Abstract
Aims
The interest in unicompartmental knee arthroplasty (UKA) for medial osteoarthritis has increased rapidly but the long-term follow-up of the Oxford UKAs has yet to be analysed in non-designer centres. We have examined our ten- to 15-year clinical and radiological follow-up data for the Oxford Phase III UKAs.
Patients and Methods
Between January 1999 and January 2005 a total of 138 consecutive Oxford Phase III arthroplasties were performed by a single surgeon in 129 patients for medial compartment osteoarthritis (71 right and 67 left knees, mean age 72.0 years (47 to 91), mean body mass index 28.2 (20.7 to 52.2)). Both clinical data and radiographs were prospectively recorded and obtained at intervals. Of the 129 patients, 32 patients (32 knees) died, ten patients (12 knees) were not able to take part in the final clinical and radiological assessment due to physical and mental conditions, but via telephone interview it was confirmed that none of these ten patients (12 knees) had a revision of the knee arthroplasty. One patient (two knees) was lost to follow-up.
Results
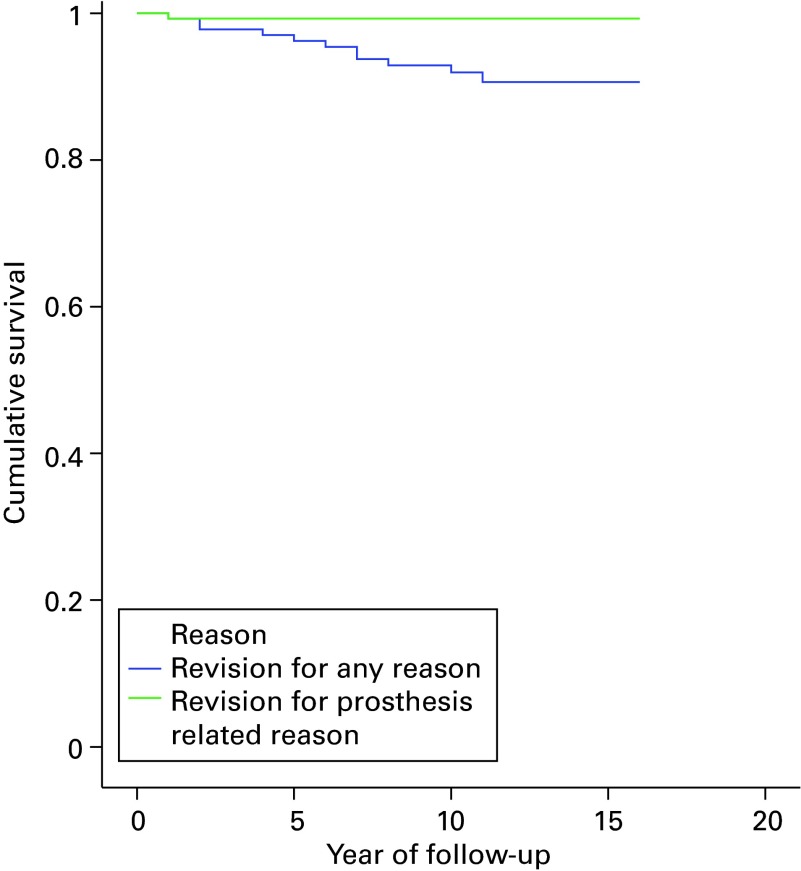
The mean follow-up was 11.7 years (10 to 15). A total of 11 knees (8%) were revised. The survival at 15 years with revision for any reason as the endpoint was 90.6% (95% confidence interval (CI) 85.2 to 96.0) and revision related to the prosthesis was 99.3% (95% CI 97.9 to 100). The mean total Knee Society Score was 47 (0 to 80) pre-operatively and 81 (30 to 100) at latest follow-up. The mean Oxford Knee Score was 19 (12 to 40) pre-operatively and 42 (28 to 55) at final follow-up. Radiolucency beneath the tibial component occurred in 22 of 81 prostheses (27.2%) without evidence of loosening.
Conclusion
This study supports the use of UKA in medial compartment osteoarthritis with excellent long-term functional and radiological outcomes with an excellent 15-year survival rate.
Cite this article: Bone Joint J 2016;98-B(10 Suppl B):41–7.
