis, shielding was associated with a significant decrease in the likelihood of excellent image quality (adjusted odds ratio, 0.31 [95% confidence interval: 0.11, 0.86]; P = .03).
Table 4.
Unadjusted and Adjusted Odds Ratio of an Excellent CT Angiography Image Quality Rating
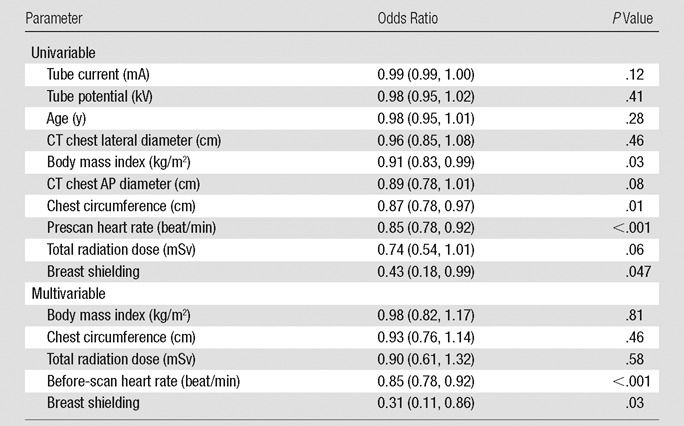
Note.—Data in parentheses are 95% confidence interval range. AP = anterior-posterior.
Manual versus Software Enumeration of γ-H2AX Foci
To ensure accurate quantification of γ-H2AX foci, we performed Pearson correlation between manual and software methods to enumerate γ-H2AX, which demonstrated excellent correlation between these techniques (r = 0.81; Fig E3 [online]). By Bland-Altman analysis, there was no relevant systematic difference between manual and software enumeration (mean difference, −0.18 foci per cell [95% confidence interval: −0.61, 0.25]; Fig E4 [online]).
Discussion
This single-center, prospective, randomized controlled trial was designed to compare the effect of breast shielding on DNA damage at breast-tissue level during coronary CT angiography, and the effect of breast shielding on CT image quality. In this study, we observed no significant effect of breast shields on excess γ-H2AX foci in blood samples exposed to in vivo radiation during CT angiography. This finding is noteworthy amid concerns that shielding may increase scatter radiation (23), and incorporates the effect of tissue attenuation on in vivo radiation. Further, we observed that shielding had no effect on γ-H2AX foci that resulted from ex vivo radiation of blood samples at breast-tissue level.
Although previous biodosimetry and phantom studies demonstrated the potential for shielding to reduce radiation exposures to breast tissue by 30%–60% (8–10), the risks versus benefits of breast shielding remain controversial. In a summary of the literature, nine studies or reviews supported breast shielding, while five studies recommended the use of alternative techniques to lower radiation or discouraged breast shields altogether (24). By incorporating available evidence, the Society of Cardiovascular CT recommends against the routine use of breast shielding (5,11), while an American College of Radiology white paper (25) recommends that technologists may need to use individualized shielding.
The primary concern with shielding remains the potential to impair image quality. In a study of coronary CT angiography scans obtained before 2010 from our institution, breast shielding increased image noise by 14% versus no shielding among scans performed with retrospective gating (radiation dose, 13.4–16.1 mSv) (12). By comparison, 99.0% (100 of 101) of scans in the current study were acquired with prospective electrocardiogram triggering, with a median radiation dose of 3.4 mSv (interquartile range, 2.5–4.2 mSv). At present relatively low radiation exposures, breast shielding yielded a 33% increase in image noise and a 30% decrease in signal-to-noise ratio. After adjustment for patient and CT angiography factors, breast shielding contributed to a statistically significant decrease in the likelihood of an excellent quality rating.
In summary, breast shielding impaired coronary CT angiography image quality but had no statistically significant effect on γ-H2AX foci levels among blood samples exposed to ex vivo radiation under breast shields or in vivo radiation of circulating lymphocytes. Though reasons why breast shielding did not affect γ-H2AX foci levels in our study remain unclear, there is concern that shielding may cause attenuation of photons both entering and exiting shields at breast level. Moreover, optimal shielding should not change the abundance of characteristic peaks, while removing higher and lower energy photons. However, the optimal shield type and thickness needed to target variable CT settings (80–120 kV) and scanners (single vs dual source, and dual energy) remains unclear. With advancements in CT hardware and software, strategies aimed at optimizing CT settings remain a primary tool to lower radiation. In our study, predictors of excess γ-H2AX foci included total radiation dose, increasing tube potential, and tube current. Similarly, Brand et al (26) found a statistically significant decrease in γ-H2AX foci levels with low-radiation-dose CT angiography protocols by comparison to a low-pitch helical method.
