Significance
We have discovered that a mammalian species, a bat called the Honduran white bat Ectophylla alba, displays a yellow carotenoid pigment called lutein in its bare skin. Even though carotenoid-based coloration has been found in birds, fish, amphibians, and reptiles, there are no reports of any extant mammals showing these pigments in their skin or hair. The implications of this finding may be profound for human health, as carotenoids are essential micronutrients. Lutein in particular is involved in the preservation of the macula of the eye. The Honduran white bat, with its ability to assimilate and deposit lutein in its bare skin, may be the sought-after mammalian model needed for enhancing studies on carotenoid function and metabolism.
Keywords: bats, carotenoids, macular degeneration, skin coloration
Abstract
Animals cannot synthesize carotenoid pigments de novo, and must consume them in their diet. Most mammals, including humans, are indiscriminate accumulators of carotenoids but inefficiently distribute them to some tissues and organs, such as skin. This limits the potential capacity of these organisms to benefit from the antioxidant and immunostimulatory functions that carotenoids fulfill. Indeed, to date, no mammal has been known to have evolved physiological mechanisms to incorporate and deposit carotenoids in the skin or hair, and mammals have therefore been assumed to rely entirely on other pigments such as melanins to color their integument. Here we use high-performance liquid chromatography (HPLC) in combination with time-of-flight mass spectrometry (HPLC-TOF/MS) to show that the frugivorous Honduran white bat Ectophylla alba colors its skin bright yellow with the deposition of the xanthophyll lutein. The Honduran white bat is thus a mammalian model that may help developing strategies to improve the assimilation of lutein in humans to avoid macular degeneration. This represents a change of paradigm in animal physiology showing that some mammals actually have the capacity to accumulate dietary carotenoids in the integument. In addition, we have also discovered that the majority of the lutein in the skin of Honduran white bats is present in esterified form with fatty acids, thereby permitting longer-lasting coloration and suggesting bright color traits may have an overlooked role in the visual communication of bats.
Carotenoids are photosynthetic pigments animals use to avoid cellular oxidative damage and to color external structures (1–3). The inability of animals to synthesize de novo carotenoids means these pigments must be obtained only from dietary sources (4). Among vertebrates, the limiting nature of carotenoids is particularly marked in mammalian species, which exhibit a variable, but generally low, efficiency for carotenoid assimilation (5, 6). Here we show a mammal, the frugivorous Honduran white bat Ectophylla alba, that deposits amounts of carotenoids in the skin that are high enough to generate a conspicuous coloration. Indeed, up to this time, no mammal has been known to incorporate significant visible amounts of carotenoids in the integument (7, 8). This has prevented adequate animal models being found for carotenoid assimilation in the skin that would enable improvement of the bioavailability of carotenoids in the diet of humans (9), where these pigments are of utmost importance for health by having provitamin A value and fulfilling antioxidant and immunostimulatory functions (10, 11). Using high-performance liquid chromatography (HPLC) in combination with time-of-flight mass spectrometry (HPLC-TOF/MS), we found that the characteristic bright yellow coloration of the bare skin of the nose-leaf and ears in these bats is generated by the xanthophyll lutein in both free and esterified forms. This indicates that the Honduran white bat represents a change of paradigm in animal physiology, showing that some mammals actually have the capacity to accumulate dietary carotenoids in the integument. It is thus a mammalian model that may help in developing strategies to improve the assimilation of lutein in humans (12), who depend on dietary contents to avoid macular degeneration (13) and skin oxidative damage (14).
The nocturnal Honduran white bat is a small phyllostomid distributed exclusively in lowland rainforests of Mesoamerica that exhibits bright yellow coloration in the bare skin of the nose-leaf, ears, and wings (Fig. 1 A and B). This distinctive coloration resembles the one generated by some carotenoids, mainly lutein and zeaxanthin, in the integument of other vertebrates, such as the bare integument and feathers of birds (4, 15). To the best of our knowledge, such yellow hues are not found in other mammals, which mostly rely on darker endogenous melanins to color their pelage and skin (16), with the exception of other tropical bats (see Evolutionary Implications). To determine the origin of the yellow color, we conducted in-depth chemical analyses of the bare skin of the ears and nose-leaf and of the liver of two male Honduran white bat specimens from Costa Rica.
Fig. 1.
