Significance
Guanine nucleotide-binding (G) protein α subunit (Gα)-interacting vesicle-associated protein (GIV)/Girdin has previously been shown to serve as a guanine nucleotide exchange factor (GEF) for the Gα activity-inhibiting polypeptide 1 (Gαi) via a conserved motif in its C terminus. Here we show that this motif serves as a guanine nucleotide dissociation inhibitor (GDI) for Gαs. Sequential phosphorylation of two serine residues that flank this motif by two kinases, cyclin-dependent kinase 5 and PKCθ, ensures that GIV exerts its GEF and GDI activities on Gαi and Gαs, respectively, in a temporally and spatially segregated manner. Through its bifunctional role as GEF and GDI, GIV serves as a pleiotropically acting G-protein modulator that integrates, reinforces, and compartmentalizes signals downstream of both growth factors and G proteins and orchestrates migration–proliferation dichotomy.
Keywords: heterotrimeric G proteins, cAMP, cancer invasion, growth factor receptor tyrosine kinase, guanine nucleotide dissociation inhibitor
Abstract
We previously showed that guanine nucleotide-binding (G) protein α subunit (Gα)-interacting vesicle-associated protein (GIV), a guanine-nucleotide exchange factor (GEF), transactivates Gα activity-inhibiting polypeptide 1 (Gαi) proteins in response to growth factors, such as EGF, using a short C-terminal motif. Subsequent work demonstrated that GIV also binds Gαs and that inactive Gαs promotes maturation of endosomes and shuts down mitogenic MAPK–ERK1/2 signals from endosomes. However, the mechanism and consequences of dual coupling of GIV to two G proteins, Gαi and Gαs, remained unknown. Here we report that GIV is a bifunctional modulator of G proteins; it serves as a guanine nucleotide dissociation inhibitor (GDI) for Gαs using the same motif that allows it to serve as a GEF for Gαi. Upon EGF stimulation, GIV modulates Gαi and Gαs sequentially: first, a key phosphomodification favors the assembly of GIV–Gαi complexes and activates GIV’s GEF function; then a second phosphomodification terminates GIV’s GEF function, triggers the assembly of GIV–Gαs complexes, and activates GIV’s GDI function. By comparing WT and GIV mutants, we demonstrate that GIV inhibits Gαs activity in cells responding to EGF. Consequently, the cAMP→PKA→cAMP response element-binding protein signaling axis is inhibited, the transit time of EGF receptor through early endosomes are accelerated, mitogenic MAPK–ERK1/2 signals are rapidly terminated, and proliferation is suppressed. These insights define a paradigm in G-protein signaling in which a pleiotropically acting modulator uses the same motif both to activate and to inhibit G proteins. Our findings also illuminate how such modulation of two opposing Gα proteins integrates downstream signals and cellular responses.
The guanine nucleotide-binding (G) protein α subunit (Gα)-interacting vesicle-associated protein (GIV, also known as “Girdin”) is a multimodular signal transducer and a nonreceptor guanine nucleotide exchange factor (GEF) for Gα activity-inhibiting polypeptide 1 (Gαi) (1). GIV binds G proteins and other signaling molecules and thereby couples G proteins to diverse signaling pathways [e.g., Akt and PI3K (2, 3)] and cell functions. Mechanistically, the best-studied aspect of GIV is its role in coupling growth factor signaling to Gi signaling (reviewed in refs. 4 and 5): Upon stimulation with growth factors such as EGF, GIV uses an SH2-like module to bind directly to the autophosphorylated cytoplasmic tail of the EGF receptor (EGFR) (6) and recruits and transactivates Gi in the vicinity of ligand-activated receptors (7). GIV is also directly phosphorylated by receptor and nonreceptor tyrosine kinases, allowing GIV to bind and activate PI3K directly (8) and enhance Akt signaling (2, 3) to trigger cell migration. Upon endocytosis of EGFR, GIV binds Gαs and follows EGFR to endosomes (9) where it facilitates down-regulation of EGFR and limits cell proliferation. Thus GIV is ubiquitously expressed (10), can be recruited to different subcellular compartments (reviewed in ref. 4), binds to both Gαi and Gαs (9–11), and affects a number of important physiologic and pathologic processes.
Mechanistically, it is known that GIV triggers the migration of diverse cell types in a variety of contexts, e.g., tumor cell motility and invasion, among others (reviewed in ref. 5). Migration is triggered when GIV activates Gαi via an evolutionarily conserved Gα-binding and activating (GBA) motif and releases “free” Gβγ heterodimers, which stimulate motogenic signals such as the PI3K–Akt pathway (reviewed in ref. 5). However, selective ablation of GIV’s GBA motif not only disables cells’ ability to migrate in response to a stimulus but also results in an unexpected gain in phenotype, i.e., cells begin to proliferate (11–13). How GIV’s GBA motif actively suppresses cell proliferation remained unclear. Subsequent work showed that GIV also binds Gαs; the GIV–Gαs interaction and the presence of Gαs in an inactive state that promotes maturation of endosomes shuts down the mitogenic MAPK–ERK1/2 signals from endosomes and suppresses cell proliferation (9). In the absence of Gαs or in cells expressing a constitutively active mutant Gαs, EGFR stays longer in endosomes, MAPK–ERK1/2 signals are enhanced, and cells proliferate (9).
Although the published work led us to conclude that a main function of GIV is to orchestrate the migration–proliferation dichotomy by enhancing cell migration and inhibiting mitosis (9, 11, 12) and that GIV must dually couple to both Gαi and Gαs to orchestrate such dichotomy, several questions remained unanswered. For example, what determines GIV’s preference for one or the other G protein, and how does dual coupling to Gαi and Gαs impact signals downstream of both G proteins and receptor tyrosine kinases and cellular responses to growth factors? Here we show that GIV accomplishes the migration–proliferation dichotomy by interacting sequentially with and paradoxically modulating the nucleotide exchange of two different G proteins with opposing roles: GIV uses an evolutionarily conserved bifunctional GBA motif that serves as a GEF to activate Gαi at the plasma membrane (PM) and trigger cell migration and that serves as a guanine nucleotide dissociation inhibitor (GDI) to inhibit Gαs at endosomes and limit cell proliferation. Such bifunctional GEF and GDI activities were previously reported for the synthetic KB-752 peptide (14, 15), whose sequence led to the identification of the GBA motif in GIV and other members of the GIV family (reviewed in ref. 5). By identifying the naturally occurring bifunctional sequence in GIV, this study illustrates a paradigm in paradoxical G-protein signaling in which, depending on the G-protein substrate, a versatile modulator uses the same motif to accelerate or inhibit nucleotide exchange. We also define the key phosphomodifications that trigger the GEF or GDI functions of GIV, thereby ensuring robustness amid versatility.
Results
GIV Binds Sequentially to Two G Proteins, First to Gαi and then to Gαs.
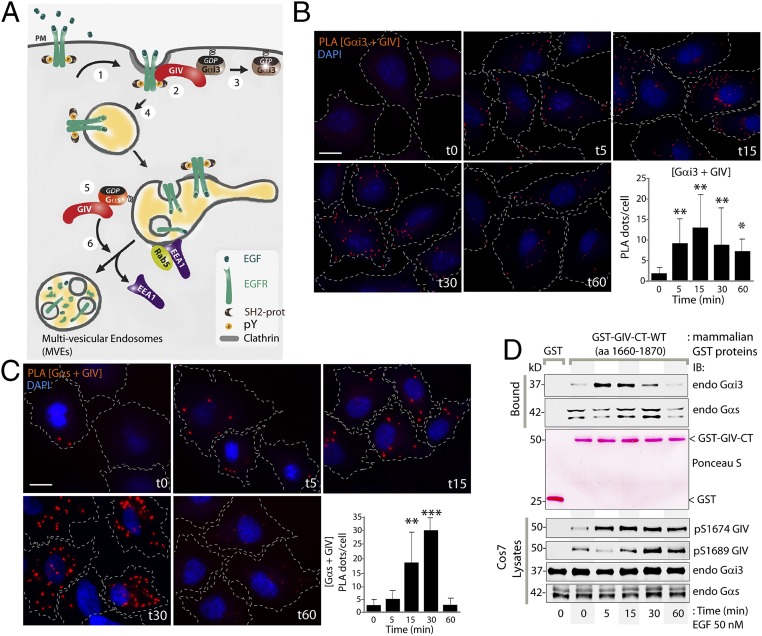
Work to date has shaped the current working model (Fig. 1A) for how GIV coordinates the cellular response to EGF by binding two Gα subunits at two places in cells: GIV interacts with Gαi at the PM (7) and with Gαs on endosomes (9). To determine if GIV can bind both G proteins at any time or binds sequentially to one or the other, we performed proximity ligation assays (PLA) (16) to detect in situ GIV–Gαi3 (Fig. 1B) and GIV–Gαs (Fig. 1C) complexes in HeLa cells. PLA signals between endogenous GIV and both Gαi3 and Gαs were detected most prominently after EGF stimulation, indicating that they interact [i.e., the maximum distance between the two is ≤30–40 nm (16)] and that such interactions are ligand dependent. A quantitative analysis of the abundance of GIV–Gαi and GIV–Gαs complexes, as determined by the number of PLA dots per cell, revealed that GIV interacts with Gαi and Gαs sequentially: The assembly of GIV–Gαi complexes is triggered within 5 min after EGF stimulation (t5) (see the graph in Fig. 1B). Although some GIV–Gαs complexes were detected in starved cells, their ligand-dependent assembly is triggered later, at 15 min after EGF stimulation (t15), and peaks at 30 min after EGF stimulation (t30), and their disassembly occurs by 60 min after EGF stimulation (t60) (see the graph in Fig. 1C). The sequential nature of in situ interactions of GIV with Gαi/s was also confirmed biochemically using protein–protein interaction assays (Fig. 1D) by analyzing endogenous Gαi/s proteins bound to a GST-tagged C-terminal fragment of GIV (GST-GIV-CT) expressed in Cos7 cells. GIV preferentially bound Gαi by t5, and the interaction decreased by t30 (Fig. 1D and Fig. S1). Consistent with our observation using PLA assays (Fig. 1C), GIV maximally bound Gαs at t30 (Fig. 1D and Fig. S1). FRET-based assays using a previously validated CFP-tagged GIV-CT probe (7) also confirmed that GIV–Gαs interaction occurs at t30 on vesicular structures, presumably endosomes (Fig. S2). Taken together, these results demonstrate that upon EGF stimulation GIV–Gαi complexes form first, followed by GIV–Gαs complexes, suggesting that the shift in the composition of GIV–Gα complexes may be orchestrated by some sequential regulatory event(s).
Fig. 1.
Upon EGF stimulation GIV binds Gαi3 and Gαs in a sequential manner. (A) Schematic summarizing GIV’s interactions with Gαi3 and Gαs at various steps in EGFR signaling and trafficking (modified from ref. 9). Upon EGF stimulation, GIV is recruited to the PM (step 1), assembles an EGFR–GIV–Gαi3 complex at the PM (step 2), activates Gαi3 (step 3), and prolongs the association of EGFR with the PM. Upon internalization, EGFR traffics to APPL endosomes (step 4) and then to EEA1 endosomes where GIV binds inactive Gαs (step 5), promoting dissociation of EEA1 and endosome maturation to multivesicular endosomes (step 6) to facilitate EGFR down-regulation, thus shutting off endosome-based proliferative signaling. (B and C) Serum-starved (0.2% FBS, overnight) HeLa cells were stimulated with EGF at the indicated time points and were fixed and analyzed for interactions between GIV and Gαi3 (B) or GIV and Gαs (C) by in situ PLA (red). Nuclei are stained with DAPI (blue). Cell boundaries were traced with interrupted lines by superimposing bright-field microscopy images. Results are expressed as mean ± SEM; n = 3. (Scale bar, 15 µm.) (Lower Right) The number of PLA dots per cell was quantified from a total of 25–30 cells per experiment. (D) Cos7 cells expressing GST-GIV-CT (amino acids 1660–1870) were stimulated with EGF at the indicated time points and were lysed and incubated with glutathione-Sepharose beads. (Top) Bound proteins were analyzed for endogenous Gαi3 and Gαs by immunoblotting (IB). (Middle) Equal loading of GST and GST-GIV-CT proteins was confirmed by Ponceau-S staining. (Bottom) Cell lysates were analyzed for pS1674-GIV, pS1689-GIV, Gαi3, and Gαs by immunoblotting and were quantified using LI-COR Odyssey (Fig. S1). *P < 0.05, **P < 0.01, ***P < 0.001.
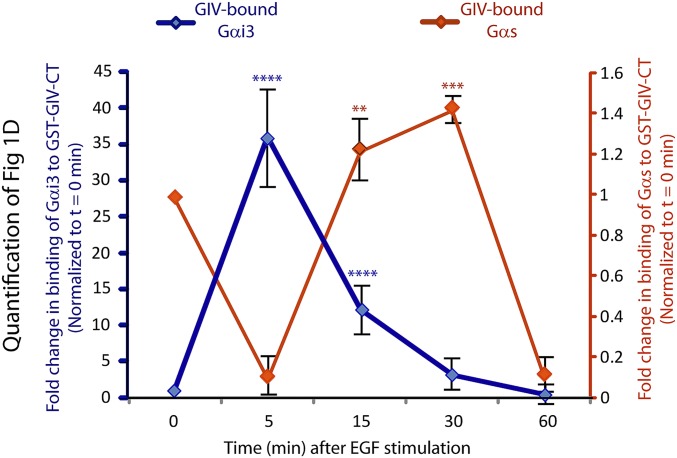
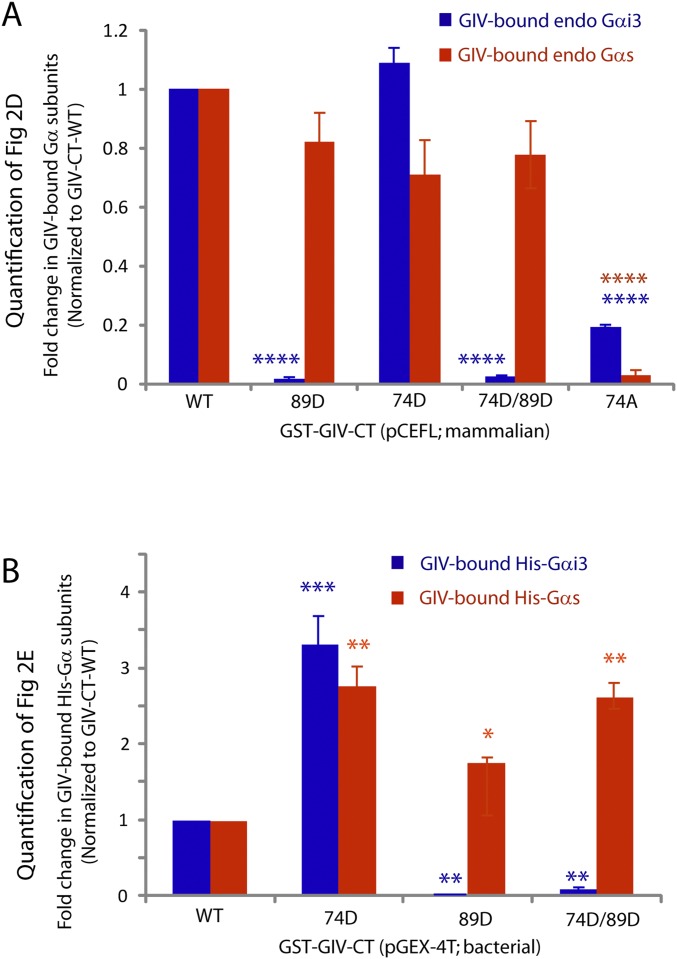
Fig. S1.
