Significance
This study quantifies sex differences in the diurnal and circadian variation of sleep and waking while carefully controlling for menstrual cycle phase and hormonal contraceptive use. Compared to men, rhythms of core body temperature, sleep, and subjective alertness are advanced in women during both the midfollicular and midluteal phases. Our study also reveals lower alertness levels at night in women. These sex differences are important for understanding the increased female susceptibility to sleep and wake disturbances.
Keywords: sex difference, circadian variation of sleep, circadian variation of alertness, core body temperature, melatonin
Abstract
This study quantifies sex differences in the diurnal and circadian variation of sleep and waking while controlling for menstrual cycle phase and hormonal contraceptive use. We compared the diurnal and circadian variation of sleep and alertness of 8 women studied during two phases of the menstrual cycle and 3 women studied during their midfollicular phase with that of 15 men. Participants underwent an ultradian sleep–wake cycle (USW) procedure consisting of 36 cycles of 60-min wake episodes alternating with 60-min nap opportunities. Core body temperature (CBT), salivary melatonin, subjective alertness, and polysomnographically recorded sleep were measured throughout this procedure. All analyzed measures showed a significant diurnal and circadian variation throughout the USW procedure. Compared with men, women demonstrated a significant phase advance of the CBT but not melatonin rhythms, as well as an advance in the diurnal and circadian variation of sleep measures and subjective alertness. Furthermore, women experienced an increased amplitude of the diurnal and circadian variation of alertness, mainly due to a larger decline in the nocturnal nadir. Our results indicate that women are likely initiating sleep at a later circadian phase than men, which may be one factor contributing to the increased susceptibility to sleep disturbances reported in women. Lower nighttime alertness is also observed, suggesting a physiological basis for a greater susceptibility to maladaptation to night shift work in women.
A meta-analysis has indicated an overall increased risk ratio of 1.41 in women vs. men for experiencing insomnia, and this risk ratio increases to 1.64 when considering high-quality studies with rigorous methodology (1). The etiology of sex-based differences in vulnerability to sleep disturbances remains to be fully elucidated, but evidence points to a role for sex-based differences in sleep, its timing, and circadian rhythms as potential contributors (2).
There is sufficient evidence to support a role for circadian factors in the pathophysiology of chronic insomnia, as the timing of sleep relative to the endogenous circadian system can substantially affect sleep initiation and maintenance (3). Interestingly, morphological differences, as well as variations in circulating hormones and their receptors, have been reported between sexes and can affect circadian physiology. For example, the localization of sex steroid receptors to the suprachiasmatic nucleus (SCN) and a sex difference in the expression of androgen and estrogen receptors there indicate a direct and differential role of specific gonadal steroid hormones in the circadian system (4). Moreover, a sexual dimorphism in structure and sex steroid receptor expression also exists in efferent targets of the SCN, including the preoptic area of the hypothalamus, which is known to influence sleep (4). In a recent postmortem brain study, the circadian variation of PER2, PER3, and ARNT1 peaked significantly earlier (∼5–7 h) in the dorsolateral prefrontal cortex of deceased women than men (5).
In humans, the timing of sleep shows sex-based differences. Prior studies using the Horne and Östberg Morningness–Eveningness Questionnaire (6) or the midpoint of sleep based on the Munich Chronotype Questionnaire (7) have shown earlier chronotypes in women than men. Interestingly, the sex difference in chronotype disappears around the age of menopause (7), indicating a likely role of hormones in circadian preference.
A study by Mongrain et al. (8) revealed that, for a similar chronotype, young women had an earlier timing of dim-light melatonin onset (DLMO) vs. young men as well as a larger phase angle between DLMO and bedtime. In that study, sleep duration tended to be the same despite sleep occurring at a later circadian phase in women, suggesting there could be a sex difference in the circadian variation of sleep.
Earlier phase of core body temperature (CBT) and melatonin rhythms relative to habitual sleep time was reported by other groups in women vs. men (9–11). Duffy et al. also reported that women have a shorter intrinsic circadian period of CBT and melatonin vs. men (12), which might contribute to these observations.
Unfortunately, many prior studies that documented sex differences in circadian physiology have not done so while controlling for menstrual cycle and hormonal contraceptive use in female participants. This is crucial, because we previously demonstrated, under controlled conditions, that the expression of circadian thermoregulatory mechanisms and sleep patterns in unmedicated healthy ovulating women is affected by menstrual cycle phase (13, 14). To our knowledge, no prior study has compared the circadian variation of sleep propensity and waking between men and women while controlling for menstrual cycle phase and hormonal contraceptive use. This is the aim of the current study. We used an ultradian sleep–wake cycle (USW) procedure in men and in women selectively studied during the midfollicular (MF) phase of the menstrual cycle to quantify sex differences in the diurnal and circadian variation of sleep propensity and organization, as well as CBT and salivary melatonin, under highly controlled laboratory conditions. A subgroup of women also participated in the USW procedure during their midluteal (ML) phase, which allowed further between-sex comparisons.
Results
We first explored sex differences by studying women during their MF phase. The study of women during their ML phase was limited to a subgroup in which data were available. Unless stated otherwise, results are based on the MF phase data.
Baseline Sleep.
