Significance
Wood-decomposing fungi are key players in the carbon cycle and are models for making energy from lignocellulose, sustainably. Our study focuses on brown rot fungi that selectively remove carbohydrates, leaving most lignin behind. These fungi often decompose wood faster than their lignin-degrading white rot ancestors, despite losses in genes involved in plant cell wall hydrolysis. To explain brown rot, many have implicated reactive oxygen species (ROS) in facilitating hydrolysis, with microenvironmental gradients partitioning ROS from enzymes. By spatially colocalizing gene expression and enzyme activities as Postia placenta colonizes wood, we provide evidence of an oxidative-hydrolytic two-step mechanism controlled by differential expression, not microenvironments, and we highlight 549 genes (∼4% of the genome) that are upregulated during this unique pretreatment.
Keywords: biodegradation, bioconversion, decomposition, lignocellulose, cellulase
Abstract
Wood-degrading brown rot fungi are essential recyclers of plant biomass in forest ecosystems. Their efficient cellulolytic systems, which have potential biotechnological applications, apparently depend on a combination of two mechanisms: lignocellulose oxidation (LOX) by reactive oxygen species (ROS) and polysaccharide hydrolysis by a limited set of glycoside hydrolases (GHs). Given that ROS are strongly oxidizing and nonselective, these two steps are likely segregated. A common hypothesis has been that brown rot fungi use a concentration gradient of chelated metal ions to confine ROS generation inside wood cell walls before enzymes can infiltrate. We examined an alternative: that LOX components involved in ROS production are differentially expressed by brown rot fungi ahead of GH components. We used spatial mapping to resolve a temporal sequence in Postia placenta, sectioning thin wood wafers colonized directionally. Among sections, we measured gene expression by whole-transcriptome shotgun sequencing (RNA-seq) and assayed relevant enzyme activities. We found a marked pattern of LOX up-regulation in a narrow (5-mm, 48-h) zone at the hyphal front, which included many genes likely involved in ROS generation. Up-regulation of GH5 endoglucanases and many other GHs clearly occurred later, behind the hyphal front, with the notable exceptions of two likely expansins and a GH28 pectinase. Our results support a staggered mechanism for brown rot that is controlled by differential expression rather than microenvironmental gradients. This mechanism likely results in an oxidative pretreatment of lignocellulose, possibly facilitated by expansin- and pectinase-assisted cell wall swelling, before cellulases and hemicellulases are deployed for polysaccharide depolymerization.
Brown rot wood-degrading fungi release sequestered carbon from lignocellulose in forests (1) and have the unique ability to accomplish this without significantly removing the recalcitrant lignin that encases the structural polysaccharides. Accordingly, their decay mechanisms may provide a model for new biomass conversion technologies that not only function despite the presence of lignin but also yield lignin as a potentially useful coproduct (1–3). Deviating from their white rot ancestors, brown rot fungi have evolved mechanisms that are generally faster (4, 5) and more polysaccharide-specific because they circumvent lignin (4, 6–8). This enhanced efficiency is coupled with losses, not expansions, of key white rot genes, including many linked to lignin degradation and processive cellulose hydrolysis. For example, few brown rot fungi produce the cellobiohydrolases that are included in commercial synergistic glycoside hydrolase (GH) mixtures (9–12). These observations imply that brown rot fungi harbor novel pathways to improve saccharification yields.
To explain why brown rot fungi are so efficient, despite their minimal toolkit of biodegradative enzymes, low-molecular-weight (LMW) oxidative agents have been proposed to operate in tandem with the enzymes. There is considerable evidence that extracellular Fenton reactions play a key role during brown rot, producing highly oxidizing hydroxyl radicals (H2O2 + Fe2+ → OH− + Fe3+ + •OH) or similarly reactive iron–oxo complexes. These oxidants apparently modify the lignocellulose to make it more susceptible to enzymatic saccharification by the limited set of GHs that brown rot fungi have retained in their genomes (13–18). In agreement, modifications in the lignin of brown-rotted wood indicate that limited oxidative cleavage of the polymer has occurred (19–21).
It is debated, however, how brown rot fungi direct Fenton reagents, which exhibit negligible selectivity for targets, to react with lignocellulose without damaging fungal hyphae or extracellular GHs. A long-standing hypothesis proposes that the fungi produce microenvironmental gradients that spatially partition the oxidants from the GHs in wood cells. A common model has Fe3+ at low pH (<3) in the vicinity of the hyphae strongly chelated by secreted oxalate, hindering its reduction to the Fe2+ required for Fenton chemistry. Diffusion of these chelates into a higher pH (>5) wood cell wall, where GHs cannot penetrate and the oxalate concentration is proposed to be lower, then results in their disassociation. With reactive Fe3+ thus available, LMW reductants secreted by the fungus could generate Fe2+ within the lignocellulose matrix and at a safe distance from hyphae and GHs (22).
Validating this hypothesis has proven difficult, and an underlying weakness lies in the high diffusivity of both H+ ions and small metal ion chelates, which would limit significant concentration gradients between fungal hyphae and the wood cell wall (23). A more feasible way to separate an oxidative pretreatment from enzymatic saccharification would be via differential expression, and this staggered approach might have added benefit in withholding metabolically costly GHs until they can operate effectively. There is evidence that fungal methoxyhydroquinones with a role in reducing Fe3+ to initiate Fenton chemistry are mostly secreted at the outset of brown rot, and then decrease as decay progresses (7, 16, 24, 25). There is also evidence of inducible expression and secretion of enzymes among brown rot fungi (9, 26), once characterized as having constitutive cellulase production (27). However, it has so far not been possible to determine whether the oxidative and GH systems are temporally separated by these fungi on wood. A major impediment to progress has been the heterogeneous nature of fungal colonization and attack on wood, during which adjacent regions of the substrate are frequently at different stages of decay.
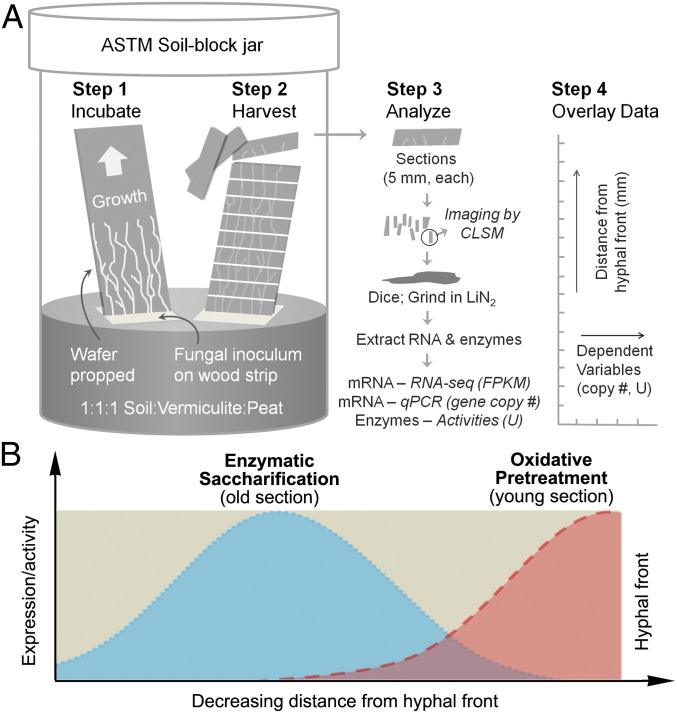
To address this problem, we elicited growth of the brown rot fungus Postia placenta in one direction along thin wood specimens. This approach spatially separated the stages of decay linearly along the substrate. We then sectioned the wood and analyzed individual sections for gene expression at the whole-transcriptome level, as well as enzyme activities they encode [defined here as lignocellulose oxidation (LOX) genes and GHs; SI Materials and Methods], with a particular focus on lignocellulolytic genes relevant to both oxidative and GH aspects of the brown rot mechanism. The reassembled data show temporal trends in expression, extending back from the advancing hyphal front, on a finer scale than is possible using conventional culture methods on wood. Our results reveal evidence that staggered gene expression has a role in regulating the progress of brown rot.