Keywords: Knee, Unicompartmental, Minimally invasive surgical technique, Long-term outcome, Non-designer
Interest in unicompartmental knee arthroplasty (UKA) for medial osteoarthritis has increased rapidly over the last two decades.1 The main reasons for its rising popularity are the introduction of minimally invasive surgical (MIS) techniques2,3 with modified surgical instruments, the publication of the excellent medium- and long-term results of the Oxford Phase II arthroplasty (Zimmer Biomet Ltd, Swindon, United Kingdom)4-7 and the well documented improved polyethylene wear characteristics of the mobile bearing device.8 Medial osteoarthritis of the knee is considered to be a unicompartmental disease and, when left untreated, may later progress to involve the other knee compartments.9 This has given rise to the rationale for treatment of only one compartment, either with a high tibial osteotomy (HTO) or a UKA. We describe our experience of using the Oxford Phase III (Zimmer Biomet Ltd) prosthesis, with a minimally invasive technique, implanted by a single surgeon and focuses on post-operative knee function, number and reason for revision operations, pain and radiological results. The medium-term outcome of the Oxford Phase IIIUKA is reported in other studies.10-13 We hypothesise that this study demonstrates the effectiveness and safety of a minimally invasive surgical approach for implanting the Oxford UKA with good to excellent long-term follow-up. This is the first study that reports the survival, clinical and radiological outcomes of the Oxford Phase III UKA after a minimum of ten years follow-up.
Materials and Methods
Between January 1999 and January 2005, 138 medial Oxford Phase III arthroplasties (129 patients) were performed in a district general hospital by a single surgeon (AEL). There were no one-stage bilateral UKAs. All patients were diagnosed with medial compartment osteoarthritis of the knee based on history, physical examination and radiographs: short-length weight-bearing anteroposterior (AP), lateral, axial patellar view and tunnel view. Stress radiographs were done on indication when clinical examination showed some medial collateral ligament stiffness. The strict indication criteria for UKA were followed.14,15 Osteoarthritis of the patellofemoral joint and obesity were not considered contraindications for this procedure. The patients’ demographic details are shown in Table I. Medium-term (mean follow-up 4.2 years, 1 to 10.4) results of this Oxford Phase III cohort were reported in 2011.13This report is a follow-up study of the original patient cohort with a minimal ten years’ follow-up.
Table I.
Demographic baseline characteristics of 138 knees in 129 patients treated by means of unicompartmental knee arthroplasty for medial compartment osteoarthritis
| Number of prosthesis | n = 138 (129 patients) |
|---|---|
| Side | 71 right; 67 left |
| Age (yrs), median (range), IQR | 72.0 (47 to 91), IQR 12.0 |
| Body mass index (kg/m2), mean sd (range), IQR | 28.2 sd 4.8 (20.7 to 52.2), IQR 5.2 |
| Operation time (mins) mean sd (range), IQR | 71.5 sd 13.7 (50 to 120), IQR 10.0 |
IQR, interquartile range; sd, standard deviation
A total of 32 patients (32 knees) died in the study period (mean 6.7 years post-operatively, 1 to 11.5), none of them as a result of the surgery. These patients were analysed until the latest follow-up recorded. Among these patients one UKA was revised to a total knee arthroplasty (TKA) for disease progression of the lateral compartment. A total of ten patients (12 knees) did not attend the outpatient clinic for their last follow-up due to general health related reasons. These patients or their relatives were subsequently interviewed by telephone and none of them had undergone a revision operation. One patient (two knees) was considered as lost to follow-up. A total of 11 patients (11 knees) were revised to TKA. In total 75 patients (81 knees) were assessed at the outpatient clinic for a final follow-up at a minimum ten years. This study was performed as routine follow-up and examination was performed in accordance with generally accepted practice. Approval was obtained from our institutional review board.
Surgical technique
The cemented Oxford Phase III UKA consists of cobalt chromium molybdenum spherical femoral and flat tibial component on which a fully congruent polyethylene mobile bearing is seated. The MIS operation technique has been described in detail by Price et al.16 The instruments available not only allow better component positioning compared with the Phase II implant, but also create a reproducible balance of the flexion and extension gap to achieve improved stability. Before cementing, pulsed lavage is used to rinse the subchondral bone. Full weight-bearing was allowed immediately post-operatively and thromboprophylaxis (Fraxiparine 2850 IU, GlaxoSmithKline, Zeist, The Netherlands) was prescribed for six weeks.
Outcome measures
The clinical follow-up consisted of a routine physical examination of the knee with range of movement (ROM) and stability testing, registration of pain and satisfaction with the visual analogue scale (VAS, 0 to 10 best to worst), complications and a standard series of radiographs: short-length weight-bearing AP, lateral and axial patellar views. Patients attended the routine follow-up assessments in the outpatient clinic scheduled at six weeks, six months, and two, five, ten and 15 years. Revision was defined as any surgical procedure that resulted in the removal or exchange of any of the arthroplasty components. Pain, function and health-related quality of life were evaluated pre- and post-operatively by patient- and assessor-based outcome scores validated in Dutch. The Western Ontario and McMaster Universities Arthritis Index (WOMAC Score),17 Oxford Knee Score,18,19 the Knee Society Score (KSS)20,21 and VAS for pain and satisfaction were used.22,23A limiting factor in the study design was that the pre-operative pain VAS was not included from the start. We continued to only use the VAS post-operatively once it was added to the study protocol.