Honduran white bat images. (A and B) Details of the bright yellow coloration of the bare skin in the ears, nose-leaf, and wings of a Honduran white bat from Costa Rica. (C) A Honduran white bat feeding on the ripe fruit of a fig tree F. colubrinae. Photographs by Sharlene Santana (A and B) and Marco Tschapka (C).
The integument of mammals represents a striking example of the difference in the bioavailability of carotenoids among animals. Carotenoids are widespread pigments in the integument of all vertebrates except mammals, as only small amounts of carotenoids are found in the skin of humans and other mammalian species (7, 8), and in contrast to the feathers and scales of reptiles and fish, there seems to be a complete lack of carotenoids in mammalian hair (15). In humans, and aside from pathological conditions in which carotenoids accumulate in the skin (i.e., carotenodermia), these pigments only occur in low amounts in the skin (∼200 ng/g tissue) (7), where they interact with the color generated by keratin, melanins, and blood (17). Carotenoids are present at higher concentrations in internal tissues (human macula: 1 mM; serum: 0.1–1 μM) (18). Therefore, the contribution of dietary carotenoids to the perceived variation in human skin coloration is negligible. To date, the role of carotenoids as the primary pigments responsible for the generation of distinctive patches of bright color in the integument, as in other vertebrates (4, 15), has never been reported in mammals. Beyond increasing our understanding of the evolution of animal coloration and appearance, finding mammals capable of coloring their integument with carotenoids would open new opportunities to obtain mammalian models that help improve our bioavailability of dietary carotenoids for a better fulfillment of human physiological functions (19).
Mammals obtain carotenoids through eating green plant products. Once ingested, these pigments are released from the food matrix by the action of bile salts and pancreatic lipases, which form lipid micelles that incorporate carotenoids and facilitate their transport to the mucosal cells of the duodenum by passive diffusion or through facilitated transport via scavenger receptor class B, type 1 (20). The capacity to assemble micelles with incorporated carotenoids partly determines the efficiency of carotenoid absorption, which has led to the advent of functional food technologies searching for food matrices that optimize the absorption of supplemented carotenoids in farmed fish (5, 21) and human diets (22). Once absorbed, carotenoids are transported by lipoproteins through the bloodstream to the target tissues, a process in which the abundance and nature of lipoproteins limit the capacity to obtain carotenoids (15). Furthermore, some animals can metabolize carotenoids into other forms, and occasionally esterify them with long-chain fatty acids (20). The result is that several factors, such as amounts and species of carotenoids ingested, molecular linkage and food matrix composition, nutrient status of the host, and genetic components, influence the bioavailability of carotenoids (23), creating large differences between species in their capacity to obtain these pigments from their diets and make physiological use of them (15).
Results and Discussion
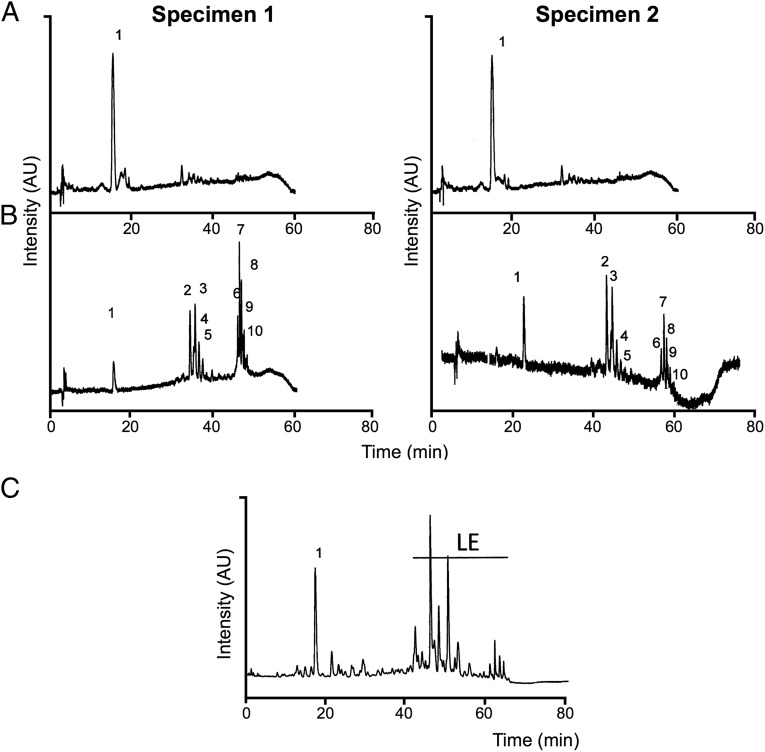
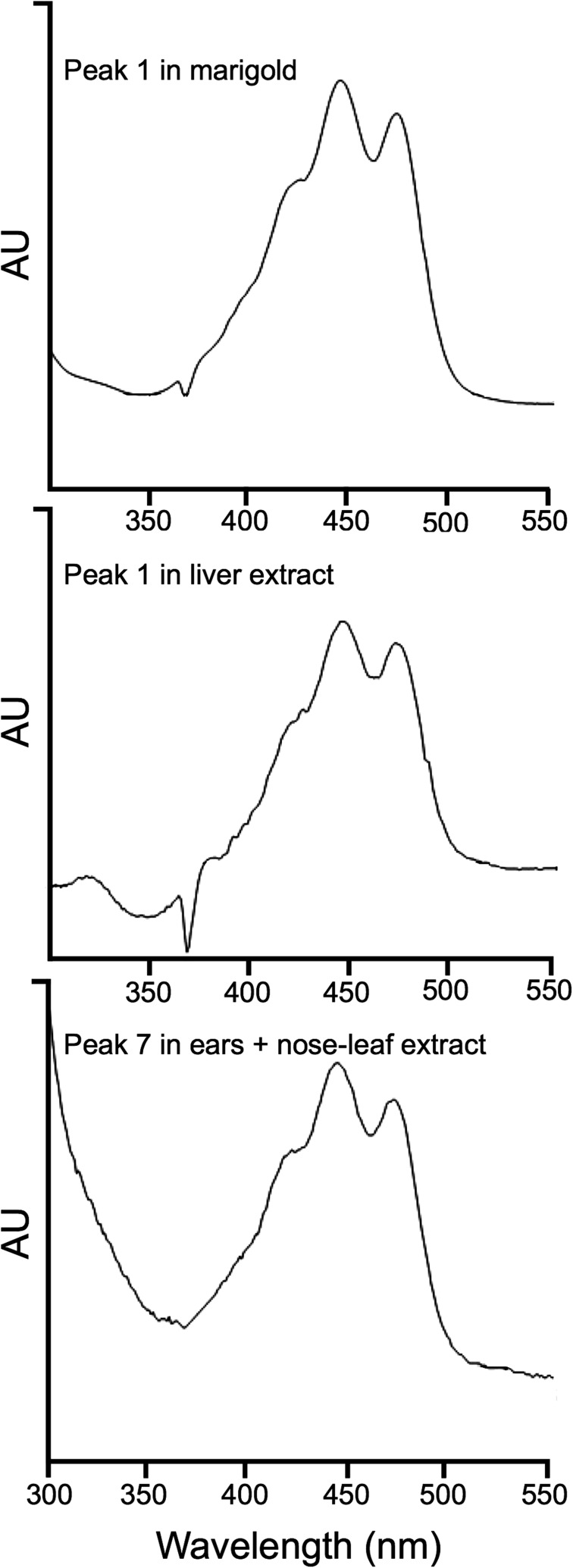
Our chromatographic analyses, performed with extracts from the ears and nose-leaf of Honduran white bats, clearly showed that their bright yellow color is caused by the xanthophyll lutein, including trans and cis isomers, and its epoxy derivative, which exclusively forms the carotenoid profile in the extracts of the two specimens analyzed. Thus, both specimens contained the same qualitative carotenoid profile. The identification was based on the chromatographic features, UV-visible absorbance spectra, and MS characteristics, including MS and MS2 spectral behavior, of the molecular ion, which is in line with the data available in the literature (24). Fig. 2 depicts the HPLC analyses of the extracts from liver (Fig. 2A), ears, and nose-leaf tissue (Fig. 2B) for both specimens, whereas Fig. 3 shows the UV-visible absorbance spectra of the main peaks detected in the analyzed tissues. We compared the experimental data with those obtained from the analysis of lutein standard isolated from a vegetable source, obtaining equivalent results (Figs. 2C and 3). Unexpectedly, most of the lutein content in the ears and nose-leaf is present as esterified forms with saturated and unsaturated fatty acids, whereas almost all lutein content in liver is present as free form (Table 1). The probable source of dietary lutein for Honduran white bats is the fig tree Ficus colubrinae, the ripe red fruit of which is reported to be the bat’s main food item (25) (Fig. 1C). The main carotenoids in fresh cultivated figs, from the congeneric species fig tree Ficus carica, are lutein followed by zeaxanthin, both of which are concentrated in the fruit peel (26).
