Significance
Narcolepsy with cataplexy is a sleep disorder characterized by excessive daytime sleepiness and sudden loss of muscle tone. These clinical manifestations are the result of selective loss of a neuronal population producing orexin. The etiology of the disease remains elusive, although converging evidence points to a key involvement of the immune system. We developed an animal model to study the autoimmune processes at play in narcolepsy. We demonstrate that cytotoxic CD8 T cells, but not Th1 CD4 cells, are able to target and destroy orexinergic neurons. This selective neuronal loss is responsible for clinical signs mimicking human narcolepsy. By identifying potential immune effectors of the immunopathological process in narcolepsy, these findings offer a rationale for the use of immunotherapies.
Keywords: autoimmunity, narcolepsy, CD8 T cells, sleep disorders, orexin
Abstract
Narcolepsy with cataplexy is a rare and severe sleep disorder caused by the destruction of orexinergic neurons in the lateral hypothalamus. The genetic and environmental factors associated with narcolepsy, together with serologic data, collectively point to an autoimmune origin. The current animal models of narcolepsy, based on either disruption of the orexinergic neurotransmission or neurons, do not allow study of the potential autoimmune etiology. Here, we sought to generate a mouse model that allows deciphering of the immune mechanisms leading to orexin+ neuron loss and narcolepsy development. We generated mice expressing the hemagglutinin (HA) as a “neo-self-antigen” specifically in hypothalamic orexin+ neurons (called Orex-HA), which were transferred with effector neo-self-antigen–specific T cells to assess whether an autoimmune process could be at play in narcolepsy. Given the tight association of narcolepsy with the human leukocyte antigen (HLA) HLA-DQB1*06:02 allele, we first tested the pathogenic contribution of CD4 Th1 cells. Although these T cells readily infiltrated the hypothalamus and triggered local inflammation, they did not elicit the loss of orexin+ neurons or clinical manifestations of narcolepsy. In contrast, the transfer of cytotoxic CD8 T cells (CTLs) led to both T-cell infiltration and specific destruction of orexin+ neurons. This phenotype was further aggravated upon repeated injections of CTLs. In situ, CTLs interacted directly with MHC class I-expressing orexin+ neurons, resulting in cytolytic granule polarization toward neurons. Finally, drastic neuronal loss caused manifestations mimicking human narcolepsy, such as cataplexy and sleep attacks. This work demonstrates the potential role of CTLs as final effectors of the immunopathological process in narcolepsy.
Narcolepsy with cataplexy, referred to as type 1 narcolepsy (T1N), is a rare and chronic neurological disease characterized by excessive daytime sleepiness, sudden loss of muscle tone triggered by emotions (cataplexy), sleep paralysis, hypnagogic hallucinations, and fragmented nocturnal sleep (1). T1N is caused by a defective neurotransmission by the orexin/hypocretin neuropeptide and is associated with a selective and almost complete loss (85–100%) of orexinergic neurons in the hypothalamus (2, 3). The mechanisms leading to this neuronal loss are not yet elucidated, although current evidence points to an autoimmune process. Indeed, T1N is tightly associated with the human leukocyte antigen (HLA) HLA-DQB1*06:02 allele, carried by 98.4% of patients vs. 17.7% of the general European population (4). An independent association with HLA class I alleles was recently revealed in two independent studies (5, 6). Additionally, an association with polymorphisms in the T-cell receptor (TCR) α chain locus was found and replicated (7, 8). Moreover, autoantibodies recognizing different antigenic targets expressed in the central nervous system (CNS) have been identified in the serum and cerebrospinal fluid (CSF) of narcoleptic patients (9–11). Finally, a dramatic increase in the incidence of T1N has been observed in Northern Europe during the 2009–2010 vaccination campaigns against pandemic H1N1 influenza virus using the Pandemrix vaccine (12–14). The immune mechanisms involved remain unknown, although molecular mimicry is strongly suspected (9, 15). However, recent results demonstrate that a H1N1 virus could have, by itself, a cytolytic impact on orexinergic neurons, but also on adjacent or more distant neuronal subsets (16).
To date, mouse models of T1N are based on genetic disruption of the orexinergic neurotransmission or the destruction of orexin+ neurons through the expression of a deleterious gene (17–19). These models have well documented the key role of the orexinegic system for sleep/wake behavior and architecture and for muscular tonus, but they do not allow the analysis of the etiology and mechanisms of orexin+ neuron destruction.
In the present work, we investigated whether an autoimmune process could lead to T1N development and deciphered the effector mechanisms responsible for the selective loss of orexin+ neurons. To this end, we generated mice expressing a “neo-self-antigen” selectively in orexin+ neurons and adoptively transferred neo-self-antigen–specific effector T cells in these mice. We show that both antigen-specific Th1 CD4 cells and cytotoxic CD8 T cells (CTLs) were able to cause hypothalamic inflammation. However, only CTLs were capable of triggering a selective loss of orexin+ neurons mimicking human T1N. The data also support direct and antigen-dependent CTL-mediated cytotoxicity of the orexin+ neurons as the mechanism of neuronal demise. Moreover, this neuronal loss leads to a narcoleptic-like phenotype. Our results thus emphasize that CTLs could play a central role in the final steps of narcolepsy immunopathogenesis.
Results
Expression of HA as a Neo-Self-Antigen Selectively in Orexin+ Neurons.
To test a potential autoimmune basis of T1N, we generated a mouse line, named Orex-HA, expressing the H1N1 influenza virus HA as a neo-self-antigen specifically in orexinergic neurons. To this end, the Rosa26tm(HA)1Lib mice (20) were crossed with the Orex-Cre animals, expressing Cre under the control of the human orexin promoter (21).
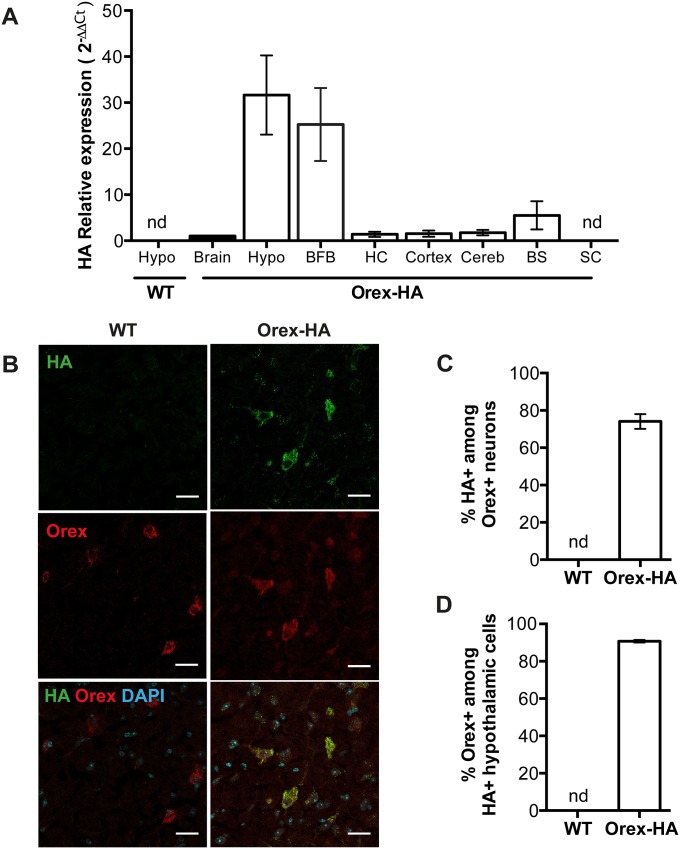
To evaluate the expression pattern of HA in the Orex-HA mice, we monitored the transcription of HA in different parts of the brain by quantitative RT-PCR (Fig. S1A). HA mRNA was highly enriched in the hypothalamus and the basal forebrain, a site of intense projection from orexinergic neurons (22, 23), compared with the rest of the brain (Fig. S1A). HA expression was not detected in any other tissue tested by RT-PCR, except for the heart. As expected, HA was not expressed in wild-type (WT) littermate control mice (Fig. S1A). HA protein expression was detected by immunohistofluorescence in ∼75% of orexin+ neurons from Orex-HA mice, but not in WT littermates (Fig. S1 B and C). Only few hypothalamic cells expressing HA (≤7%) were not immunostained for orexin (Fig. S1D). Collectively, these results show that HA is expressed as a neo-self-antigen selectively in the orexin+ neurons of Orex-HA mice.
