Plant cells are equipped with a collection of membrane surface molecular “antennas” specifically sensitive to different signals. They are mostly represented by hundreds of receptor-like kinases (RKs): about 600 encoded in the Arabidopsis genome (1), which allow plants to react swiftly to signals related to the progress of their own ontogeny (intercellular communication) and also to environmental changes, including stress situations or pathogen attack. Such surface alertness is especially important for sessile organisms bound to be born and die at the same single spot. Not surprisingly, study of plant RK regulation is among the most important current fields of plant research. RKs involved in pathogen presence recognition via specific binding of pathogen activity-related molecular species—pattern-recognition receptor kinases (PRKs)—are also studied for practical reasons of plant protection (for a recent overview, see refs. 2–4). RKs, as most other components of the plasmalemma (PM), are not static. Even without activation, RKs undergo constitutive recycling to and from the PM by insertion (exocytosis) and removal (endocytosis), often involving the trans-Golgi network/early endosome (TGN/EE). Kinetics and steady-state localization differs for individual RK species (5, 6). Until recently, there was only very limited insight into what happens to PRKs in plant cells upon the arrival of the signal: that is, after specific ligand binding resulting in an intracellular kinase domain activation and signaling initiation. Two side-by-side reports in PNAS (7, 8) show that three different PRKs [PEP receptor 1 (PEPR1), EF-TU RECEPTOR (EFR), and FLAGELLIN SENSING 2 (FLS2)], involved in biotic defense interactions activated by three different ligands, are all removed from the cell surface via clathrin-mediated endocytosis (CME). An interacting coreceptor, BRI1-ASSOCIATED KINASE 1 (BAK1), is necessary for the internalization of all these activated receptors. Requirement for the active RK domain was demonstrated for the FLS2 PRK, but this might be a more general feature also valid for other activated PRK internalization mechanisms (7).
In a pathway seemingly bypassing the TGN/EE, all three internalized ligand-bound receptors converge on the same ARA7/RabF2b marker-positive late-endosomal prevacuolar compartment (PVC), which is identical to the multivesicular body (MVB) and destined finally for the vacuole (7, 8) (Fig. 1). Interestingly, temporal dynamics for endocytotic internalization of the steroid receptor BRI1 were speedier when compared with pathogen-related pattern recognition ligand-bound FLS2 or PEPR1 receptors, but the final destination was the same, indicating a possible existence of the late-endosomal compartment as a general depot for internalized activated RKs in plant cells (6–8).
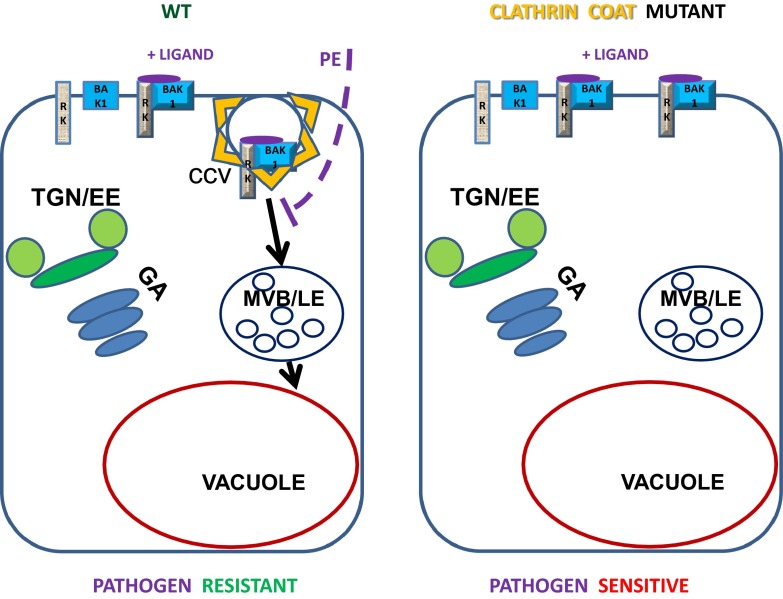
Fig. 1.
In the WT (Left), the activated complex of PRK with the BAK1 coreceptor is formed upon binding the pathogen-derived ligand (small violet ellipse), and the clathrin-dependent endocytosis (CCV) is initiated, resulting in the transport of the ligand–receptor complex to the vacuole via a late endosomal (LE) MVB compartment bypassing the Golgi (GA) or TGN/EE compartments. Pathogens might try to block this defense mechanism by injection of effector proteins (PE and dashed violet line). In clathrin mutants (Right), this process is inhibited, and in the case of FLS2-dependent defense, this results in an increased sensitivity to Pseudomonas syringae infection.