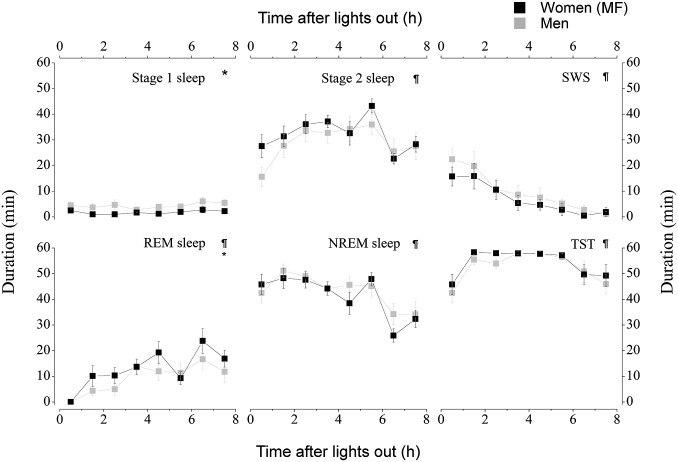
As detailed in SI Appendix, Table S2, bedtime was comparable between groups (mean ± SEM; women, 0024 hours ± 15 min; men, 0010 hours ± 11 min; P = 0.46) as well as wake time (women, 0818 hours ± 14 min; men, 0810 hours ± 11 min; P = 0.63). SI Appendix, Table S3 presents the average polysomnography (PSG) sleep parameters measured during the nocturnal 8-h sleep period in women and men. During the nocturnal sleep period, stage 1 sleep [minutes and percentage of total sleep time (TST); P ≤ 0.036] and non-REM (NREM) sleep (percentage of TST only; P = 0.009) were significantly shorter in women than men. Rapid eye movement (REM) sleep (minutes and percentage of TST; P ≤ 0.009) was also significantly longer for women than men. No sex difference was detected for TST, sleep efficiency (SE), sleep onset latency (SOL), REM sleep onset latency (ROL), stage 2 sleep, and slow-wave sleep (SWS) (P ≥ 0.113).
The analysis of the PSG parameters throughout the nocturnal sleep periods revealed a significant time effect for all PSG parameters measured (P ≤ 0.026), except for stage 1 sleep (P = 0.18; Fig. 1). Furthermore, across the night, women presented shorter stage 1 sleep (P = 0.02) and longer REM sleep (P = 0.01) than men. No other significant sex effect (P ≥ 0.23) or interaction was observed (P ≥ 0.49) for sleep parameters across the night.
Fig. 1.
Sex differences in nocturnal baseline sleep. Women were studied during their MF phase. *, sex differences (P < 0.05); ¶, time effects (P < 0.05). All values are means ± SEM.
Diurnal and Circadian Variation of Sleep.
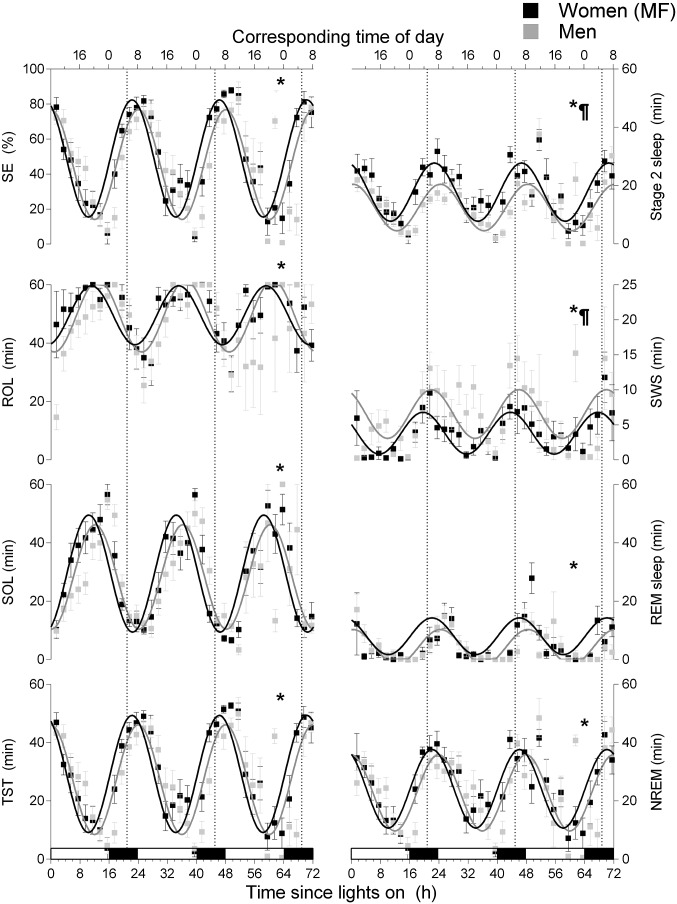
A significant diurnal rhythm was observed for all sleep parameters when USW nap data were aligned by the time since lights on (P < 0.0001; Fig. 2 and Table 1). The nonlinear mixed-effect analysis revealed significant, but small, sex differences in the diurnal mesor (the average level throughout the day) of stage 1 sleep (−1.2 min in women, P = 0.02; not illustrated), stage 2 sleep (+5.2 min in women, P = 0.001), and SWS (−2.8 min in women, P = 0.003). Women had a slightly lower stage 1 sleep amplitude (−1.1 min, P = 0.001) vs. men. The acrophase of all sleep parameters was advanced (by +1.7 to +2.3 h, P ≤ 0.03; Table 1) in women vs. men.
Fig. 2.