Fig. 2.
Chromatogram traces. The graphs correspond to the analyses of liver (A) and ears+nose-leaf (B) extracts from two specimens of Honduran white bats, and to the analysis of marigold Tagetes erecta extract (C). Peak identification is the same as in Table 1. LE, lutein esters.
Fig. 3.
UV-visible spectra of the main peaks detected in chromatographic analysis of marigold, liver, and ears+nose-leaf extract from Honduran white bats.
Table 1.
Chromatographic, UV-visible, and MS characteristics of main carotenoids from tissues of Honduran white bats E. alba
| Peak | Carotenoid | tR (min) | λmax (nm) | [M+H]+* | Main product ions† |
| 1 (L, E+n-l)‡ | Lutein | 15.7 | 420, 444, 472 | 569.4335 (−3.1/28.8) | 551.4281 [M+H-18]+; 495.3642 |
| 2 (E+n-l) | Dilauroyl-5,6-epoxy-lutein | 33.8 | 418, 443, 469 | 949.7668 (2.6/42.3) | 931.7601 [M+H-H2O]+; 749.5791 [M+H-FA]+ |
| 549.4184 [M+H-2FA]+ | |||||
| 3 (E+n-l) | Unknown¶ | 35.3 | 420, 444, 472 | — | — |
| 4 (E+n-l) | Lauroyl-myristoyl-5,6-epoxy-lutein | 36.2 | 418, 443, 469 | 977.7970 (−1.4/39.2) | 959.7912 [M+H-H2O]+; 777.6150 [M+H-FA]+ |
| 749.5890 [M+H-FA]+; 549.4179 [M+H-2FA]+ | |||||
| 5 (E+n-l) | Myristoyl-oleyl-5,6-epoxy-lutein | 37.1 | 418, 443, 469 | 1,059.8695 (−2.9/45.6) | 1,041.8592 [M+H-H2O]+; 831.6697 [M+H-FA]+ |
| 777.6196 [M+H-FA]+;549.4162 [M+H-2FA]+ | |||||
| 6 (E+n-l) | Lauroyl-linoleoyl-lutein | 45.5 | 420, 444, 472 | 1,013.8339 (−1.96/29.3) | 995.8237 [M+H-H2O]+; 813.6589 [M+H-FA]+ |
| 733.5929 [M+H-FA]+; 533.4187 [M+H-2FA]+ | |||||
| 7 (E+n-l) | Palmitoyl-linoleoyl-lutein§ | 46.3 | 420, 444, 472 | 1,067.8813 (1.74/30.2) | 1,049.9804 [M+H-H2O]+; 811.6524 [M+H-FA]+ |
| 789.6613 [M+H-FA]+; 533.4249 [M+H-2FA]+ | |||||
| 8 (E+n-l) | Palmitoyl-linoleoyl-lutein§ | 47.0 | 420, 444, 472 | 1,067.8859 (1.84/29.5) | 1,049.9797 [M+H-H2O]+; 811.6537 [M+H-FA]+ |
| 789.6595 [M+H-FA]+; 533.4262 [M+H-2FA]+ | |||||
| 9 (E+n-l) | Palmitoyl-linoleoyl-lutein§ | 47.5 | 420, 444, 472 | 1,067.8806 (1.06/32.8) | 1,049.9992 [M+H-H2O]+; 811.6529 [M+H-FA]+ |
| 789.6604 [M+H-FA]+; 533.4236 [M+H-2FA]+ | |||||
| 10 (E+n-l) | Oleyl-linoleoyl-lutein | 48.4 | 420, 444, 472 | 1,095.9183 (1.35/19.8) | 1,077.9015 [M+H-H2O]+; 813.6593 [M+H-FA]+ |
| 815.6758 [M+H-FA]+;533.4278 [M+H-2FA]+ |
m/z experimental value of the molecular ion. Numbers in parenthesis are mass error in ppm and SigmaFit value.
Main products ion arising from MS2-based reactions.
Peak present in liver extract (L) and/or in ears+nose-leaf (E+n-l) extract.
Peak not identified.