Quantification of the profile of binding of endogenous Gαi3 (blue) and Gαs (red) proteins to GST-GIV-CT expressed in mammalian cells. Signal intensities of bands in the immunoblots displayed in Fig. 1D were quantified using LI-COR Odyssey software, normalized to the starved sample (t0), and displayed here as fold-change in binding (y axis) at various times after EGF stimulation (x axis). Such quantification confirmed that GIV–Gαi complexes (blue) were maximally assembled at t5, whereas GIV–Gαs complexes (red) were maximally assembled at t15–t30. Data are presented as mean ± SEM; n = 3. **P < 0.01, ***P < 0.001, ****P < 0.0001.
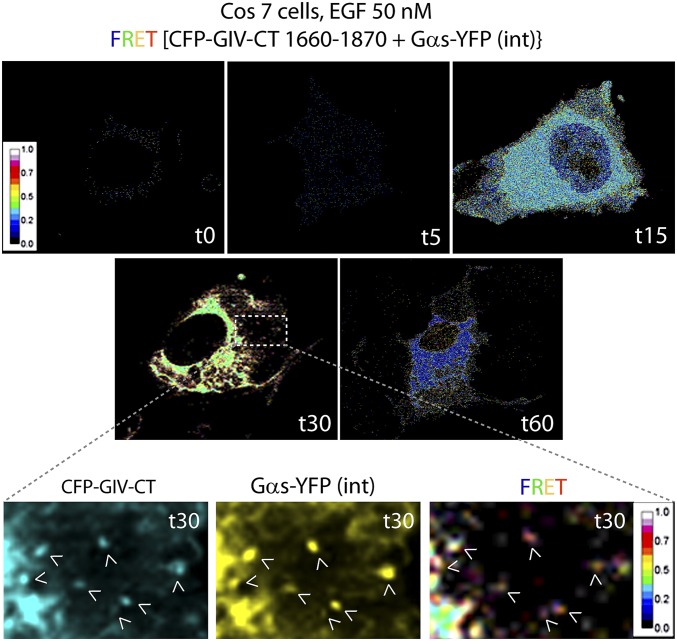
Fig. S2.
Spatiotemporal dynamics of GIV–Gαs interaction after EGF stimulation, as determined by FRET imaging. Cos7 cells were first cotransfected with internally tagged Gαs-YFP [Gαs-YFP(int)] and a previously validated functional CFP-tagged GIV-CT (amino acids 1660–1870) probe which retains key properties of full-length GIV (i.e., the ability to couple G-protein signaling to ligand-activated growth factor receptors). Cells were starved overnight (0% FBS), stimulated with EGF, and analyzed for FRET by confocal live-cell imaging at different time points (t0–t60). Representative images from different time points are displayed. Images show the intensities of acceptor emission triggered by energy transfer in each pixel. Maximum interaction between GIV and Gαs, as determined by increased FRET, occurred at t30 in a perinuclear compartment and on endosome-like structures (Insets). (Magnification, 63×.)
GIV’s Ability to Bind Gαi3 and Gαs Sequentially Is Regulated by Two Key Phosphomodifications.
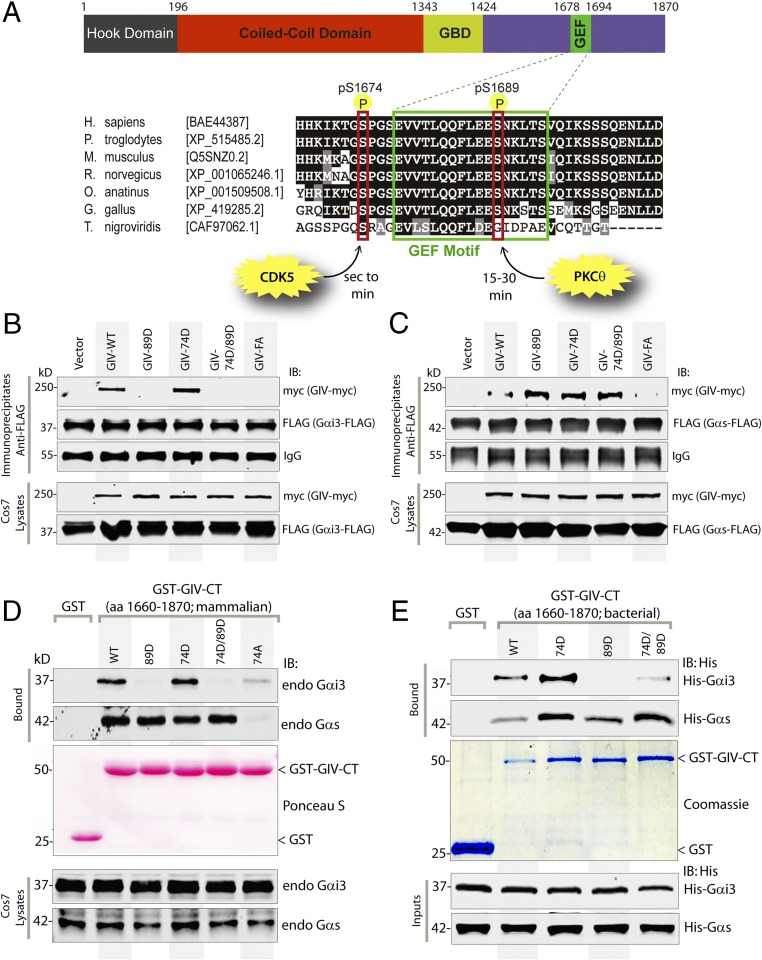
It is noteworthy that GIV’s C terminus (GIV-CT; amino acids 1660–1870) containing the evolutionarily conserved GBA motif (amino acids 1678–1694) (Fig. 2A) that binds and activates Gαi could recapitulate the sequential profile of the binding of full-length GIV to Gαi and Gαs (Fig. 1D). Based on this observation, we hypothesized that phosphorylation of one or more key serine/threonine residues that flank the GBA motif may regulate GIV’s ability to bind both Gαi and Gαs sequentially. In this regard, we recently reported that cyclin-dependent kinase 5 (CDK5) phosphorylates S1674 just upstream of GIV’s GBA motif within seconds to minutes after EGF stimulation and triggers GIV to bind both Gαi and Gαs, albeit with a higher affinity for Gαi (11). Another kinase, PKCθ, phosphorylates S1689 just downstream of the GIV’s GBA motif at approximately t15–t30 and terminates GIV’s ability to bind and/or activate Gαi (13). Analysis of these key phosphomodifications on the G-protein–bound GST-GIV-CT protein (Fig. 1D, Bottom) demonstrated that GIV-CT was phosphorylated at S1674 by t5, coinciding with maximal binding to Gαi3. Phosphorylation at S1689 peaked at t30, coinciding with a selective loss of GIV’s ability to bind Gαi and enhanced binding to Gαs (Fig. 1D). Because phosphorylation at S1674 alone increased GIV’s binding to both Gα subunits, but GIV continues to bind Gαi at a higher affinity (11), we reasoned that this phosphoevent cannot account for the shift in GIV’s preference from Gαi to Gαs. Instead the shift is likely to be brought about by the second phosphoevent at S1689, because phosphorylation at that site disrupts binding to Gαi without affecting binding to Gαs.
Fig. 2.
Sequential phosphorylation of GIV by CDK5 and PKCθ triggers sequential GIV–Gαi3 and GIV–Gαs interactions. (A) Schematic of the domain architecture of GIV and sequence alignment of its C-terminal GEF motif. (Upper) Various domains of GIV are shown. Residue numbers marking the boundaries of each domain are shown. (Lower) The sequence encompassing the GEF motif (green rectangle) and surrounding residues was aligned among various species (accession numbers are shown in brackets) using ClustalW. Conserved residues are shaded in black, and similar residues are shaded in gray. The two phosphorylated residues (S1674 and S1689 in human GIV) that respectively activate (11) and inactivate (13) the GEF function are boxed in red. The timing of these phosphoevents after EGF stimulation and the kinases responsible for these two regulatory phosphoevents [CDK5 (11) and PKCθ (13)] are shown also. (B and C) Immunoprecipitation was carried out with anti-FLAG antibody on equal aliquots of lysates of Cos7 cells coexpressing Gαi3-FLAG (B) or FLAG-Gαs (C) with full-length Myc-tagged GIV (WT and mutants), followed by incubation with protein-G Sepharose beads. Immunoprecipitates (Upper) and lysates (Lower) were analyzed for GIV-Myc and Gαi3-FLAG (B) or Gαs-FLAG (C) by immunoblotting. (D) Lysates of Cos7 cells expressing WT or mutant GST-GIV-CT were incubated with glutathione-Sepharose beads. Bound proteins (Upper) and lysates (Lower) were analyzed by immunoblotting for endogenous (endo) Gαi3 and Gαs and were quantified using band densitometry (Fig. S3A). Equal loading of GST proteins was confirmed by Ponceau-S staining. (E) Equimolar amounts of purified His-Gαi3 (Upper) or His-Gαs (Lower) were incubated with GST or GST-GIV-CT proteins (WT and mutants) immobilized on glutathione-Sepharose beads (Coomassie). Bound His-Gαi3 or His-Gαs was analyzed by immunoblotting using anti-His mAb and was quantified using band densitometry (Fig. S3B). Equal loading of inputs was confirmed by immunoblotting using anti-His mAb.
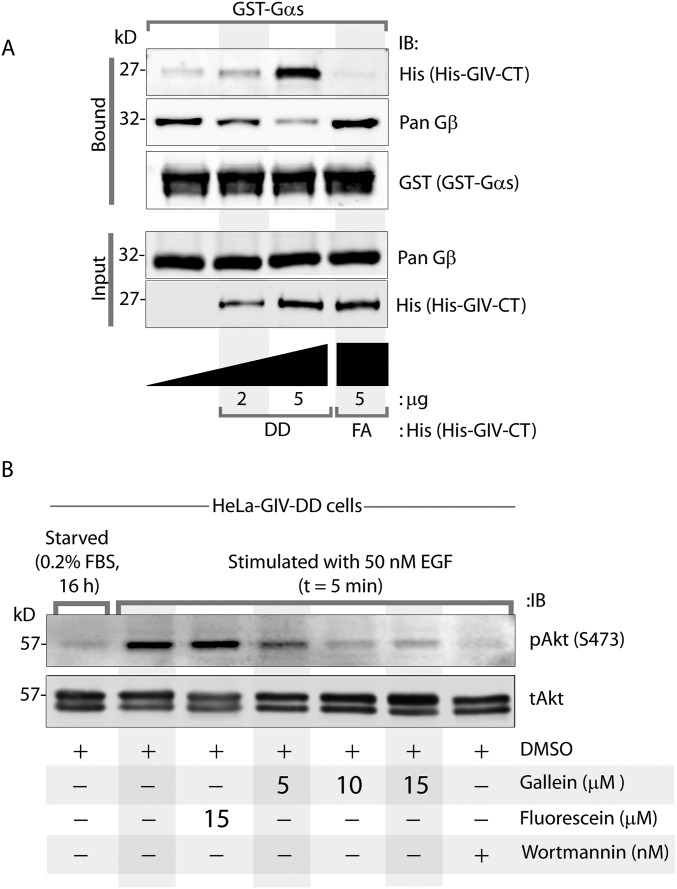
To investigate if phosphorylation of S1674 and/or S1689 affects GIV’s ability to bind Gαi/s, we generated several phosphomimicking mutants of full-length GIV or GST-tagged GIV-CT by replacing serine (S) with aspartate (D) at S1674 alone (74D), at S1689 alone (89D), or at both sites (74D/89D, hereafter “DD”) and tested their ability to bind Gαi3 and Gαs in a series of protein–protein interaction assays (Fig. 2 B–E). First we immunoprecipitated FLAG-tagged Gαi3 (Fig. 2B) or Gαs (Fig. 2C) and looked for binding to Myc-tagged full-length GIV-WT and mutants. In the case of Gαi3 (Fig. 2B), binding was detected with GIV-WT and was slightly augmented with the phosphomimetic 74D mutant, exactly as reported previously (11). In keeping with previous work (13), binding was undetectable with the phosphomimetic 89D mutant (Fig. 2B). Binding also was undetectable with the double-phosphomimetic DD mutant, indicating that inhibitory phosphorylation at S1689 likely overrides the augmentation brought about by phosphorylation at S1674. Gαs binding to GIV-74D was enhanced compared with GIV-WT (Fig. 2C), exactly as reported previously (11). However, unlike Gαi, Gαs continued to bind GIV-89D and GIV-DD mutants, indicating that dually phosphorylated GIV can bind Gαs exclusively. Similar results were obtained when we expressed various phosphomimetic GST-GIV-CT mutants in mammalian cells and tested their ability to bind endogenous Gαi3 and Gαs (Fig. 2D and Fig. S3A). Once again, the GIV-89D and GIV-DD mutants exclusively bound Gαs but not Gαi. The GEF-deficient F1685A (GIV-FA) mutant (Fig. 2 B and C) (1) or the phosphorylation-deficient S1674A (hereafter, “74A”) mutant (Fig. 2D) (11) showed little to no binding to either Gαi3 or Gαs, indicating that an intact GEF motif is required for both interactions. Finally, we expressed the same mutant GST-GIV-CT constructs in bacteria and carried out in vitro pulldown assays with recombinant 6×His-tagged Gαi3 or Gαs (Fig. 2E and Fig. S3B). Once again, compared with GIV-WT, binding to GIV-74D was enhanced for both Gαi and Gαs, whereas only Gαs bound to the GIV-89D and GIV-DD mutants. It is noteworthy that the increase in the binding of both G proteins to GIV-74D (relative to GIV-WT) is not appreciated when the pulldown assays are carried out on cell lysates (Fig. 2D and Fig. S3A) rather than on purified recombinant proteins (Fig. 2E and Fig. S3B). This discrepancy likely stems from the S1674 residue in GIV-CT-WT being abundantly phosphorylated (∼35%) in cells at steady state, as determined by mass spectrometry (11). These findings demonstrate that, despite key differences in how phosphorylation of two serine residues (1674 and 1689) that flank the GEF motif may affect GIV’s ability to bind Gαi vs. Gαs, the binding nonetheless relies on the presence of an intact GEF motif. These findings also support the hypothesis that sequential phosphorylation, first at S1674 and then at S1689, is sufficient to orchestrate a shift in the composition of GIV–Gα complexes from Gαi3 to Gαs. It is phosphorylation at S1674 that serves as a key determinant of GIV’s ability to bind Gαi and Gαs in vitro, but it is phosphorylation at S1689 that reduces GIV’s ability to bind Gαi without affecting its ability to bind Gαs in vivo. These results also provide a set of tools (specific mutants) to interrogate further GIV’s interplay with the two G proteins: GIV-WT physiologically binds both G proteins sequentially, GIV-FA binds neither, and GIV-DD is the physiologically relevant mutant that is expected to bind Gαs selectively and maximally, but not Gαi. No mutants could be designed that selectively bind Gαi. Thus we used the DD and FA mutants as the GDI-proficient and GDI-deficient GIV mutants, respectively, and analyzed them alongside GIV-WT in all subsequent assays. GIV-FA, rather than the nonphosphorylatable 74A mutant, was the preferred GDI-deficient mutant, either alone or in combination, for three reasons: (i) it has been characterized extensively in prior work, including work investigating the GIV–Gαs interaction (9), and therefore preserves continuity and enables comparisons; (ii) its specificity for disrupting GIV–Gα interactions while allowing binding to receptor or other downstream effectors (6) has been confirmed; and (iii) its design and effect on GIV–Gα interaction are guided and explained by a strong structural rationale, whereas the disruptive effect of the 74A mutation remains incompletely understood (11).