Fig. S1.
HA is expressed in orexin+ neurons in Orex-HA mice. (A) Detection of HA mRNA expression by quantitative RT-PCR within different brain structures of Orex-HA and WT animals. BFB, basal forebrain; BS, brainstem; Cereb, cerebellum; HC, hippocampus; Hypo, hypothalamus; SC, spinal cord. Results are expressed as mean ± SEM from 5–11 mice per CNS region, from five independent experiments. nd, not detected. (B) Representative immunofluorescence analysis of HA and orexin-A expression in the hypothalamus of WT and Orex-HA animals. Nuclei are labeled with DAPI. Red and green stainings reveal orexin and HA, respectively. (Scale bars: 25 μm.) (C and D) Percentages of HA+ cells among orexin+ neurons (C) and of orexin+ neurons among HA+ cells in the hypothalamus (D). Results are expressed as mean ± SEM of 4–13 mice per group from four independent experiments. nd, not detectable.
The Orex-HA mice presented a normal phenotype and exhibited a sleep architecture and numbers of orexin+ neurons similar to those of WT littermates.
Neo-Self-Antigen–Specific Th1 CD4 T Cells Infiltrate the Hypothalamus of Orex-HA Mice, but Fail to Elicit Neuronal Demise in the Absence of Autoantibodies.
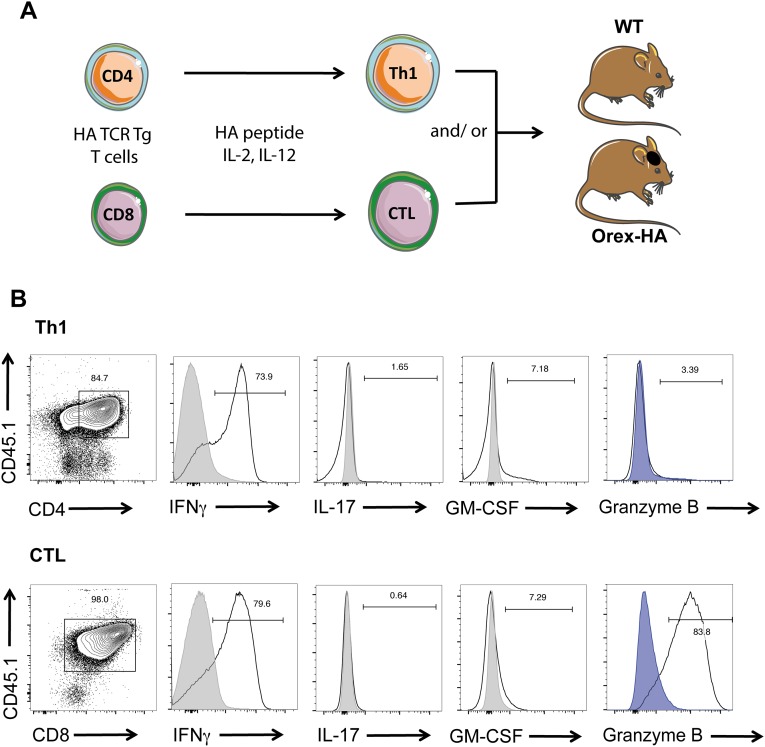
A remarkable association has been found worldwide between T1N and the HLA-DQB1*06:02 allele, reaching an odds ratio of >250 (4), suggesting the contribution of CD4 T cells to the disease process. Furthermore, recent studies reported an increase in IFN-γ or IFN-γ–induced chemokines in the serum or CSF of T1N patients (24, 25). We therefore evaluated the ability of in vitro differentiated neo-self-antigen–specific Th1 cells to trigger the destruction of orexin+ neurons in Orex-HA mice by adoptive transfer experiments (Fig. S2 A and B).
Fig. S2.
Transfer of HA-specific Th1 and/or CTLs in Orex-HA and WT animals. (A) Naive HA-specific CD4 and CTLs isolated from TCR transgenic mice were differentiated in vitro toward Th1 cells and CTLs, respectively, before their adoptive transfer i.v. into Orex-HA or WT nontransgenic littermates. HA-specific Th1 cells (CD45.1+ CD4+) or CTLs (CD45.1+ CD8+) comprise 80–98% of the transferred cells. (B) Before injection, the quality of T-cell differentiation was verified by flow cytometry based on the expression of IFN-γ, IL-17, GM-CSF, and granzyme B after phorbol 12-myristate 13-acetate /ionomycin stimulation. Gray curves delineate staining on unstimulated T cells, and blue curves delineate staining with isotype control antibodies.
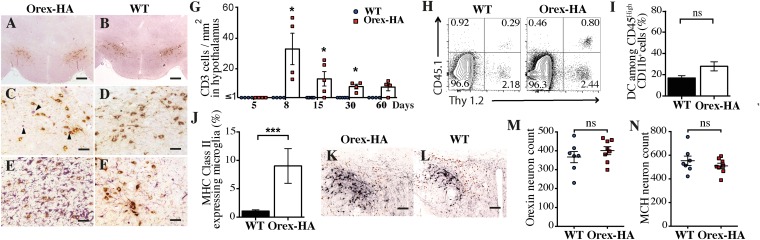
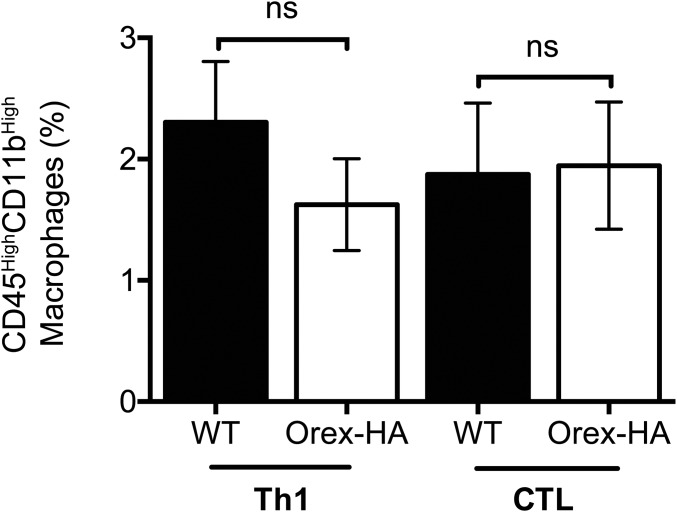
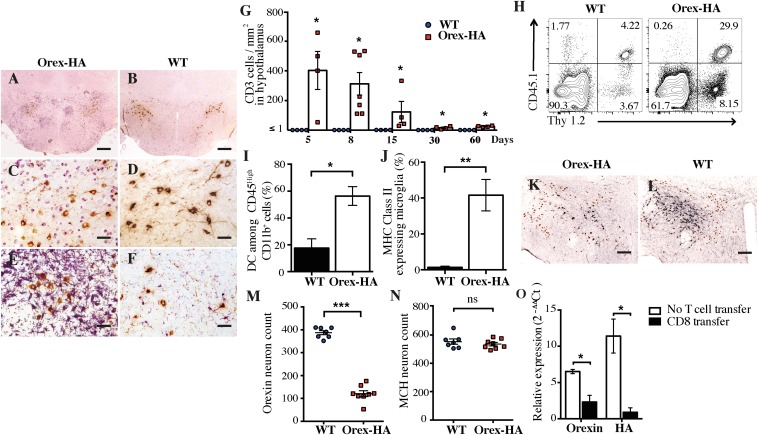
To determine whether the antigen-specific Th1 cells could reach the antigen-expressing region of the brain, we analyzed CD3 and Orexin-A staining on the hypothalamus of Orex-HA and control mice 8 d after transfer. We detected CD3+ T cells in the hypothalamus of Orex-HA (Fig. 1 A and C), but not control (Fig. 1 B and D), mice, indicating that the presence of the neo-self-antigen was required for the infiltration and/or retention of T cells in the parenchyma. This T-cell infiltration spared the rest of the brain (Fig. S3 A–D) and was accompanied by marked microglial activation (Fig. 1 E and F), with up-regulation of major histocompatibility complex (MHC) class II molecules (Fig. 1J). No significant recruitment of dendritic cells or macrophages was noted (Fig. 1I and Fig. S4). To evaluate the kinetics of T-cell infiltration, we quantified the number of CD3+ T cells in the hypothalamus of Orex-HA mice at different time points after Th1 injection and noted that T-cell infiltration peaked at day 8 after transfer and declined progressively thereafter (Fig. 1G). Based on the CD45.1 congenic marker exclusively expressed by transferred T cells, we demonstrated that antigen-specific Th1 cells were among the CNS-infiltrating T cells in Orex-HA mice (Fig. 1H). Interestingly, localized inflammation due to both T cells (Fig. S5A) and activated microglia (Fig. S5C) persisted for >60 d in close vicinity to the hypothalamic orexin+ neurons. Despite this chronic inflammation, there was surprisingly no reduction in the number of orexin+ neurons in Orex-HA (Fig. 1 K and M) compared with WT mice (Fig. 1 L and M). The number of hypothalamic melanin-concentrating hormone+ (MCH) neurons, which are intermingled with but different from orexin+ neurons, also remained unchanged (Fig. 1 K, L, and N).