SI Materials and Methods
RNA-Seq.
RNA extraction.
To isolate Postia placenta RNA, fresh wood wafer sections were snap-frozen and ground to fine powder in liquid N2 with a mortar and pestle, an extraction enabled somewhat by using thin wafers and also by the orientation of wood cells in our design. Approximately 50 mg of powder was used for RNA extraction in 1 mL of TRIzol (Life Technologies). On-column DNA digestion was subsequently performed with DNase treatment.
RNA degradation was minimal and was monitored using denaturing RNA electrophoresis and an Agilent Bioanalyzer 2100 (Agilent Technologies). RNA samples with the RNA integrity number (RIN) > 8 were used for the downstream RNA-seq and quantitative PCR analysis. DNA-contaminated samples were excluded if the introns were still present in PCR verification.
RNA-seq and data analysis.
For RNA-seq, nine barcoded TruSeq RNA v2 libraries with ∼200-bp average insertions were created and sequenced on a 125-bp paired-end run on the HiSeq 2500 System (Illumina, Inc.) using v4 chemistry and the standard protocols from Illumina. Three samples at sections 0–5 mm, 15–20 mm, and 30–35 mm from aspen wafers were included, with three bioreplicates for each sample. A total of ≥220 million pass filter reads were generated for all nine libraries in a single sequencing flow cell lane. RNA-seq was performed at the University of Minnesota Genomics Center.
The RNA-seq data analyses were performed on the Galaxy platform (https://usegalaxy.org) through University of Minnesota according to the routine pipeline of Trapnell et al. (59). Raw reads were first cleaned up with Trimmomatic (v0.3) by setting the parameters as follows: java -jar trimmomatic-0.30.jar PE –phred33 input_forward.fq.gz input_reverse.fq.gz output_forward_paired.fq.gz output_forward_unpaired.fq.gz output_reverse_paired.fq.gz output_reverse_unpaired.fq.gz ILLUMINACLIP: TruSeq2-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36. The qualities of the trimmed reads were further verified by FastQC (Galaxy Version 0.63). Then, the cleaned reads were mapped against the genome of P. placenta MAD 698-R (v1.0) (Postia_placenta.fasta) with Tophat (v2.0.13) as follows: tophat -r 0 -i 20 -I 5000 –min-segment-intron 20 –max-segment-intron 5000 -G Postia_placenta_FilteredModels2.gff -o tophat_out_? $INPUT/Pospl1 trimmomatic/Ppl_?.R1.PE.fastq trimmomatic/Ppl_?.R2.PE.fastq. The statistical results of the cleaning and mapping process for RNA-seq reads are summarized in Table S3.
Table S3.
Cleaning of RNA-seq reads, and mapping of clean reads to the P. placenta genome
| Samples* | Reads before trimming | Clean paired reads† | Concordant pair alignment rate,‡ % | Multiple alignments | Discordant alignments |
| 0–5 mm_0 | 30,753,156 | 29,884,512 (97.18%) | 87.0 | 5,777,018 (21.9%) | 424,076 (1.6%) |
| 0–5 mm_1 | 28,731,524 | 27,999,535 (97.45%) | 86.3 | 5,587,014 (22.8%) | 377,835 (1.5%) |
| 0–5 mm_2 | 31,600,067 | 30,805,100 (97.48%) | 86.8 | 5,961,761 (21.9%) | 439,525 (1.6%) |
| 15–20 mm_0 | 31,077,840 | 30,304,779 (97.51%) | 87.7 | 6,435,079 (23.8%) | 435,145 (1.6%) |
| 15–20 mm_1 | 28,128,765 | 27,433,981 (97.53%) | 87.2 | 5,784,636 (23.8%) | 382,660 (1.6%) |
| 15–20 mm_2 | 31,365,488 | 30,531,214 (97.34%) | 87.2 | 6,422,959 (23.7%) | 426,672 (1.6%) |
| 30–35 mm_0 | 29,726,771 | 28,927,543 (97.31%) | 86.4 | 6,403,817 (25.2%) | 390,088 (1.5%) |
| 30–35 mm_1 | 29,527,548 | 28,787,751 (97.49%) | 87.1 | 6,143,924 (24.1%) | 390,410 (1.5%) |
| 30–35 mm_2 | 32,265,990 | 31,454,831 (97.49%) | 86.5% | 6,801,996 (24.6%) | 448,067 (1.6%) |
The reference genome of P. placenta MAD 698-R v1.0 was used in this work (genome.jgi.doe.gov/Pospl1/Pospl1.home.html).
The 0, 1, and 2 represent three bioreplicates.
Clean reads were obtained after trimming with Trimmomatic (v0.3 in Galaxy platform through University of Minnesota).
The cleaned reads were mapped against the genome of P. placenta MAD 698-R (v1.0) (Postia_placenta.fasta) with Tophat (v2.0.13) (methods are provided in SI Materials and Methods).
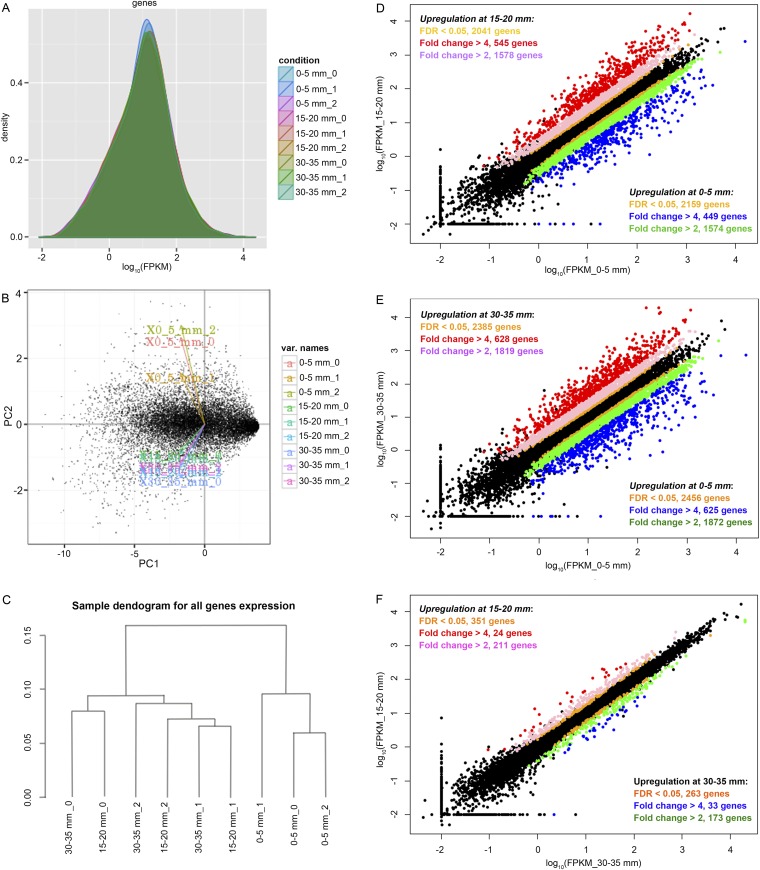
By using the reference transcript models Postia_placenta_FilteredModels2.gtf from the JGI Genome Portal (genome.jgi.doe.gov/Pospl1/Pospl1.home.html), expression levels and difference significances were calculated by comparing each pairwise combination of the three section samples (Dataset S1). The Cuffdiff output (e.g., all gene expression density distribution, principal component analysis, and sample dendrogram for all gene expression) was visualized by cummerbund (Galaxy Version 1.0.1). Comparisons of gene expression from each of two sections were presented as scatter plots by using RStudio (Version 0.99.491) (Fig. S3).