The accuracy of implant positioning (varus, valgus, flexion and extension of the implant) was determined by short-length weight-bearing AP and lateral knee radiographs on first outpatient assessment and then at routine outpatient clinic visits. A fluoroscopic-centred technique, in which the x-ray beam was perfectly aligned to be perpendicular to the implant interfaces as described by Gulati et al,24 was applied by the senior author (AEL) to assess any (partial or complete) radiolucency at the bone-cement interface above the femoral component and under the tibial component. A radiolucent line < 2 mm width with a sclerotic line beneath the tibial component was considered to be physiological. Any line > 2 mm without a thin sclerotic bordering line was considered as a pathological radiolucency.25Partial or complete radiolucency refers to the extent of the line bordering the component.24The presence and extent of radiolucency were investigated in 75 available patients (81 knees).
Statistical analysis
A survival table was constructed and the cumulative rates were calculated using the Kaplan–Meier survival analysis26with a 95% confidence interval (CI).27 Failure was defined as the removal of any component of the implant during the follow-up. A distinction was made between revision prosthesis and non-prosthesis related. Prosthesis related was due to component malposition/dislocation. Except for age, the data were not normally distributed. Pre- and post-operative data are represented with descriptive statistics. The median or mean and the range are presented as appropriate. The tibiofemoral angles were compared using the non-parametric Wilcoxon signed-rank test with a level of significance at p < 0.05. Data were analysed using SPSS software (SPSS 22.0, SPSS Inc., Chicago, Illinois).
Results
The mean follow-up was 11.7 years (10 to 15). Pre- and post-operative outcomes are summarised in Table II. In all, 77% of knees (n = 62) had a good or excellent clinical outcome score according to the KSS. The survival at 15 years with revision for any reason as the endpoint was 90.6% (95% CI 85.2 to 96.0) and prosthesis related revision was 99.3% (95% CI 97.9 to 100; Fig. 1). A total of 11 knees (8%) (138 knees at risk) underwent revision surgery after a mean follow-up of 5.7 years (0.5 to 11). In four patients the revision surgery was within four years post-operatively because of surgical error (n = 1; combination of malalignment femoral component and flexion-extension gap mismatch) or due to failure to adhere to the strict indication criteria for the Oxford UKA (n = 3) the details of which are reported in Table III. A total of seven knees were revised between five and 11 years follow-up: two because of consistent unexplained pain (1.5%) and five (3.6%) due to progression of osteoarthritis in the lateral compartment. There were no revisions due to infection, wear, implant fracture or loosening of the components.
Fig. 1.
Kaplan-Meier Estimates of Survival Function. Survival based on revisions for any reason and for prosthesis specific reasons. Revisions due to pain or disease progression were not considered prosthesis related. Five year survival based on revision for any reason: 96.2% (95% confidence interval (CI): 93.0 to 99.5%). Seven year survival based on revision for any reason: 93.8% (95% CI: 89.6 to 98.0%). Ten year survival based on revision for any reason: 91.6% (95%CI: 87.1 to 96.8%). 12 year survival based on revision for any reason: 90.6% (95%CI: 85.2 to 96.0%).
Table II.
Outcome results of 81 patients treated by means of unicompartmental knee arthroplasty for medial compartment osteoarthritis with minimum ten years’ follow-up (mean with standard deviation (sd))
| Pre-operative | 6 mths post-operative | 5 yrs post-operative | Final follow-up (mean 11.7 yrs; 9 to 16) | |
|---|---|---|---|---|
| Oxford Knee Score | 19.4 (sd 6.8) | 36.9 (sd 8.4) | 38.8 (sd 8.3) | 41.9 (sd 6.4) |
| VAS satisfaction (cm) | NA | 0.8 (sd 0.8) | 1.4 (sd 1.2) | 1.5 (sd 1.3) |
| VAS pain (cm) | NA | 1.3 (sd 1.1) | 1.8 (sd 1.4) | 1.8 (sd 1.4) |
| WOMAC pain score | 45.6 (sd 17.2) | 85.4 (sd 15.7) | 86.4 (sd 17.0) | 92.9 (sd 10.4) |
| WOMAC stiffness score | 49.4 (sd 20.7) | 72.7 (sd 20.8) | 77.0 (sd 21.1) | 89.5 (sd 12.5) |
| WOMAC function score | 47.3 (sd 20.7) | 81.5 (sd 20.8) | 83.7 (sd 17.5) | 89.4 (sd 11.9) |
| KSS total score | 47.0 (sd 17.5) | 89.7 (sd 15.3) | 84.1 (sd 19.5) | 81.0 (sd 20.7) |
| ROM (degrees) | 121.9 (sd 10.7) | 125.7 (sd 10.8) | 129 (sd 9.6) | 125.0 (sd 7.8) |
NA, item not available; VAS, visual analogue score; WOMAC, Western Ontario and McMaster Universities Arthritis Index; KSS, Knee Society Score; ROM, range of movement
Table III.