However, is the TGN/EE compartment bypassed by this general internalization pathway, or is there a specific subcompartment of the TGN/EE destined to mature into the PVC/MVB lacking conventional TGN markers (8)? Uncovering probable cell type-specific functional subcompartmentalization of the TGN/EE compartment in plants is a difficult yet imminent task that will need to be resolved in the future.
Substantial differences with respect to the importance of CME for the initiation of early signaling pathways were observed between FLS2 versus PEPR RKs. Although flg22-induced/FLS2 RK-dependent transient activation of reactive oxygen species production (via RBOH NADPH oxidase/NOX) and MAPK signaling was not inhibited concomitantly with clathrin endocytosis inhibition (7), in the case of Pep1-induced/PEPR-dependent activation, MAPK signaling output was strongly inhibited by endocytosis down-regulation (8). As in the case of FLS2, activated BRI1 internalization is not necessary for the signaling; in contrast, retention of activated brassinosteroid-bound BRI1 at the PM stimulated signal output (6). Although early MAPK/NOX response to FLS2 activation is not affected by CME inhibition, following callose deposition and stomata closure, defense responses are inhibited (7). This distinction clearly indicates specific features of different signaling pathways, despite activated receptor similarity in spatiotemporal endomembrane dynamics.
Testing the sensitivity of Arabidopsis mutant plants with compromised CME toward infection by different Pseudomonas bacteria strains was straightforward in the case of activated FLS2-dependent defense reactions (7). Plants with disturbed CME are more sensitive to Pseudomonas than WT, regardless of the presence or absence of Pseudomonas functional type III secretion system (important for bacterial effector injection into the host cells) or coronatin production (reopening stomata to support infection). This finding indicates the general importance of CME in plant defense against bacteria (7). There are known effectors delivered into host plant cells by pathogens, which interfere with the endocytosis factors, resulting in the mislocalization of FLS2 receptors (2, 9), which indicates a possibility that some pathogen effectors are interfering with this internalization pathway to overcome host cell defense (7) (Fig.1).
Interestingly, only clathrin heavy chain 2 (CHC2)-dependent endocytosis affects the internalization of the activated receptors; down-regulation of CHC1 has no effect (7, 8). FLS2-dependent flg22-induced stomata closure is impaired in chc2 mutants, whereas ABA-imposed closure still can take place. However, in chc1 mutants FLS2-dependent flg22-induced stomata closure is not affected (7). In chc2 mutants, FLS2-dependent Pseudomonas- (or flg22-) induced callose deposition is also severely impaired, whereas wound-induced callose deposition is not (7). Thus, in this context the CHC2, but not CHC1, function is relevant for pathogen resistance. Although the clathrin adaptor complex 2 is involved in BRI1 endocytosis, it is not in the case of PEPR (5, 8). How are different and specific clathrin coats, including the adaptor complexes, involved in the specific cargo molecules internalization? This is one of the interesting questions to be solved in the future, not only in the context of phytopathology.
Not too long ago, arguments against the mere possibility of clathrin-dependent endocytosis in normally turgescent plant cells based on physics were put forward (10), and an important experiment proving extensive constitutive endocytosis in all plant cells was published only a little over 10 y ago (11). Partially because of the advent of endocytosis monitoring using fluorescence probes, as well as pharmacological and genetic interventions into endocytosis, our understanding of endocytosis in plant cells has made a distinct leap forward over the last decade, not the least because of the successful implementation of chemical genetics screens uncovering new endocytosis-compromising substances (12). As in other eukaryotic cell types, clathrin-dependent endocytosis assisted by several adaptor complexes seems to be a prevalent mechanism of PM and apoplastic cargo internalization, despite the clear presence of clathrin-independent endocytosis in plants as well (13).