Fig. S3.
Comparisons of whole-genome transcription along the advancing mycelium in aspen wafers. (A) Gene expression density distribution, showing that all nine libraries (three bioreplicates of three sections) have similar density distributions. Principal component (PC) analysis (B) and a sample dendrogram (C) verify that samples clustered according to experimental conditions. Samples from 15–20 mm and 30–35 mm grouped together, indicating these samples have similar expression patterns. The 0, 1, and 2 represent three bioreplicates. (D–F) Scatter plots show comparisons of the transcription levels of all genes between each two sections at 0–5, 15–20, and 30–35 mm.
Definition of DEGs.
Given the high expression similarity of two older sections, samples at 15–20 and 30–35 mm were both used to make the comparison with the section at 0–5 mm. Specifically, for genes that were deemed significantly up-regulated during early decay, at least one of the following two conditions was satisfied: (i) FPKM_0–5 mm vs. FPKM_15–20 mm > fourfold and FPKM_0–5 mm vs. FPKM_30–35 mm > twofold and (ii) FPKM_0–5 mm vs. FPKM_30–35 mm > fourfold and FPKM_0–5 mm vs. FPKM_15–20 mm > onefold. For genes that were deemed significantly up-regulated during late decay, at least one of the following two conditions was satisfied: (i) FPKM_15–20 mm vs. FPKM_0–5 mm > fourfold and FPKM_30–35 mm > FPKM_0–5 mm and (ii) FPKM_30–35 mm vs. FPKM_0–5 mm > fourfold and FPKM_15–20 mm > FPKM_0–5 mm. To then be defined as DEGs, FDR < 0.05 and FPKM > 5 were satisfied. An FPKM >5 was generally >100 read counts in the corresponding mapping area.
Hierarchical clustering, gene annotation, and GO enrichment.
Hierarchical clustering of the DEGs was performed to visualize gene expression patterns more clearly during early and late decay. The gene expression levels were standardized using a standard normal distribution N (0, 1) and used for heat map construction with the average linkage (unweighted pair group method with arithmetic mean) method using HCE v3.0 software.
Gene annotations were assigned with Blast2GO (v3.2.7) for Pospl1_FilteredModels2_proteins by using default sets and the following steps: run Blast → run InterProScan (merge InterProScan GOs to annotation) → run Mapping → run Annotation. In total, 63% genes were annotated using this pipeline.
GO term enrichment analyses were subsequently tested with Fisher’s exact test for the DEGs of either early decay (0–5 mm) or late decay (15–20 mm and 30–35 mm) with Blast2GO. The term filter model FDR < 0.05 was applied for significance analysis.
Gene categories.
Genes associated with lignocellulose utilization were categorized according to their functions. The LOX_Fenton category includes the genes that were proposed to function in Fenton chemistry (9, 57): quinone redox cycling and hydroquinone biosynthesis genes (e.g., quinone reductase, phenol monooxygenase, phenylalanine ammonia lyase), glycopeptides, glucose-methanol-choline oxidoreductase (GMC) family genes (e.g., pyranose oxidase, alcohol oxidase, aryl-alcohol oxidase, other GMC enzymes), copper radical oxidases, amino acid/amine oxidases, and iron reduction and homeostasis genes (e.g., ferric iron reductase, iron permease, ferroxidase). Other lignin/aromatic compound modification genes (e.g., laccase, heme-thiolate peroxidase, ∆9/12-fatty acid desaturase, fatty acid synthase, GST, aromatic ring dioxygenase), oxalate metabolism genes (e.g., oxaloacetate acetylhydrolase, glycolate oxidase, d-lactate dehydrogenase, oxalate decarboxylase), polysaccharide monooxygenation genes (e.g., GH61), and genes for protection from reactive oxygen species (e.g., superoxide dismutase, catalase) were categorized as LOX_Others; CAZy category was assigned according to www.cazy.org, including GH, carbohydrate esterases, glycosyltransferases, other cellulose-binding module proteins, and feruloyl esterases; and genes named as cytochrome P450 and aldo keto reductase (AKR) by Blast2GO were categorized as P450s and AKRs, respectively. Sugar transporters, amino acid transporters, oligopeptide transporters, and other general substrate transporters in facilitator superfamily (MFS) were grouped into the transporter category. Sugar metabolism genes are those genes functioning in sugar utilization processes such as glycogenesis and TCA cycle, and the protease category includes the enzymes named as protease/peptidase by Blast2GO.
Quantitative PCR.
Gene targets.
To test the two-step oxidative-enzymatic hypothesis (Fig. 1B), eight brown-rotting relevant oxidoreductases and eight major GH genes in P. placenta were selected for qRT-PCR analysis using a spruce wafer, which was thought to be a more likely native wood substrate for brown rot fungi in nature.
Fig. 1.
Schematic of wafer setup and staggered mechanism hypothesis. (A) P. placenta was cultured in modified American Society for Testing and Materials (ASTM) soil-block microcosms, with wood wafers propped to force fungi to grow vertically. This setup generated a spatiotemporal gradient along the advancing mycelium, delineated by the visible advancing hyphal front and reconstructed from sections. LIN2, liquid nitrogen. (B) Data from RNA-seq, quantitative PCR (qPCR), confocal laser scanning microscopy (CLSM), and enzymatic activity assays were layered (i.e., stacked) in specific zones relative to the hyphal front and used to test the hypothesis that brown rot fungi stagger the expression of genes and extracellular enzymes during wood decay.
The oxidoreductases tested in this work included those oxidoreductases linked to H2O2 production [glucose oxidase (Gox1, Ppl108489/113276), methanol oxidase (Aox1, Ppl118723), aryl alcohol oxidase (Aao1, Ppl55496), and copper radical oxidase (Cro5, Ppl56703/99632)], as well as those oxidoreductases putatively involved in either/both H2O2 production and iron reduction [quinone reductase (Qrd1, Ppl124517), a LMW glycopeptide (Glp1, Ppl128974/128371), and ferric reductase (Ferd1, Ppl130027/135071)]. Laccase (Lac1, Ppl111314) was also included because of its potential to generate biodegradative reactive oxygen species, as well as its presence in the P. placenta genome (24). These oxidoreductases were either previously shown to be secreted by P. placenta or have relatively high expression levels (9, 26). Comparatively, the GHs chosen were proteins mainly responsible for hydrolyzing cellulose and hemicellulose, including three endoglucanases (Cel5A Ppl115648/135019, Cel5B Ppl103675/117690, and Cel12A Ppl121191/112658), a putative β-glucosidase (Bgl1, Ppl128500/128225), a putative endomannanase (Man5A, Ppl121831/57321), two putative endoxylanases (Xyn10A-1 Ppl113670/90657 and Xyn10A-2 Ppl105534), and a β-xylosidase (Bxl1 Ppl134890/51213). The β-actin gene (Ppl108207/118729) was used as an endogenous control to normalize gene expression levels, because previous work demonstrates that it is constitutively expressed in P. placenta (9, 26).