Details of revisions to primary total knee arthroplasty (TKA)
| Revision | Indication for revision | Operative findings | Time to revision (yrs) | Procedure | Outcome |
|---|---|---|---|---|---|
| 1 | 2nd bearing dislocation | Flexion-extension gap mismatch†, Malrotation femoral component | 0.51 | Primary TKA | Good |
| 2 | Pain | Insufficient ACL, Chondropathy lateral compartment* | 2.06 | Primary TKA | Good |
| 3 | Disease progression | Lateral compartment OA*, previous HTO | 2.46 | Primary TKA | Poor |
| 4 | Pain | PFJ-OA* | 3.69 | Primary TKA | Poor |
| 5 | Disease progression | PFJ-OA and lateral compartment OA | 5.49 | Primary TKA | Good |
| 6 | Pain | No cause found | 5.74 | Primary TKA | Good |
| 7 | Disease progression | Lateral compartment OA | 6.8 | Primary TKA | Good |
| 8 | Disease progression | Lateral compartment OA | 7.49 | Primary TKA | Good |
| 9 | Disease progression | Lateral compartment OA | 7.82 | Primary TKA | Good |
| 10 | Disease progression | Lateral compartment OA | 10.16 | Primary TKA | Good |
| 11 | Pain | No cause found | 11.39 | Primary TKA | Good |
* Failure to adhere to the strict indication criteria for the Oxford unicompartimental knee arthroplasties † Prosthesis related failure ACL, anterior cruciate ligament; PFJ, patellofemoral joint; OA, osteoarthritis; HTO, high tibial osteotomy
Radiology
A total of 81 knees were available for radiological examination. Radiolucency was identified in 27.2% of all available UKAs. Complete physiological radiolucency (< 2 mm) was observed in five (6.2%) tibial components. In all, 15 (18.5%) tibial components had only partial physiological radiolucent lines. All these physiological radiolucencies (total 24.7%) in 20 knees were visible at year one post-operatively and remained unchanged in extent and thickness at later follow-up. In two knees (2.5%), pathological signs of radiolucency beneath the tibial component were observed. These arthroplasties were still not revised and functioning well at final (greater than ten years) follow-up. No radiolucency was found in relation to the femoral component.
Progression of medial facet patellofemoral joint osteoarthritis (PFJ-OA) as seen on axial patellar view in the presence of patellofemoral joint narrowing was observed in two non-symptomatic knees. The occurrence of lateral facet PFJ-OA was observed in two patients, of whom one knee in each patient was symptomatic and was revised. The mean tibiofemoral angle measured on weight-bearing short-length AP knee views at six months was 5.0° valgus, (-2 to 15) and decreased at final follow-up to 4.7° valgus (-6 to 16) (p = 0.001). Long-length standing radiographs were not available at the institution.