Fig. 1.
Neo-self-antigen–specific Th1 cells trigger focal hypothalamic inflammation but no loss of orexinergic neurons. (A–D) Immunohistochemistry staining for CD3 (violet) and orexin-A (brown) in Orex-HA mice (A and C) and WT littermate control mice (B and D) 8 d after transfer of 3 × 107 neo-self-antigen–specific Th1 cells. (E and F) Immunohistochemistry staining for Iba-1 (violet) and orexin+ neurons (brown) in Orex-HA (E) and WT (F) mice. Representative results from four or five mice per group are shown. Arrowheads in C point to infiltrating CD3+ cells. [Scale bars: 200 μm (A and B) or 50 μm (C–F).] (G) Quantification of CD3+ T cells in the hypothalamus of WT or Orex-HA mice at different time points after Th1 injection. Each symbol represents an individual mouse. Results are expressed as mean ± SEM of four or five mice per group for each time point. (H) Representative FACS plots of brain-infiltrating cells from WT or Orex-HA animals at day 8 after Th1 transfer. Representative results from three independent experiments are shown. (I and J) Frequency of CD11c+ among CD45high CD11b+ cells was assessed by flow cytometry (I) and MHC class II expression on CD45dim CD11b+ Thy1.2− microglia (J). Results are expressed as mean ± SEM of 12 mice per group from three independent experiments. (K and L) At 60 d after Th1 cell transfer, double immunohistochemistry, staining for orexin+ neurons (black) and MCH+ neurons (brown) in Orex-HA mice (K) and WT controls (L). Representative results from seven or eight mice per group are shown. [Scale bars: 125 μm (K and L).] (M and N) The density of orexin+ neurons (M) and MCH+ neurons (N) in Orex-HA and WT mice is shown at day 60 after Th1 injection. Results are expressed as mean ± SEM of seven or eight mice per group from two independent experiments. Statistical analysis was performed by using the Mann–Whitney u test. ns, not significant. *P < 0.05; ***P < 0.001.
Fig. S3.
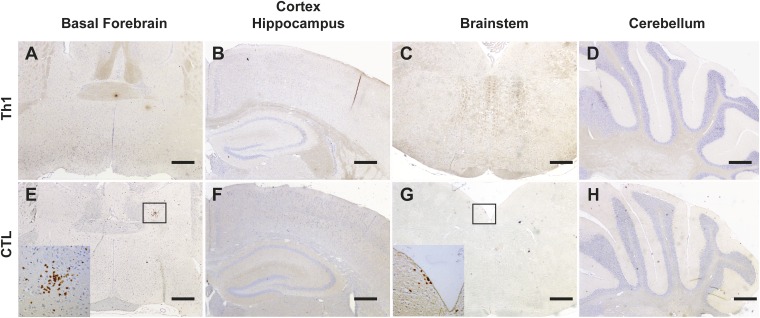
Mild or no T-cell infiltration is detected outside the hypothalamus in Orex-HA mice injected with HA-specific Th1 or CTL. Immunohistological staining of brain sections of Orex-HA mice at day 8 after Th1 (A–D) or CTL transfer (E–H) to detect T-cell infiltration (CD3, brown) in extrahypothalamic regions including basal forebrain (A and E), cortex, and hippocampus (B and F), brainstem (C and G), and cerebellum (D and H) is shown. Insets highlight rare CD3+ cells in the brain parenchyma. Nuclear counterstaining was performed with hematoxylin (blue). Representative results from four or five mice per group are shown. (Scale bars: 200 μm.)
Fig. S4.
T-cell infiltration in the hypothalamus is not associated with recruitment of macrophages. The proportion of macrophages (CD45High CD11bHigh) among living cells extracted from the brains of WT mice (filled bars) and Orex-HA (open bars) 8 d after Th1 or CTL transfer was assessed by flow cytometry. Results are expressed as mean ± SEM of 6–11 mice per group from three independent experiments. Statistical analysis was performed by using the Mann–Whitney u test. ns, not significant.
Fig. S5.
Persistence of inflammation within the hypothalamus after transfer of HA-specific Th1 cells or CTLs. (A–D) Staining for T cells (CD3, violet) and orexin+ neurons (brown) (A and B) or microglia (Iba1; violet) and orexin+ neurons (brown) (C and D) in Orex-HA (A and C) and WT (B and D) mice, 60 d after Th1 transfer. (E–H) Staining for T cells (CD3, violet) and orexin+ neurons (brown) (E and F), or microglia (Iba1; violet) and orexin+ neurons (brown) (G and H) in Orex-HA (E and G) and WT (F and H) mice, 60 d after CTL transfer. Arrowheads in A and E point to infiltrating CD3+ T cells. Representative results from four to eight mice per group are shown. (Scale bars: 50 μm.)
Collectively, these data indicate that autoreactive Th1 cells can infiltrate the hypothalamus, but are unable to cause the destruction of orexin+ neurons.