Trans and cis isomers.
Potential Applications for Human Health.
The presence of esterified lutein and epoxy-lutein in the yellow skin of Honduran white bats, but not in the liver, suggests bats possess a physiological mechanism that allows them to esterify those xanthophylls that accumulate in the integument. The bioavailability of xanthophylls to humans partly depends on the efficiency with which the esterified forms of these carotenoids are hydrolyzed by enzymes during digestion, and in fact only free xanthophylls are normally found in human serum and peripheral tissues (27). Although the nutritional use of xanthophylls may require esters to be metabolized (27), the fact that bats are able to esterify them into their integument opens up interesting possibilities for improving the benefits of xanthophylls for human health. This is because, in plants, the esterification of xanthophylls enhances their stability (27). Together with zeaxanthin, lutein forms the macular pigment and plays a prominent role in human vision by acting as a filter of pro-oxidant short-wavelength light and as an antioxidant to prevent damage of free radicals in the retina. Lutein in the human macula is not esterified (28), but if humans had the mechanism to esterify this carotenoid as bats have, it could be speculated that the stability of macular lutein, and therefore its capacity to avoid eye damage, may be improved. A recent study in birds shows that the genes involved in the conversion of dietary yellow carotenoids to red ketocarotenoids are expressed in both integumentary tissues that are red colored by these pigments and in the retina, where they fulfill a role in color vision by forming red oil droplets (29). Similarly, if esterified lutein also had a visual function in Honduran white bats, it would be expected that the genes regulating the metabolic transformation of lutein, and particularly its esterification, were expressed in both the integument and the eye. Almost nothing is known about the physiological mechanisms by which some animals esterify carotenoids (30), but proving that genes involved in esterification are expressed in the macula of Honduran white bats would represent a body of evidence of the physiological value of this metabolic transformation. This would be the precursor of future treatments in humans that may consider modifying the levels of the proteins that are involved.
The Honduran white bat therefore represents a potential mammalian model that could be used to understand these mechanisms, which may help improve the stability and bioavailability of carotenoids, and thus their benefits for human health. Although Honduran white bats should not be expected to become laboratory animals, they can be maintained in captivity if provided with adequate specific conditions (31). Furthermore, this species is considered as near threatened (i.e., not threatened with extinction) by the International Union for Conservation of Nature and is locally abundant, despite its restricted distribution (32), meaning it should be a valid model species for at least pioneering studies on the genetic basis of carotenoid esterification.
Evolutionary Implications.
The esterification of lutein that likely occurs directly in the skin in Honduran white bats suggests this process has evolved because it provides some adaptive value. Given the stability that esterified xanthophylls are known to confer to plants, Honduran white bats may benefit from achieving a longer-lasting skin coloration, which may be related to a role in visual communication of the bats’ skin, as suggested for the bare integument of birds (30). Honduran white bats construct tents using large plant leaves, under which several individuals roost together (25), and gregarious behavior is known to promote the evolution of conspicuous color traits that facilitate visual communication (33). At this stage, we can only speculate about the reasons that have led to the evolution of yellow skin coloration in Honduran white bats, but we can assert that at least one bat species has evolved the capacity to use dietary carotenoids to generate bright skin pigmentation. However, the existence of other bat species that also display vivid yellow color in the skin and hair suggests our finding may not be limited to the Honduran white bat and could have evolved in different bat lineages distributed in tropical regions. This is the case with the MacConnell’s bat Mesophylla macconnelli, a species sympatric with and phylogenetically close to the Honduran white bat that also feeds on the fruits of fig trees (25) and has yellow bare skin. African tropical bats such as the insectivorous yellow-winged bat Lavia frons and the Sierra Leone collared fruit bat Myonycteris leptodon also show integumentary yellow coloration. Although our study represents one report of integument primarily pigmented by carotenoids in mammals, recent findings indicate that a functional M/LWS opsin gene tuned to long-wavelength light is functional even in species of bats that use echolocation and have a long evolutionary history of nocturnality (34). Altogether, these results call into question the commonly neglected role of vision in bat communication and suggest microbats may have evolved color traits despite constraints imposed by nocturnal habits.
Materials and Methods
Animals.