Primers.
Primers for target genes were designed with Primer Premier 5.00 according to the P. placenta genome database (genome.jgi.doe.gov/Pospl1/Pospl1.home.html), and their amplification efficiencies (E) were calculated with the slopes (Y) of linear standard curves (R2 ≥ 0.99) generated using four serial cDNA dilutions (i.e., E = 10−1/Y − 1; Table S4). In this study, the amplification efficiencies of all of the tested primer pairs were around 90–110% and were assumed to be 100% when calculating gene expression levels.
Table S4.
Primers used for qRT-PCR in this work
| Protein ID | Primers | Sequences 5′–3′ | Product length, bp | Amplification efficiency,* % |
| Actin (108207/118729) | PpAct(real)-S | AGAACTTGACGGAGCGTGGGTA | 229 | 97.72 |
| PpAct(real)-A | AGGAATGCGGGCTGGAAGA | |||
| Cel5A (115648/135019) | PpCel5A(real)-S | CGTCTCCTTTTCGGCGTCAT | 155 | 98.62 |
| PpCel5A(real)-A | GGGAATGTTTGGGCAGAGGT | |||
| Cel5B (103675/117690) | PpCel5B(real)-S | ACTCGTCTGGGTGGTGTCAAC | 147 | 90.3 |
| PpCel5B(real)-A | GGCAAACGGTATGCGGTAG | |||
| Cel12A (121191/112658) | PpCel12A(real)-S | TTTCAGGCGAAGGAGGCATT | 84 | 91.06 |
| PpCel12A(real)-A | CCCAGAGATTCCAGGTGTAGCC | |||
| Man5A (121831/57321) | PpMan5A(real)-S | GCTGACTGGCACCGACTACC | 87 | 100.57 |
| PpMan5A(real)-A | CCCACGAACGCATCCAAATAG | |||
| Xyn10A-1 (113670/90657) | PpXyn10A1(real)-S | CTTCGGCTCTGCTACGGACAA | 218 | 94.96 |
| PpXyn10A1(real)-A | ACCATACGCAGTTGTGTCCTCT | |||
| Xyn10A-2 (105534) | PpXyn10A2(real)-S | TCGGAGCCTGAGCCATTTGT | 150 | 97.07 |
| PpXyn10A2(real)-A | TGCTGCGGTGTAATTGTTGG | |||
| Bgl1 (128500/128225) | PpBgl1(real)-S | AGGCACAAGCCAAGTCGTCA | 166 | 95.71 |
| PpBgl1(real)-A | CTTGGCAATCGTGAAAGTGGT | |||
| Bxl1 (134890/51213) | PpXyl1(real)-S | GTGCGTTTCCCGACTGTGC | 139 | 96.05 |
| PpXyl1(real)-A | GCGGTGTTGCCGGTATTGT | |||
| Qrd1 (124517) | PpOrd1(real)-S | CGACGACAAGCCCAACAAGG | 238 | 90.66 |
| PpOrd1(real)-A | GGCGTGCATGAGCTTGAGGAT | |||
| Aox1 (118723) | PpAox1(real)-S | ACACCAAGGAGGACGACGAG | 218 | 91.43 |
| PpAox1(real)-A | GACGAGCAAGGCAGACGAGTA | |||
| Glp1 (128974/128371) | PpGlp1(real)-S1 | AATGGCGAAGACTGCACCCTC | 130 | 99.35 |
| PpGlp1(real)-A1 | CGTAGTAGGCAAAGGACGACCACA | |||
| Cro5 (56073/99632) | PpCro5(real)-S1 | CGGCGATGTTTCGGACGTTAT | 135 | 105.38 |
| PpCro5(real)-A1 | CCGCCATTCCAATAGTAGAGC | |||
| Lac1 (111314) | PpLac1(real)-S1 | CGATTCGGTTCAGGACGGATA | 140 | 100.03 |
| PpLac1(real)-A1 | CCAGGATTCAGAGGGATGGACTA | |||
| Gox1 (108489/113276) | PpGox1(real)-S1 | TCCTCGAAGCTGGAGACACCG | 111 | 101.24 |
| PpGox1(real)-A1 | CGGTCTCGTAAGCCCAGTCGTAG | |||
| Aao1 (55496) | PpAAO1(real)-S2 | GGCGTTGGCCAGAATCTGA | 77 | 95.18 |
| PpAAO1(real)-A2 | TCGTCATTAGTGTTCGTGGAGTTTA | |||
| Ferd1 (130027/135071) | PpFerd1(real)-S2 | TCGACCTCCTACTGCGTTTC | 109 | 98.33 |
| PpFerd1(real)-A2 | CATCCTGCATTGAGCGTTG |
Amplification efficiency was calculated using as template in series dilutions.
Quantitative reverse transcription PCR.
To quantify the gene expression levels, ∼100 ng of total RNA was reverse-transcribed to cDNA with the PrimeScript RT Reagent Kit (Clontech Laboratories). Quantitative PCR was performed in an Applied Biosystems 7900HT system (Thermo Fisher Scientific, Inc.). All PCR assays were carried out in three technical replicates in 15-μL reaction mixtures containing 7.5 μL of iTaq Universal SYBR Green Supermix (Bio-Rad), 0.5 μM forward and reverse primers, and 5 μL of 10-fold diluted cDNA template. The PCR reactions were as follows: 30 s initial denaturation at 95 °C, followed by 40 cycles of 15 s at 95 °C and then 60 s at 60 °C. A melting curve analysis was performed with a temperature increment of 0.1 °C⋅s−1 from 65 °C to 95 °C. Applied Biosystems SDS 2.4.1 software was applied to calculate cycle threshold (Ct) values by using the same fluorescence threshold for all of the target genes. Transcription levels of the target genes, in arbitrary units, were normalized against the levels of the internal reference β-actin gene by using the equation (2Ct for β-actin − Ct for target gene) × 104 (60).
Using spruce (a gymnosperm, softwood) data to guide analyses, we further duplicated our methods using wafers of aspen (an angiosperm, hardwood) as the substrate. Three sections (0–5, 15–20, and 30–35 mm) were sampled to represent early to late decay stages, targeting the whole-genome transcription analysis. In aspen, xylan is more prevalent than mannan in the hemicellulose fraction and side-chain hemicellulose (arabinan and galactan) can be 60% lower than in spruce (3, 41). On both wood types, this approach allowed us to recreate a temporal sequence of brown rot and to test the hypothesis (including its genetic basis) that brown rot fungi stagger oxidative Fenton-based pretreatments ahead of hydrolytic saccharification (Fig. 1B).
Enzyme Assays.
Protein extraction from wafers.
To verify the gene expression levels, the presence and activities of relevant enzymes were determined for the extracellular proteins extracted from P. placenta-colonized aspen wafers. Twelve separate aspen wafers were sectioned and pooled per location (0–5 mm, 15–20 mm, and 30–35 mm) for protein extraction. As an example, an initial razor cut along the advancing hyphal front and again 5 mm behind the front produced a section that was pooled with 11 other 0- to 5-mm sections.
The pooled wafers for each location along the wood section were coarsely chopped with a razor blade and extracted in 10 mL of 0.05 M acetate buffer. Two bioreplicates were included in this work.
Enzyme activity assays.