Discussion
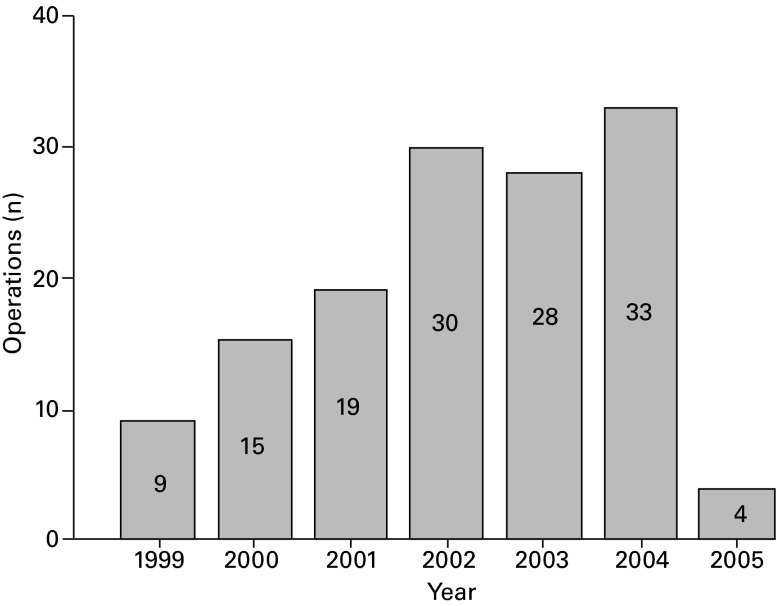
The most important findings of this study were the excellent28 long-term clinical outcome scores of the Oxford Phase III UKA with a cumulative survival rate with revision for any reason as endpoint of 90.6% (95% CI 85.2 to 96.0) at 15 years follow-up obtained in a district general hospital. Price et al29 and Clement et al30 also reported high medium-term (seven to ten year) survival rates. The first two years were considered as the learning curve period. These patients are included in the study. The average number of procedures that were performed annually in this series was 28 (Fig. 2). According to Liddle et al31 28 per year would account for a medium volume (eight to 30 per year). After the learning curve period in this study, high volumes were obtained annually. In another study Liddle et al32 showed that low-usage surgeons tend to have high revision rates and recommend that at least 20% of their arthroplasties should be UKAs to achieve higher survival rates. The importance of high-volume units for the technically demanding Oxford arthroplasty was stressed by Koskinen et al33 who reported high failure rates in their Finnish Arthroplasty Register study in low number surgeons/clinics. To our knowledge this is the first study, which describes the results of the Oxford Phase III UKA after a minimum of ten years follow-up for a single non-designer surgeon with large volume. Svärd6 also described the long-term (mean 12.5 years; 10.1 to 15.6) results of the Oxford prosthesis (Phase I and II) but by a standard open procedure. Their ten-year cumulative survival was 95.0% (95% CI 90.8 to 99.3). The series by Svärd and Price7 showed very few revisions in the second decade after the index procedure and suggested that the implant is durable in this period after implantation. Recently, Pandit et al34 reported similar long-term (mean 10.3 years; 5.3 to 16.6) outcomes in the designer’s group of 1000 implants with a 15-year survival rate (with all implant-related re-operations considered as failures) of 91% (95% CI 83.0 to 97.9) and 79% of knees with a good or excellent clinical outcome score.28
Fig. 2.
Annual number of Oxford Phase III arthroplasties performed by the single surgeon.
The present study reports the outcome of patients with a long-term follow-up. We observed that functional recovery is almost reached after one year and does not improve significantly thereafter. This finding is also stated by Pandit et al.35 When any surgery related factors are involved, revisions occur mostly within two years after primary surgery.6,36,37 Late revisions in our series occurred due to the presence of symptomatic lateral compartment arthritis after a mean follow-up of 7.5 years (3.6%; Table III). Progression of lateral compartment OA is the most common cause of revision in our series and this corresponds with Pandit et al11 and Price, Waite and Svärd.38 Pandit et al34 showed that 2.5% of their revisions were due to lateral compartment OA. Emerson and Higgins10 reported 12.7% of total revisions including 7.3% (n = 4) of revisions due to lateral OA after a mean follow-up of 10.2 years in a series of 55 UKAs. They did not find any correlation between revision and post-operative alignment of the limb. On the other hand some similar studies report that the incidence of disease progression of the lateral compartment is low and even rare: Saldanhaet al39 reported 1.3%, Kim et al12 reported 0.6% and Faour-Martín et al40 reported none in their series. Overall, in the present study the revision rate for lateral compartment OA is slightly higher than previously reported. Apart from overcorrection into valgus in one case with minimal lateral compartment chondropathy pre-operatively, we do not have an explanation for this slightly higher revision rate.