In these two PNAS studies, genetic down-regulation of clathrin-dependent endocytosis was achieved either by knock down of six clathrin heavy-chain genes in a Nicotiana benthamiana transient expression system (7), by using chc2 Arabidopsis mutant plants (7, 8), or by overexpression of the clathrin coat disassembly cochaperone Auxilin2 (8, 14). Parallel evidence from different approaches
Two side-by-side reports in PNAS show that three different PRKs [PEP receptor 1 (PEPR1), EF-TU RECEPTOR (EFR), and FLAGELLIN SENSING 2 (FLS2)], involved in biotic defense interactions activated by three different ligands, are all removed from the cell surface via clathrin-mediated endocytosis (CME).
in two different laboratories is a very valuable added value of this side-by-side publication (7, 8), reciprocally strengthening evidence for clathrin-dependent endocytosis involvement. However, both clathrin and auxilin are known to have extra-endocytotic functions in animal or yeast cells (including signal transduction or the early secretory pathway), and we cannot exclude that similar features of these proteins might partially contribute to observed effects of clathrin and auxilin deregulation in plants (15, 16). In any case, identifying and characterizing possible noncanonical clathrin functions in plants is an important task for the future.
As in many previous studies of the plant secretory pathway, brefeldin A (BFA) intervention into the normal secretion was also used in the case of the PEPR receptor resulting in the formation of PEPR1–GFP containing BFA bodies (8). However, careful controls using a cycloheximide block of de novo protein synthesis very clearly demonstrated that, contrary to the prevailing interpretation of BFA bodies as containing mostly endocytosed/internalized PM material, the population of the PEPR1–GFP receptor captured in BFA bodies is coming from de novo biosynthetic anterograde membrane transport (8). Pathway of PEPR1 receptor exocytosis, but not its endocytotic internalization and further transport, requires BFA-sensitive ARF GTPase GDP/GTP exchange factor function, and exocytosis seems to be the main factor controlling the incidence of inactive PEPRs at the PM (8). This observation is congruent with recent reinterpretation of PIN-FORMED2 (PIN2)-containing BFA bodies as also mostly coming from de novo biosynthetic anterograde PIN2 membrane transport (17). It is now clear that at least some of the published data based on the use of BFA secretion block and “recycling” from BFA bodies after the BFA washout need to be reinterpreted. Obviously, various cargo proteins might behave very differently in this context, and critical re-evaluation of BFA experiments in plant cell biology is certainly necessary.
A new era in plant ligand–receptor interaction studies is indicated by the use of signaling-competent fluorescent ligand analogs [5′-carboxytetramethylrhodamine at the N-terminal (TAMRA)-modified ligands in these two PNAS reports (7, 8)] to monitor real-time dynamics of ligand binding to receptors and subsequent internalization by CME, demonstrating the feasibility of this approach in plant cell signaling studies (6–8). Despite the general functioning of the BAK1 coreceptor in all activated receptor complexes studied in both reports (7, 8), specific receptor–ligand interactions result in exclusive activation of a single pathway in response to a particular ligand. There is no bleed-through; a mixture of elicitors is necessary to activate several receptors concomitantly (7). Nevertheless, all activated RKs colocalize in the same late-endosomal compartments upon CME in such situations (7, 8).
The question of signaling endosome presence in plants, not specifically addressed in the two reports (7, 8), still remains open. Once proposed for brassinosteroid signaling via BRI1 (18), this theory was disproved in a study involving the first-time use of a fluorescent-labeled low molecular-weight ligand in plants, which has proven that internalization of the activated receptor is a negative regulation step, whereas ligand-activated BRI1 receptors are signaling-competent at the PM (6). However, observed clathrin dependency of PEP1-activated PEPR1 MAPK kinase signaling suggests active signaling from the ligand-activated PEPR1 receptors after they are internalized into an endosomal compartment (8). It should also be considered that clathrin is equally important for sorting vacuolar cargos at the TGN/EE, and thus might contribute by removal of the putative negative regulator of PEPR-Pep signaling (8). Conclusive evidence for the functioning of a signaling endosome in plants is currently missing, but might be expected for some of many potential receptor-dependent signaling pathways (2).
Generally available tools to study endocytosis in plant cells (especially fluorescent probes but also good artificial endocytosis cargos) catalyzed substantial progress in this area, whereas the exocytosis phase of plant PM dynamics still remains in the shadow of attention to this day. However, it is impossible to really understand one without the other. As indicated in these two PNAS reports (7, 8), sensitivity of plant cells to elicitors might be regulated mostly by exocytosis, because it is primarily secretion, not endocytosis, that regulates the pool of alert, nonactivated PEPR receptors at the cell surface (8). Development of new approaches for analyzing molecular mechanisms of exocytosis in plant cells is possibly currently the biggest challenge in this field.