Fig. S3.
Quantification of the amount of endogenous (A) or His-tagged (B) Gαi3 (blue) or Gαs (red) proteins bound to WT or various phosphomimicking mutant GST-GIV-CT proteins. Signal intensities of bands in the immunoblots displayed in Fig. 2 D and E were quantified using LI-COR Odyssey software, normalized to GIV-CT-WT, and displayed here as fold-change in binding (y axis) with various GIV-CT constructs (x axis). Any mutant GIV-CT construct in which the serine at 1689 was mutated to aspartate (89D) showed significantly less binding to Gαi but not to Gαs. Data are presented as mean ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
GIV Serves as a GDI for Gαs via the Same Motif It Uses to Activate Gαi.
To determine the consequence of GIV–Gαs interaction and the impact of GIV phosphorylation on Gαs activity, we carried out enzymatic assays using recombinant, bacterially expressed WT and mutant His-GIV-CT (Fig. 3A) and His-Gαs proteins in vitro. When we measured the steady-state GTPase activity of Gαs alone or in the presence of different concentrations of His-GIV-CT WT or various phosphomimetic mutants (74D, 89D, or DD) at a fixed time point (t15), we found that although all inhibited steady-state Gαs GTPase activity in a dose-dependent manner, the dual phosphomimetic DD mutant was significantly more efficient than GIV-WT or any of the other mutants at each concentration tested (from ∼20–25% to ∼50% inhibition at 2 µM) (Fig. 3B). No significant difference was observed between GIV WT and either of the two single mutants, 74D or 89D. Time-course experiments (Fig. 3C) confirmed these inhibitory effects and validated that the dose-dependence experiments were carried out under conditions in which the GTPase activity is linear, therefore reflecting bona fide changes in activity rates. To mitigate concerns about the integrity and purity of His-tagged GIV-CT used in these experiments, we synthesized WT and dual phosphomimetic GIV-DD peptides (SI Experimental Procedures) and tested their ability to inhibit Gαs in steady-state GTPase assays. Much like the purified His-tagged proteins, WT peptide showed a weak inhibitory effect (∼20% at 5 µM), whereas the DD mutant peptide was significantly more potent (∼50% at 5 µM) (Fig. 3D). Although diminished steady-state GTPase activity of Gαs suggests inhibition of nucleotide exchange, the reduced activity also could be caused by inhibition of the intrinsic rate of GTP hydrolysis. We ruled out the latter possibility by carrying out GTPγS-binding assays that directly measure nucleotide exchange rates. These experiments showed that WT peptide reduced GTPγS binding by ∼20%, and the DD mutant peptide inhibited binding by ∼50%, whereas the control FA mutant peptide, which does not bind Gαs (Fig. 2 B and C), had no effect (Fig. 3E). Taken together, these results indicate that GIV has modest GDI activity on Gαs and that this activity is enhanced by dual phosphorylation at S1674 and S1689.
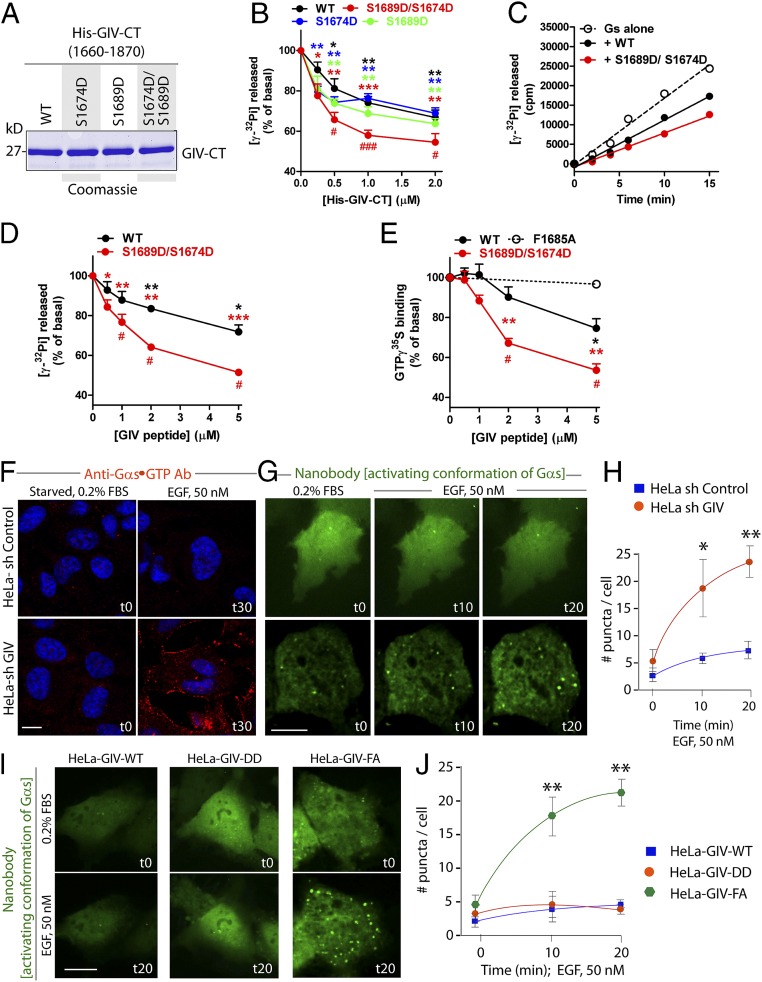
Fig. 3.
GIV serves as a GDI for Gαs. (A) His-tagged WT C-terminal (amino acids 1660–1870; His-GIV-CT WT) and various phosphomimetic mutants of GIV were purified from bacteria, analyzed by SDS/PAGE, and stained with Coomassie blue. (B) The steady-state GTPase activity of His-Gαs (50 nM) was determined at t15 in the presence of increasing concentrations of purified His-GIV-CT WT (black), S1674D (blue), S1689D (green), or S1674D/S1689D (red). Gαs activity is expressed as percent of the steady-state GTPase activity of Gαs alone in the absence of His-GIV-CT protein. Results are expressed as ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with no GIV; #P < 0.05, ##P < 0.01 compared with GIV WT at the same concentration. (C) The steady-state GTPase activity of His-Gαs (50 nM) was determined by measuring (in counts per minute) the release of radiolabeled phosphate at different time points in the absence (open circles) or presence of 1 μM purified His-GIV-CT WT (solid black circles) or His-GIV-CT S1674D/S1689D (solid red circles). One experiment representative of three is shown. (D) The steady-state GTPase activity of His-Gαs (50 nM) was determined at t15 in the presence of increasing concentrations of a synthetic GIV-derived peptide (amino acids 1671–1705) of WT sequence (black) or containing the S1674D/S1689D mutations (red). Gαs activation is expressed as percent of the steady-state GTPase activity of Gαs alone in the absence of any peptide. Results are expressed as ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with no GIV; #P < 0.05 compared with GIV WT at the same concentration. (E) GTPγS binding by His-Gαs (50 nM) was determined by measuring the incorporation of 35S-radiolabeled nucleotide at t15 in the presence of increasing concentrations GIV-derived peptide (amino acids 1671–1705) of WT sequence (black), containing the DD mutations (red), or the FA mutation (G-protein binding–deficient negative control; dashed line). Results are expressed as ± SEM; n = 3. *P < 0.05, **P < 0.01 compared with no GIV; #P < 0.05 compared with GIV WT at the same concentration. (F) Serum-starved control (shControl) and GIV-depleted (shGIV) HeLa cells were stimulated with 50 nM EGF for 30 min and then were fixed and stained for active Gαs using anti-Gαs⋅GTP (red) and DAPI (to stain the nucleus; blue) and were analyzed by confocal microscopy. (Scale bars, 10 µm.) (G–J) Control (shControl) and GIV-depleted (shGIV) HeLa cells (G and H) or HeLa cells stably expressing GIV-WT or the DD or FA mutants (I and J) expressing GFP-tagged anti–Gαs-GTP (activating) conformation-specific nanobodies were serum starved overnight, stimulated with 50 nM EGF, and analyzed by live-cell imaging using a Leica scanning disk microscope for 20 min. Freeze frames from representative cells are shown in G and I. Bright puncta indicate active Gαs. (Scale bars, 10 µm.) Graphs in H and J show the average number of puncta per cell (y axis) at the indicated time points (x axis) in the experiments shown in G and I, respectively. Results are expressed as ± SEM; n = 3. *P < 0.05, **P < 0.01.
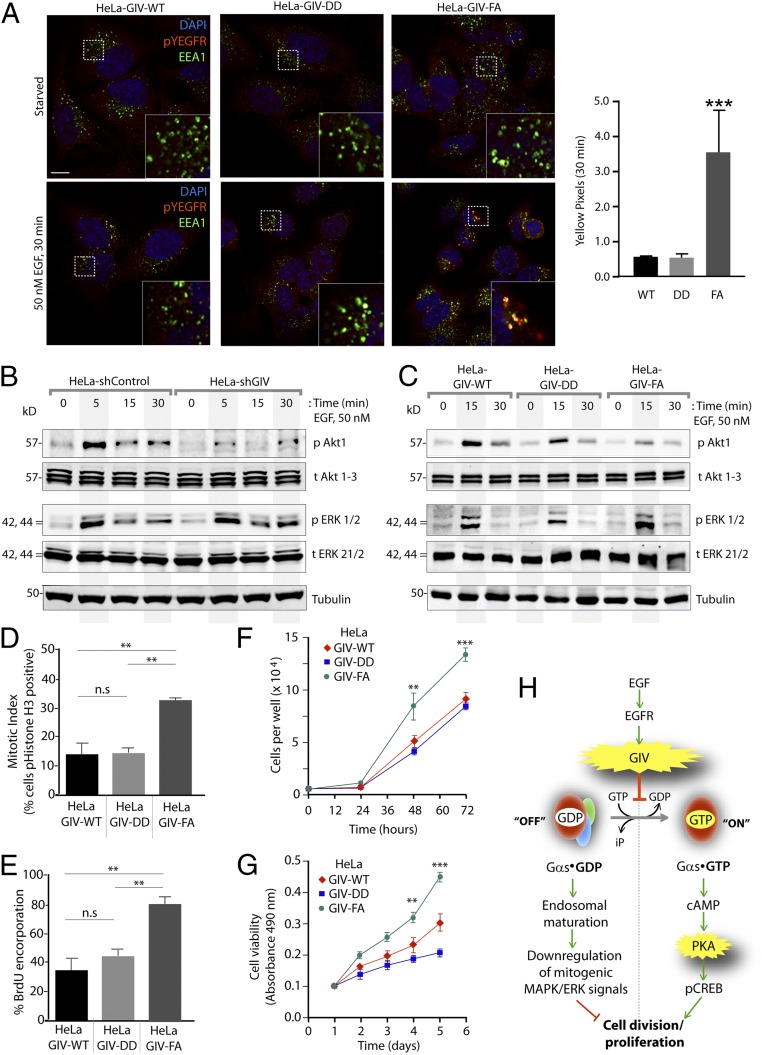
To determine if GIV functions as a GDI and inhibits Gαs activity in HeLa cells, we used two different approaches, immunofluorescence on fixed cells and fluorescence live-cell imaging using two different conformation-specific anti-Gαs antibodies. For immunofluorescence we used a commercially obtained mouse monoclonal IgG that specifically recognizes the active GTP-bound conformation of Gαs (anti–Gαs-GTP) (Fig. 3F). In control cells, no significant Gαs activity was detected either before or after ligand stimulation, indicating either that Gαs is not activated after EGF stimulation or that its activity is efficiently suppressed by some modulator for sustained periods of time. In GIV-depleted cells [80–85% depletion of endogenous GIV by shRNA sequence targeting the 3′ UTR (SI Experimental Procedures and Fig. S4)] Gαs activity was easily detected exclusively after ligand stimulation (Fig. 3F, Lower), indicating that GIV is required for the suppression of Gαs activity. A similar pattern also was observed by live-cell imaging using the extensively well-validated Gαs conformational biosensor, nanobody Nb37-GFP that binds and helps detect the nucleotide-free intermediate during Gαs activation (17). Little or no Gαs activity was seen in control HeLa cells responding to EGF; in GIV-depleted cells, however, a significant increase in Gαs activity was seen on vesicular structures that are likely to be endosomes (Fig. 3 G and H and Movies S1 and S2). These findings demonstrate that GIV is required for the inhibition of Gαs activity after EGF stimulation. Next we carried out similar analyses on HeLa cell lines stably expressing GIV-WT, GIV-DD (phosphomimetic, GDI-proficient), and GIV-FA (GDI-deficient) mutants at physiologic levels (Fig. S4). In GIV-WT and GIV-DD Hela cells responding to EGF little or no Gαs activity was seen; however, in GIV-FA Hela cells a significant increase in Gαs activity was seen on vesicular structures that are likely to be endosomes at t20 (Fig. 3 I and J and Movies S3–S5). These results obtained in live cells using conformation-sensitive antibodies are in agreement with our in vitro enzymatic assays: Both approaches point to GIV’s GDI function having a role in the inhibition of Gαs activity.