Pre-existent PFJ-OA is considered not to be a contraindication for performing UKA. According to the designer group of the Oxford prosthesis this implant can be used for medial replacement even when PFJ-OA changes are present.3Kang et al41 reported in their series of 195 knees that degenerative changes of the patellofemoral joint should not be considered a contraindication for medial Oxford UKA. They did not see significant difference in scores between those patients who had patellofemoral osteoarthritis pre-operatively and those who did not. However, Beard et al42 stated that the presence of lateral facet PFJ-OA might negatively influence the outcome of the UKA and that caution in these cases should be observed. We report two patients with symptomatic lateral facet PFJ-OA who were revised to TKA, one with poor and the other with good results. Two of the patients with progression of medial patellofemoral facet degeneration are still doing well after 11.3 and 12.3 years follow-up and we believe that the presence of medial facet PFJ-OA has no influence on the outcome of medial UKA. This report shows that the progression of symptomatic PFJ-OA in medial UKAs is rare and is supported by Weale et al.43
Dislocation of the mobile bearing in the Oxford knee primarily occurs shortly after implantation44 as seen in our single case. It was the result of an error producing a mismatch in the extension and flexion gap and malposition of the components. Conversion to a standard condylar type TKA led to good clinical outcome. No revisions were performed due to deep infection, primary polyethylene wear, fracture of the bearing or loosening of the components. In contrast to the present study, the most common reason for revision in a series of 1819 UKAs from the Finnish Arthroplasty Register implanted between 1985 and 2003 as described by Koskinen et al33 was aseptic loosening. As reported by others we also conclude that right indication criteria and a meticulous surgical technique are the key factors for success of the arthroplasty.45
When compared with previous studies a low incidence (27.2%) of radiolucency was found. Pandit et al11 reported radiolucent lines in 70% of their UKAs (40% complete and 60% partial). From our experience we agree with previous authors that these radiolucent lines have no clinical relevance.45 Our use of thorough pulsed lavage and a dry surgical field before cementing in the procedures might contribute to the low incidence of radiolucency we found. This is supported by the studies of Faour-Martín et al40 and Clarius et al.46 However, we acknowledge that the surgeon also undertook the fluoroscopic examination and this might be prone to bias.
Regarding the survival and clinical outcome scores the scores in this report are fairly similar to the scores presented by others. Overall results of medial UKA according to the KSS showed 96% excellent or good outcome for knees in the report by Faour-Martín et al,40 compared with 79% and 77% in a report from Pandit et al34and the present study respectively. The mean Oxford Knee Scores were 40 and 42 in Pandit et al’s34 series and our series respectively. The mean age in these three reports is 59, 66 and 72 years and mean follow-up 10.4, 10.6 and 11.7 years, respectively. Survival was 96.3% (ten years), 91% (15 years) and 90.6 (15 years), respectively. The age and follow-up duration might be factors that explain the differences in outcome scores.
Short-term follow-up results of UKA47demonstrate predictably better results comparable with those of TKA, but longer follow-up data that make this comparison are not yet available. Liddle et al32 showed better patient-reported outcomes measures (PROMS) in UKA compared with TKA in the short-term (six months) using data from a large national joint registry. They stated that the higher revision rate in UKAs compared with TKAs might be due to the fact that UKAs can be revised more easily despite possible better functional outcome in the longer term. Difference in revision rates may not be because of differences in functional outcomes alone. Clarification of risk factors for failure still need to be assessed in the near future. With appropriate patient selection, prosthetic design and surgical technique a trained surgeon can achieve good outcomes in patients with UKA. Patients may experience a rapid recovery after UKA with use of the MIS technique.48
In conclusion, this independent prospective study showed a high survival rate of the Oxford Phase III UKA performed by a single surgeon with good to excellent outcome scores. The major complication rate was similar to other reports after a minimum of ten years follow-up. In our opinion excellent, durable and reproducible results can be expected for this minimally invasive surgical procedure in the long-term with appropriate case selection. The Oxford Phase III prosthesis has proven to be a reliable implant for patients with anteromedial OA and can be recommended as long as the strict indications for UKA are observed.
Take home message: This independent prospective study showed a high survival rate of the unicompartmental knee prosthesis performed by a single surgeon with a low major complication rate and when strict indication criteria are followed, excellent, durable and reliable results can be expected for this minimally invasive surgical procedure in the long-term.
References
- 1.Ritter MA, Faris PM, Thong AE, et al. Intra-operative findings in varus osteoarthritis of the knee. An analysis of pre-operative alignment in potential candidates for unicompartmental arthroplasty. J Bone Joint Surg [Br] 2004;86-B:43–47. [PubMed] [Google Scholar]
- 2.Repicci JA, Eberle RW. Minimally invasive surgical technique for unicondylar knee arthroplasty. J South Orthop Assoc 1999;8:20–27. [PubMed] [Google Scholar]
- 3.Murray DW, Goodfellow JW, O’Connor JJ. The Oxford medial unicompartmental arthroplasty: a ten-year survival study. J Bone Joint Surg [Br] 1998;80-B:983–989. [DOI] [PubMed] [Google Scholar]
- 4.Goodfellow J, O’Connor J, Murray DW. The Oxford meniscal unicompartmental knee. J Knee Surg 2002;15:240–246. [PubMed] [Google Scholar]
- 5.Rajasekhar C, Das S, Smith A. Unicompartmental knee arthroplasty. 2- to 12-year results in a community hospital. J Bone Joint Surg [Br] 2004;86-B:983–985. [DOI] [PubMed] [Google Scholar]
- 6.Svärd ULong term results after partial knee arthroplasty with the oxford knee [thesis]. University of Gothenburg, 2009.