Diurnal variation of sleep parameters during the USW procedure aligned by the time since lights on. Women were studied during their MF phase. The vertical dotted lines correspond to the CBT minimum. The bottom x axis represents the time since lights on, and the top x axis represents the corresponding clock time. Black bars along the x axis represent the time of projected habitual nocturnal sleep episodes. ¶, sex differences in mesor (P < 0.05); *, sex differences in phase (P < 0.05). All values are means ± SEM.
Table 1.
Diurnal parameters of sleep measures, alertness, CBT, and melatonin during the USW procedure aligned by the time since lights on
| Sleep recording | Variables aligned on lights on | |||||
| Mesor | Amplitude | Acrophase, h | ||||
| Women | Men | Women | Men | Women | Men | |
| SE, % | 48.9 ± 1.8 | 45.5 ± 1.6 | 33.4 ± 1.9 | 31.3 ± 1.8 | 22.3 ± 0.2* | 0.0 ± 0.2* |
| SOL, min | 29.4 ± 12.8 | 28.3 ± 11.0 | 20.0 ± 1.2 | 17.9 ± 1.1 | 10.4 ± 0.2* | 12.1 ± 0.2* |
| ROL, min | 49.5 ± 10.1 | 49.2 ± 8.7 | 10.1 ± 1.3 | 12.2 ± 1.2 | 11.1 ± 0.5* | 12.9 ± 0.4* |
| Stage 1, min | 2.7 ± 0.3* | 3.9 ± 0.3* | 1.2 ± 0.2* | 2.3 ± 0.2* | 23.3 ± 0.6* | 1.4 ± 0.3* |
| Stage 2, min | 17.7 ± 1.1* | 12.5 ± 1.0* | 10.0 ± 0.8 | 8.0 ± 0.8 | 22.7 ± 0.3* | 0.3 ± 0.4* |
| SWS, min | 3.8 ± 0.6* | 6.6 ± 0.5* | 3.0 ± 0.6 | 3.5 ± 0.6 | 19.6 ± 0.8* | 21.9 ± 0.6* |
| REM, min | 8.0 ± 1.8 | 4.3 ± 0.5 | 6.3 ± 0.7 | 6.0 ± 0.7 | 22.1 ± 0.4* | 0.4 ± 0.5* |
| NREM, min | 24.1 ± 8.2 | 22.8 ± 7.0 | 13.5 ± 1.1 | 13.1 ± 1.1 | 22.1 ± 0.3* | 23.9 ± 0.3* |
| TST, min | 29.2 ± 8.7 | 27.3 ± 7.5 | 20.1 ± 1.1 | 18.8 ± 1.1 | 22.3 ± 0.2* | 0.0 ± 0.2* |
| Alertness, 0–10 | 6.4 ± 0.3 | 7.1 ± 0.3 | 1.8 ± 0.1* | 1.2 ± 0.1* | 9.5 ± 0.3* | 11.7 ± 0.4* |
| CBT, °C | 37.13 ± 0.04 | 37.08 ± 0.04 | 0.31 ± 0.01 | 0.30 ± 0.01 | 9.0 ± 0.1* | 10.1 ± 0.1* |
| Melatonin, pg/mL | 10.7 ± 2.3 | 10.0 ± 1.6 | 4.9 ± 0.7 | 4.9 ± 0.5 | 19.7 ± 0.5 | 20.2 ± 0.4 |
Women were studied during their MF phase.
Sex difference (P < 0.05).
A significant circadian rhythm was observed for all PSG parameters when USW nap data were aligned by the CBT minimum (P ≤ 0.0005; SI Appendix, Fig. S2 and Table S4). A significant sex difference was observed for the circadian mesor of NREM sleep (+2.8 min in women, P = 0.033), stage 2 sleep (+4.9 min in women, P = 0.002), and SWS (−2.8 min in women, P = 0.01). No sex difference of circadian amplitude was observed for sleep parameters. The acrophase of SE (+16.0° or 64 min, P = 0.004), stage 2 sleep (+17.1° or 68 min, P = 0.047), and TST (+16.0° or 64, P = 0.006) was advanced in women vs. men.
Diurnal and Circadian Rhythm of Subjective Alertness.
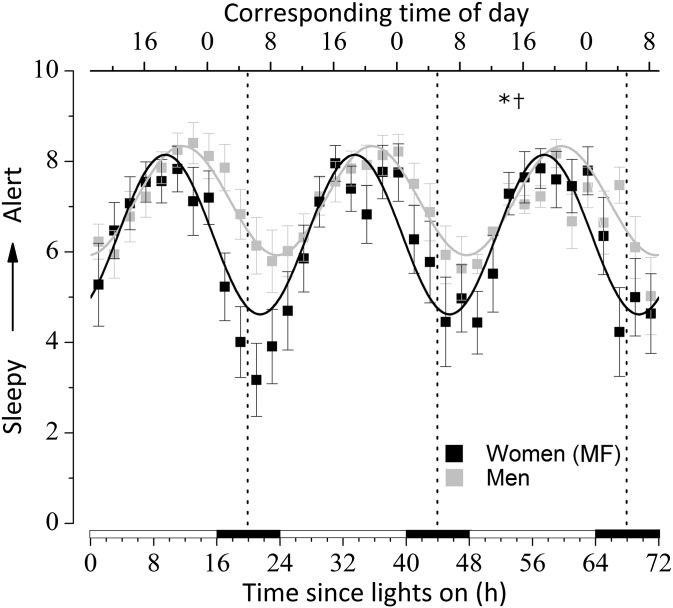
When data were aligned by the time since lights on, the diurnal rhythm of subjective alertness was significant (P < 0.0001; Fig. 3 and Table 1). This rhythm was significantly advanced (P < 0.001), with higher amplitude (P = 0.002) but no mesor differences (P = 0.10) in women vs. men. The higher amplitude in women was the result of lower nocturnal alertness scores. When data were aligned by the CBT minimum (SI Appendix, Fig. S3 and Table S4), rhythms were significant (P < 0.0001), phase-advanced (P = 0.037), and of greater amplitude (P = 0.020) in women vs. men but with no mesor differences (P = 0.11).