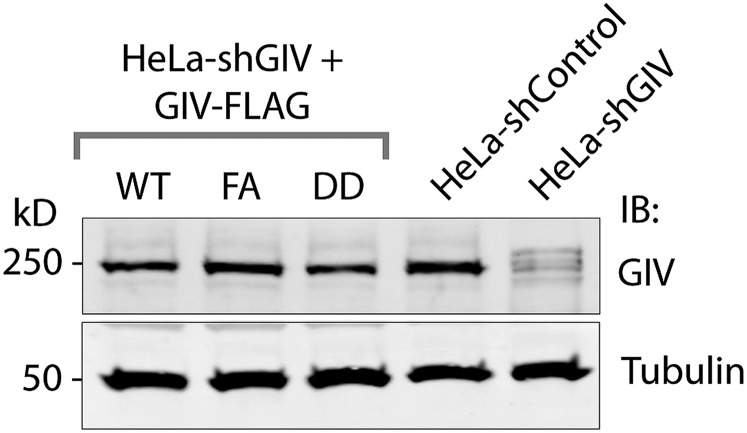
Fig. S4.
Expression of GIV in various HeLa cell lines used in this study. Equal aliquots of whole-cell lysates of control (shControl), GIV-depleted (shGIV), and GIV-depleted HeLa cells stably expressing GIV-WT, GIV-DD, and GIV-FA constructs that are insensitive to the action of shRNA were analyzed for GIV and tubulin by immunoblotting. The efficacy of GIV depletion by shRNA was analyzed by band densitometry using LI-COR Odyssey and was determined to be >90% compared with control cell lysates. Both GIV-WT and mutants were expressed equally well and at levels similar to endogenous GIV.
GIV Suppresses the cAMP→PKA→cAMP Response Element-Binding Protein Pathway via Its Ability to Inhibit Gαs.
Next we analyzed the impact of GIV’s GDI function on signaling pathways downstream of Gαs, i.e., the cAMP→PKA→cAMP response element-binding protein (CREB) pathway. First, we assessed cellular levels of cAMP using a previously well-characterized TEpacVV FRET probe (described in SI Experimental Procedures) that detects submicromolar changes in the second messenger (18). We found that in the presence of GIV (shControl HeLa cells), EGF stimulation was accompanied by suppression of cellular cAMP in a bimodal pattern, as determined by the increase in intramolecular FRET (Fig. 4A). An early wave of cAMP suppression was observed at approximately t5–t6, followed by a late wave of suppression at approximately t15–t45 (Fig. 4A); both events are delayed compared with the rapid (i.e., within a few seconds) suppression observed with the same FRET probes in the setting of canonical activation of Gi by Gi-coupled receptors (GPCRs) (19). However, as we reported previously (7), in the absence of GIV (i.e., in GIV-depleted cells) no suppression of cAMP was observed in response to EGF. We hypothesized that the first wave of suppression is a consequence of GIV’s ability to bind and activate Gαi, whereas the later wave is a consequence of GIV’s ability to bind and inhibit Gαs. To test this hypothesis, cAMP was assessed by FRET in HeLa cells stably expressing GIV-WT or the GDI-proficient/GEF-deficient GIV-DD or GDI/GEF-deficient GIV-FA mutants. We found that GIV-WT and GIV-FA Hela cells showed a pattern similar to that observed in shControl and shGIV cells, respectively (Fig. 4B). GIV-DD Hela cells differed from both, in that they failed to suppress cAMP early on but suppressed it efficiently later (Fig. 4B). The patterns of changes observed in cAMP are consistent with GIV’s role as a GEF for Gαi early and as a GDI for Gαs later in cells responding to EGF.
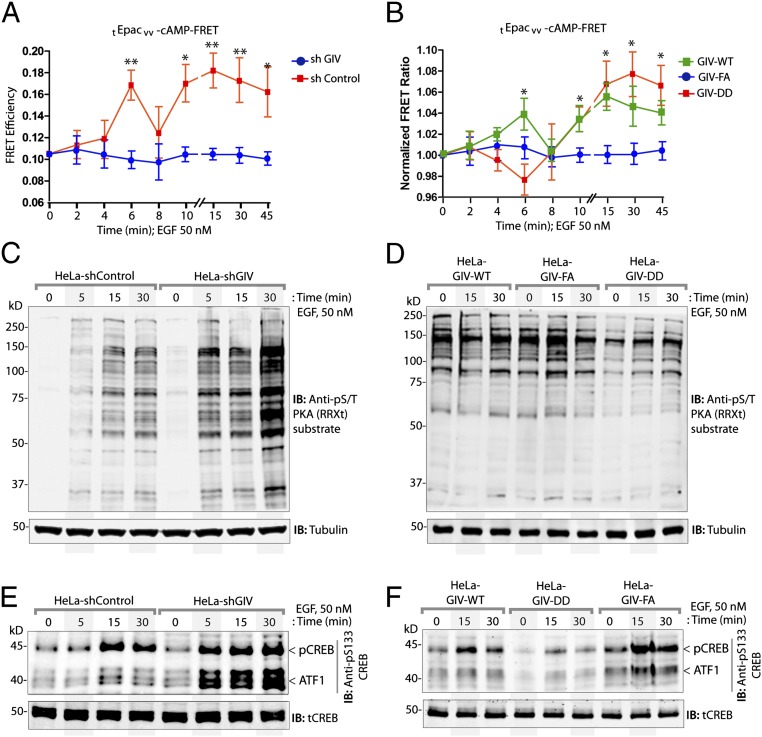
Fig. 4.
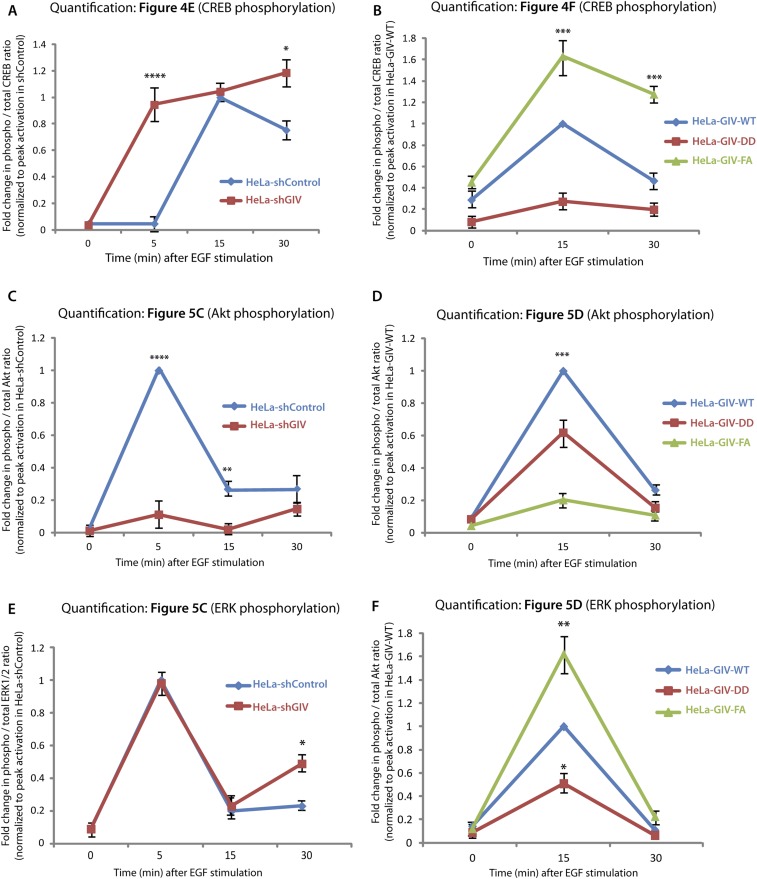
GIV inhibits the Gαs–GTP→cAMP→PKA→CREB pathway. (A and B) Control (shControl) and GIV-depleted (shGIV) HeLa cells (A) or GIV-WT, GIV-DD, or GIV-FA Hela cell lines (B) expressing the tEpacvv-cAMP FRET probe were serum starved (0.2% FBS) and subsequently stimulated with 50 nM EGF and analyzed for ratiometric FRET imaging using a confocal microscope (increase in cAMP = loss of FRET, and vice versa). Graphs display the change in FRET efficiency (y axis) over time (x axis). Results are expressed as ± SEM; n = 3. *P < 0.05, **P < 0.01. (C and D) Serum-starved control (shControl) or GIV-depleted (shGIV) HeLa cell lines (C) or GIV-depleted HeLa cells stably expressing GIV-WT, GIV-DD, or GIV-FA (D) were stimulated with 50 nM EGF at the indicated time points before lysis. Equal aliquots of whole-cell lysates were analyzed for PKA activity by immunoblotting using anti–phospho-serine/threonine–PKA substrate-specific antibody and tubulin. (E and F) HeLa cell lysates in C and D were analyzed for pCREB, phospho-ATF1, and total CREB (tCREB) by immunoblotting and were quantified by band densitometry (Fig. S5 A and B).
Further downstream, GIV-depleted cells also showed elevated levels of cellular PKA activity, as determined by an antibody that recognizes the abundance of phosphosubstrates of the kinase (Fig. 4C) and, more specifically, by analyzing the levels of phosphorylated CREB and the closely related substrate, activating transcription factor-1 (ATF1) (Fig. 4E and Fig. S5A). HeLa cells stably expressing the GDI-proficient GIV-DD mutant effectively suppressed PKA activity (Fig. 4D) and phosphorylation of CREB and ATF1 (Fig. 4F and Fig. S5B). By contrast, cells expressing the GDI-deficient GIV-FA mutant (Fig. 4B) displayed elevated PKA activity at t15 (Fig. 4D) and phosphorylation of CREB and ATF1 at t15 and t30 (Fig. 4F and Fig. S5B). These analyses revealing contrasting patterns of signaling in control vs. GIV-depleted and in GDI-proficient (GIV-DD) vs. GDI-deficient (GIV-FA) cells demonstrate the role of GIV and its GDI function in the suppression of Gαs GTP→cAMP→PKA→CREB signals.
Fig. S5.
Quantification of CREB and pCREB (A and B), Akt and pAkt (C and D), and ERK1/2 and pERK1/2 (E and F) signaling. Signal intensities of phosphoprotein and total protein bands in the immunoblots displayed in Figs. 4 E and F and 5 C and D were quantified using LI-COR Odyssey software, normalized to peak signals observed in shControl (A, C, and E) or GIV-WT (B, D, and F) Hela cells, and displayed here as fold-change (y axis) at various time points after EGF stimulation (x axis). Data are presented as mean ± SEM; n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
GIV Promotes Endosomal Maturation and Down-Regulates Mitogenic MAPK→ERK1/2 Signals via Its Ability to Inhibit Gαs.
Prior studies (9) have shown that GIV’s ability to bind Gαs and the maintenance of Gαs in the inactive state promotes endosomal maturation and thereby accelerates the transit of ligand-activated EGFR through the early endocytic compartment. Conversely, when GIV cannot bind Gαs or when Gαs is active, the transit of ligand-activated EGFR through early endosomes is prolonged. Based on these findings, we expected EGFR trafficking to be accelerated in cells expressing the GDI-proficient GIV-DD mutant, which inhibits Gαs activity, and to be slowed in HeLa cells expressing the GDI-deficient GIV-FA mutant which neither binds nor modulates Gαs signaling. Indeed we found that by t30 ligand-activated autophosphorylated EGFR, as determined by anti-EGFR pY1068, was no longer seen in endosomes expressing early endosome antigen 1 (EEA1) in GIV-WT and GIV-DD cells, but pY1068EGFR still persisted on EEA1+ endosomes in GIV-FA cells (Fig. 5A). Together, these findings indicate that the GDI-proficient GIV-DD mutant, which inhibits Gαs activation, ensures rapid clearance of the ligand-activated receptor through endosomes and the finiteness of signaling from that site. By contrast, the GDI-deficient GIV-FA mutant, which impairs binding to Gαs, delays endosomal maturation and prolongs the transit time and signaling from ligand-activated EGFR on EEA1+ endosomes (Fig. 5A).
Fig. 5.