- 7.Svärd UC, Price AJ. Oxford medial unicompartmental knee arthroplasty. A survival analysis of an independent series. J Bone Joint Surg [Br] 2001;83-B:191–194. [DOI] [PubMed] [Google Scholar]
- 8.Kendrick BJ, Longino D, Pandit H, et al. Polyethylene wear in Oxford unicompartmental knee replacement: a retrieval study of 47 bearings. J Bone Joint Surg [Br] 2010;92-B:367–373. [DOI] [PubMed] [Google Scholar]
- 9.Sharma L, Song J, Felson DT, et al. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001;286:188–195. [DOI] [PubMed] [Google Scholar]
- 10.Emerson RH Jr, Higgins LL. Unicompartmental knee arthroplasty with the oxford prosthesis in patients with medial compartment arthritis. J Bone Joint Surg [Am] 2008;90-A:118–122. [DOI] [PubMed] [Google Scholar]
- 11.Pandit H, Jenkins C, Barker K, Dodd CA, Murray DW. The Oxford medial unicompartmental knee replacement using a minimally-invasive approach. J Bone Joint Surg [Br] 2006;88-B:54–60. [DOI] [PubMed] [Google Scholar]
- 12.Kim KT, Lee S, Kim JH, et al. The Survivorship and Clinical Results of Minimally Invasive Unicompartmental Knee Arthroplasty at 10-Year Follow-up. Clin Orthop Surg 2015;7:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lisowski LA, van den Bekerom MP, Pilot P, van Dijk CN, Lisowski AE. Oxford Phase 3 unicompartmental knee arthroplasty: medium-term results of a minimally invasive surgical procedure. Knee Surg Sports Traumatol Arthrosc 2011;19:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyes GW, Carr AJ, Miller RK, Goodfellow JW. The radiographic classification of medial gonarthrosis. Correlation with operation methods in 200 knees. Acta Orthop Scand 1992;63:497–501. [DOI] [PubMed] [Google Scholar]
- 15.Goodfellow JW, Kershaw CJ, Benson MK, O'Connor JJ. The Oxford Knee for unicompartmental osteoarthritis. The first 103 cases. J Bone Joint Surg [Br] 1988;70-B:692–701. [DOI] [PubMed] [Google Scholar]
- 16.Price AJ, Webb J, Topf H, et al. Rapid recovery after oxford unicompartmental arthroplasty through a short incision. J Arthroplasty 2001;16:970–976. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840. [PubMed] [Google Scholar]
- 18.Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg [Br] 1998;80-B:63–69. [DOI] [PubMed] [Google Scholar]
- 19.Haverkamp D, Breugem SJ, Sierevelt IN, Blankevoort L, van Dijk CN. Translation and validation of the Dutch version of the Oxford 12-item knee questionnaire for knee arthroplasty. Acta Orthop 2005;76:347–352. [PubMed] [Google Scholar]
- 20.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthoped Relat Res 1989;248:13–14. [PubMed] [Google Scholar]
- 21.Van Der Straeten C, Witvrouw E, Willems T, Bellemans J, Victor J. Translation and validation of the Dutch new Knee Society Scoring System. Clin Orthop Relat Res 2013;471:3565–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freyd M. The graphic rating scale. J Educ Psychol 1923;14:83–102. [Google Scholar]
- 23.Van der Kloot WA, Vertommen H, eds. De MPQ-DLV, een standaard nederlandstalige versie van de McGill Pain Questionnaire: Achtergronden en handleiding. Lisse: Swets & Zeitlinger; 1989.