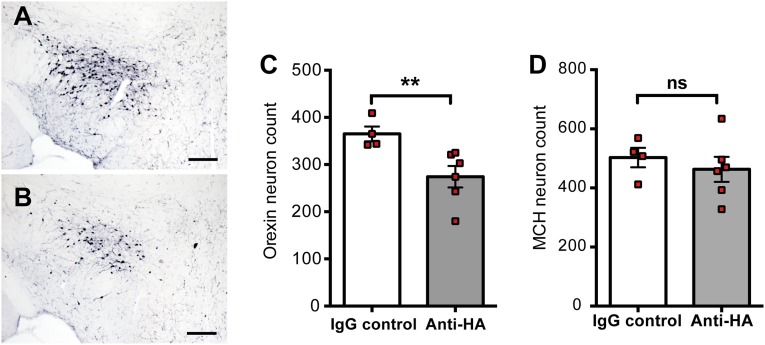
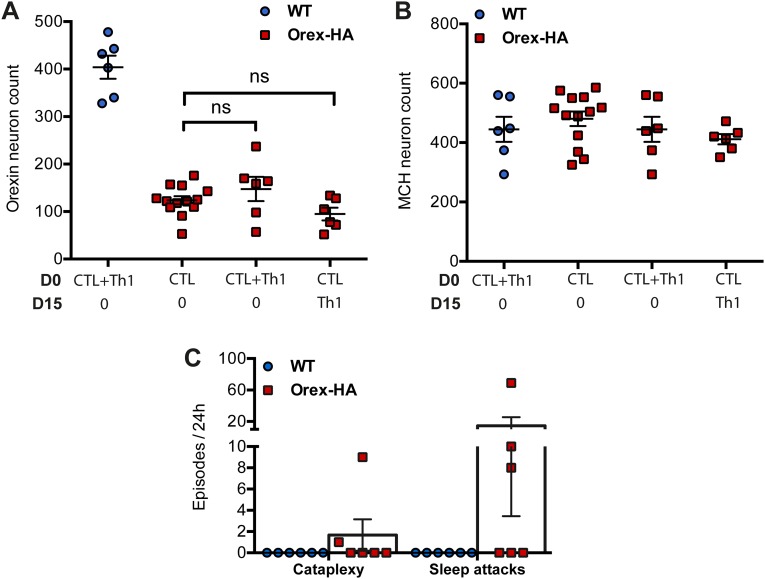
Several studies (9–11) described the presence of circulating autoantibodies directed against hypothalamic neurons in narcolepsy. We thus investigated if antibodies directed against HA could trigger neuronal death in the context hypothalamic inflammation induced by Th1 cells. Orex-HA mice received either anti-HA antibodies or control IgG between day 5 and 15 after the transfer of neo-self-antigen–specific Th1 cells. A mild loss of orexin+ neurons (mean = 22.6%, range 8.3–49.2%) was observed 60 d after Th1 cell transfer in mice receiving anti-HA antibodies, in contrast to mice injected with control IgG (Fig. S6 A, C, and D). MCH+ neurons were unaffected (Fig. S6B). This result suggests that synergy between cellular and humoral immunity could contribute to the pathogenesis of narcolepsy.
Fig. S6.
Anti-HA antibodies induce mild loss of orexin+ neurons in the context of Th1 inflammatory lesions. Enumeration of orexin+ neurons 60 d after transfer of 3 × 107 Th1 cells in Orex-HA mice by Immunohistochemistry. (A and B) Mice received either 200 μg of control IgG (A) or anti-HA antibodies (B) every 2 d, from day 5 to day 15 after Th1 transfer. (Scale bars: 150 μm.) (C and D) Quantification of orexin+ (C) and MCH+ (D) neuronal cell bodies in the hypothalamus of Orex-HA mice that received the control IgG (white bars) or the anti-HA antibodies (gray bars). Results are expressed as mean ± SEM of four to six mice per group. Statistical analysis was performed by using the Mann–Whitney u test. **P < 0.01. ns, not significant.
Cytotoxic CTLs Induce Selective Loss of Orexin+ Neurons.
Because CTLs have been implicated in neuronal destruction in other neurological disorders (26), we investigated whether autoreactive CTLs could be the immune effectors of orexin+ neuron destruction in T1N. To this aim, in vitro-differentiated neo-self-antigen–specific CTLs were transferred into Orex-HA and WT littermates (Fig. S2 A and B). Histological analyses revealed a dense T-cell infiltration in the hypothalamus of Orex-HA mice (Fig. 2 A and C), but not in controls (Fig. 2 B and D). This robust T-cell infiltration peaked early, at day 5–8 after transfer (Fig. 2G), and scarcely involved CNS structures other than the hypothalamus (Fig. S3 E–H). The vast majority of brain-infiltrating T cells were the transferred antigen-specific CTLs marked by the CD45.1 congenic surface molecule (Fig. 2H). Marked activation of microglia (Fig. 2 E, F, and J) and recruitment of dendritic cells (Fig. 2I) were also salient features of this model. However, no significant increase in the number of CD45high CD11b+ macrophages was noted (Fig. S4).
Fig. 2.
Orex-HA animals develop massive hypothalamic inflammation and marked orexin neuron loss after transfer of neo-self-antigen–specific CTLs. (A–D) Immunohistochemistry staining for CD3 (violet) and orexin-A (brown) in Orex-HA mice (A and C) and WT mice (B and D) 8 d after adoptive transfer of 3 × 107 CTLs. (E and F) Immunohistochemistry staining of microglial (Iba1; violet) and orexin+ neurons (brown) in Orex-HA (E) and WT (F) mice. Representative results from four to seven mice per group are shown. [Scale bars: 200 μm (A and B) or 50 μm (C–F).] (G) Quantification of T cells in the hypothalamus of Orex-HA and WT mice at different time points after CTL injection. Results are expressed as mean ± SEM of four to seven mice per group for each time point. (H) Representative FACS plots of brain-infiltrating cells from Orex-HA and WT littermates on day 8 after CTL transfer. (I and J) The proportion of CD11c+ among CD45high CD11b+ cells (I) and the expression of MHC class II molecules on CD45dim CD11b+ Thy1.2− cells microglia (J) were assessed by flow cytometry. Results are expressed as mean ± SEM of six to eight mice per group from three independent experiments. Statistical analyses were performed by using the Mann–Whitney u test. *P < 0.05; **P < 0.01, comparing the Orex-HA group with the respective WT controls. (K and L) At 60 d after CTL transfer, immunohistochemistry staining for orexin+ neurons (black) and MCH+ neurons (brown) in Orex-HA animals (K) and WT mice (L). [Scale bars: 125 μm (K and L).] (M and N) Quantification of orexin+ (M) and MCH+ (N) neuronal cell bodies in the hypothalamus of Orex-HA mice compared with WT animals 60 d after CTL transfer. Results are expressed as mean ± SEM of seven or eight mice per group from two independent experiments. (O) Expression of orexin and HA mRNA in the hypothalamus of Orex-HA mice that received neo-self-antigen–specific CTLs or have been left untreated. Results are expressed as mean ± SEM of four or five mice per group from two independent experiments. Statistical analyses were performed by using the Mann–Whitney u test. *P < 0.05; **P < 0.01; ***P < 0.001. ns, not significant.
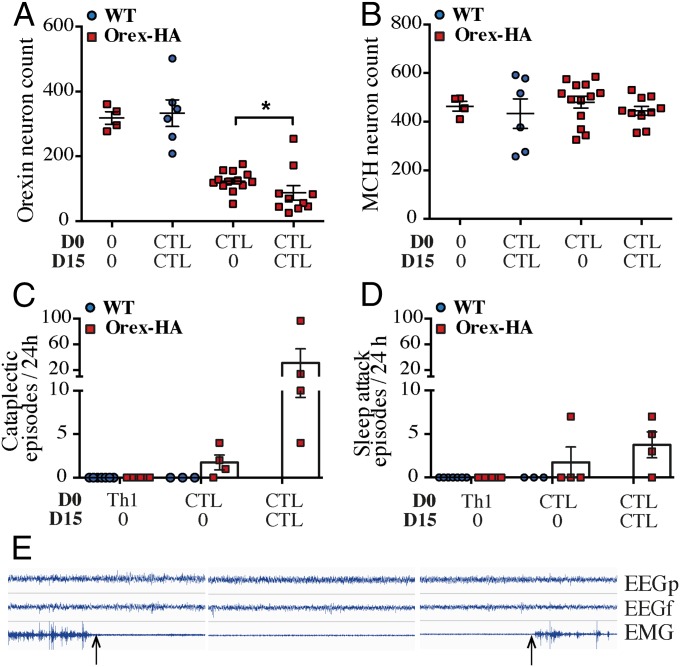
T-cell infiltration recessed from day 8 onward (Fig. 2G), although local inflammation lasted for at least 60 d in the vicinity of orexin+ neuronal cell bodies (Fig. S5 E–H). At this late time point, a profound loss in orexin+ neurons (mean = 68.8%; range 54.5–86.3%) was revealed in Orex-HA animals (Fig. 2 K and M), compared with WT mice (Fig. 2 L and M). A concomitant decrease of orexin and HA transcripts was found in the hypothalamus of these Orex-HA mice, arguing further in favor of the orexin+ neuron death, rather than a defect in orexin expression (Fig. 2O). Importantly, MCH+ neurons were not destroyed, underlining the antigen-specific and selective nature of the neuronal destruction (Fig. 2 K, L, and N).