GIV’s ability to bind and inhibit Gαs is essential for EGFR trafficking and down-regulation of mitogenic signals triggered by EGF. (A, Left) Serum-starved (0.2% FBS, overnight) HeLa cells stably expressing GIV-WT, GIV-DD, or GIV-FA were stimulated with 50 nM EGF for 30 min before fixation. Fixed cells were stained for ligand-activated EGFR (determined by staining for Y1068 phospho-EGFR (pEGFR, Y1068) (red), EEA1 (green), and DAPI (to stain the nucleus; blue) and were examined by confocal microscopy. Insets display magnified boxed regions. (Scale bar, 10 μm.) (Right) Bar graphs display quantification of yellow pixels indicating colocalization of active EGFR within EEA1+ endosomes in each set of cells at t30. ***P < 0.001. (B and C) Control (shControl) or GIV-depleted (shGIV) HeLa cells (B) or GIV-depleted HeLa cells stably expressing GIV-WT, GIV-DD, or GIV-FA mutants (C) were starved and stimulated with 50 nM EGF before lysis. Equal aliquots of whole-cell lysates were analyzed for phospho-Akt (pAkt), phospho-ERK (pERK), total Akt (tAkt), total ERK (tERK), and actin by immunoblotting and were quantified by band densitometry (Fig. S5 C–F). (D) Bar graphs display the mitotic index (Experimental Procedures), as determined by the percentage of each HeLa GIV cell line with nuclear phosphorylated (S28)-histone H3 (y axis). (E) Bar graphs display the percentage of each HeLa GIV cell line stained with anti-BrdU mAb after a BrdU uptake assay. Data in both graphs are presented as mean ± SEM; n = 3. **P < 0.01. Representative images for D and E are shown in Fig. S7. (F and G) Graphs display the rates of proliferation of various GIV HeLa cell lines, as determined by cell counting (F) and cell viability assays (G). Results are presented as mean ± SEM; n = 3. **P < 0.01, ***P < 0.001. (H) Schematic summary of G-protein and EGF signaling data presented in Figs. 3–5. Upon EGF stimulation, GIV serves as a GDI for Gαs and maintains Gαs GDP in the inactive state. (Left) The inactive state favors rapid down-regulation of endosome-based mitogenic (MAPK–ERK1/2) signals, as is consistent with prior findings that the presence of inactive Gαs on endosomes accelerates endosomal maturation and EGFR degradation (9). (Right) As a direct consequence of GIV-GDI, the Gαs GTP→cAMP→PKA→pCREB signaling pathway is inhibited; this inhibition is a key trigger for cell cycle-progression.
Endosomes are accepted as a legitimate compartment for the propagation of mitogenic MAPK–ERK1/2 signals downstream of growth factors (20, 21). Because GIV’s GDI function was required for accelerating the transit time of activated receptors through those compartments, we asked if GIV and, more specifically, its GDI function also affect mitogenic MAPK/ERK1/2 signals. Compared with control cells, GIV-depleted cells showed sustained phosphorylation of ERK1/2 but, as anticipated, failed to enhance phosphorylation of Akt (Fig. 5B and Fig. S5C). Compared with GIV-WT cells, peak phosphorylation of ERK1/2 was higher in the GDI-deficient GIV-FA cells and was lower in the GDI-proficient GIV-DD cells (Fig. 5C and Fig. S5D). These findings indicate that GIV requires its GDI function to suppress the MAPK/ERK1/2 signaling axis. Akt phosphorylation, on the other hand, was suppressed in GIV-DD cells compared with GIV-WT cells and, as shown previously (11, 12), was significantly decreased in GIV-FA cells. This result indicates that the GDI-proficient GIV-DD mutant, which does not bind Gαi, can enhance Akt signaling to levels intermediate between those of GIV-WT and GIV-FA. Akt enhancement in the GDI-proficient GIV-DD mutant required free Gβγ, because such activation was inhibited by gallein, a small molecule that blocks Gβγ binding to PI3K (22, 23), but not by fluorescein, its inactive analog (Fig. S6).
Fig. S6.
GIV displaces Gβγ from Gs[αβγ] trimers, and free Gβγ is required for the enhancement of Akt phosphorylation in response to EGF observed in GDI-proficient HeLa GIV-DD cells. (A) Equal aliquots of GST-Gαs–Gβγ preformed complexes (assembled by incubating bacterially expressed GST-Gαs overnight with lysates of HEK cells as source for Gβγ heterodimers, followed by washing off excess proteins) immobilized on glutathione beads were incubated with increasing concentrations of His-GIV-CT DD or FA mutants. Bound proteins were analyzed by immunoblotting. Increasing concentrations of the GDI-proficient GIV-CT-DD mutant, but not the GDI-deficient GIV-CT-FA mutant, were associated with increased binding of GIV to Gαs and a concomitant decrease in bound Gβ, indicating that GIV displaces Gβγ subunits from Gαs. (B) HeLa GIV-DD cells were serum starved for 16 h, pretreated (+) or not (−) with DMSO, gallein (a small-molecule inhibitor of Gβγ hot spots), fluorescein (an inactive analog of gallein), or Wortmannin (an irreversible potent inhibitor of PI3K), as indicated, for 30 min and subsequently stimulated for 5 min with 50 nM EGF before lysis. Equal aliquots of whole-cell lysates were analyzed for pAkt and tAkt by immunoblotting. Akt phosphorylation was enhanced in response to EGF (compare lanes 1 and 2), as expected. Such activation was virtually abolished in a dose-dependent manner with increasing dose of gallein (lanes 4–6) but not with fluorescein (lane 3). Akt phosphorylation also was inhibited in the presence of the positive control Wortmannin (lane 7). Together, these results indicate that GIV’s ability to serve as a GDI for Gαs is associated with release of free Gβγ subunits from Gs trimers, which in turn enhance PI3K–Akt signaling.
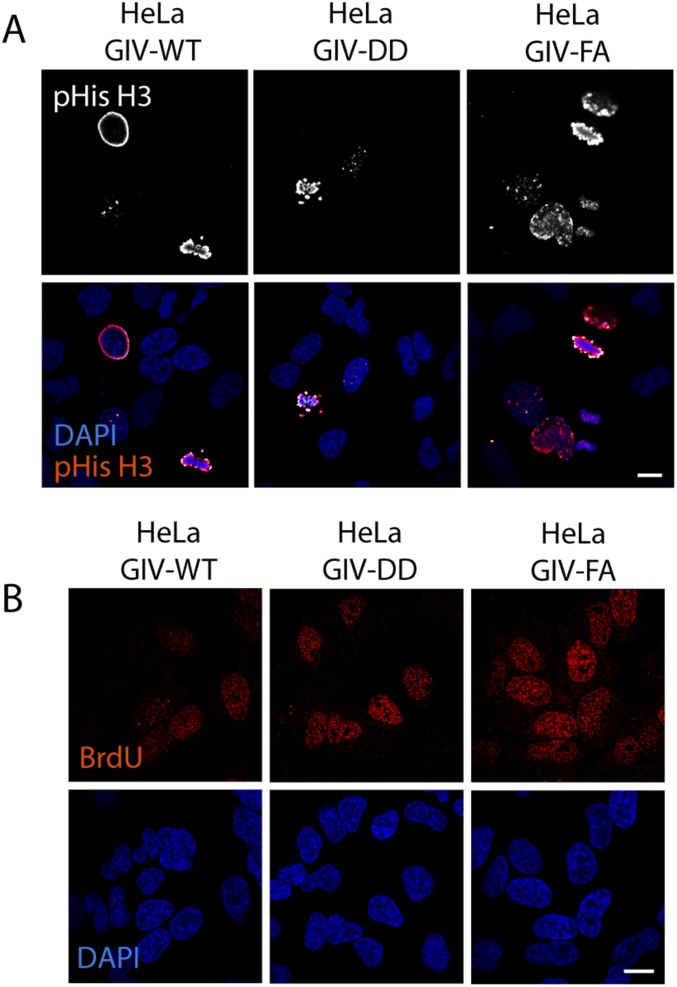
Consistent with GDI-proficient GIV-DD cells suppressing and GDI-deficient GIV-FA cells enhancing mitogenic MAPK/ERK and phospho-CREB (pCREB) signals, the rate of proliferation of these cells, as determined by four different approaches—nuclear localization of phosphohistone H3 (Fig. 5D and Fig. S7A); BrdU uptake (Fig. 5E and Fig. S7B); cell counting (Fig. 5F); and cell viability (Fig. 5G) assays—also was decreased in GIV-DD cells by ∼50% compared with GIV-FA cells. These findings support the following model in which EGF stimulation activates both the GEF and GDI functions of GIV via key phosphomodifications flanking the same motif. This bifunctional role of GIV maintains Gαs in the GDP-bound inactive state (Fig. 5H); inhibition of Gαs speeds up endosome maturation and down-regulates mitogenic MAPK–ERK and the cAMP→PKA→CREB signaling axes. Consequently, GIV-dependent inhibition of Gαs primarily suppresses mitosis.
Fig. S7.
GDI-proficient HeLa GIV-DD cells proliferate more slowly than the GDI-deficient HeLa GIV-FA cells. (A) GIV-depleted HeLa cells expressing GIV-WT or DD or FA mutants were grown in 2% (vol/vol) FBS before fixation. Fixed cells were stained for phosphorylated (S28)-histone H3 (red) and DAPI (to stain the nucleus; blue) and were analyzed by confocal microscopy. Images of representative fields are shown. (Scale bar, 10 µm.) Quantification of positively stained cells is displayed as bar graphs in Fig. 5D. (B) HeLa cell lines in A were grown in 2% (vol/vol) FBS overnight, incubated with BrdU for 30 min, fixed and stained for anti-BrdU mAb (red) and DAPI (to stain the nucleus; blue), and analyzed by confocal microscopy. Images of representative fields are shown. (Scale bar, 10 µm.) Quantification of positively stained cells is displayed as bar graphs in Fig. 5E.
GIV Inhibits Anchorage-Dependent Growth via Its Ability to Inhibit Gαs.
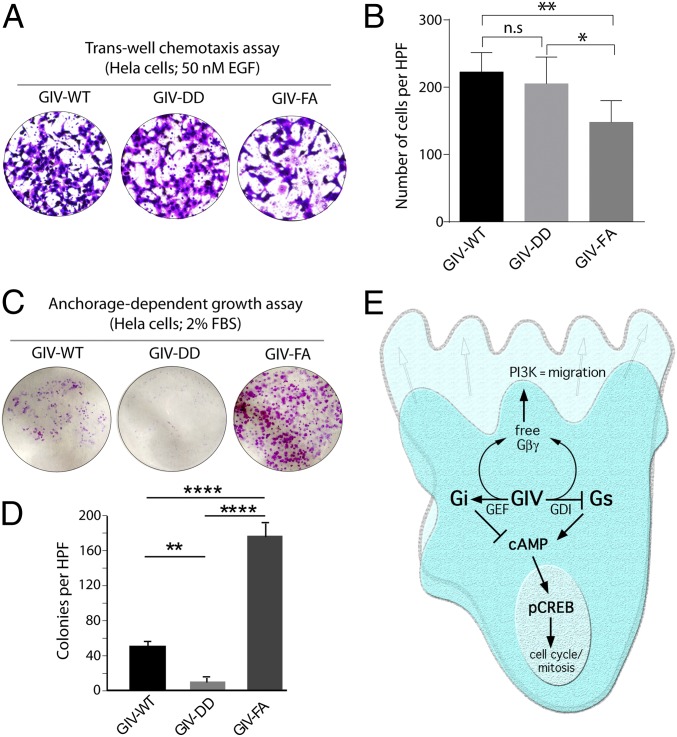
Prior studies have demonstrated that the presence or absence of a functional GBA motif in GIV, through which GIV activates Gαi, is a critical determinant of migration–proliferation dichotomy, a hallmark of invasive cancer cells (24). Because the presence or absence of a functional GDI motif in GIV affected the relative levels of motogenic PI3K–Akt signals (which trigger cell migration) and mitogenic MAPK–ERK1/2 signals (which trigger proliferation), we hypothesized that inhibition of Gαs by GIV’s GBA motif also contributes to migration–proliferation dichotomy. We found that the GDI-proficient GIV-DD cells migrated as efficiently as GIV-WT cells, as determined by Transwell chemotaxis assays (Fig. 6 A and B), but failed to proliferate into colonies, as determined by anchorage-dependent cell growth assays (Fig. 6 C and D). By contrast, GDI-deficient GIV-FA cells were less motile (Fig. 6 A and B) and instead preferentially grew into colonies (Fig. 6 C and D). These results demonstrate that the antiproliferative aspect of migration–proliferation dichotomy requires GIV’s ability to inhibit Gαs.
Fig. 6.
Inhibition of Gαs signaling by GIV inhibits anchorage-dependent tumor growth but does not affect cell motility/chemotaxis in HeLa cells. (A and B) GIV HeLa cell lines were analyzed for chemotaxis toward EGF using a Transwell migration assay (Experimental Procedures). After 6 h, membranes were fixed and stained with toluidine blue and imaged at 20×. (A) Representative images of high-power fields (HPF). (B) Bar graphs display the number of migrating cells per HPF (y axis) averaged from 20 HPFs per experiment; n = 3. Data are presented as mean ± SEM; *P < 0.05, **P < 0.01. (C and D) The HeLa cell lines used in A were analyzed for anchorage-dependent growth on six-well plastic plates (Experimental Procedures). After 2 wk, cells were fixed and stained with crystal violet. (C) Representative images of the crystal violet-stained single wells of a six-well plate. (D) Bar graphs display the average number of colonies per HPF (y axis), as determined using the colony-counter feature of ImageJ; colonies in 20 HPFs per well from two wells per experiment were counted, n = 4. Results are expressed as ± SEM; **P < 0.01, ****P < 0.0001. (E) Schematic summarizing the findings of the current work integrated with previous literature on GIV’s role in the modulation of G-protein and growth factor signaling during cell migration. Previously published work (on the left) has shown that GIV triggers the activation of Gαi via an evolutionarily conserved, C-terminally located GBA motif (1). Activation of Gi has two major consequences: (i) free Gβγ heterodimers released from Gi activate the PI3K–Akt pathway (1), and (ii) activation of Gαi suppresses cellular cAMP (7). This work (on the right) shows that GIV’s GBA motif also serves as a GDI for Gαs and maintains the G protein in an inactive Gαs-GDP state. Inhibition of Gαs by GIV also releases free Gβγ (Fig. S6) and suppresses cellular cAMP, thereby synergistically potentiating both consequences of Gαi activation. Overall, GIV’s GBA motif suppresses mitogenic MAPK/ERK1/2 signals and cAMP→pCREB–mediated cell-cycle progression and enhances promigratory PI3K–Akt signals by paradoxically activating and inhibiting two opposing G proteins.