Each protein extract was tested for activity on model substrates by detecting increases in reducing sugars. Reactions contained 0.1 M acetate buffer at pH 4.8 and one of three model substrates (carboxylmethyl cellulose, birchwood xylan, or locust bean gum) to assess endoglucanase, xylanase, and mannanase activities, respectively. Prewarmed substrate solutions (0.75 mL each) were mixed with equivalent volumes of crude enzyme extract and incubated at 50 °C for 30 min. Reactions were stopped by adding 2 mL of a dinitrosalicylic acid developing reagent (54) and boiled before detection of reducing sugars. Standard curves were made with solutions of glucose, xylose, or mannose, depending on the substrate. Reducing sugars were determined by measuring the absorbance at 540 nm on an iMark microplate reader (Bio-Rad). One unit of activity was defined as the amount of enzyme needed to liberate 1 μmol of sugar equivalent per minute.
β-Glucosidase, β-xylosidase, β-mannosidase, and β-arabinofuranosidase activities were measured (55). The solutions of p-Nitrophenol (pNP) were used to construct the standard curve. One unit of activity was defined as the amount of enzyme needed to liberate 1 μg of pNP per minute.
Catalase activities were measured as the loss of H2O2 during a reaction containing the following reagents: 50 μL of properly diluted crude enzymes, 30 μL of 1 mM H2O2, and 100 μL of 0.1 M (pH 6.4) citric buffer. After a defined incubation time at room temperature, the reactions were stopped and 2 units of horseradish peroxidase and 20 μL of 5 mM ABTS were added to measure the residue H2O2 by measuring the oxidation of ABTS at OD420. One unit of catalase activity was defined as the amount of enzyme needed to degrade 1 nmol H2O2 per min. Laccase was assessed by measuring the changes of OD420 in reactions that contained equivalent volumes of crude enzyme extract and 0.5 mM ABTS in 50 mM sodium citrate buffer (pH 3.0) (55). The reactions were run at room temperature for 24 h.
All enzyme activities were normalized to fungal biomass, assessed indirectly using total fungal proteins measured as described in Fungal biomass determination.
Western blotting.
The presence of secreted endoglucanase PpCel5B (∼37 kDa, protein ID 103675/117690) was detected along the advancing hyphal front using Western blotting, according to Renart et al. (61). Equal amounts of each crude protein extract from different decay stages (5-mm, 15-mm, and 30-mm distance to the hyphal front) were separated by SDS/PAGE using a 5% (wt/vol) polyacrylamide stacking gel and a 12% (wt/vol) polyacrylamide resolving gel. After transferring the proteins to a polyvinylidene difluoride membrane using a Transblot cell (Bio-Rad), the presence of PpCel5B was detected with polyclonal antibodies that had been raised in rabbits against recombinant PpCel5B, which had been expressed in Pichia pastoris and then purified. The purification of recombinant PpCel5B and preparation of polyclonal antiserum to it are described below. The target PpCel5B signal on the membrane was then detected by using alkaline phosphatase-conjugated goat anti-rabbit IgG secondary antibody (Life Technologies). Negative controls were extracts from no-fungus wood, whereas deglycosylated rCel5B (+) purified from P. pastoris was used as a positive control. Blotting signals were measured with a Gel-Pro analyzer and normalized to the fungal biomass.
Heterologous expression of rPpCel5B.
The development of the recombinant strain of P. pastoris KM71H that heterologously expresses rPpCel5B is described by Ryu et al. (29). Fermentation and purification of rPpCel5B were performed by the Biotechnology Resource Center at the University of Minnesota, St. Paul. Induction of the recombinant strain was performed as described for KM71H strains in the EasySelect Pichia Expression Kit (Life Technologies) protocol. Briefly, a single glycerol stock vial containing the transgenic strain of P. pastoris was used to inoculate two 6-L baffled shake flasks (BSFs) containing 1.5 L of buffered glycerol complex medium. The flasks were incubated at 30 °C and shaken at 200 rpm. At 46 h postinoculation, the OD600 had reached a suitable level for induction. Cultures were centrifuged at 3,000 × g for 5 min, and pellets were resuspended in 100 mL of buffered methanol complex (BMMY) induction medium. Resuspended cells were placed into two 6-L BSFs (each containing 1.4 L of BMMY induction medium), incubated at 30 °C, and shaken at 250 rpm for 46 h. About 7.5 g of methanol was added at 0 and 24 h to maintain induction. After 46 h, the contents of both flasks were centrifuged at 6,000 × g for 10 min, cell pellets were discarded, and ∼2.7 L of supernatant was retained and frozen at −20 °C.
Purification of rPpCel5B.
Thawed supernatant was centrifuged at 10,000 × g for 30 min to remove precipitate. Retained supernatant was filtered through a sterile 0.22-μm filter and concentrated down to 200 mL. Concentrate was diafiltered into DEAE buffer A (6.89 g⋅L−1 NaH2PO4·H2O) with 4 × 850-mL volumes using a 10-kDa molecular mass cutoff membrane. Membranes were washed with 50 mL of the DEAE buffer A and pooled with diafiltered concentrate. About 250 mL of diafiltered concentrate was loaded onto a DEAE column (Capto DEAE resin, 4-mL bed volume), washed with five column volumes of DEAE buffer A at 5 mL⋅min−1, and eluted with a gradient to 100% DEAE buffer B [23.34 g⋅L−1 NaCl, 6.89 g⋅L−1 NaH2PO4·H2O (pH 6.0)]. Fractions were analyzed by SDS/PAGE to identify those fractions containing protein with an approximate molecular mass of 50 kDa. Combined fractions of 150 mL were concentrated by ultrafiltration to 12 mL and dialyzed back into DEAE buffer A overnight in 1 L of buffer. The yield of total purified rPpCel5B was about 5.8 mg with a concentration of 0.48 mg⋅mL−1.
Development of antiserum.
Polyclonal antiserum to rPpCel5B was developed by Pacific Immunology (Ramona, CA) as an analytical service. An aliquot of 2.4 mg of purified protein was supplied for immunization of two New Zealand White rabbits (PAC-8255 and PAC-8256). After preimmune serum collection, each rabbit was immunized with rPpCel5B mixed 1:1 with complete Freund’s adjuvant. Rabbits were immunized again after 3, 6, and 10 wk with rPpCel5B mixed 1:1 with incomplete Freund’s adjuvant. Rabbits were bled at 7, 9, 11, and 13 wk after initial immunization. Exsanguination bleeds were obtained 14 wk after initial immunization. ELISAs conducted on serum obtained from 7-wk bleeds indicated an antibody titer of ∼1:500,000 for both rabbits.
Fungal Growth Determination.
Fungal biomass determination.
The fungal growth along the aspen sections was determined by measuring the amount of the NaOH-extractable proteins (56). The corresponding wood from each wafer section was washed twice with ddH2O, ground into fine powder in liquid N2, and then extracted with 10 mL of 0.05 M NaOH for 1 h at 4 °C. The fungal total proteins were subsequently measured with a Bio-Rad Protein Assay Kit (Bio-Rad).
Staining of hyphae.