Acknowledgments
V.Ž.’s research is supported by the Czech Science Foundation (Grants GA15-14886S and GF16-34887L), and Ministry of Education Youth and Sport of the Czech Republic Project NPUI LO1417.
Footnotes
References
- 1.Shiu SH, Bleecker AB. Plant receptor-like kinase gene family: Diversity, function, and signaling. Sci STKE. 2001;2001(113):re22. doi: 10.1126/stke.2001.113.re22. [DOI] [PubMed] [Google Scholar]
- 2.Bar M, Avni A. Endosomal trafficking and signaling in plant defense responses. Curr Opin Plant Biol. 2014;22:86–92. doi: 10.1016/j.pbi.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Bartels S, Boller T. Quo vadis, Pep? Plant elicitor peptides at the crossroads of immunity, stress, and development. J Exp Bot. 2015;66(17):5183–5193. doi: 10.1093/jxb/erv180. [DOI] [PubMed] [Google Scholar]
- 4.Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immun. August 1, 2016;16(9):537–552. doi: 10.1038/nri.2016.77. [DOI] [PubMed] [Google Scholar]
- 5.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012;24(10):4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani NG, et al. Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol. 2012;8(6):583–589. doi: 10.1038/nchembio.958. [DOI] [PubMed] [Google Scholar]
- 7.Mbengue M, et al. Clathrin-dependent endocytosis is required for immunity mediated by pattern recognition receptor kinases. Proc Natl Acad Sci USA. 2016;113:11034–11039. doi: 10.1073/pnas.1606004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortiz-Morea FA, et al. Danger-associated peptide signaling in Arabidopsis requires clathrin. Proc Natl Acad Sci USA. 2016;113:11028–11033. doi: 10.1073/pnas.1605588113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaparro-Garcia A, et al. Phytophthora infestans RXLR-WY effector AVR3a associates with dynamin-related protein 2 required for endocytosis of the plant pattern recognition receptor FLS2. PLoS One. 2015;10(9):e0137071. doi: 10.1371/journal.pone.0137071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gradmann D, Robinson DG. Does turgor prevent endocytosis in plant cells? Plant Cell Environ. 1989;12(2):151–154. [Google Scholar]
- 11.Meckel T, Hurst AC, Thiel G, Homann U. Endocytosis against high turgor: Intact guard cells of Vicia faba constitutively endocytose fluorescently labelled plasma membrane and GFP-tagged K-channel KAT1. Plant J. 2004;39(2):182–193. doi: 10.1111/j.1365-313X.2004.02119.x. [DOI] [PubMed] [Google Scholar]
- 12.Hicks GR, Raikhel NV. Plant chemical biology: Are we meeting the promise? Front Plant Sci. 2014;5:455. doi: 10.3389/fpls.2014.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paez Valencia J, Goodman K, Otegui MS. Endocytosis and endosomal trafficking in plants. Annu Rev Plant Biol. 2016;67:309–335. doi: 10.1146/annurev-arplant-043015-112242. [DOI] [PubMed] [Google Scholar]
- 14.Ezaki B, Kiyohara H, Matsumoto H, Nakashima S. Overexpression of an auxilin-like gene (F9E10.5) can suppress Al uptake in roots of Arabidopsis. J Exp Bot. 2007;58(3):497–506. doi: 10.1093/jxb/erl221. [DOI] [PubMed] [Google Scholar]
- 15.Ding J, et al. Auxilin facilitates membrane traffic in the early secretory pathway. Mol Biol Cell. 2016;27(1):127–136. doi: 10.1091/mbc.E15-09-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky FM, Sosa RT, Ybe JA, O’Halloran TJ. Unconventional functions for clathrin, ESCRTs, and other endocytic regulators in the cytoskeleton, cell cycle, nucleus, and beyond: Links to human disease. Cold Spring Harb Perspect Biol. 2014;6(9):a017004. doi: 10.1101/cshperspect.a017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jásik J, et al. Effects of auxins and auxin transport inhibitors on PIN-FORMED2 (PIN2) dynamics in Arabidopsis epidermal root cells are not mediated by inhibiting PIN2 endocytosis. Plant Physiol. August 1, 2016 doi: 10.1104/pp.16.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21(13):1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]