In summary (Fig. 6E), we provide evidence that GIV can bind both Gαi and Gαs in a sequential manner after growth factor stimulation. Although binding to both Gα subunits is mediated by the same evolutionarily conserved GBA motif in GIV’s C terminus, the binding of the two G proteins has opposite outcomes: GIV serves as a GEF for Gαi and as a GDI for Gαs. Because Gαi and Gαs have opposing roles (inhibitory and stimulatory, respectively) in the regulation of cellular cAMP, GIV’s paradoxical ability to activate Gαi and inhibit Gαs results in an overall synergistic down-regulation of cellular cAMP and the cAMP-driven PKA→CREB axis of signaling (Fig. 6E). In addition, serving as both a GEF (for Gαi) and a GDI (for Gαs), GIV is capable of releasing free Gβγ heterodimers that can synergistically enhance the Gβγ-driven PI3K→Akt signaling axis (Fig. 6E). Although high PI3K–Akt signaling triggers migration, suppression of CREB stalls entry into the cell cycle. We propose that GIV’s ability to modulate the two opposing G proteins dually and differentially in a sequential manner is the key property of this signaling circuitry that orchestrates a cohesive, sustained, and robust phenotypic response, one that favors persistent migration without repeated interruption resulting from entry into the cell cycle.
SI Experimental Procedures
Antibodies and Reagents.
All reagents and chemicals were of analytical grade and were purchased from Sigma unless otherwise indicated. Cell culture media, EGF, and DAPI were purchased from Life Technologies. Gallein and fluorescein were purchased from TCI Chemicals. BrdU and Wortmannin were obtained from Sigma. MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was obtained from Thermo-Fisher. Pfu Ultra DNA polymerase was purchased from Agilent. All restriction endonucleases and Escherichia coli strain DH5α were purchased from New England Biolabs. E. coli strain BL21 (DE3) and DAPI were purchased from Invitrogen. Puromycin was purchased from Gibco, and the neomycin analog G418 was purchased from CellGro. Paraformaldehyde (PFA) 16% was purchased from Electron Microscopy Sciences. Mouse anti-FLAG (M2), anti-polyhistidine, β-actin, and α-tubulin were from Sigma. Rabbit anti-CREB (total and phospho), Gαs, pEGFR (Y1068), total and pAkt (S473), pERK (p44/42), and mouse anti-Myc were from Cell Signaling. Rabbit anti-GIV CT (T-13), anti-Gαi3, total ERK, and anti-GST antibodies were from Santa Cruz Biotechnology. Phospho antibodies against Ser1689 and Ser1674 on GIV were generated commercially in collaboration with 21st Century Biochemicals. Mouse anti-EEA1 and anti-BrdU were from BD Biosciences. Goat anti-rabbit and goat anti-mouse Alexa Fluor 680 and IRDye 800 F(ab)2 used for Odyssey infrared imaging were from LI-COR Biosciences. Goat anti-rabbit Alexa Fluor 488 and goat anti-mouse Alexa Fluor 594 for immunofluorescence were purchased from Life Technologies.
Plasmid Constructs, Mutagenesis, and Gene Depletion.
Cloning of GIV, Gαi3, and Gαs into p3×FLAG-CMV-14 plasmid (GIV-FLAG, Gαi3-FLAG, and Gαs-FLAG), pET28b plasmid [6×His-GIV-CT (amino acids 1660–1870), 6×His-Gαi3, and 6×His-Gαs short], and pGEX-4T1-GIV CT (amino acids 1660–1870) (GST-GIV-CT) have been described previously (1, 9, 11, 12, 25, 26). Cloning of CFP-tagged GIV-CT has been described previously (7). Cloning of full-length GIV cDNA with an Myc tag at the C terminus in pcDNA3.1 has been described previously (11). Cloning of GST-tagged GIV-CT proteins for mammalian expression and use in protein–protein interaction assays has been described previously (7, 11). GIV constructs harboring the 74D, 89D, and DD mutations were created using the QuikChange II site-directed mutagenesis kit (Agilent Technologies). All mutations and the integrity of the rest of the inserts were confirmed by sequencing. Gαs-YFP (internal tag; Addgene plasmid 55781) was a kind gift from Catherine Berlot, Weis Center for Research, Danville, PA (38). The nanobody for detection of the active conformation of Gαs was previously validated (17). The third-generation cAMP FRET biosensor mTurq-EPAC-Venus (tEPACvv) was a generous gift from Kees Jalink, Nederlands Cancer Institute, Amsterdam (18). tEPACvv encompasses mTurquoise as donor and mVenus-mVenus as dual acceptor, which has superior quantum yield and longer lifetime, making it the most advanced probe for monitoring submicromolar changes in cellular cAMP concentration.
GIV was stably depleted from cells using an shRNA approach described previously (11). Briefly, shRNA for the 3′ UTR of GIV (GIV shRNA: CCGGGCTTTCATTACCAGCTCTGAACTCGAGTTCAGAGCTGGTAATGAAAGCTTTTTTG) was cloned into pLKO.1 (TRCN0000130452) or control vector TRC1.5-pLKO.1-puro. Lentiviral packaging was performed in HEK 293T cells by cotransfecting the shRNA constructs with psPAX2 and pMD2G plasmids (4:3:1 ratio), using TransIT-LT1 (Mirus Bio). The medium was changed after 24 h. Virus-containing medium was collected after 36–48 h and was centrifuged and filtered through a 0.45-μm filter. psPAX2 and pMD2G plasmids were a generous gift from Christopher K. Glass, University of California, San Diego.
Cell Culture, Transfection, Generation of Stable Cell Lines, and EGF Stimulation.
Cells were cultured according to American Type Culture Collection (ATCC) guidelines. Briefly, HeLa and Cos7 cells were grown in DMEM (Invitrogen) supplemented with 10% (vol/vol) FBS (HyClone) and penicillin-streptomycin-glutamine (Invitrogen). All transient transfections were performed using TransIT-LT1 (Mirus Bio). HeLa cells were stably depleted of endogenous GIV by shRNA as described previously (11). GIV-depleted HeLa cell lines stably expressing shRNA-resistant full-length GIV-WT and various mutants were generated by transfecting cells with the desired GIV-FLAG construct and selection in the presence of G418 (800 μg/μL) for 4–6 wk. The resultant multiclonal pool was subsequently maintained in the presence of 500 μg/mL G418. GIV expression was verified to be ∼1–1.5× endogenous levels by immunoblotting using anti-GIV antibody.
For EGF stimulation experiments, cells were first starved overnight (∼16–18 h) in DMEM with either 0.2% FBS (HeLa cells) or 0% FBS (Cos7 cells). Cells were stimulated with 50 nM EGF for 0–30 min. For signaling assays, cells were directly lysed in 1× Laemmli sample buffer by heating at 95 °C for 5 min and were analyzed by SDS/PAGE and immunoblotting. For immunofluorescence and PLA assays, cells were prepared as described below.
Recombinant Protein Expression and Purification.
Protein expression and purification were carried out as described previously (25, 26, 33). Briefly, plasmids encoding His-Gαi3, His-Gαs, or GST-GIV-CT (amino acids 1660–1870; WT and 74D mutant) fusion constructs were used to express these proteins in E. coli strain BL21 (DE3) (Invitrogen). Cells were grown in LB medium containing an appropriate antibiotic at 37 °C until OD600 reached ∼0.5–0.7; protein expression was induced by adding 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and growing cultures overnight at 25 °C. For His-tagged Gαi3 and GIV-CT, bacteria pelleted from 1 L of culture were resuspended in 10 mL His-lysis buffer [50 mM NaH2PO4 (pH 7.4), 300 mM NaCl, 10 mM imidazole, 1% Triton X-100 (vol/vol), 1× Complete Protease Inhibitor Mixture (Roche)], sonicated four times (50% power, 20-s on/20-s off), and centrifuged at 12,000 × g for 20 min at 4 °C. Solubilized protein was purified using a cobalt resin (Pierce), eluted with 250 mM imidazole, and dialyzed in 1× PBS. His-tagged bovine Gαs, (pHIS6-Gαs) was purified from BL21 (DE3) codon-positive bacteria by nickel-affinity chromatography followed by ion exchange chromatography as described in ref. 37. Briefly, bacteria were grown at 37 °C until the OD600 reached ∼0.5, and His-Gαs expression was induced by the addition of 0.1 mM IPTG followed by overnight incubation at 23 °C. Bacteria were centrifuged for 10 min at 4,000 × g, and pellets were resuspended in 50 mM Tris⋅HCl (pH 8.0), 50 mM NaCl, 5 mM MgCl2, 50 μM GDP, and 5 mM β-mercaptoethanol supplemented with a SigmaFAST EDTA-free protease inhibitor mixture in ice. After sonication (4 times with 30 sec bursts), the lysate was cleared by centrifugation at 12,000 × g for 30 min at 4 °C. The supernatants were collected and adjusted to 500 mM NaCl and 20 mM imidazole before incubation with Ni-NTA resin (Qiagen) for 90 min at 4 °C. The resin was washed four times in the same buffer by cycles of centrifugation and resuspension, and proteins were eluted with 100 mM imidazole. The eluted fraction was adjusted to buffer exchanged into 50 mM Tris⋅HCl (pH 8.0), 50 mM NaCl, 5 mM MgCl2 using Pierce concentrators with a 9-kDa cutoff and was loaded onto a HiTrap Q HP column (GE Healthcare) connected to an ÄKTA FPLC. Proteins were eluted by applying a 50–500 mM NaCl gradient, and fractions containing His-Gαs were pooled and supplemented with 10 µM GDP and 5 mM β-mercaptoethanol before concentration in 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 5 mM MgCl2, 5% (wt/vol) glycerol, 10 µM GDP, and 5 mM β-mercaptoethanol and storage at −80 °C.
For GST-tagged proteins, bacteria were pelleted from 500 mL of culture, resuspended in 20 mL of lysis buffer [50 mM Tris⋅HCl (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture (Roche)], sonicated, and centrifuged to remove insoluble material. Affinity purification of solubilized proteins was carried out using glutathione-Sepharose 4B beads (GE Healthcare).
PLA.
In situ interactions of endogenous GIV with Gαi3 or Gαs were detected using a Duolink proximity ligation assay kit (Olink Biosciences) per the manufacturer’s instructions. Fixation, permeabilization, and blocking were done as described for whole-cell immunofluorescence. Cells incubated with only secondary antibodies were used as a negative control.
In Vitro and in Cellulo GST Pull-Down Assays.
The in vitro and in cellulo pull-down assays were carried out exactly as described previously (11). For in vitro pull-down assays, equimolar amounts of bacterially expressed and purified GST-GIV-CT proteins (amino acids 1660–1870) or GST alone immobilized on glutathione-Sepharose beads were incubated with purified His-tagged Gαi3 or Gαs in binding buffer [50 mM Tris⋅HCl (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 5 mM EDTA, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture (Roche)] for 4 h with constant tumbling at 4 °C. Beads were washed three times with 1 mL of binding buffer and were eluted by heating to 42 °C in 1× Laemmli sample buffer for 5 min. Binding between GIV-CT and His-Gα proteins was analyzed by immunoblotting.
For in cellulo pull-down assays, Cos7 cells were grown in 10-cm plates and were transfected with pCEFL-GST-GIV-CT WT and mutants. After 48 h, cells were washed once with cold 1× PBS and were harvested by scraping in 0.5 mL of binding buffer [25 mM Hepes (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 30 μM GDP, 2 mM DTT, 0.5% Triton X-100, 1× Complete Protease Inhibitor Mixture (Roche), and 1× Phosphatase Inhibitor Mixtures 1 and 2 (Sigma)]. Cell lysates were incubated with glutathione-Sepharose beads with constant tumbling at 4 °C for 4 h. Beads were washed four times with 1 mL of lysis buffer and were eluted in 1× Laemmli sample buffer by heating to 95 °C for 5 min. Binding of endogenous Gα proteins was analyzed by immunoblotting.
Coimmunoprecipitation Assays.
Coimmunoprecipitation assays were performed as described previously (11). Briefly, Cos7 cells grown on 10-cm plates were cotransfected with either Gαi-FLAG or Gαs-FLAG and pcDNA3.1, GIV-Myc WT, or various GIV mutant constructs. After 48 h, cells were washed once with cold 1× PBS and were harvested by scraping into 0.5 mL of immunoprecipitation buffer [20 mM Hepes (pH 7.4), 5 mM Mg-acetate, 125 mM K-acetate, 0.4% Triton X-100, 1 mM DTT, 1× Complete Protease Inhibitor Mixture (Roche), and 1× Phosphatase Inhibitor Mixtures 1 and 2 (Sigma)] on ice. Cell lysates were incubated for 10 min at 4 °C and were centrifuged at 12,000 × g for 10 min. Clarified cell lysates were incubated with anti-mouse FLAG-M2 overnight at 4 °C, followed by incubation with equilibrated protein G-Sepharose beads for 1 h at 4 °C. Beads were washed four times with cold immunoprecipitation buffer and were eluted by heating at 95 °C for 5 min in 1× Laemmli sample buffer. Bound proteins were analyzed by SDS/PAGE and immunoblotting.
Immunofluorescence Microscopy.
Trafficking of activated EGFR was tracked as previously described (11). Briefly, cells grown on coverslips were fixed at room temperature with 3% (wt/vol) PFA for 30 min, washed three times with 1× PBS, blocked and permeabilized for 45 min, and then incubated for 1 h each with primary and secondary antibodies. For staining with anti-pEGFR (pY1068), coverslips were incubated in the primary antibody overnight at 4 °C. For staining with EEA1, coverslips were incubated in the primary antibody for 1 h at room temperature. DAPI (1:10,000) (Invitrogen) was used to stain the nuclei. ProLong Gold (Life Technologies) was used as mounting medium. Images were taken using a Leica DMI400B confocal microscope equipped with a Leica Hamamatsu 9100-02 camera and the LAS AF SPE software (Leica) using a 63× oil-immersion objective and 488, 561, and 405 laser lines for excitation. The settings were optimized, and the final images were scanned with line-averaging of three scans. Images shown are representative of ∼85–90% of cells that were evaluated across three independent experiments.
Peptide Synthesis.