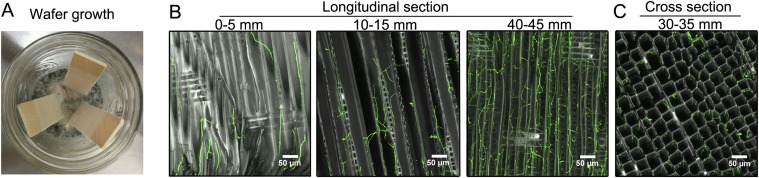
To stain hyphae and image hyphae growing within spruce wafers, a chitin-specific fluorophore and wood autofluorescence were used to stain and image the hyphae and the wood, respectively (62). This procedure was used to compare, at various magnifications, the hyphal growth patterns among recently colonized and older wood sections. Samples were soaked in 25% (vol/vol) non–crystal-forming Tissue Freezing Medium (Fisher Scientific), vacuum-impregnated for 20 min, frozen in a dry ice/ethanol slurry, and sectioned to ∼40 μm at −20 °C with a microtome-cryostat. Internal areas of these sections were imaged using a Nikon A1 Spectral Confocal Microscope with Z-stacking after 10 min of staining with 10 μg⋅mL−1 wheat germ agglutinin/tetramethylrhodamine conjugate (Life Technologies) specific for chitin. Excitation/emission wavelengths of 561/580 nm were used to identify the hyphae, whereas excitation/emission wavelengths of 488/520 nm were used to observe lignin autofluorescence in the wood. ImageJ (NIH) calculation (as overlays, scaled to micrometers using the scale bar) of total hyphal lengths for only the particular sections imaged here (a very preliminary assessment) were 1.57 mm for the 0- to 5-mm sections, 3.23 mm for the 15- to 20-mm sections, and 8.36 mm for the 30- to 35-mm sections. Hyphal tip numbers per image frame were 6, 19, and 52, respectively, and branching frequency (as branch points per millimeter of hyphae) were 3.83, 10.53, and 14.00, respectively.
Results
Wafer Colonization.
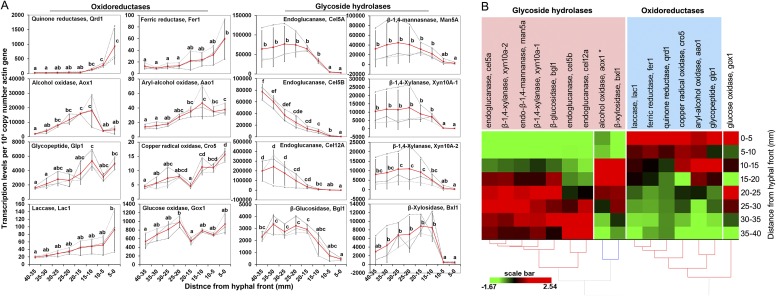
Decay microcosms elicited directional growth by P. placenta on both aspen and spruce wood wafers, resulting in distinct fronts of advancing hyphae that could be sectioned spatially to reconstitute a well-resolved temporal sequence (Fig. S1A). Mycelial growth rates were ∼2.5 mm⋅d−1; thus, the 0- to 5-mm sections used to represent early decay represented a ca. 48-h window. Confocal microscopy verified that the hyphal front within the wood matrix coincided with the visible front on the surface (methods are provided in SI Materials and Methods). Fungal biomass increased in the wood over time, with more branching near the wafer base in older hyphae, but with tips and branching evident in all wafer sections (Fig. S1B). Once colonization had extended ∼50 mm up the 60-mm wafers, triplicate wafers with uniform hyphal fronts (horizontal and linear) were cut into sections to assess the hypothesis of a staggered oxidative-hydrolytic mechanism (Fig. 1), with the hyphal front assigned a position of 0 mm. Among these sections, preliminary quantitative PCR analysis done on colonized spruce wood showed staggered gene regulation patterns, with transcripts for six oxidoreductases relatively more abundant at the hyphal front and transcripts for eight (hemi)cellulases accumulating distally (Fig. S2).
Fig. S1.
Imaging of P. placenta growth in wood wafers. (A) Colonization of wood wafers by P. placenta. (B) Confocal laser scanning micrographs of P. placenta in longitudinal wafer sections at different distances from the advancing hyphal front. (C) Transverse section showing that P. placenta cells stained using a chitin-specific fluorophore adhered to the S3 layer of adjacent wood cell lumens, with growth parallel to the grain. Hyphal cells are shown in green, whereas the wood autofluorescence is shown in gray.
Fig. S2.
Quantitative PCR analysis of gene expression in spruce wafers. (A) Differential expression of oxidoreductase and GH genes in P. placenta as a function of distance from the hyphal front in wafers. (B) Heat map showing that transcripts of oxidoreductases accumulated ahead of GHs as P. placenta-colonized wafers. Transcription levels were assessed using qRT-PCR in samples from eight contiguous wafer sections behind the visible hyphal front. Expression levels of each gene were normalized against β-actin gene and presented as the average of three independent bioreplicates (Note: RNA-seq revealed actin up-regulated 1.8-fold at the front, but could not be applied to normalize quantitative PCR data). Each gray dashed line in A indicates the expression data from one independent wafer, whereas red lines show the mean values. Error bars represent SDs. Significance of differences in expression levels of each gene along the advancing mycelium were calculated with a Tukey honest significant difference (HSD) test (α = 0.05) by using log2 transformation of the data when normalization was required. Letters in A indicate a significant difference between two means (P < 0.05) for each gene. The average expression data for each gene along the advancing hyphal front were normalized and used for hierarchical clustering analysis.
Staggered Gene Expression During Brown Rot.
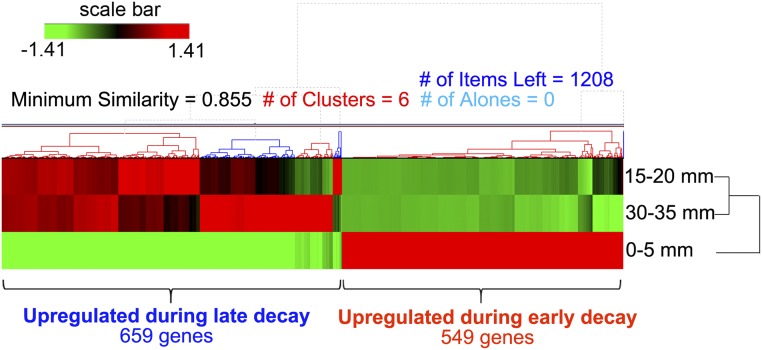
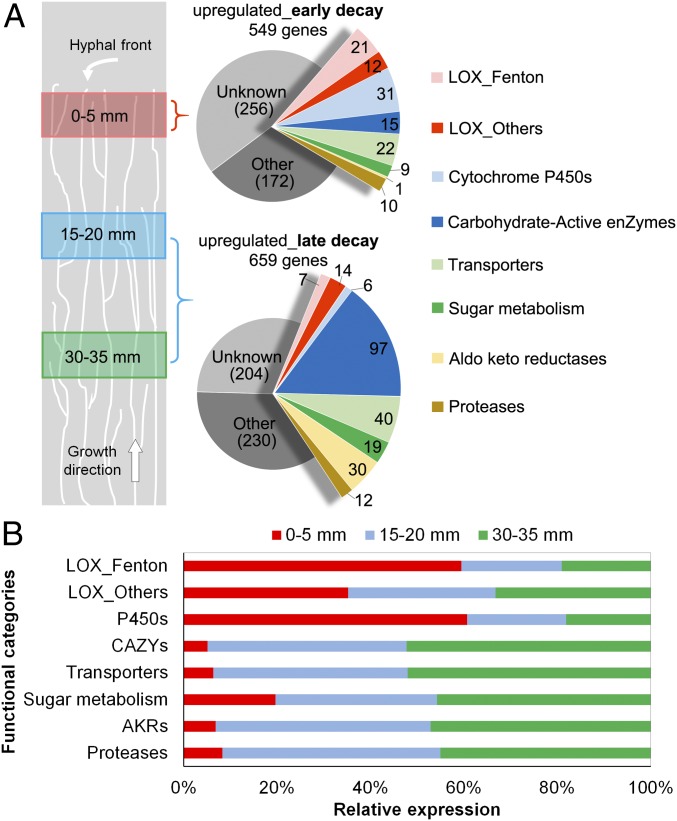
Whole-transcriptome shotgun sequencing (RNA-seq) analysis showed differential expression of genes involved in substrate oxidation vs. polysaccharide hydrolysis, based on analyses of aspen wafer sections at three locations (0–5, 15–20, and 30–35 mm). The overall gene expression profiles were similar for older mycelium on the two sections at 15–20 and 30–35 mm, whereas they were clearly different for the younger mycelium at 0–5 mm (Fig. S3 and Dataset S1; Gene Expression Omnibus database accession no. GSE84529). Considering the 0- to 5-mm sections as representative of early decay and the two older sections together as representative of late decay, differentially expressed genes (DEGs) were screened for greater than fourfold changes in transcript accumulation [false discovery rate (FDR) < 0.05 and fragments per kilobase unique exon sequence per megabase of library mapped (FPKM) > 5; DEG definition is provided in SI Materials and Methods]. For brevity, we arbitrarily refer to genes meeting this criterion as “upregulated.” In total, 549 DEGs were up-regulated during early decay, and sequences that encode oxidoreductase activities, such as incorporation or reduction of O2 and heme/iron binding, were significantly enriched [FDR < 0.05 for Gene Ontology (GO) enrichment]. In contrast, 659 DEGs were up-regulated during late decay, and, among them, sequences that encode GH activities were significantly enriched (FDR < 0.05; Fig. S4 and Dataset S2).