Though not the focus of our investigation, some advocate for the displacement of mobile breast tissue outside the CT scan range as an alternative to breast shielding (27,28). By this approach, breast displacement may lower direct radiographic exposure to breast tissue while reducing photon attenuation and minimizing the dose required to achieve a diagnostic CT angiography. Previously, breast displacement reduced effective radiation exposure by approximately 33% when combined with an automated tube potential and tube current algorithm in women who underwent coronary CT angiography (27). Similarly, Foley et al (28) randomized patients who underwent coronary CT angiography to a control group (n = 16), breast displacement (n = 22), or breast displacement and shielding (n = 16). Compared with control patients, mean breast surface dose with thermoluminescent dosimetry was reduced by 23% with breast displacement versus a 36% reduction by the combination of breast displacement and shielding.
Our study has several inherent limitations. Patients were enrolled at a single institution, and thus our findings may not be generalizable to other CT scanners or populations. Consequently, larger studies across multiple centers are needed. For comparison with previous studies, we used four-ply bismuth breast shields for adult patients. Variable shield types (eg, tungsten-antimony) and shield sizes were not studied. By design, ex vivo blood samples in our study provided a surrogate measure of the biologic effects of radiation at breast-tissue level. Consequently, further work is needed to examine tissue-specific responses (eg, breast, thyroid, and gonads) to radiation and shielding. Though thermoluminescent dosimetry badges were not available, future examinations may also benefit from correlation of direct biologic markers with surface measures of local radiation exposure. While γ-H2AX is a well-studied technique to estimate DNA damage with a linear dose response to radiation, alternative methods to quantify cellular changes were not performed (29). We excluded patient factors that may affect γ-H2AX foci and handled all samples in a similar fashion, blinded to patient data. Nonetheless, unobserved patient and technical confounders may contribute to individual variations in DNA damage, repair, and detection (30). Finally, while previous studies estimated the lifetime attributable risk of cancer from low-level diagnostic radiation exposure, the long-term risk of diagnostic imaging on cancer development remains uncertain. Hence, our study reports data regarding the potential effect of breast shielding on DNA damage, but it does not equate to cancer risk and should be interpreted with caution.
Advances in Knowledge
■ There was no statistically significant effect of breast shields on γ-H2AX foci in blood samples exposed to ex vivo radiation under shields at breast-tissue level during coronary CT angiography (unadjusted regression: β = .08; P = .43, shielding vs no shielding).
■ There was no statistically significant effect of breast shields on γ-H2AX foci in blood samples exposed to in vivo radiation during coronary CT angiography (unadjusted regression: β = −.07; P = .50, shielding vs no shielding).
■ Significant predictors of increased γ-H2AX foci levels during coronary CT angiography included total radiation dose, increasing tube potential, and tube current.
■ Compared with no shielding, breast shielding increased image noise by 33% at current radiation exposure levels (median dose, 3.4 mSv), and the rate of CT studies rated excellent decreased by 19% with breast shielding.
Implication for Patient Care
■ This study supports current efforts to minimize radiation exposures by primary attention to the optimization of CT settings, but found no evidence of a direct biologic benefit with breast shielding.
APPENDIX
SUPPLEMENTAL FIGURES
Received October 28, 2015; revision requested December 14 and received January 23, 2016; accepted February 3; final version accepted February 5.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
M.K.C. supported by American Medical Association Foundation. W.M.B. supported by Radiation Nuclear Countermeasure Program (National Institute of Allergy and Infectious Diseases). W.M.B. supported by Intramural Research Program of the Center for Cancer Research (National Cancer Institute, National Institutes of Health, Bethesda, Maryland).
M.K.C. and C.E.R. contributed equally to this work.
Disclosures of Conflicts of Interest: The opinions herein and assertions contained are the authors’ alone, and do not constitute endorsement by the United States Army, Department of Defense, United States Army Office of the Surgeon General, United States Government, or the National Institutes of Health. M.K.C. disclosed no relevant relationships. C.E.R. disclosed no relevant relationships. A.S.B. disclosed no relevant relationships. A.S.K. disclosed no relevant relationships. J.B. disclosed no relevant relationships. D.M. disclosed no relevant relationships. H.B. disclosed no relevant relationships. A.P. disclosed no relevant relationships. P.W. disclosed no relevant relationships. P.D. disclosed no relevant relationships. B.N. disclosed no relevant relationships. W.M.B. disclosed no relevant relationships. T.C.V. disclosed no relevant relationships.