Peptides corresponding to GIV amino acids 1671–1705 WT sequence (1671KTGSPGSEVVTLQQFLEESNKLTSVQIKSSSQENL1705) or bearing the S1674D/1689D mutations (1671KTGDPGSEVVTLQQFLEEDNKLTSVQIKSSSQENL1705) were synthesized using the in situ neutralization protocol for Boc-Solid Phase Peptide Synthesis (Boc-SPPS) (39) on a p-methylbenzhydrylamine (MBHA) resin (0.67 mmol/g, 100–200 mesh) (Novabiochem). Following chain elongation, the peptide was cleaved from the resin using a solution of hydrofluoric acid containing 5% (vol/vol) anisole for 1 h at 0 °C. Next, the solution was removed under vacuum, and the resulting residue was crushed out with Et2O and filtered. The collected solid was redissolved in a 50% (vol/vol) CH3CN/H2O solution containing 0.1% TFA, frozen down, and lyophilized. The crude peptide was purified by reverse-phase HPLC in a Waters X-Bridge C18 (19 × 100 mm) column at a flow of 20 mL/min using H2O (0.1% TFA) and CH3CN (0.1% TFA) as eluents. The final purity of the peptide was analyzed by analytical reverse-phase HPLC and mass spectrometry (electrospray ionization-TOF) with yields higher than 97%.
Steady-State GTPase Assay.
The steady-state GTPase assay was performed as described previously (26, 37) with minor modifications. Briefly, His-Gαs (100 nM) was preincubated with different concentrations of His-GIV-CT (amino acids 1660–1870) or GIV peptides (WT or mutants) for 60 min at 4 °C in assay buffer [20 mM Na-Hepes (pH 8), 100 mM NaCl, 1 mM EDTA, 2 mM MgCl2, 1m M DTT, 0.05% (wt/vol) C12E10]. GTPase reactions were initiated at 20 °C by adding an equal volume of assay buffer containing 1 μM [γ-32P]GTP (∼50 cpm/fmol). Duplicate aliquots (25 μL) were removed at the required times, and reactions were stopped with 975 μL ice-cold 5% (wt/vol) activated charcoal in 20 mM H3PO4, pH 3. Samples then were centrifuged for 10 min at 10,000 × g, and 500 μL of the resultant supernatant was scintillation counted to quantify released [32P]Pi. Data were expressed as raw counts per minute for the time-course experiments. In the dose–response experiments, the background [32P]Pi detected in the absence of G protein was subtracted from each reaction, and the data were expressed as the percentage of the basal G-protein activity in the absence of His-GIV-CT or GIV peptides.
GTPγS-Binding Assay.
The GTPγS-binding assay was performed as described previously (26, 37) with minor modifications. Briefly, His-Gαs (100 nM) was preincubated with different concentrations of His-GIV-CT (amino acids 1660–1870) or GIV peptides (WT or mutants) for 60 min at 4 °C in assay buffer [20 mM Na-Hepes (pH 8), 100 mM NaCl, 1 mM EDTA, 2 mM MgCl2, 1 mM DTT, 0.05% (wt/vol) C12E10]. GTPase reactions were initiated at 20 °C by adding an equal volume of assay buffer containing 1 μM [35S]GTPγS (∼50 cpm/fmol). Duplicate aliquots (25 μL) were removed at the required times, and reactions were stopped by rapid filtration through nitrocellulose filters. Filters were washed, dried, and scintillation counted to quantify the amount of bound [35S]GTPγS. Background [35S]GTPγS binding to the filters detected in the absence of G protein was subtracted from each reaction, and the data were expressed as the percentage of the basal G-protein activity in the absence of His-GIV-CT or GIV peptides.
FRET Studies for Measuring Cellular cAMP.
FRET studies for measuring cellular cAMP were carried out exactly as before using the third-generation cAMP FRET biosensor tEPACvv (18). Briefly, various HeLa cell lines were grown to 60–70% confluence in sterile 35-mm MatTek glass-bottomed dishes, transfected with the cAMP biosensor, stimulated with EGF, and imaged using an Olympus FV1000 inverted confocal laser scanning microscope (University of California, San Diego Neuroscience Core Facility). Statistical analyses were performed on 7–10 regions of interest per cell in four or five cells per experiment (n = 4 biological repeats).
Live-Cell Confocal Imaging Using Gαs Active Conformation-Specific GFP-Nanobody.
Live-cell imaging for visualizing Gαs activation via conformational-specific GFP-nanobodies was done using a PerkinElmer spinning disk confocal microscope in live HeLa cells plated on glass-bottomed dishes in a 37 °C temperature- and CO2-controlled chamber. Final images were analyzed and frames were selected using ImageJ software.
Cell Proliferation Assays.
Two complementary methods were used for cell proliferation assays. For cell-counting assays, cells were seeded at 1 × 104 per well in Ham’s F-12 medium with 10% (vol/vol) FBS and were grown at 37 °C in a humidified 5% CO2 incubator. At various times cells were trypsin released and manually counted with a hemocytometer. Results are shown as means ± SEM (n = 3). For cell viability assays, cells were seeded into 96-well plates at 2 × 105 cells/mL and were cultured in DMEM/Ham’s F-12 medium supplemented with 2% (vol/vol) FBS for 1–5 d. Cell growth was documented every 24 h via a colorimetric assay using an MTT assay (Sigma). Absorbance values were collected at 490 nm using a SpectraMax 190 microplate reader (Molecular Devices). Control samples were treated with vehicle (0.1% DMSO or ethanol in DMEM/Ham’s F-12 culture medium). In each individual experiment, proliferation was determined in triplicate, and the overall experiment was repeated at least three times.
Anchorage-Dependent Colony Formation Assay.
Anchorage-dependent growth was monitored on a solid (plastic) surface as described previously (11). Briefly, 5,000 HeLa cells stably expressing various GIV constructs were grown in six-well tissue-culture plates at 37 °C for 2 wk in medium supplemented with 2% (vol/vol) FBS before staining with 0.005% crystal violet for 1 h.
Transwell Migration Assay.
Cell migration was assessed using Costar Transwell inserts with 8-μm pores in 24-well plates. Cells were serum starved (DMEM supplemented with 0.4% FBS) overnight followed by harvesting using trypsin/EDTA and were resuspended in DMEM containing 0.4% FBS. Then 5 × 104 cells in a volume of 300 μL were loaded in the upper well, and the lower well was filled with 750 μL DMEM with 0.4% FBS plus 50 nM EGF. The plates were incubated at 37 °C for 12 h before the remaining cell suspension was removed. The migration insert was placed in a clean well containing 4% (wt/vol) PFA for 1 h at room temperature, stained with Giemsa for 1 h, and washed three times in PBS. Cells on the upper side of the filters were removed with cotton-tipped swabs, and the number of migrated cells on the bottom side of the filter were counted in five randomly chosen fields at 200× magnification and averaged. All experiments were performed in triplicate, and each experiment was repeated at least three times.
Discussion
One Motif Serves as a GEF and GDI: Genesis of Pleiotropy in G-Protein Signaling.
The major finding in this work is the identification of GIV as a GDI for Gαs. Together with its previously well-characterized ability to bind Gαi (1, 25, 26), GIV joins several other receptor GEFs, i.e., GPCRs (27–30), that can dually couple to both Gi and Gs, two G proteins with opposing effects on adenylyl cyclase and the generation of cellular cAMP. In all instances in which dual coupling of GPCRs to Gi and Gs has been reported, ligand stimulation leads to the activation of both G proteins. By contrast, GIV appears to be a unique modulator that can modulate the two G proteins paradoxically, activating Gαi and inactivating Gαs.
A series of studies (reviewed in ref. 31) has established GIV as a bona fide GEF for Gαi. Here, using enzymatic assays with recombinant proteins, we show that GIV inhibits the rate of Gαs nucleotide exchange. Consistent with the properties of other known GDIs, prior studies have confirmed that GIV cannot bind Gαs subunits in the active GTP-bound conformation (9–11). It is noteworthy that both the GEF and GDI functions of GIV are mediated via the same evolutionarily conserved C-terminal short GBA motif, as reported previously in the case of the synthetic KB-752 peptide (14, 15). Such a role is analogous to the bifunctional [GTPase-activating proteins (GAP) and GDI] role of the regulators of G protein signaling (or RGS) domain of Drosophila Double hit (Dhit) (32).
How can the same motif serve as both a GEF and a GDI? Although mechanistic insights are lacking at the atomic level, multiple studies (1, 26, 33) have provided important structural insights into the assembly of the GIV–Gα complex by using a combination of homology modeling [based on the X-ray structure of the KB-752 peptide bound to Gαi1 (14)] and site-directed mutagenesis (1, 17). These studies have revealed that conserved hydrophobic residues that align on one side of a short aliphatic helix in GIV dock onto a hydrophobic cleft between the switch II and the α3 helix of Gαi/s. The structure of the KB-752 peptide-bound Gαi1 (14) also sheds light on how the homologous short stretch in GIV can accelerate nucleotide exchange rates of Gαi subunits: The peptide appears to alter switch I and II regions of Gαi, which were proposed to create a feasible exit route for GDP (14). Thus, well-validated homology models of GIV-bound Gαi3 built using the KB-752–bound Gαi1 as a template allow us to rationalize how GIV, like the KB-752 peptide, serves as a GEF for Gαi subunits. By contrast, as yet we have no clear rationale as to why GIV may serve as a GDI for Gαs. That both the KB-752 peptide (15) and GIV do exert a GDI-like effect on Gαs suggests that both may inhibit one or more of the conformational steps believed to facilitate GDP release by Gαs (34), i.e., unhinging of the Ras-like and helical domains, destabilization of the GDP-binding site, or movement of the α5-helix and/or the α4–β6 loop. Insight at an atomic level by X-ray crystallography is required to resolve this question.
Finally, in light of the findings of this work, we propose a nomenclature, “guanine-nucleotide exchange modulators” (GEMs), to describe the GIV family of G-protein modulators, all of which share homology with the KB-752 peptide. GEMs such as GIV or the synthetic KB-752 peptide are distinct from the other nonreceptor GEFs (such as Ric8A/B or AGS1) because they have a well-defined GBA motif (35) that can display a propensity for pleiotropy; i.e., the motif can either accelerate or inhibit nucleotide exchange and thereby serve as a GEF or a GDI, depending on the G-protein substrate and posttranslational modifications flanking the motif. Whether other GIV-related G-protein regulators, such as Daple (36) and Calnuc (35), which serve as GEFs for Gαi via GBA motifs that are similar to those in KB-752 and GIV, also serve as GDIs for Gαs remains to be explored.
Two Key Phosphoevents Shift GIV’s Preference from Gαi to Gαs.
Another finding in this work is the sequential nature of GIV–Gαi and GIV–Gαs interactions after ligand stimulation. GIV–Gαi complexes are assembled within 5 min and are disassembled ∼15–30 min after EGF stimulation. Although their assembly is triggered by phosphorylation of GIV at S1674, an event catalyzed by CDK5 within seconds to minutes after EGF stimulation (11), their disassembly is triggered by phosphorylation of GIV at S1689, an event catalyzed by PKCθ (13). GIV–Gαs complexes are assembled at approximately t15–t30 and are disassembled by approximately t60 after EGF stimulation, coinciding with the abundance of phosphorylation of GIV at S1689. Because GIV phosphorylated at S1674 binds both Gαi and Gαs but has an ∼5- to 10-fold higher affinity for Gαi, and because GIV that is dually phosphorylated at S1674 and S1689 can bind only Gαs, it is likely that the second phosphoevent (at S1689 catalyzed by PKCθ) is the key trigger that shifts GIV’s preference from Gαi to Gαs. We conclude that two sequential phosphomodifications on GIV that are catalyzed by two kinases, CDK5 and PKCθ, ensure that GIV exerts its GEF and GDI activities on Gαi and Gαs, respectively, in a temporally and spatially segregated manner.
Pleiotropic G-Protein Signaling Triggered by GIV Integrates Downstream Signals and Reinforces a Promigratory Phenotype.
We also show here the consequences of Gi activation and Gs inhibition by GIV on downstream signaling pathways and the cellular response to growth factors. Previous studies have shown that binding and activating Gαi prolongs the time that EGFRs spend on the cell surface and enhances PM-based promigratory PI3K–Akt signals (12). Binding Gαs, on the other hand, and maintaining it in the GDP-bound inactive state shortens the time EGFRs spend in endosomes and diminishes the endosome-based promitotic Ras/Raf/MAPK/ERK pathway (9). Consequently, when GIV’s GBA motif can bind and modulate both Gα subunits, cells enhance PI3K–Akt signals from the PM and suppress mitogenic MAPK–ERK1/2 signals from endosomes by ensuring rapid transit through endocytic compartments; as a consequence, cells preferentially migrate and suppress proliferation. When GIV’s GBA motif cannot bind or modulate either G protein, cells suppress PI3K–Akt signals at the PM and enhance mitogenic MAPK–ERK1/2 signals from endosomes by delaying the transit time of EGFR through endocytic compartments; consequently, cells are poorly motile and preferentially divide. This phenotype is seen in cells expressing either GIV-FA or GIV-74A, a mutant that cannot be phosphorylated by CDK5 and therefore cannot bind either G protein in cells (11). Because the inhibitory effects of GIV-WT on Gαs activity, on mitogenic MAPK–ERK1/2 signals, and on cell proliferation can be fully recapitulated in cells expressing GIV-DD, a phosphomutant that exclusively binds and inhibits Gαs but cannot bind or activate Gαi, we conclude that the GIV’s ability to bind and inhibit Gαs is required and is primarily responsible for GIV’s antiproliferative effects.