Fig. S4.
Hierarchical clustering analysis of DEGs along the advancing mycelium in aspen wafers. As shown by RNA-seq analysis, 659 genes were up-regulated during late decay (15–20 mm and 30–35 mm), whereas 549 genes were up-regulated during early decay (0–5 mm). Fold changes between early and late decay were greater than fourfold (FDR < 0.05), with FPKM > 5 for all genes included.
Oxidoreduction-Associated Genes.
RNA-seq analysis identified 33 genes up-regulated during early decay that are likely associated with redox processes, such as LOX. Of these genes, 21 (designated LOX_Fenton) may be involved in generating H2O2 or Fe2+ to support biodegradative Fenton chemistry (Fig. 2 and Table S1). These LOX_Fenton genes encode enzymes that putatively include glucose oxidases [protein IDs 44331 and 128830 from the Joint Genome Institute (JGI) database, P. placenta v1.0], alcohol oxidases (55972 and 129158/126217), glyoxal oxidase (46390), amino acid/amine oxidases (47008 and 110689), iron reductases (130025 and 130030/130043), and Fe2+ transporters (52765/43266). In addition, eight genes with likely roles in the biosynthesis of aromatic metabolites were up-regulated during early decay [e.g., benzoquinone reductases (124517/64069), phenylalanine ammonia lyase (111514), aromatic ring monooxygenases (54109/46071, 23052/62058, and 129128)]. The encoded enzymes may be involved in the biosynthesis of secreted methoxyhydroquinones, which drive extracellular Fenton chemistry by reducing Fe3+ to Fe2+ (7, 16, 24, 25). One O-methyltransferase (52307) putatively involved in methoxyhydroquinone synthesis was also up-regulated by about twofold (P < 0.05) (Dataset S1).
Fig. 2.
Gene transcription patterns along the advancing hyphal front in aspen wafers as measured by RNA-seq. (A) Distribution of DEGs. Relative to sections at 15–20 mm and 30–35 mm, which represent late decay, 549 genes were up-regulated in sections at 0–5 mm, which represent early decay. Conversely, 659 genes were up-regulated during late decay. Data were obtained from three bioreplicates at each position. (B) Relative expression levels in different gene categories. The relative FPKM levels of DEGs in each category were compared among the sections of the three lengths. For DEGs, the fold change was >4 (FDR < 0.05) and the FPKM was >5. The LOX_Fenton category includes enzymes that may support extracellular Fenton chemistry (e.g., via extracellular H2O2 generation, iron reduction and homeostasis, hydroquinone biosynthesis, quinone redox cycling). LOX_Others includes genes that may be involved in lignin/aromatic compound modification, oxalate metabolism, polysaccharide monooxygenation, and protection from reactive oxygen species (9, 57). CAZy genes were assigned according to the CAZy database (www.cazy.org) (58). The assignment of other gene categories is provided in Dataset S3. AKR, aldo keto reductase.
Table S1.
Differential transcription of genes likely involved in LOX during wood decay by P. placenta
| Protein ID | Sequence description* | Comments† | FPKM | Ratio,‡ log2 (early/late) | ||
| 0–5 mm | 15–20 mm | 30–35 mm | ||||
| Genes up-regulated during early decay | ||||||
| 121561/115965 | Glyoxylate dehydrogenase | Oxalate production | 907.92 | 15.14 | 12.94 | 6.01 |
| 112832/124688 | Oxaloacetate acetylhydrolase | Oxalate production; TM | 1415.05 | 39.08 | 25.82 | 5.45 |
| 54109/46071 | Salicylate hydroxylase | Hydroquinone biosynthesis (F) | 80.24 | 2.74 | 1.57 | 5.22 |
| 44331/none | Glucose oxidase-like protein | AA3_4 (F) | 73.42 | 3.23 | 2.33 | 4.72 |
| 128830/none | Glucose oxidase-like protein§ | AA3_4; SignalP (F) | 99.50 | 4.72 | 3.66 | 4.57 |
| 47008¶ | d-amino acid oxidase | (F) | 20.60 | 1.31 | 0.99 | 4.16 |
| 110668¶ | ∆-12 Fatty acid desaturase | TM | 184.03 | 20.55 | 23.77 | 3.05 |
| 129128¶ | Phenol 2-monooxygenase | Hydroquinone biosynthesis (F) | 20.02 | 2.33 | 2.60 | 3.02 |
| 55972/none | Alcohol oxidase-like protein | AA3_3 (F) | 26.08 | 4.45 | 3.04 | 2.80 |
| 110689¶ | Amine oxidase | SignalP (F) | 57.83 | 9.01 | 8.11 | 2.76 |
| 124517/64069 | Benzoquinone reductase | AA6; Hydroquinone biosynthesis (F) | 136.36 | 13.98 | 26.95 | 2.74 |
| 23052/62058 | Salicylate hydroxylase | Hydroquinone biosynthesis (F) | 12.86 | 2.38 | 1.58 | 2.70 |
| 52765/43266 | Mn2+ and Fe2+ transporter | TM (F) | 60.28 | 10.20 | 9.36 | 2.62 |
| 127397/none | Putative heme-thiolate peroxidase | TM | 46.25 | 7.43 | 9.48 | 2.45 |
| 46390¶ | Glyoxal oxidase | AA5_1 (F) | 5.14 | 0.95 | 0.93 | 2.45 |
| 61132/none | Oxalate decarboxylase | Oxalate degradation; SignalP | 43.88 | 8.55 | 7.78 | 2.43 |
| 129158/126217 | Alcohol oxidase-like protein | AA3_3 (F) | 181.10 | 39.65 | 34.48 | 2.29 |
| 62913/none | d-lactate dehydrogenase | Oxalate production | 6.40 | 1.89 | 0.97 | 2.16 |
| 124964/none | GST | — | 91.61 | 24.17 | 17.86 | 2.12 |
| 129212/none | Fatty acid synthase | — | 99.99 | 24.21 | 23.86 | 2.06 |
| 111514/none | Phenylalanine ammonia lyase | Hydroquinone biosynthesis (F) | 57.67 | 20.40 | 7.42 | 2.05 |
| 130025/none | Ferric reductase-like protein | AA8; TM (F) | 91.90 | 33.97 | 22.66 | 1.70 |
| 25391/61079 | Heme-thiolate peroxidase | SignalP; TM | 29.69 | 7.20 | 11.73 | 1.65 |
| 130030/130043 | Ferric reductase-like protein | AA8; SignalP; TM (F) | 66.60 | 59.33 | 7.03 | 1.01 |
| Genes up-regulated during late decay | ||||||
| 59021/none | Catalase | — | 2.34 | 10.85 | 39.52 | −3.43 |
| 54008/none | Aryl-alcohol oxidase-like protein | AA4 (F) | 3.72 | 48.80 | 26.18 | −3.33 |
| 99098/none | Catalase | — | 4.24 | 17.04 | 60.50 | −3.19 |
| 121565/61477 | Glycolate oxidase | Oxalate production | 81.82 | 559.23 | 650.37 | −2.89 |
| 110493/111797 | Superoxide dismutase | SignalP; TM | 60.49 | 470.68 | 404.56 | −2.85 |