Fig. 3.
Diurnal variation of subjective alertness during the USW procedure aligned by the time since lights on. Women were studied during their MF phase. See the legend for Fig. 2 for more details. *, sex differences in phase; †, sex differences in amplitude (P < 0.05). All values are means ± SEM.
Diurnal and Circadian Phase Markers.
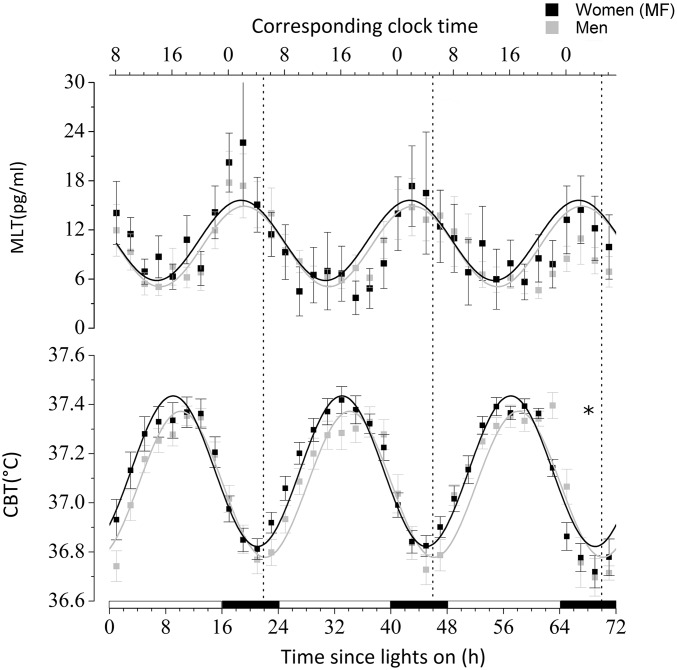
The average CBT minimum during the USW procedure occurred at 0455 hours ± 24 min in women and 0521 hours ± 15 min in men (P = 0.34; SI Appendix, Table S2). The average phase angle (wake time–time of CBT minimum) for women (3.41 ± 0.35 h) was longer than that for men (2.81 ± 0.27 h), but not significantly (P = 0.15). When data were aligned by the time since lights on, a significant diurnal rhythm was observed for CBT and melatonin (P < 0.0001; Fig. 4 and Table 1). The CBT acrophase was 66 min advanced in women vs. men (P < 0.0001) but the melatonin acrophase was similar (P = 0.48). No other difference was observed in CBT and melatonin rhythms. When melatonin and CBT data were aligned by the CBT minimum, no significant mesor, amplitude, or phase difference was observed (P ≥ 0.08; SI Appendix, Table S4).
Fig. 4.
Diurnal variation of salivary melatonin (MLT) and CBT during the USW procedure aligned by the time since lights on. Women were studied during their MF phase. See the legend for Fig. 2 for more details. *, sex differences in phase (P < 0.05). All values are means ± SEM.
Menstrual Phase Effect.
Results of between-sex comparisons for the ML phase data available for 8 out of 11 women are detailed in SI Appendix, Figs. S4–S9 and Tables S5–S7. Results are essentially the same whether the ML or MF phase data were used. Due to the reduced sample size, some significant differences became a trend or became nonsignificant. This was especially the case when USW data were aligned by the CBT minimum, as the effect size was smaller (SI Appendix, Table S7). Of note, women in the ML phase had higher CBT mesor and reduced amplitude of CBT vs. men when data were aligned by the time since lights on (P ≤ 0.004; SI Appendix, Table S5) or by the CBT minimum (P ≤ 0.004; SI Appendix, Table S7). As illustrated in SI Appendix, Fig. S7, higher nocturnal CBT values in women during their ML phase mainly contribute to these results.
Discussion
To our knowledge, no prior study has examined sex differences in the diurnal and circadian variation of sleep and waking under highly controlled conditions while simultaneously controlling for menstrual cycle phase and hormonal contraceptive use. Our use of the USW procedure allowed for a quantification of PSG-derived sleep measures at all circadian phases while reducing the confounding effects of changes in environmental light, posture, and food intake as well as for a comparison between sexes while studying naturally cycling women.
In both women and men, SE, SOL, ROL, TST, stage 1 sleep, stage 2 sleep, SWS, REM sleep, and/or NREM sleep significantly varied with time of day, as previously reported for a subset of participants (13–15) and with others who used imposed experimental non–24-h sleep–wake cycle procedures to desynchronize the sleep–wake and circadian cycles (16, 17). The greatest and lowest sleep propensity occurred near the trough and crest of the CBT rhythm, respectively.