Mechanism of CTL-Mediated Loss of Orexin+ Neurons.
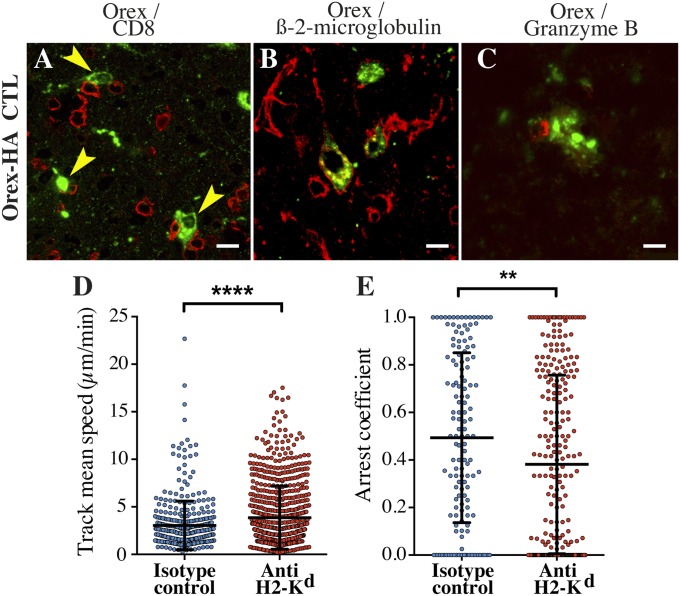
In favor of a direct killing of neurons by the neo-self-antigen–specific CTLs, CTLs were localized at early time points after transfer in close contact to orexin+ neurons (Fig. 3A), which expressed MHC class I molecules (Fig. 3B). We also revealed that this interaction led to the polarization of the CTL cytolytic granules toward orexin+ neurons (Fig. 3C). To further investigate the T-cell/neuron interactions in situ, we used two-photon laser-scanning microscopy on explanted brain tissue of Orex-HA mice, also expressing a green fluorescent protein in orexin+ neurons, after transfer of fluorescently labeled antigen-specific CTLs. Dynamic as well as static physical interactions between neo-self-antigen–specific CTLs and orexin+ neurons were revealed (Movie S1). When CTL motility was assessed in the presence of a monoclonal antibody blocking the MHC class I-presenting molecule Kd, we noted enhanced mean track velocities (Fig. 3D) and decreased arrest coefficient (Fig. 3E), revealing a clear inhibition of CTL/orexin+ neuron interactions. These data indicate that the direct interaction between CTLs and orexin+ neurons are antigen-specific and MHC class I-dependent.
Fig. 3.
CTL-mediated cytotoxicity contributes to orexin+ neuron loss in Orex-HA mice. (A and B) Representative confocal micrographs of CTLs (anti-CD8; red) and orexin+ neurons (anti–orexin-A; green) (A) or MHC class I expression (anti–β2-microglobulin; red) and orexin+ neuron (anti-orexin A; green) in Orex-HA mice (B) 8 days after CTL transfer. (C) Immunofluorescence analysis of granzyme B-containing granules (red) and orexin+ neurons (green) in Orex-HA mice. [Scale bars: 10 μm (A–C).] Analysis of the behavior of CellTrace violet-labeled neo-self-antigen–specific CTLs interacting with orexinergic neurons from ZsGreen Orex-HA mice by two-photon laser scanning microscopy in ex vivo slices of hypothalamus. (D) The mean speed of labeled T cells was analyzed in the presence of anti-H2-Kd mAb (red circles; 776 cells) or isotype control IgG (blue circles; 403 cells). (E) The proportion of time spent arrested (<2 μm/min) in contact with ZsGreen+ orexinergic neurons was determined for the CTLs that have once been in contact with ZsGreen+ orexinergic neuron cell bodies. This subgroup analysis included 263 cells in the presence of anti–H2-Kd mAb and 139 cells in the presence of isotype control IgG. Circles represent individual CTLs in five mice per group from two independent experiments. Results are expressed as mean ± SD, and statistical analyses were performed by using the unpaired Student t test. **P < 0.01; ****P < 0.0001.
We next tested whether Th1 cells could potentiate the neuronal destruction mediated by CTLs. We thus transferred neo-self-antigen–specific Th1 cells in addition to neo-self-antigen–specific CTLs in Orex-HA mice, either concomitantly or 15 d apart. In both conditions, however, the level of orexin+ neuron loss was of similar magnitude (mean = 63.6%; range 41.4–85.9%) than when CTLs were transferred alone (Fig. S7 A and B), suggesting that effector Th1 cells do not play a significant role in the demise of orexin+ neurons. To investigate whether repeated waves of effector immune cells could potentiate destruction of orexin+ neurons, Orex-HA mice received CTLs twice, 15 d apart. This experimental approach led to a profound loss of orexin+ neurons (mean = 73.8%, range 23.7–92.5%) in Orex-HA animals (Fig. 4A). Here again, no significant loss of MCH+ neurons was observed (Fig. 4B). These data strongly argue for the key contribution of effector CTLs, but not of Th1 cells, in the final pathogenic steps of this immune-mediated narcolepsy model.
Fig. S7.
HA-specific Th1 cells do not potentiate the cytotoxic effect of CTLs. (A and B) Enumeration of orexin+ (A) and MCH+ (B) neurons 60 d after transfer of either CTLs alone, concomitant transfer of CTLs and Th1, or transfer of CTLs and Th1 15 d apart in Orex-HA (red squares) or WT (blue circles) mice. The type of transferred cells (and timing of injection) is indicated in the bottom of the graph. The group of Orex-HA mice receiving only CTLs is identical to that shown in Fig. 4. Results are expressed as mean ± SEM of 6–13 mice per group. Statistical analyses were performed by using one-way ANOVA, P values were adjusted for multiple comparisons by using Sidak’s test. ns, not significant. (C) Enumeration of cataplexy episodes and sleep attacks during 24 h in WT mice (blue circles) and Orex-HA animals (red squares) after concomitant transfer of CTLs and Th1 cells. Results are expressed as mean ± SEM of six mice per group from two independent experiments.
Fig. 4.
Repeated injections of CTLs aggravate the loss of orexin+ neurons in Orex-HA animals and lead to a narcolepsy-like phenotype. (A and B) Quantification of orexin+ neurons (A) and MCH+ neurons (B) in Orex-HA mice (red squares) that received no injection, CTLs once, or CTLs twice (15 d apart) 60 days after adoptive transfer. The results of WT mice (blue circles) that received CTLs twice (15 d apart) are also shown. Results for Orex-HA mice receiving CTLs once are already partially depicted in Fig. 2. Results are expressed as mean ± SEM of 4–13 mice per group. Statistical analyses were performed by using the Mann–Whitney u test. *P < 0.05. (C and D) Enumeration of cataplexy episodes (C) and sleep attacks (D) during 24 h in Orex-HA animals (red squares) and WT mice (blue circles) that were injected with Th1 cells or with CTLs once or twice (15 d apart). The behavioral arrests were subdivided into sleep attacks or cataplexy according to their EEG/EMG features. Results are expressed as mean ± SEM of four mice per group. (E) Example of cataplexy with typical EEG/EMG characteristics. Arrows demarcate the onset and termination of the cataplexy episode.