What is the primary role of GIV’s ability to bind and activate Gαi? Although a mutant GIV that can bind and activate Gαi but cannot bind and inhibit Gαs is yet to be identified, the role of Gi activation by GIV is apparent from a subtractive analysis of readouts of GIV-WT cell lines, which can modulate both Gi/s, vs. phosphomutant GIV-DD cell lines, which can modulate only Gs. Such analysis shows that the cells expressing the GIV-DD mutant have an intermediate level of peak Akt phosphorylation, lower than that of GIV-WT, which has an intact GBA motif, but higher than that of GIV-FA, which has no functional GBA motif (Fig. S5D). This finding indicates that GIV’s ability to inhibit Gαs can account for only part of the observed role of GIV’s GBA motif in the enhancement of Akt signals and that activation of Gi is the likely contributor of the remaining part. Because GIV displaces Gβγ from Gαs (Fig. S6A) and because Akt signals enhanced by GIV’s GBA motif are inhibited by gallein (Fig. S6B) (1), a compound that blocks Gβγ interactions with PI3Kγ by binding to a protein–protein interaction hot spot on the Gβ subunit (22), we conclude that the mechanism of PI3K–Akt enhancement brought about by GIV’s GBA motif involves the release of free Gβγ heterodimers from both Gi and Gs trimers. Thus, by triggering the activation of Gi and inhibiting Gs, GIV accomplishes one common goal, i.e., the release of free Gβγ and the activation of PI3K–Akt signals, thereby integrating and reinforcing the downstream signaling response (Fig. 6E).
Another final common goal that can be accomplished by the paradoxical activation of Gi and inhibition of Gs is the suppression of cellular cAMP. Prior studies using a FRET-based approach have confirmed that activation of Gi by GIV is critical for the suppression of cellular cAMP at approximately t5 in cells pretreated with forskolin (7). Analyses of signaling pathways in control vs. GIV-depleted cells responding to EGF underscore the importance of GIV in suppressing both PKA activation and the phosphorylation of CREB by approximately t5 and continuing to do so up to approximately t30–t45. This timeline suggests that much of the early suppression of the cAMP→PKA→pCREB axis we observe is likely to be contributed by GIV-dependent activation of Gαi, which occurs early (at approximately t5) and excludes any significant contribution from GIV-dependent inhibition of Gαs which occurs later (at approximately t15–t30). Conversely, the sustained suppression of the cAMP→PKA→pCREB axis until t30–t45 is likely contributed exclusively by the GIV-dependent inhibition of Gαs, because GIV can no longer bind or activate Gαi at these delayed time points. We propose that by triggering paradoxical activation of Gi and inhibition of Gs, GIV’s GBA motif integrates and reinforces another common goal, the suppression of the cAMP→PKA→pCREB axis (Fig. 6E).
We propose that the cellular response to paradoxical signaling that is triggered by GIV is likely to be shaped by both the sequential and spatiotemporally segregated nature of GIV-dependent modulation of Gαi and Gαs: The sequential triggering likely ensures pulses of early (Gαi-dependent) and late (Gαs-dependent) signals, whereas the spatiotemporally segregated triggering likely ensures the compartmentalization of those pulses.
In conclusion, we provide evidence of pleiotropic G-protein signaling in physiology in which one protein can both accelerate and inhibit the guanine nucleotide exchange rates of two opposing G proteins, Gαi and Gαs, using the same module. These insights provide clues into how GIV may achieve sustained and coordinated responses through pulses of compartmentalized signals.
Experimental Procedures
Detailed methods are described in SI Experimental Procedures.
Cell Culture, Transfection, Immunoblotting, Immunofluorescence, and Protein–Protein Interaction Assays (GST Pulldowns and Immunoprecipitations).
Cell culture, transfection, immunoblotting, immunofluorescence, and protein–protein interaction assays were carried out exactly as described previously (1, 9, 11, 12, 33). All transfections were performed using TransIT-LT1 reagent (Mirus Bio). All Western blotting (Odyssey–LI-COR) images were processed and assembled for presentation using Image Studio Lite, Photoshop, and Illustrator software (Adobe).
PLA.
In situ interactions of endogenous GIV with Gαi3 or Gαs were detected using a Duolink proximity ligation assay kit (Olink Bioscience) per the manufacturer’s instructions, as done previously (11).
Single-Turnover and Steady-State GTPase Assays.
Single-turnover and steady-state GTPase assays were performed as described previously (26, 37), with minor modifications outlined in SI Experimental Procedures.
Live-cell imaging for visualizing Gαs activation using conformational-specific GFP nanobodies on HeLa cells was done using a PerkinElmer spinning disk confocal microscope as described previously (17).
BrdU Incorporation, Phosphohistone H3 Staining, and Estimation of Mitotic Index.
BrdU incorporation, phosphohistone H3 staining, and estimation of mitotic index were performed as described previously (9, 11, 12).
Anchorage-Dependent Colony Formation Assay.
Anchorage-dependent growth was monitored on a solid (plastic) surface as described previously (11). Briefly, 1,000 GIV-depleted HeLa cells stably expressing WT, 74D, and 74A GIV-FLAG constructs were grown in six-well tissue-culture plates at 37 °C for 2 wk in medium supplemented with 0.2% FBS before staining with 0.005% crystal violet for 1 h. Images were acquired by light microscopy.
Transwell Migration Assay.
Transwell migration assays were carried out using Costar Transwell inserts with 8-μm pores in 24-well plates exactly as described previously (11).
Statistical Analysis.
Each experiment presented in the figures is representative of at least three independent experiments. Statistical significance between the differences of means was calculated by unpaired Student’s t test. A two-tailed P value of < 0.05 at a 95% confidence interval is considered statistically significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. All graphical data presented were prepared using GraphPad or Matlab software.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants CA100768 (to M.G.F. and P.G.) and CA160911 and DK099226 (to P.G.). Support was also provided by a Career Award for Medical Scientists award from the Burroughs Wellcome Fund (to P.G.); American Cancer Society Grant ACS-IRG 70-002 (to P.G.); the Moores Cancer Center of the University of California, San Diego (P.G.); American Cancer Society Grant RSG-13-362-01-TBE (to M.G.-M.); NIH Grant R01GM108733 (to M.G.-M.); Susan G. Komen Grant PDF14298952 (to K.K.M.); and American Heart Association Fellowship AHA 14POST20050025 (to I.L.-S.). D.B. was supported by NIH Building Infrastructure Leading to Diversity (BUILD) Research Stimulation Grant (UL1MD009601) and California State University Program for Education and Research in Biotechnology (CSUPERB) New Investigator Grant (GF00631143).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609502113/-/DCSupplemental.
References
- 1.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV is a nonreceptor GEF for G alpha i with a unique motif that regulates Akt signaling. Proc Natl Acad Sci USA. 2009;106(9):3178–3183. doi: 10.1073/pnas.0900294106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enomoto A, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9(3):389–402. doi: 10.1016/j.devcel.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Anai M, et al. A novel protein kinase B (PKB)/AKT-binding protein enhances PKB kinase activity and regulates DNA synthesis. J Biol Chem. 2005;280(18):18525–18535. doi: 10.1074/jbc.M500586200. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh P. G protein coupled growth factor receptor tyrosine kinase: No longer an oxymoron. Cell Cycle. 2015;14(16):2561–2565. doi: 10.1080/15384101.2015.1066538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aznar N, Kalogriopoulos N, Midde KK, Ghosh P. Heterotrimeric G protein signaling via GIV/Girdin: Breaking the rules of engagement, space, and time. BioEssays. 2016;38(4):379–393. doi: 10.1002/bies.201500133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin C, et al. Structural basis for activation of trimeric Gi proteins by multiple growth factor receptors via GIV/Girdin. Mol Biol Cell. 2014;25(22):3654–3671. doi: 10.1091/mbc.E14-05-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Midde KK, et al. Multimodular biosensors reveal a novel platform for activation of G proteins by growth factor receptors. Proc Natl Acad Sci USA. 2015;112(9):E937–E946. doi: 10.1073/pnas.1420140112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin C, et al. Tyrosine phosphorylation of the Gα-interacting protein GIV promotes activation of phosphoinositide 3-kinase during cell migration. Sci Signal. 2011;4(192):ra64. doi: 10.1126/scisignal.2002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beas AO, et al. Gαs promotes EEA1 endosome maturation and shuts down proliferative signaling through interaction with GIV (Girdin) Mol Biol Cell. 2012;23(23):4623–4634. doi: 10.1091/mbc.E12-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le-Niculescu H, Niesman I, Fischer T, DeVries L, Farquhar MG. Identification and characterization of GIV, a novel Galpha i/s-interacting protein found on COPI, endoplasmic reticulum-Golgi transport vesicles. J Biol Chem. 2005;280(23):22012–22020. doi: 10.1074/jbc.M501833200. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari D, et al. Cyclin-dependent kinase 5 activates guanine nucleotide exchange factor GIV/Girdin to orchestrate migration-proliferation dichotomy. Proc Natl Acad Sci USA. 2015;112(35):E4874–E4883. doi: 10.1073/pnas.1514157112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh P, et al. A Galphai-GIV molecular complex binds epidermal growth factor receptor and determines whether cells migrate or proliferate. Mol Biol Cell. 2010;21(13):2338–2354. doi: 10.1091/mbc.E10-01-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.López-Sánchez I, et al. Protein kinase C-theta (PKCθ) phosphorylates and inhibits the guanine exchange factor, GIV/Girdin. Proc Natl Acad Sci USA. 2013;110(14):5510–5515. doi: 10.1073/pnas.1303392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston CA, et al. Structure of Galpha(i1) bound to a GDP-selective peptide provides insight into guanine nucleotide exchange. Structure. 2005;13(7):1069–1080. doi: 10.1016/j.str.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston CA, et al. A bifunctional Galphai/Galphas modulatory peptide that attenuates adenylyl cyclase activity. FEBS Lett. 2005;579(25):5746–5750. doi: 10.1016/j.febslet.2005.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söderberg O, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3(12):995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 17.Irannejad R, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495(7442):534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klarenbeek JB, Goedhart J, Hink MA, Gadella TW, Jalink K. A mTurquoise-based cAMP sensor for both FLIM and ratiometric read-out has improved dynamic range. PLoS One. 2011;6(4):e19170. doi: 10.1371/journal.pone.0019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponsioen B, et al. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. EMBO Rep. 2004;5(12):1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugh JM. Localization of receptor-mediated signal transduction pathways: The inside story. Mol Interv. 2002;2(5):292–307. doi: 10.1124/mi.2.5.292. [DOI] [PubMed] [Google Scholar]
- 21.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: A legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106(42):17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonacci TM, et al. Differential targeting of Gbetagamma-subunit signaling with small molecules. Science. 2006;312(5772):443–446. doi: 10.1126/science.1120378. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann DM, Seneviratne AM, Smrcka AV. Small molecule disruption of G protein beta gamma subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol Pharmacol. 2008;73(2):410–418. doi: 10.1124/mol.107.041780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fedotov S, Iomin A. Migration and proliferation dichotomy in tumor-cell invasion. Phys Rev Lett. 2007;98(11):118101. doi: 10.1103/PhysRevLett.98.118101. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Marcos M, et al. Functional characterization of the guanine nucleotide exchange factor (GEF) motif of GIV protein reveals a threshold effect in signaling. Proc Natl Acad Sci USA. 2012;109(6):1961–1966. doi: 10.1073/pnas.1120538109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Marcos M, Ghosh P, Ear J, Farquhar MG. A structural determinant that renders G alpha(i) sensitive to activation by GIV/girdin is required to promote cell migration. J Biol Chem. 2010;285(17):12765–12777. doi: 10.1074/jbc.M109.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao RP. Beta-adrenergic signaling in the heart: Dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci STKE. 2001;2001(104):re15. doi: 10.1126/stke.2001.104.re15. [DOI] [PubMed] [Google Scholar]
- 28.Herrlich A, et al. Involvement of Gs and Gi proteins in dual coupling of the luteinizing hormone receptor to adenylyl cyclase and phospholipase C. J Biol Chem. 1996;271(28):16764–16772. doi: 10.1074/jbc.271.28.16764. [DOI] [PubMed] [Google Scholar]
- 29.Michal P, Lysíková M, Tucek S. Dual effects of muscarinic M(2) acetylcholine receptors on the synthesis of cyclic AMP in CHO cells: Dependence on time, receptor density and receptor agonists. Br J Pharmacol. 2001;132(6):1217–1228. doi: 10.1038/sj.bjp.0703931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonhaus DW, Chang LK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: Evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287(3):884–888. [PubMed] [Google Scholar]
- 31.Garcia-Marcos M, Ghosh P, Farquhar MG. GIV/Girdin transmits signals from multiple receptors by triggering trimeric G protein activation. J Biol Chem. 2015;290(11):6697–6704. doi: 10.1074/jbc.R114.613414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin C, et al. Double suppression of the Gα protein activity by RGS proteins. Mol Cell. 2014;53(4):663–671. doi: 10.1016/j.molcel.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Marcos M, Ear J, Farquhar MG, Ghosh P. A GDI (AGS3) and a GEF (GIV) regulate autophagy by balancing G protein activity and growth factor signals. Mol Biol Cell. 2011;22(5):673–686. doi: 10.1091/mbc.E10-08-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dror RO, et al. Signal transduction. Structural basis for nucleotide exchange in heterotrimeric G proteins. Science. 2015;348(6241):1361–1365. doi: 10.1126/science.aaa5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Marcos M, Kietrsunthorn PS, Wang H, Ghosh P, Farquhar MG. G Protein binding sites on Calnuc (nucleobindin 1) and NUCB2 (nucleobindin 2) define a new class of G(alpha)i-regulatory motifs. J Biol Chem. 2011;286(32):28138–28149. doi: 10.1074/jbc.M110.204099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aznar N, et al. Daple is a novel non-receptor GEF required for trimeric G protein activation in Wnt signaling. eLife. 2015;4:e07091. doi: 10.7554/eLife.07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leyme A, Marivin A, Casler J, Nguyen LT, Garcia-Marcos M. Different biochemical properties explain why two equivalent Gα subunit mutants cause unrelated diseases. J Biol Chem. 2014;289(32):21818–21827. doi: 10.1074/jbc.M114.549790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yost EA, Mervine SM, Sabo JL, Hynes TR, Berlot CH. Live cell analysis of G protein beta5 complex formation, function, and targeting. Mol Pharmacol. 2007;72(4):812–825. doi: 10.1124/mol.107.038075. [DOI] [PubMed] [Google Scholar]
- 39.Schnölzer M, Alewood P, Jones A, Alewood D, Kent SB. In situ neutralization in Boc-chemistry solid phase peptide synthesis. Rapid, high yield assembly of difficult sequences. Int J Pept Protein Res. 1992;40(3-4):180–193. doi: 10.1111/j.1399-3011.1992.tb00291.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.