When comparing the diurnal variation of sleep between sexes by aligning our results on the habitual time of awakening, we observed a significant advance of +1.7 to 2.3 h of the rhythm of all sleep measures in women vs. men. By aligning our results on the time of CBT minimum, we observed this advance in women to be significant, although smaller (of +1.0–1.1 h), for the circadian variation of SE, stage 2 sleep and TST. When the sex difference in CBT acrophase (+1.1 h earlier in women vs. men) is added to the circadian variation of sleep, it explains the diurnal variation of sleep. Similar observations are made for the diurnal variation of subjective alertness. When the phase angle difference in CBT minimum is added to the circadian variation of alertness (+1.1 h in women vs. men), it explains the diurnal variation of alertness (+2.2 h in women vs. men).
The phase advance of the alertness rhythm in women vs. men is consistent with a prior study (18), although alertness and sleepiness were only assessed from 0800 to 2100 hours and not under controlled conditions. An advance in the drive for alertness may conceivably explain the higher prevalence of difficulty maintaining sleep (19, 20) and early-morning awakenings (20) reported in women vs. men. Additionally, we currently report that the amplitude of the variation was significantly increased in women vs. men due to a larger decline of alertness scores at night. This observation may partially explain the lower tolerance to shift work (21), greater fatigue and sleepiness levels (22), and increased risk of work-related injuries (23) in female shift workers, although contradictory results have been reported (24) and other nonphysiological factors (e.g., marital and parental status, socioeconomic factors) could be involved. Current results indicate lower values of alertness in the night and in the early morning in women, which is likely related to their higher prevalence of feeling unrefreshed upon awakening (19). These results are consistent with the lower nighttime performance scores in women compared with men reported by Santhi et al. (25). In that study, women also rated themselves as less sleepy than men in the late afternoon and evening. Methodological differences in recruitment criteria, study protocol, and experimental measures might contribute to differences between their sleepiness results and ours.
As for the menstrual cycle effect, the earlier diurnal variation of sleep and alertness was still observed in a subgroup of women studied during the ML phase vs. men. Due to our reduced sample size, these differences were not always significant, especially for the analyses aligned by the CBT minimum, whose size effect was smaller than those aligned by the time since lights on. Nevertheless, these sex differences remained in the same direction. We cannot totally exclude the possibility that small menstrual-related differences in the circadian variation of sleep might exceed the limit of detectability of this experimental protocol and our prior one (13). This possibility is reinforced by the observation that the size of between-sex differences in the phase of the diurnal variation of sleep and alertness is reduced by 4.3 to 95.2% when ML phase data are considered instead of MF phase data. Similarly, this reduction is on the order of 18.6 to 88.6% for the circadian variation of sleep and alertness, except for NREM sleep (+34.2%) and SWS (+19.2%), for which sex differences in phase are increased during the ML vs. MF phases.
Our observed sex-based differences in the phase of the CBT rhythm are similar to that reported by Cain et al. (9) but our amplitude data are not. When considering MF phase data, no sex difference was observed in CBT amplitude, although there was for ML phase data. Although Cain et al. did not track menstrual cycle phase, they speculate that more women were studied during the luteal than the follicular phase, which could account for the reduced CBT amplitude observed (9). Our prior findings of a reduced CBT amplitude during the ML vs. MF phase together with the current observations support their assertion (13). Moreover, Baker et al. (26) compared sex and menstrual differences in 24-h CBT profiles and observed an increase in the nocturnal CBT minimum during the luteal vs. follicular phase, as we did previously (14), and with men, as we do here. Therefore, it appears that the sex difference in circadian CBT amplitude is limited to the ML phase and is minimized when women are selectively studied during their preovulatory follicular phase.
It is well-established that body temperature changes are closely linked to the sleep–wake cycle and that sleep is initiated on the falling limb of the CBT curve (27). Thus, the advanced CBT rhythm in women may be partially driving the earlier sleep and sleepiness rhythms. Our study does not indicate that changes in the timing of melatonin secretion significantly contribute to sex differences in the diurnal and circadian variation of sleep and waking. These are consistent with a recent study reporting no sex difference in melatonin onset relative to bedtime (28) but differ with others who did observe a phase advance and increased circadian amplitude of plasma melatonin in women vs. men (9) or of advanced salivary DLMO (10, 11). However, it should be pointed out again that the previous studies (9–11) did not control for menstrual cycle phase. Another important consideration that was controlled for here is hormonal contraceptive use. This is important, because women taking hormonal contraceptives appear to have increased melatonin secretion, but no changes in circadian timing, compared with naturally cycling women in both the follicular and luteal phases of the menstrual cycle (29). This may explain the recent findings by Santhi et al. (25), which, in contrast to current observations, reported higher melatonin secretion in women vs. men despite no sex difference in the circadian timing of melatonin secretion. Indeed, in that study, half of the female participants were using hormonal contraceptives, and menstrual cycle phase was not controlled for (25). Nevertheless, it should be noted that our sample size is smaller than in these studies, which could have prevented us from detecting between-sex differences. Similar to current findings, a subsequent study using a forced desynchrony protocol also failed to detect a statistically significant sex difference in the circadian phase of melatonin secretion (12). Our prior controlled study found no menstrual phase difference in the circadian melatonin profile (13), although there have been other reports of decreases (30), increases (31), phase delays (32), or no change (29) in melatonin secretion during the luteal vs. follicular phase. Therefore, more work should be done to determine whether and how menstrual and/or sex-based differences affect melatonin secretion, and what the practical implications of this could be.