Orex-HA Mice Exhibit a Narcoleptic-Like Phenotype After Cytotoxic CTL Transfer.
Given the similarity between the histopathological features observed in our mouse model and those of patients with T1N (2, 3), we next determined whether Orex-HA mice that had received neo-self-antigen–specific T cells develop a narcolepsy–cataplexy phenotype. At 60 d after T-cell transfer, we monitored electroencephalography (EEG)/electromyography (EMG) activities concomitantly with video recording over a 24-h period in Orex-HA and control mice. Orex-HA mice that received CTLs alone or in combination with Th1 cells exhibited pathologic behavioral arrests. These behavioral arrests were composed of cataplexy (Fig. 4 C and E) and sleep attack (Fig. 4D), as defined (27). These narcoleptic-like events were present in 86% of animals (three of four mice injected with CTLs once; five of six mice injected with CTL+Th1; and all four mice injected twice with CTLs) (Fig. 4 C and D and Fig. S7C). Interestingly, the severe loss of orexin+ neurons, achieved with two injections of CTLs, was accompanied by a marked increase in the number of cataplectic events (Fig. 4C).
Discussion
We have established a mouse model to investigate the immunopathogenesis of T1N. In this model, antigen-specific CTLs triggered the specific destruction of orexin+ neurons, whereas antigen-specific Th1 cells did not. In addition, the loss of orexin+ neurons after CTL transfer in Orex-HA mice was associated with both sleep attacks and cataplectic episodes.
Surprisingly, although effector Th1 cells migrated to the hypothalamus in Orex-HA mice, this did not promote any loss of orexin+ neurons. This result may be due to the lack of MHC class II expression on neurons, leading to the absence of cognate interactions between specific CD4 T cells and the neurons expressing HA as a neo-self-antigen. However, myelin-specific Th1 cells induce experimental autoimmune encephalomyelitis (28). Thus, the pathogenic contribution of Th1 in CNS autoimmune diseases may depend on the considered model. Indeed, the pathogenic impact of myelin-specific Th1 was shown to depend strongly on the recruitment of macrophages (29), a feature absent in our model. Interestingly, a tendency for higher levels of Th1-related cytokines/chemokines was detected in the CSF or serum of narcoleptic patients, in particular soon after disease onset (24, 25). The data have been interpreted as pointing to the importance of Th1 cells in narcolepsy, although other immune cell types could secrete these mediators. It may also be important to assess the involvement of other Th subtypes, such as Th17 cells that play important roles in other neuroinflammatory diseases, such as multiple sclerosis and neuromyelitis optica (30, 31), although no evidence currently points to their implication in narcolepsy.
Our hypothesis that CTLs play a key role in the destruction of orexin+ neurons (32) is supported by data showing that infiltration of the hypothalamus by CTLs is prominent in rare cases of “symptomatic” T1N, such as anti-Ma2 antibody-associated encephalitis (33). In addition, HLA class I alleles have recently been associated with narcolepsy (5, 6). Consistent with this hypothesis, we showed that neo-self-antigen–specific CTLs could readily infiltrate into the hypothalamus and induce a specific loss of orexin+ neurons. This neuronal loss likely involves a cognate interaction between granzyme B+ CTLs and orexin+ neurons (34), although we cannot rule out a possible contribution of additional cytotoxic pathways such as Fas/FasL or TNF/TNFR1. In any event, the neuronal destruction is clearly antigen-dependent, because the intermingled MCH+ neurons were spared. Our demonstration of the expression of MHC class I molecules by neurons, likely driven by IFN-γ and TNF-α released from the CTLs (and possibly activated microglia), as well as the inhibitory effect of Kd-blocking antibodies, strongly argues for a direct presentation of neo-self-antigen peptide by the orexin+ neurons to the antigen-specific CTLs.
CD4 T cells contribute at several steps to the development of an optimal antigen-specific CTL response. First, naive CTLs are attracted to sites of productive interaction between CD4+ T cells and antigen-presenting dendritic cells through the local release of CCL3 and CCL4 chemokines (35). In addition, after interaction with activated CD4 T cells, antigen-presenting dendritic cells up-regulate CD80/CD86 costimulatory molecules and produce cytokines, thereby enhancing CTL activation and differentiation (36–38). Finally, antigen-specific CD4 T cells are at the center of an elaborate immune cell interaction, resulting in optimal development of effector CD8+ T-cell responses and the acquisition of robust immune memory (39). As such, CD4 T cells could make a key contribution in T1N pathogenesis, by initiating and maintaining pathogenic effector CTL (and possibly also B-cell) responses, without behaving as final effectors.
The drastic loss of orexin+ neurons led to clear narcoleptic episodes (i.e., cataplexy and sleep attack) in most Orex-HA mice that received neo-self-antigen–specific CTLs. The phenotype was particularly striking in mice transferred twice with CTLs, in which ∼90% of orexin+ neurons were lost. This finding is reminiscent of the clinical observations made in T1N patients, which may result from a chronic multistep process. Indeed, most patients first develop excessive daytime sleepiness, whereas cataplectic episodes appear months or years later (40).
Our data indicate that immune effector mechanisms other than CTLs, in particular autoantibodies, could also contribute to T1N pathogenesis, especially in the context of T-cell–triggered hypothalamic inflammation. Antibodies directed against several intracellular or membrane-bound neuronal autoantigens have been detected in the serum of narcolepsy patients (9–11). In particular, a recent study reported that 85% of Finnish T1N patients carrying the HLA-DQB1*06:02 allele and vaccinated with Pandemrix presented antibodies against the orexin receptor type 2. Importantly, these autoantibodies also cross-reacted with the H1N1 influenza nucleoprotein (9). However, these autoantibodies were also highly prevalent (55%) in a cohort of Finnish children with neither T1N nor pandemic H1N1 vaccination (8). Another recent study has shown the presence of autoantibodies in the sera of some narcoleptic patients reacting to hypothalamic antigens on rat brain slices (10). Although these autoantibodies did not target orexin+ neurons, intracerebral injection of autoantibody-containing IgG induced alterations in the sleep architecture, but not a narcolepsy-like phenotype. Antibodies directed against Trib2, found in autoimmune uveitis, have also been described in narcolepsy. Our results underline the potential pathogenic relevance of circulating autoantibodies in an inflammatory context (41). Further studies are needed, however, to narrow down the contribution of B cells and autoantibodies in the pathogenesis of T1N, especially in the post-H1N1 vaccine context. In addition, it is currently unknown whether autoantibodies precede or are the consequence of brain tissue destruction in narcolepsy (42).
To conclude, our study highlights the central role of CTLs as final effectors in immune-mediated narcolepsy. This observation does not negate the contribution of other immune cell types in the disease process. Indeed, activated CD4 T cells could have a major impact on the initiation and development of the pathogenic immune response, involving CTLs and/or autoantibodies. In that respect, the development of hypothalamic autoimmunity in response to influenza virus vaccination remains to be investigated. By providing direct evidence that immune targeting of orexin+ neurons can model clinical and neuropathological aspects of T1N, our study uncovers important clues. Further studies should address whether T1N patients, related or not with Pandemrix vaccine, also exhibit altered cellular immune responses.
Materials and Methods
Mouse Strains.
The (BALB/cJ × C57BL/6J) F1-Orex-HA mice were generated by crossing the BALB/cJ-Rosa26tm(HA)1Lib mice (20) with the C57BL/6J Orex-Cre mice, a gift from T. Sakurai, Kanazawa University, Kanazawa, Japan (21). In the resulting double-transgenic Orex-HA mice, the expression of HA of an H1N1 influenza virus, which is otherwise prevented by an upstream floxed Stop cassette, was induced because of the expression of the Cre recombinase specifically in orexin+ neurons. The (BALB/cJ × C57BL/6J) F1-CL4-TCR and F1-6.5-TCR mice have been described (43, 44). To perform two-photon laser microscopy experiments, we generated Orex-HA-ZsGreen triple-transgenic mice by the cross of C57BL/6J Orex-Cre mice, C57BL/6J.Cg-Gt(ROSA)26Sortm6(CAG-ZsGreen1)Hze (The Jackson Laboratory) (45), and BALB/cJ-Rosa26tm(HA)1Lib. These mice were kept in pathogen-free conditions within the UMS006 animal facility, Toulouse, France. All animal experiments were performed in accordance with the European Union guidelines following approval of the local ethics committee (13-U1043 RL/RV-16).