Abbreviation:
- DNA
- deoxyribonucleic acid
References
- 1.Taylor AJ, Cerqueira M, Hodgson JM, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol 2010;56(22):1864–1894. [DOI] [PubMed] [Google Scholar]
- 2.Bhargavan M. Trends in the utilization of medical procedures that use ionizing radiation. Health Phys 2008;95(5):612–627. [DOI] [PubMed] [Google Scholar]
- 3.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA 2012;307(22):2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith-Bindman R. Environmental causes of breast cancer and radiation from medical imaging: findings from the Institute of Medicine report. Arch Intern Med 2012;172(13):1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliburton SS, Abbara S, Chen MY, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5(4):198–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raff GL. Radiation dose from coronary CT angiography: five years of progress. J Cardiovasc Comput Tomogr 2010;4(6):365–374. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan KM, Boura JA, DePetris A, et al. Progressive radiation dose reduction from coronary computed tomography angiography in a statewide collaborative quality improvement program: results from the Advanced Cardiovascular Imaging Consortium. Circ Cardiovasc Imaging 2013;6(5):646–654. [DOI] [PubMed] [Google Scholar]
- 8.Geleijns J, Salvadó Artells M, Veldkamp WJ, López Tortosa M, Calzado Cantera A. Quantitative assessment of selective in-plane shielding of tissues in computed tomography through evaluation of absorbed dose and image quality. Eur Radiol 2006;16(10):2334–2340. [DOI] [PubMed] [Google Scholar]
- 9.Parker MS, Kelleher NM, Hoots JA, Chung JK, Fatouros PP, Benedict SH. Absorbed radiation dose of the female breast during diagnostic multidetector chest CT and dose reduction with a tungsten-antimony composite breast shield: preliminary results. Clin Radiol 2008;63(3):278–288. [DOI] [PubMed] [Google Scholar]
- 10.Chung JJ, Cho ES, Kang SM, Yu JS, Kim DJ, Kim JH. Usefulness of a lead shielding device for reducing the radiation dose to tissues outside the primary beams during CT. Radiol Med (Torino) 2014;119(12):951–957. [DOI] [PubMed] [Google Scholar]
- 11.Abbara S, Arbab-Zadeh A, Callister TQ, et al. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3(3):190–204. [DOI] [PubMed] [Google Scholar]
- 12.Hulten E, Devine P, Welch T, et al. Comparison of coronary CT angiography image quality with and without breast shields. AJR Am J Roentgenol 2013;200(3):529–536. [DOI] [PubMed] [Google Scholar]
- 13.Rothkamm K, Horn S. gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita 2009;45(3):265–271. [PubMed] [Google Scholar]
- 14.Redon CE, Nakamura AJ, Zhang YW, et al. Histone gammaH2AX and poly(ADP-ribose) as clinical pharmacodynamic biomarkers. Clin Cancer Res 2010;16(18):4532–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redon CE, Weyemi U, Parekh PR, Huang D, Burrell AS, Bonner WM. γ-H2AX and other histone post-translational modifications in the clinic. Biochim Biophys Acta 2012;1819(7):743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett 2012;327(1-2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Löbrich M, Rief N, Kühne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102(25):8984–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. γ-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res 2009;43(8):1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redon CE, Nakamura AJ, Sordet O, et al. γ-H2AX detection in peripheral blood lymphocytes, splenocytes, bone marrow, xenografts, and skin. Methods Mol Biol 2011;682:249–270. [DOI] [PubMed] [Google Scholar]
- 20.Cai Z, Chen Z, Bailey KE, Scollard DA, Reilly RM, Vallis KA. Relationship between induction of phosphorylated H2AX and survival in breast cancer cells exposed to 111In-DTPA-hEGF. J Nucl Med 2008;49(8):1353–1361. [DOI] [PubMed] [Google Scholar]
- 21.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA 2009;301(5):500–507. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KF, Altman DG, Moher D; Group CONSORT. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geleijns J, Wang J, McCollough C. The use of breast shielding for dose reduction in pediatric CT: arguments against the proposition. Pediatr Radiol 2010;40(11):1744–1747. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Oates ME. CT bismuth breast shielding: is it time to make your own decision? J Am Coll Radiol 2012;9(12):856–858. [DOI] [PubMed] [Google Scholar]
- 25.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol 2007;4(5):272–284. [DOI] [PubMed] [Google Scholar]
- 26.Brand M, Sommer M, Achenbach S, et al. X-ray induced DNA double-strand breaks in coronary CT angiography: comparison of sequential, low-pitch helical and high-pitch helical data acquisition. Eur J Radiol 2012;81(3):e357–e362. [DOI] [PubMed] [Google Scholar]
- 27.Vadvala H, Kim P, Mayrhofer T, et al. Coronary CTA using scout-based automated tube potential and current selection algorithm, with breast displacement results in lower radiation exposure in females compared to males. Cardiovasc Diagn Ther 2014;4(6):470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley SJ, McEntee MF, Achenbach S, Brennan PC, Rainford LS, Dodd JD. Breast surface radiation dose during coronary CT angiography: reduction by breast displacement and lead shielding. AJR Am J Roentgenol 2011;197(2):367–373. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen PK, Lee WH, Li YF, et al. Assessment of the radiation effects of cardiac CT angiography using protein and genetic biomarkers. JACC Cardiovasc Imaging 2015;8(8):873–884. [Published correction appears in JACC Cardiovasc Imaging 2015;8(11):1350.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojewodzka M, Sommer S, Kruszewski M, et al. Defining blood processing parameters for optimal detection of γ-H2AX foci: a small blood volume method. Radiat Res 2015;184(1):95–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.