Because habitual bed and wake times were similar between women and men, our currently observed advanced CBT rhythm in women confirms the hypothesis that women are initiating sleep at a later circadian phase than men. This delay in the timing of habitual sleep initiation relative to the timing of the endogenous circadian system may be one factor contributing to the increased susceptibility to sleep disturbances in women, although this was not the case for our study group, which did not show a sex difference in SE during the baseline sleep episode. It is possible that healthy young women living on a conventional day-oriented schedule are less affected by such slight circadian and/or diurnal differences, although these might become important in particular situations or vulnerable populations. For instance, sex differences in the circadian/diurnal variation of sleep could affect the ability to sleep at atypical times of day, with potential applications for jet lag or shift work adaptation. Indeed, prior studies do indicate that circadian parameters are important for individuals’ susceptibilities to developing insomnia (3).
The clinical implications of the small sex differences in mesor and amplitude of sleep parameter rhythms are unclear. These could have been affected by the number of baseline sleep periods preceding the USW procedure. Indeed, a lower mesor of stage 1 and SWS and higher mesor of stage 2 were observed in men having one vs. two baseline nights. These were also the differences observed in women vs. men. Because 91% of women (10 out of 11) had only one baseline night compared with 40% of men (6 out of 15), the number of baseline nights could have affected the slight sex differences observed in mesor of these sleep stages. However, when we compared results of the USW sleep data in men who had one vs. two baseline nights, we observed that the phase, its relationship to lights on, and the diurnal rhythm amplitude were unaffected by the number of baseline nights. An effect of the study protocol on mesor and amplitude of sleep rhythms is also possible. Indeed, small differences in amplitude and mesor were observed when we excluded data from participants who had a blood pressure measure taken during their nap.
Despite these uncertainties concerning mesor and amplitude differences, in all cases, sex differences in circadian phase were consistent. We believe our approach, which required participants to maintain a regular 8-h sleep schedule for at least 2 wk before laboratory entry, is adequate to stabilize the phase angle between the endogenous circadian system and the sleep–wake schedule and is not affected by the number of baseline nights or the differences in experimental measurements across the study groups.
Interestingly, the phase advance of sleep propensity rhythms in women vs. men is reminiscent of findings from a study that also used a USW procedure to compare circadian sleep rhythms in old and young participants (17). Therefore, because young adult women are already demonstrating a circadian variation of sleep propensity that is advanced in a way similar to that of older adults, the diurnal variation of sleep in women may become worsened with age. This may explain why the already high prevalence of insomnia in women continues to disproportionally increase after menopause (2), and why a higher prevalence of insomnia in women vs. men persists in old age (1).
Several limitations in the current work should be discussed. This study included a relatively limited sample size, which was smaller than a prior study investigating sex differences in circadian physiology (9). The assessment of baseline sleep, before the USW procedure, was limited by the concomitant screening for sleep disorders. Also, the present paper combines data from three different studies that were not perfectly balanced across sexes. Finally, melatonin data were available in a smaller sample of participants, which might have prevented us from detecting sex differences.
In the current protocol, the diurnal/circadian variation of sleep was quantified using the USW procedure. By design, our protocol was developed to specifically address the circadian variation of sleep while minimizing the effects of the homeostatic buildup of sleep propensity. Using this sensitive and controlled protocol, we did observe sex differences in the expression of the diurnal/circadian variation of sleep, although we are limited in our investigation of the homeostatic regulation of sleep. Using a forced desynchrony protocol, Santhi et al. recently described higher slow-wave activity in women during the biological night compared with men (25). However, as described above, that study suffered from a lack of control for menstrual cycle as well as hormonal contraceptive use in a substantial proportion of female participants, which could contribute to their findings. Future studies are necessary to more fully characterize sex differences in the interaction between circadian and homeostatic sleep regulation.
We cannot totally exclude the possibility that sleep inertia could have partially influenced subjective alertness measured 30-min postawakening, although this is thought to be minimal. Indeed, sleep inertia is generally believed to be dissipated within that time frame (33). Controlling for both circadian phase and homeostatic sleep pressure using a forced desynchrony protocol, Scheer et al. (34) demonstrated that the effect of sleep inertia on performance was gone within 20 min across all circadian phases. Conversely, others, albeit using protocols that did not assess throughout all circadian phases (35) or with infrequent assessments postawakening (36), have reported that sleep inertia can take longer periods to dissipate (between 2 and 4 h). We also did not include an objective measure of performance, although our results are consistent with those of others who did (25).
Despite these limitations, our stringent inclusion/exclusion criteria, preexperimental baseline sleep–wake tracking, and strict control of menstrual cycle phase and hormonal contraceptive use added strength. Moreover, studying naturally cycling women during the follicular and luteal phases of their menstrual cycle provided the necessary control to conduct nonconfounded sex-based comparisons. Overall, the results point consistently in the same direction of advanced diurnal and circadian rhythms of sleep and waking in women vs. men. The current findings are important, because an interaction between the menstrual and circadian cycles can influence sleep, particularly during the luteal phase, which is often characterized by sleep complaints (38).
In conclusion, we observed an advance in the diurnal and circadian rhythms of sleep and alertness in women vs. men during both the MF and ML phases of the menstrual cycle. This is explained by an earlier circadian variation of sleep and waking superimposed on advanced circadian rhythms. The acrophase of SE and TST in women was observed 1.0 to 1.7 h before their habitual time of waking, whereas it was observed at the habitual time of waking in men. Thus, the last 2 h of women’s habitual sleep period have a weaker circadian drive for sleep at a time when the homeostatic drive is also low. Even though participants in the present study did not present sleep disturbances, our current findings are particularly relevant for helping to explain women’s susceptibility to early-morning awakenings. Because diurnal and circadian factors are relevant for the physiopathology of insomnia (3), it is important to consider the timing of sleep and circadian rhythms for the treatment of sleep complaints in women (1).