T-Cell Differentiation and T-Cell Transfer.
HA-specific CD8+ CTL and HA-specific CD4+ Th1 were generated from F1 CL4-TCR mice and F1 6.5-TCR mice, respectively, as described (46, 47). After culture, living cells were collected by Ficoll density separation. CTL and Th1 preparations routinely contained >95% of CD8+Vβ8+ or CD4+Vβ8+, respectively, and >70% were IFN-γ/TNF-α producers when assessed by flow cytometry. Then, 3 × 107 CTLs and/or Th1 were adoptively transferred i.v. to Orex-HA mice or WT littermate controls.
Histopathology.
Tissues were fixed in 4% (wt/vol) paraformaldehyde, conserved in 70% (vol/vol) ethanol, and embedded in paraffin. Immunohistochemical or immunofluorescence staining of 5-μm-thick coronal sections was performed by using the following antibodies: rabbit anti-orexin A (Phoenix Pharmaceuticals), rabbit anti-MCH (Phoenix Pharmaceuticals), rabbit anti-CD3 (SP7; Zytomed), rat anti-CD8a (4SM15; eBioscience), rabbit anti–Iba-1 (Wako), anti-granzyme B (ab4059, AbCam), anti–β2-microglobulin, and anti-HA hybridoma (37, 38) supernatant. Image acquisition was performed with confocal microscope Leica SP8 (Leica Microsystems) or 3DHistec Panoramic 250 slide scanner (3DHistec Ltd). For neuronal enumeration, brains were fixed in 4% paraformaldehyde and 30-μm-thick coronal free-floating sections performed at cryostat. Immunohistochemical detection of orexin+ and MCH+ neurons was performed by using the antibodies described above. Neuron enumeration was performed on 1/12th of the entire hypothalamus (seven sections per brain) by using Mercator software (Explora Nova).
Statistical Analyses.
All statistical analyses were made by using GraphPad Prism software (Version 6.0). Results were compared by using one-way ANOVA, Mann–Whitney u test, or unpaired Student t test. Statistical significance was expressed as *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001.
SI Material and Methods.
Details regarding RT-PCR, purification of CNS-infiltrating mononuclear cells, FACS analyses, injection of anti-HA antibodies, two-photon laser scanning microscopy, and polysomnographic analyses are displayed in Supporting Information.
SI Materials and Methods
RT-PCR.
To assess HA and orexin gene expression, total RNA was extracted with TRIzol reagent, followed by reverse transcription (SuperScript III first-strand synthesis system for RT-PCR; Invitrogen), as recommended by the manufacturer. The cDNA was used as template for quantitative PCR using SYBR Green I master mix (Roche) and assessed in a thermocycler (LightCycler 480; Roche). mRNA expression was normalized to that of HPRT mRNA. Specific primers were used to amplify HA (forward, 5′-AAACTCTTCGCGGTCTTTCCA-3′; reverse, 5′-GATAAGGTAGCTTGGGCTGC-3′), orexin (forward, 5′-GACGACGGCCTCAGACTTCTTTG-3′; reverse, 5′-GAACCTTTGTAGAAGGAAGGTTCAT-3′), and HPRT (forward, 5′TGGTTAAGCAGTACAGCCCCAA-3′; reverse, 5′-AGGTCCTTTTCACCAGCAAGCT).
Purification of Central Nervous System-Infiltrating Mononuclear Cells.
Mice were deeply anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused intracardially with PBS. Brains were removed and homogenized, followed by digestion with collagenase D (1 mg/mL) and DNase I (10 mg/mL). Central nervous system (CNS)-infiltrating mononuclear cells were then collected by using Percoll density separation.
FACS Analyses.
T-cell cultures were stained by using a viability dye (eBioscience) and anti-CD8α (53-6.7; BD Pharmingen), anti-Vβ8 (F23.1; BD Pharmingen), anti-CD45.1 (A20; Biolegend), anti–IFN-γ (XMG1.2; BD Pharmingen), anti–TNF-α (MP6-XT22; BD Pharmingen), and anti-granzyme B (NGZB; eBioscience) mAbs. CNS-infiltrating mononuclear cells were stained by using a viability dye and anti-CD45 (30-F11; BD Pharmingen), anti-CD45.1 (A20; Biolegend), anti-CD45.2 (104; BD Pharmingen), anti-CD11b (M1/70; eBioscience), anti-CD11c, anti-Thy1.2 (53-2.1; eBioscience), anti-CD4 (RM4-5; BD Pharmingen), anti-CD8 (53-6.7; BD Pharmingen), or anti-MHC class II (M5/114.15.2; eBioscience) mAbs. Data were collected on a LSRII Fortessa flow cytometer (Becton Dickinson) and analyzed with FlowJo X software (Tree Star).
Injection of Anti-HA Antibodies.
Anti-HA antibodies were purified from culture supernatants of the CM1-1 hybridoma (a gift from L. Sherman, Scripps Research Institute, La Jolla, CA). Specificity was verified on an HA-expressing tumor cell line (4T1-HA). Orex-HA mice received i.p. injections of 200 μg of anti-HA mAb or mouse control IgG (Sigma-Aldrich) every other day, between days 5 and 15 after transfer of Th1 cells.
Two-Photon Laser Scanning Microscopy.
Brains were harvested from Orex-HA-ZsGreen mice, 6 d after injection of HA-specific T cells labeled with Celltrace violet (Invitrogen). After intracardial perfusion with PBS, brains were sliced in the middle of the hypothalamus with a scalpel and kept in artificial CSF (aCSF) (48) before imaging. For MHC class I blockade experiments, brain slices were incubated for 30 min in 10 μg/mL anti–H2-Kd (SF1-1.1.1; eBioscience) or mouse IgG2a isotype control (eBM2a; eBioscience). While imaging, the tissue was glued in a Petri dish and constantly perfused with oxygenated aCSF warmed to a temperature of 37 °C in a custom-made environment chamber. The 4D datasets were acquired by using an AxioImager upright microscope LSM Carl Zeiss 7MP (Carl Zeiss). A pulsed femtosecond Ti:Sapphire laser (Chameleon Ultra II; Coherent) tunable in the range of 690–1,064 nm was used as the excitation light source. Emitted light is detected through a descanned pathway leading to 5 non-descanned detectors. The 4D images were acquired in 512 × 512-pixel dimensions for each scan, with an immersion 20× plan apo 1-N.A. objective. CellTrace Violet (Invitrogen) emission was detected by using a cube with a 490-nm long-pass filter and a 485-nm short-pass filter. ZsGreen emission was detected by using a filter cube with a 500- to 550-nm band-pass filter and a 555-nm long-pass filter and by tuning the two-photon laser to 800 nm. The 3D stacks with a z-step size of 3 μm and a xyz scan field of 425 × 425 × 60 μm were captured every 35 s. Imaging data were processed with Imaris software (Version 7.7). CTLs were divided into three groups: cells in steady contact with neurons, cells never in contact with neurons, and cells in unsteady contact with neurons. Mean track velocities (micrometers per minute) was calculated for all cells and arrest coefficient for each individual T cells in steady and unsteady contact with neurons. Arrest coefficient was defined as the proportion of time spent arrested in contact with orexinergic neurons (<2 μm/min).
Polysomnographic Analyses.