Two adult male Honduran white bats were captured in the Tirimbina Biological Reserve (10°26′ N, 83°59′ W), in the province of Heredia, on the Northeastern Caribbean lowlands of Costa Rica. The specimens weighed 5 and 5.5 g and naturally died shortly after collection with no need for chemicals to sacrifice them. They were immediately flash-frozen and shipped to Spain, packaged with dry ice. Once the bats were received at the Instituto de la Grasa–Consejo Superior de Investigaciones Científicas (CSIC) (Spain), they were left at ambient temperature for 15 min. Then, the yellow ears were sliced as well as the large yellow nose-leaf. After this, the body cavity was opened and the viscera exposed by cutting down the body wall from the center of the bat until reaching the genitals. At the posterior end of the cut, two lateral incisions were made to open from the center of the bat two flaps of skin. The liver was located and eviscerated with the help of a clamp. The capture of Honduran white bats was approved by the Biodiversity Commission of the University of Costa Rica, and was conducted with the authorization SINAC-SE-GASP-PI-R-059-2015 from the Costa Rican Ministry for the Environment and Energy to B.R.-H.
Carotenoid Extraction.
Extraction of carotenoids from tissues was carried out under dim light and avoiding a longer processing time, using a previously described method (35) with the following modifications. The complete tissue (ears, nose-leaf, or liver) was hydrated with 25 mL cold deionized water, then mixed with 50 mL cold N,N-dimethylformamide and blended with an Ultra-Turrax (T-25 IKA) for 2 min. The extract was filtrated in a Buchner funnel, and the solid residue returned to the blender and reextracted with 50 mL cold N,N-dimethylformamide. The filtrates were mixed and the lipid fraction extracted with 75 mL hexane:diethyl ether (1:1) in a decanting funnel. Partition of solvents was promoted by adding 50 mL NaCl solution (10% wt/vol). The water and organic layers were allowed to separate, and the lower aqueous phase was discarded. Residual N,N-dimethylformamide was removed by washing the organic phase with 50-mL portions of deionized water. After separation, the water layer was drawn off and the organic phase filtered through a solid bed of Na2SO4, and solvent removed in a rotary evaporator. The extracted residue was reconstituted with 30 μL chloroform, vortexed for 30 s, and then diluted with 70 μL methanol and vortexed again for 30 s. Samples were stored at −80 °C until analysis.
Liquid Chromatography–MS.
The carotenoid profile in extracts from tissue samples was separated in a Dionex Ultimate 3000RS U-HPLC (Thermo Fisher Scientific), as described previously (36, 37). Briefly, a C30 reversed-phase YMC analytical column, 250 × 4.6 mm (i.d.) with 3 µm particle size, was used for separation. The mobile phases were mixtures of methanol:tert-butyl methyl ether:water (A, 81:15:4 and B, 7:90:3), starting with 10 min isocratic A (100%), then a gradient to B (50%) at 40 min, B (100%) at 50 min, A (100%) at 55 min, and isocratic A (100%) from 55 to 60 min. The flow rate was 1 mL/min, and the injection volume was 30 µL. UV-visible spectra were recorded from 350 to 600 nm with a photodiode array spectrometer. A split postcolumn of 0.4 mL/min was introduced directly onto the mass spectrometer ion source. MS was performed in a micrOTOF-QII high-resolution TOF mass spectrometer (UHR-TOF) with quadrupole (qQ)-TOF geometry (Bruker Daltonics) equipped with atmospheric pressure chemical ionization (APCI) source. The instrument was operated in positive ion mode, using a scan range of m/z 50–1,200. Mass spectra were acquired through the broadband collision-induced dissociation mode, providing MS and MS/MS spectra simultaneously. The instrument control was performed using Bruker Daltonics Hystar 3.2.
The strategy for the identification of pigment catabolites in plant tissues was adapted to the identification of carotenoids in animal tissues (38). The in-house mass database created ex professo comprises monoisotopic masses, elemental composition, and optionally, retention time and characteristic product ions for 360 carotenes, xanthophylls, and xanthophyll esters. Data evaluation was performed with Bruker Daltonics DataAnalysis 4.0. From the HPLC/TOF-MS data, an automated peak detection on the extracted ion chromatograms expected for the [M+H]+ ion of each compound in the database was performed with a script command editor. The identification of carotenoids was performed on the basis of their chromatographic behavior, UV-visible data, and high-resolution MS data. Thus, positive identification rely on mass accuracy of the protonated molecule, mass error in comparison with the calculated mass for the target compound, and comparison of the theoretical and experimental isotope patterns for the assumed protonated molecule. The latter was automatically calculated by the software module SigmaFit, included in the data evaluation software. The SigmaFit value is a measure of the goodness of fit between theoretical and experimental isotopic patterns, with smaller values indicating a better isotopic matching.