Methods
Participants.
Results from 11 healthy women in their MF phase (mean age ± SD, 25.8 ± 3.5 y) and 15 healthy men (23.4 ± 3.7 y) who participated in one of three studies [two published (13, 15)] carried out between 2004 and 2011 at the Douglas Mental Health University Institute were included in the present study (see SI Appendix, Table S1 for demographic details). Each subject provided informed consent before participation. Each study had similar screening procedures, inclusion/exclusion criteria, prestudy sleep–wake schedule requirements, and baseline in-patient study conditions, as previously reported (13–15, 37). None of our female participants were on contraceptives when we first contacted them during our recruitment procedures (see further details in SI Appendix, Methods). Data from eight women (subjects no. 1–8; SI Appendix, Tables S1 and S2) were also collected in their ML phase (13). All participants gave their informed consent. For additional details, see SI Appendix, Methods.
Study Design.
All studies had a similar protocol including at least one 8-h nocturnal baseline sleep period followed by a 72-h USW procedure (SI Appendix, Fig. S1 and Table S2), and is detailed in SI Appendix, Methods. The USW procedure consisted of 60-min wake episodes in dim light (<10 lx) alternating with 60-min nap opportunities in total darkness for a total of 36 wake and nap periods spanning 3 d in controlled conditions. All experimental procedures were approved by the Douglas Mental Health University Institute Research Ethics Board and are within the ethical standards of the Declaration of Helsinki.
Measures and Data Processing.
CBT was automatically monitored (four times per min) using a disposable rectal thermistor (Steri-Probe; Cincinnati Sub-Zero Products) inserted 10 cm into the rectum. To minimize the masking effect of the 8-h nocturnal sleep episode, the first 8 h of CBT recording during the USW procedure was excluded from analysis.
Saliva samples were collected once (5 min before lights out for 1 woman and 10 men) or twice (5 min after lights on and 5 min before lights out for 10 women and 6 men) during each wake episode and assayed for their content of melatonin using a direct saliva RIA 125I-labeled tracer. See details in SI Appendix, Methods.
Sleep was recorded by PSG across all nocturnal baseline sleep periods and nap episodes throughout the USW procedure. PSG recordings included a central and occipital electroencephalogram, an electrooculogram, and a submental electromyogram. For consistency across participants, all PSG data were visually scored according to the Rechtschaffen and Kales criteria (39) using 30-s epochs.
Subjective alertness was assessed in the middle of all wake episodes via a 10-cm bipolar visual analog scale, with 0 cm being extremely sleepy and 10 cm being extremely alert.
Data and Statistical Analysis.
CBT data were averaged into 1-min bins, and a dual-harmonic regression model (courtesy of C. A. Czeisler, Brigham and Women’s Hospital, Boston) without serial correlated noise was applied to individual CBT curves to assess the individual circadian parameters (detailed in SI Appendix, Methods).
To explore the sex differences in baseline nocturnal sleep, data from the sleep period immediately preceding the USW procedure were used for all subjects.
To evaluate the diurnal variation of sleep and alertness during the USW procedure, data were aligned by the time since lights on. This set of analyses demonstrates the temporal relationship between sleep–wake propensity and the habitual nocturnal sleep period. To clarify the circadian component of this variation, another set of analyses was done with data now aligned by the time of CBT minimum. This latter set of analyses addresses sex differences in the circadian variation of sleep and waking.
After alignment, data were averaged into 2-h bins for each participant and then across participants of each group. A nonlinear mixed-effect model was applied to 2-h–binned (i.e., 30°-binned) data using the nlmixed SAS procedure (SAS Institute) to compare circadian parameters between sexes. See SI Appendix, Methods for details. We repeated the between-sex analyses based on data collected in eight women during the ML phase of their menstrual cycle.
Materials and Data Availability.
Participants in this study have not agreed that their data be placed in a publicly accessible database. Thus, for ethical reasons, the authors cannot provide public access to these data. Nevertheless, materials, data, and associated protocols will be made available for investigation of scientific integrity if necessary. For these purposes, investigators should contact the principal investigator directly and a process involving the institutional review board of the Douglas Institute will be initiated. Readers are free to contact the principal investigator if they wish to initiate discussions regarding research collaborations to build on these published data.
Supplementary Material
Acknowledgments
We thank the research participants, staff, and students of the Centre for Study and Treatment of Circadian Rhythms for contributions to this investigation. We also thank Dr. Sylvie Rhéaume, Dr. Alain Solignac, and Abdelmadjid Azzoug, RN, for medical supervision; Francine Duquette for dietary advice; and Manon Robert, Milad Amdan, and Maryse Parenteau for assistance with sleep recording and scoring. This work was supported by operating grants from the Canadian Institute of Health Research (to D.B.B.); and salary support from the Fonds de la recherche du Québec-Santé (to D.B.B.); the Standard Life Foundation (to D.B.B.); and the Institut de Recherche Robert-Sauvé en Santé et en Sécurité du Travail (to P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524484113/-/DCSupplemental.