Anesthesia was induced by an i.p. injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Mice were placed on a stereotaxic frame (David Kopf Instruments) and were implanted with three EEG electrodes, one above the parietal (1.5 mm lateral to midline and −2.5 mm from Bregma) and frontal (1 mm lateral to midline and 1 mm anterior to Bregma) cortices, and a last one above the cerebellum (1 mm lateral to midline and 6 mm posterior to Bregma). In addition, two EMG electrodes were placed between neck muscles as described in rats (49). A s.c. injection of carprofen (5 mg/kg) was administered at the end of surgery for pain management. Mice were connected to a cable plugged in a rotating connector (Bilaney; Plastics One) to allow free movements. Unipolar EEG and bipolar EMG signals were amplified (MCP+; Alpha-Omega Engineering), digitized at 250 Hz, collected with a CED interface using Spike2 software (Cambridge Electronic Design), and analyzed offline by using custom scripts written under Spike2 software. Vigilance states were scored by using a 5-s window frame according to standard criteria (49).
Behavioral arrests were defined as total immobilization of >10 s occurring outside the nest during active wakefulness. According to the consensus definition (27), a behavioral arrest is considered as cataplexy if (i) the mouse is immobile during the whole episode, (ii) it occurs abruptly after at least 40 s of wakefulness, (iii) it lasts at least 10 s, and (iv) it ends with a brutal return to motor activity. During the attacks, the EEG is enriched in theta frequency (5–7 Hz).
Sleep attacks were defined as short episodes with EEG activity rich in high amplitude slow wave (0–4 Hz) accompanied with no or very low muscle tone. They are very similar to slow-wave sleep episodes, but occur outside the nest. Sleep attack onset is more progressive than cataplexy-like attacks.
Supplementary Material
Acknowledgments
We thank Drs. D. Gonzalez-Dunia, A. Dejean, and A. Saoudi for insightful comments on the manuscript. We are indebted to the Imaging, especially Sophie Allart, and Cytometry plateforms of Centre de Physiopathologie de Toulouse Purpan for technical help. We thank M-A. Daussion for mouse care. This work has been supported by INSERM, CNRS, and grants from Fondation Bettencourt-Schueller, Journées de Neurologie de Langue Française, Agence Nationale pour la Recherche, Toulouse University (Emergence Idex), and Région Midi-Pyrénées.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603325113/-/DCSupplemental.
References
- 1.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511. doi: 10.1016/S0140-6736(07)60237-2. [DOI] [PubMed] [Google Scholar]
- 2.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tafti M, et al. DQB1 locus alone explains most of the risk and protection in narcolepsy with cataplexy in Europe. Sleep. 2014;37(1):19–25. doi: 10.5665/sleep.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ollila HM, et al. HLA-DPB1 and HLA class I confer risk of and protection from narcolepsy. Am J Hum Genet. 2015;96(1):136–146. doi: 10.1016/j.ajhg.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tafti M, et al. Narcolepsy-associated HLA class I alleles implicate cell-mediated cytotoxicity. Sleep. 2016;39(3):581–587. doi: 10.5665/sleep.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallmayer J, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han F, et al. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS Genet. 2013;9(10):e1003880. doi: 10.1371/journal.pgen.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed SS, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7(294):294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]
- 10.Bergman P, et al. Narcolepsy patients have antibodies that stain distinct cell populations in rat brain and influence sleep patterns. Proc Natl Acad Sci USA. 2014;111(35):E3735–E3744. doi: 10.1073/pnas.1412189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cvetkovic-Lopes V, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120(3):713–719. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dauvilliers Y, et al. Narcoflu-VF study group Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136(Pt 8):2486–2496. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 13.Szakács A, Darin N, Hallböök T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80(14):1315–1321. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 14.Nohynek H, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han F, Lin L, Li J, Dong XS, Mignot E. Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol. 2013;73(4):560. doi: 10.1002/ana.23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesoriero C, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc Natl Acad Sci USA. 2016;113(3):E368–E377. doi: 10.1073/pnas.1521463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 18.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30(2):345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 19.Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: A new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saxena A, et al. Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes. J Immunol. 2008;181(3):1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki T, et al. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci USA. 2009;106(11):4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol. 2013;23(5):752–759. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadel J, Burk JA. Orexin/hypocretin modulation of the basal forebrain cholinergic system: Role in attention. Brain Res. 2010;1314:112–123. doi: 10.1016/j.brainres.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kornum BR, et al. Cerebrospinal fluid cytokine levels in type 1 narcolepsy patients very close to onset. Brain Behav Immun. 2015;49:54–58. doi: 10.1016/j.bbi.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecendreux M, et al. Impact of cytokine in type 1 narcolepsy: Role of pandemic H1N1 vaccination? J Autoimmun. 2015;60:20–31. doi: 10.1016/j.jaut.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Yshii L, Gebauer C, Bernard-Valnet R, Liblau R. Neurons and T cells: Understanding this interaction for inflammatory neurological diseases. Eur J Immunol. 2015;45(10):2712–2720. doi: 10.1002/eji.201545759. [DOI] [PubMed] [Google Scholar]
- 27.Scammell TE, Willie JT, Guilleminault C, Siegel JM. International Working Group on Rodent Models of Narcolepsy A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32(1):111–116. [PMC free article] [PubMed] [Google Scholar]
- 28.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183(11):7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lock C, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 31.Wang HH, et al. Interleukin-17-secreting T cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 2011;18(10):1313–1317. doi: 10.1016/j.jocn.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 32.Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14(3):318–328. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- 33.Dauvilliers Y, et al. Hypothalamic immunopathology in anti-Ma-associated diencephalitis with narcolepsy-cataplexy. JAMA Neurol. 2013;70(10):1305–1310. doi: 10.1001/jamaneurol.2013.2831. [DOI] [PubMed] [Google Scholar]
- 34.Huse M, Quann EJ, Davis MM. Shouts, whispers and the kiss of death: Directional secretion in T cells. Nat Immunol. 2008;9(10):1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castellino F, et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440(7086):890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 36.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393(6684):480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 37.Bennett SR, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393(6684):478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 38.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393(6684):474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 39.Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421(6925):852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 40.Dauvilliers Y, et al. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology. 2001;57(11):2029–2033. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- 41.Flach AC, et al. Autoantibody-boosted T-cell reactivation in the target organ triggers manifestation of autoimmune CNS disease. Proc Natl Acad Sci USA. 2016;113(12):3323–3328. doi: 10.1073/pnas.1519608113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. 2013;31:345–385. doi: 10.1146/annurev-immunol-020711-075041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabarrocas J, Bauer J, Piaggio E, Liblau R, Lassmann H. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur J Immunol. 2003;33(5):1174–1182. doi: 10.1002/eji.200323492. [DOI] [PubMed] [Google Scholar]
- 44.Magnusson FC, et al. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134(4):1028–1037. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 45.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheikl T, et al. Cutting edge: Neuronal recognition by CD8 T cells elicits central diabetes insipidus. J Immunol. 2012;188(10):4731–4735. doi: 10.4049/jimmunol.1102998. [DOI] [PubMed] [Google Scholar]
- 47.Piaggio E, et al. Multimerized T cell epitopes protect from experimental autoimmune diabetes by inducing dominant tolerance. Proc Natl Acad Sci USA. 2007;104(22):9393–9398. doi: 10.1073/pnas.0610423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herz J, Johnson KR, McGavern DB. Therapeutic antiviral T cells noncytopathically clear persistently infected microglia after conversion into antigen-presenting cells. J Exp Med. 2015;212(8):1153–1169. doi: 10.1084/jem.20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthaud S, et al. Paradoxical (REM) sleep deprivation in mice using the small-platforms-over-water method: Polysomnographic analyses and melanin-concentrating hormone and hypocretin/orexin neuronal activation before, during and after deprivation. J Sleep Res. 2015;24(3):309–319. doi: 10.1111/jsr.12269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.