- 24.Gulati A, Chau R, Pandit HG, et al. The incidence of physiological radiolucency following Oxford unicompartmental knee replacement and its relationship to outcome. J Bone Joint Surg [Br] 2009;91-B:896–902. [DOI] [PubMed] [Google Scholar]
- 25.Tibrewal SB, Grant KA, Goodfellow JW. The radiolucent line beneath the tibial components of the Oxford meniscal knee. J Bone Joint Surg [Br] 1984;66-B:523–528. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 27.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 1977;35:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asif S, Choon DS. Midterm results of cemented Press Fit Condylar Sigma total knee arthroplasty system. J Orthop Surg (Hong Kong) 2005;13:280–284. [DOI] [PubMed] [Google Scholar]
- 29.Price AJ, Dodd CA, Svärd UG, Murray DW. Oxford medial unicompartmental knee arthroplasty in patients younger and older than 60 years of age. J Bone Joint Surg [Br] 2005;87:1488–1492. [DOI] [PubMed] [Google Scholar]
- 30.Clement ND, Duckworth AD, MacKenzie SP, Nie YX, Tiemessen CH. Medium-term results of Oxford phase-3 medial unicompartmental knee arthroplasty. J Orthop Surg (Hong Kong) 2012;20:157–161. [DOI] [PubMed] [Google Scholar]
- 31.Liddle AD, Pandit H, Judge A, Murray DW. Effect of Surgical Caseload on Revision Rate Following Total and Unicompartmental Knee Replacement. J Bone Joint Surg [Am] 2016;98:1–8. [DOI] [PubMed] [Google Scholar]
- 32.Liddle AD, Pandit H, Judge A, Murray DW. Optimal usage of unicompartmental knee arthroplasty: a study of 41,986 cases from the National Joint Registry for England and Wales. Bone Joint J 2015;97-B:1506–1511. [DOI] [PubMed] [Google Scholar]
- 33.Koskinen E, Paavolainen P, Eskelinen A, Pulkkinen P, Remes V. Unicondylar knee replacement for primary osteoarthritis: a prospective follow-up study of 1,819 patients from the Finnish Arthroplasty Register. Acta Orthop 2007;78:128–135. [DOI] [PubMed] [Google Scholar]
- 34.Pandit H, Hamilton TW, Jenkins C, et al. The clinical outcome of minimally invasive Phase 3 Oxford unicompartmental knee arthroplasty: a 15-year follow-up of 1000 UKAs. Bone Joint J 2015;97-B:1493–1500. [DOI] [PubMed] [Google Scholar]
- 35.Pandit H, Jenkins C, Beard DJ, et al. Cementless Oxford unicompartmental knee replacement shows reduced radiolucency at one year. J Bone Joint Surg [Br] 2009;91-B:185–189. [DOI] [PubMed] [Google Scholar]
- 36.Luscombe KL, Lim J, Jones PW, White SH. Minimally invasive Oxford medial unicompartmental knee arthroplasty. A note of caution!. Int Orthop 2007;31:321–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty 2013;28(Suppl):116–119. [DOI] [PubMed] [Google Scholar]
- 38.Price AJ, Waite JC, Svärd U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res 2005;435:171–180. [DOI] [PubMed] [Google Scholar]
- 39.Saldanha KA, Keys GW, Svärd UC, White SH, Rao C. Revision of Oxford medial unicompartmental knee arthroplasty to total knee arthroplasty - results of a multicentre study. Knee 2007;14:275–279. [DOI] [PubMed] [Google Scholar]
- 40.Faour-Martín O, Valverde-García JA, Martín-Ferrero MA, et al. Oxford phase 3 unicondylar knee arthroplasty through a minimally invasive approach: long-term results. Int Orthop 2013;37:833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang SN, Smith TO, Sprenger De Rover WB, Walton NP. Pre-operative patellofemoral degenerative changes do not affect the outcome after medial Oxford unicompartmental knee replacement: a report from an independent centre. J Bone Joint Surg [Br] 2011;93-B:476–478. [DOI] [PubMed] [Google Scholar]
- 42.Beard DJ, Pandit H, Ostlere S, et al. Pre-operative clinical and radiological assessment of the patellofemoral joint in unicompartmental knee replacement and its influence on outcome. J Bone Joint Surg [Br] 2007;89-B:1602–1607. [DOI] [PubMed] [Google Scholar]
- 43.Weale AE, Murray DW, Crawford R, et al. Does arthritis progress in the retained compartments after ‘Oxford’ medial unicompartmental arthroplasty? A clinical and radiological study with a minimum ten-year follow-up. J Bone Joint Surg [Br] 1999;81-B:783–789. [DOI] [PubMed] [Google Scholar]
- 44.Pandit H, Jenkins C, Beard DJ, et al. Mobile bearing dislocation in lateral unicompartmental knee replacement. Knee 2010;17:392–397. [DOI] [PubMed] [Google Scholar]
- 45.Vardi G, Strover AE. Early complications of unicompartmental knee replacement: the Droitwich experience. Knee 2004;11:389–394. [DOI] [PubMed] [Google Scholar]
- 46.Clarius M, Hauck C, Seeger JB, et al. Pulsed lavage reduces the incidence of radiolucent lines under the tibial tray of Oxford unicompartmental knee arthroplasty: pulsed lavage versus syringe lavage. Int Orthop 2009;33:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardi AV Jr, Berend KR, Walter CA, Aziz-Jacobo J, Cheney NA. Is recovery faster for mobile-bearing unicompartmental than total knee arthroplasty? Clin Orthop Relat Res 2009;467:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berend ME, Berend KR, Lombardi AV Jr. Advances in pain management: game changers in knee arthroplasty. Bone Joint J 2014;96-B(Suppl A):7–9. [DOI] [PubMed] [Google Scholar]