References
- 1.Zhang B, Wing YK. Sex differences in insomnia: A meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 2.Mong JA, et al. Sleep, rhythms, and the endocrine brain: Influence of sex and gonadal hormones. J Neurosci. 2011;31(45):16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boivin DB, Boudreau P. Circadian rhythms and insomnia—Approaching the time barrier. Insomnia Rounds. 2014;2(4):1–8. [Google Scholar]
- 4.Bailey M, Silver R. Sex differences in circadian timing systems: Implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim AS, et al. Sex difference in daily rhythms of clock gene expression in the aged human cerebral cortex. J Biol Rhythms. 2013;28(2):117–129. doi: 10.1177/0748730413478552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adan A, Natale V. Gender differences in morningness-eveningness preference. Chronobiol Int. 2002;19(4):709–720. doi: 10.1081/cbi-120005390. [DOI] [PubMed] [Google Scholar]
- 7.Roenneberg T, et al. A marker for the end of adolescence. Curr Biol. 2004;14(24):R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J Biol Rhythms. 2004;19(3):248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- 9.Cain SW, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Reen E, et al. Sex of college students moderates associations among bedtime, time in bed, and circadian phase angle. J Biol Rhythms. 2013;28(6):425–431. doi: 10.1177/0748730413511771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229–237. doi: 10.1111/j.1365-2869.2005.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy JF, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shechter A, Boudreau P, Varin F, Boivin DB. Predominance of distal skin temperature changes at sleep onset across menstrual and circadian phases. J Biol Rhythms. 2011;26(3):260–270. doi: 10.1177/0748730411404677. [DOI] [PubMed] [Google Scholar]
- 15.Boudreau P, Yeh WH, Dumont GA, Boivin DB. Circadian variation of heart rate variability across sleep stages. Sleep. 2013;36(12):1919–1928. doi: 10.5665/sleep.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15(5 Pt 1):3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Münch M, et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol Aging. 2005;26(9):1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Adan A, Sánchez-Turet M. Gender differences in diurnal variations of subjective activation and mood. Chronobiol Int. 2001;18(3):491–502. doi: 10.1081/cbi-100103971. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg E, et al. Sleep disturbances in a young adult population: Can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387. doi: 10.1093/sleep/20.6.381. [DOI] [PubMed] [Google Scholar]
- 20.Li RH, Wing YK, Ho SC, Fong SY. Gender differences in insomnia—A study in the Hong Kong Chinese population. J Psychosom Res. 2002;53(1):601–609. doi: 10.1016/s0022-3999(02)00437-3. [DOI] [PubMed] [Google Scholar]
- 21.Saksvik IB, Bjorvatn B, Hetland H, Sandal GM, Pallesen S. Individual differences in tolerance to shift work—A systematic review. Sleep Med Rev. 2011;15(4):221–235. doi: 10.1016/j.smrv.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Ogińska H, Pokorski J, Ogiński A. Gender, ageing, and shiftwork intolerance. Ergonomics. 1993;36(1–3):161–168. doi: 10.1080/00140139308967868. [DOI] [PubMed] [Google Scholar]
- 23.Wong IS, McLeod CB, Demers PA. Shift work trends and risk of work injury among Canadian workers. Scand J Work Environ Health. 2011;37(1):54–61. doi: 10.5271/sjweh.3124. [DOI] [PubMed] [Google Scholar]
- 24.Hakola T, Härmä MI, Laitinen JT. Circadian adjustment of men and women to night work. Scand J Work Environ Health. 1996;22(2):133–138. doi: 10.5271/sjweh.121. [DOI] [PubMed] [Google Scholar]
- 25.Santhi N, et al. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci USA. 2016;113(19):E2730–E2739. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker FC, et al. Sleep and 24 hour body temperatures: A comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530(Pt 3):565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kräuchi K. The thermophysiological cascade leading to sleep initiation in relation to phase of entrainment. Sleep Med Rev. 2007;11(6):439–451. doi: 10.1016/j.smrv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Lazar AS, et al. Circadian period and the timing of melatonin onset in men and women: Predictors of sleep during the weekend and in the laboratory. J Sleep Res. 2013;22(2):155–159. doi: 10.1111/jsr.12001. [DOI] [PubMed] [Google Scholar]
- 29.Wright KP, Jr, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103(2):185–194. doi: 10.1016/s0166-4328(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 30.Shibui K, et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48(11):1062–1068. doi: 10.1016/s0006-3223(00)00912-4. [DOI] [PubMed] [Google Scholar]
- 31.Webley GE, Leidenberger F. The circadian pattern of melatonin and its positive relationship with progesterone in women. J Clin Endocrinol Metab. 1986;63(2):323–328. doi: 10.1210/jcem-63-2-323. [DOI] [PubMed] [Google Scholar]
- 32.Cagnacci A, Soldani R, Laughlin GA, Yen SS. Modification of circadian body temperature rhythm during the luteal menstrual phase: Role of melatonin. J Appl Physiol (1985) 1996;80(1):25–29. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- 33.Wesensten NJ, Balkin TJ, Belenky G. Countermeasures for mitigating fatigue in motor vehicle operators. Rev Hum Factors Ergon. 2015;10(1):115–137. [Google Scholar]
- 34.Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. J Biol Rhythms. 2008;23(4):353–361. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jewett ME, et al. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8(1):1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 36.Burke TM, Scheer FA, Ronda JM, Czeisler CA, Wright KP., Jr Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res. 2015;24(4):364–371. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boudreau P, Yeh WH, Dumont GA, Boivin DB. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int. 2012;29(6):757–768. doi: 10.3109/07420528.2012.674592. [DOI] [PubMed] [Google Scholar]
- 38.Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. doi: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Brain Inf Serv, Brain Res Inst, UCLA; Los Angeles: 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.