Acknowledgments
Sharlene Santana and Marco Tschapka kindly allowed us to reproduce their Honduran white bat photographs, shown in Fig. 1. I.G. is supported by a Ramón y Cajal Fellowship (RYC-2012-10237) from the Spanish Ministry of Economy and Competitiveness (MINECO). J.G.-F. and A.P.-G. benefited from Project AGL2013-42757-R from the same ministry.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300(5616):125–127. doi: 10.1126/science.1082142. [DOI] [PubMed] [Google Scholar]
- 2.Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24(6):345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 3.Kaspar KL, et al. Pigmented potato consumption alters oxidative stress and inflammatory damage in men. J Nutr. 2011;141(1):108–111. doi: 10.3945/jn.110.128074. [DOI] [PubMed] [Google Scholar]
- 4.Negro JJ, et al. Coprophagy: An unusual source of essential carotenoids. Nature. 2002;416(6883):807–808. doi: 10.1038/416807a. [DOI] [PubMed] [Google Scholar]
- 5.Meyers SM. Developments in world aquaculture, feed formulations, and role of carotenoids. Pure Appl Chem. 1994;66(5):1069–1076. [Google Scholar]
- 6.Arab L, Steck-Scott S, Bowen P. Participation of lycopene and beta-carotene in carcinogenesis: Defenders, aggressors, or passive bystanders? Epidemiol Rev. 2001;23(2):211–230. doi: 10.1093/oxfordjournals.epirev.a000803. [DOI] [PubMed] [Google Scholar]
- 7.Hata TR, et al. Non-invasive Raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115(3):441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee EH, et al. Dietary lutein reduces ultraviolet radiation-induced inflammation and immunosuppression. J Invest Dermatol. 2004;122(2):510–517. doi: 10.1046/j.0022-202X.2004.22227.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee CM, et al. Review of animal models in carotenoid research. J Nutr. 1999;129(12):2271–2277. doi: 10.1093/jn/129.12.2271. [DOI] [PubMed] [Google Scholar]
- 10.Demmig-Adams B, Adams WW., 3rd Antioxidants in photosynthesis and human nutrition. Science. 2002;298(5601):2149–2153. doi: 10.1126/science.1078002. [DOI] [PubMed] [Google Scholar]
- 11.Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6(2):466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lienau A, et al. Bioavailability of lutein in humans from intrinsically labeled vegetables determined by LC-APCI-MS. J Nutr Biochem. 2003;14(11):663–670. doi: 10.1016/j.jnutbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 14.Palombo P, et al. Beneficial long-term effects of combined oral/topical antioxidant treatment with the carotenoids lutein and zeaxanthin on human skin: A double-blind, placebo-controlled study. Skin Pharmacol Physiol. 2007;20(4):199–210. doi: 10.1159/000101807. [DOI] [PubMed] [Google Scholar]
- 15.McGraw KJ. Mechanics of carotenoid-based coloration. In: Hill GE, McGraw KJ, editors. Bird Coloration, Vol. I: Mechanisms and Measurements. Harvard Univ. Press; Cambridge, MA: 2006. pp. 177–242. [Google Scholar]
- 16.Bradley BJ, Mundy NI. The primate palette: The evolution of primate coloration. Evol Anthropol. 2008;17(2):97–111. [Google Scholar]
- 17.Whitehead RD, Re D, Xiao D, Ozakinci G, Perrett DI. You are what you eat: Within-subject increases in fruit and vegetable consumption confer beneficial skin-color changes. PLoS One. 2012;7(3):e32988. doi: 10.1371/journal.pone.0032988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krinsky NI, Mayne ST, Sies H. Carotenoids in Health and Disease. Marcel Dekker; New York, NY: 2004. [Google Scholar]
- 19.Nwachukwu ID, Udenigwe CC, Aluko RE. Lutein and zeaxanthin: Production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci Technol. 2016;49:74–84. [Google Scholar]
- 20.Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids. J Lipid Res. 2013;54(7):1719–1730. doi: 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbosa MJ, Morais R, Choubert G. Effect of carotenoid source and dietary lipid content on blood astaxanthin concentration in rainbow trout (Oncorhynchus mykiss) Aquaculture. 1999;176(3–4):331–341. [Google Scholar]
- 22.Xavier AAO, Mercadante AZ, Garrido-Fernández J, Pérez-Gálvez A. Fat content affects bioaccessibility and efficiency of enzymatic hydrolysis of lutein esters added to milk and yogurt. Food Res Int. 2014;65:171–176. [Google Scholar]
- 23.West CE, Castenmiller JJ. Quantification of the “SLAMENGHI” factors for carotenoid bioavailability and bioconversion. Int J Vitam Nutr Res. 1998;68(6):371–377. [PubMed] [Google Scholar]
- 24.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids Handbook. Birkhäuser. Switzerdland; Basel: 2004. [Google Scholar]
- 25.Rodríguez-Herrera B, Medellín RA, Timm RM. Neotropical Tent-Roosting Bats. Instituto de Biodiversidad; Santo Domingo, Costa Rica: 2007. [Google Scholar]
- 26.Su Q, Rowley KG, Itsiopoulos C, O’Dea K. Identification and quantitation of major carotenoids in selected components of the Mediterranean diet: Green leafy vegetables, figs and olive oil. Eur J Clin Nutr. 2002;56(11):1149–1154. doi: 10.1038/sj.ejcn.1601472. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Gálvez A, Mínguez-Mosquera MI. Esterification of xanthophylls and its effect on chemical behavior and bioavailability of carotenoids in the human. Nutr Res. 2005;25(7):631–640. [Google Scholar]
- 28.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34(6):2033–2040. [PubMed] [Google Scholar]
- 29.Mundy NI, et al. Red carotenoid coloration in the zebra finch is controlled by a cytochrome P450 gene cluster. Curr Biol. 2016;26(11):1435–1440. doi: 10.1016/j.cub.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 30.García-de Blas E, Mateo R, Viñuela J, Pérez-Rodríguez L, Alonso-Alvarez C. Free and esterified carotenoids in ornaments of an avian species: The relationship to color expression and sources of variability. Physiol Biochem Zool. 2013;86(5):483–498. doi: 10.1086/671812. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Herrera B, Víquez-R L, Cordero-Schmidt E, Sandoval JM, Rodríguez-Durán A. Energetics of tent roosting in bats: The case of Ectophylla alba and Uroderma bilobatum (Chiroptera: Phyllostomidae) J Mammal. 2016;97:246–252. [Google Scholar]
- 32.Rodríguez-Herrera B, Medellín RA, Gamba-Rios M. Roosting requirements of white tent-making bat Ectophylla alba (Chiroptera: Phyllostomidae) Acta Chiropt. 2008;10(1):89–95. [Google Scholar]
- 33.Leo Lester R, Grach C, Paul Pener M, Simpson SJ. Stimuli inducing gregarious colouration and behaviour in nymphs of Schistocerca gregaria. J Insect Physiol. 2005;51(7):737–747. doi: 10.1016/j.jinsphys.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, et al. The evolution of color vision in nocturnal mammals. Proc Natl Acad Sci USA. 2009;106(22):8980–8985. doi: 10.1073/pnas.0813201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.del Val E, et al. The liver but not the skin is the site for conversion of a red carotenoid in a passerine bird. Naturwissenschaften. 2009;96(7):797–801. doi: 10.1007/s00114-009-0534-9. [DOI] [PubMed] [Google Scholar]
- 36.Emenhiser C, Simunovic N, Sander LC, Schwartz SJ. Separation of geometrical carotenoid isomers in biological extracts using a polymeric C30 column in reversed-phase liquid chromatography. J Agric Food Chem. 1996;44(12):3887–3893. [Google Scholar]
- 37.Breithaupt DE, Wirt U, Bamedi A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta L.) and several fruits by liquid chromatography-mass spectrometry. J Agric Food Chem. 2002;50(1):66–70. doi: 10.1021/jf010970l. [DOI] [PubMed] [Google Scholar]
- 38.Ríos JJ, Roca M, Pérez-Gálvez A. Systematic HPLC/ESI-High resolution-q-TOF-MS methodology for metabolomic studies in nonfluorescent chlorophyll catabolites pathway. J Anal Methods Chem. 2015;2015:490627. doi: 10.1155/2015/490627. [DOI] [PMC free article] [PubMed] [Google Scholar]