| 119457/125432 | 3-Hydroxyanthranilate dioxygenase | — | 61.39 | 387.51 | 499.51 | −2.85 |
| 115618/126811 | Polysaccharide monooxygenase | AA9; SignalP | 28.50 | 181.18 | 162.16 | −2.59 |
| 46778/none | Oxalate decarboxylase | Oxalate degradation; SignalP | 16.35 | 49.96 | 126.63 | −2.43 |
| 43912¶ | Oxalate decarboxylase | Oxalate degradation; SignalP | 137.00 | 724.09 | 613.86 | −2.29 |
| 58266/none | Aryl-alcohol oxidase-like protein | AA4; SignalP (F) | 1.80 | 10.27 | 7.13 | −2.27 |
| 109824¶ | Fet3 ferroxidase protein | AA1_2; SignalP; TM (F) | 1.88 | 3.11 | 14.26 | −2.21 |
| 60536/none | Glutathionyl-hydroquinone reductase | Hydroquinone biosynthesis (F) | 10.96 | 42.49 | 51.41 | −2.10 |
| 118556¶ | Iron permease ftr1 | TM (F) | 1.28 | 2.10 | 8.74 | −2.08 |
| 34850¶ | Aromatic compound dioxygenase | — | 52.06 | 230.08 | 186.99 | −2.00 |
| 110414/none | GST | — | 61.57 | 198.37 | 262.33 | −1.90 |
| 127865/129444 | Ferric reductase-like protein | AA8; SignalP; TM (F) | 19.71 | 56.76 | 88.83 | −1.88 |
Differentially expressed LOX genes are shown, including LOX_Fenton and LOX_Others genes as in Fig. 2. Early expression (0–5 mm) is compared with late expression (15–20 mm or 30–35 mm). Criteria for inclusion are fold change in expression greater than fourfold (FDR < 0.05) and FPKM > 5. Data are mean values from three bioreplicates. AA, auxiliary activities; (F), LOX_Fenton genes; SignalP, secretion signal peptide; TM, transmembrane.
Genes were annotated with Blast2GO v3.2, and then were further verified by BlastP with the UniProt database via the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov/) and JGI (genome.jgi.doe.gov/Pospl1/Pospl1.home.html) databases.
Auxiliary activities are as assigned by Levasseur et al. (57).
Genes are ranked according to the ratios of early expression (0–5 mm) to late expression (average of the expression levels at 15–20 mm and 30–35 mm).
A second allele is present in these cases but is excluded because its fold change in expression was less than fourfold or its FPKM was <5.
Also relevant to LOX was the up-regulation of 31 cytochrome P450s during early decay vs. six that were up-regulated during later decay (Fig. 2 and Dataset S3). Some cytochrome P450s are likely involved in aromatic hydroxylation reactions required for methoxyhydroquinone biosynthesis, as well as the hydroxylation of lignin fragments preparatory to aromatic ring fission (28).
With regard to Fe3+ mobilization, the expression of genes associated with production of the physiological iron chelator oxalate (16) followed a pattern that suggests stimulation followed by attenuation. On the production side, we found two putative glyoxylate dehydrogenase genes (121561/115965) and one putative oxaloacetate acetylhydrolase gene (112832) that were markedly up-regulated by more than 60-fold during early decay (Table S1). This was countered during later decay by an up-regulation of likely oxalate decarboxylases (46778 and 43912) at far higher expression levels than we found for another oxalate decarboxylase (61132) that was up-regulated near the hyphal front.
Among 21 oxidoreductases that were up-regulated during later decay, there were seven genes in the LOX_Fenton category, including two aryl alcohol oxidases, a ferroxidase, and a transmembrane iron permease; two iron reductases (127865/129444); and a glutathionyl-hydroquinone reductase (60536) (Table S1). Among the other LOX genes up-regulated during later decay, there were several that might be involved in detoxification rather than lignocellulose degradation, including catalases (59021 and 99098), superoxide dismutases (110493/111797), and a glutathione S-transferase (60536). Aromatic compound dioxygenases (119457/125432 and 34850) and a polysaccharide monooxygenase (115618/126811) were also up-regulated late rather than early, presumably to cleave lignin fragments and polysaccharides, respectively, during this stage of decay.
Carbohydrate-Active Enzyme and Sugar Transporter Genes.
A total of 97 carbohydrate-active enzyme (CAZy) genes involved in lignocellulose hydrolysis were up-regulated during later decay. This list includes 70 GHs (P. placenta has 255 predicted GH genes), 15 carbohydrate esterases, 6 glycosyl transferases, 3 feruloyl esterases, and 3 carbohydrate-binding module family genes (Fig. 2). Those differentially expressed CAZys that are likely involved in lignocellulose saccharification and have been predicted as secreted enzymes (50 total, 82% of them GHs) are listed in Table S2, including well-characterized endoglucanases Cel5A 115648/108962, Cel5B 103675/117690, and Cel12A 121191/112658, a xylanase (Xyn10A-1 113670/90657), and a mannanase (Man5A 57321/121831), as shown by previous work with P. placenta (9, 26, 29). Consistent with an increase in polysaccharide hydrolysis, sugar transporters, sugar-metabolic enzymes, and aldo keto reductases were also up-regulated during later decay stages (Dataset S3).
Table S2.
Differential transcription of CAZy genes likely involved in lignocellulose saccharification during wood decay by P. placenta
| Protein ID | Sequence description* | FPKM | Ratio,† log2 (early/late) | ||
| 0–5 mm | 15–20 mm | 30–35 mm | |||
| Genes up-regulated during early decay | |||||
| 111730/43189 | GH28, polygalacturonase‡ | 1,770.99 | 215.86 | 22.67 | 3.89 |
| 126976/none | Expansin-like protein | 261.02 | 57.76 | 38.47 | 2.44 |
| 128179/none | Expansin-like protein | 150.22 | 41.98 | 15.31 | 2.39 |
| 52150§ | GH13, α-amylase | 9.07 | 1.88 | 1.75 | 2.32 |
| 48716§ | GH92‡ | 39.68 | 14.79 | 7.43 | 1.84 |
| 48548/44386 | CE16 | 19.21 | 12.22 | 3.82 | 1.26 |
| Genes up-regulated during late decay | |||||
| 121191/112658 | GH12, endo-1,4-β-glucanase (Cel12A)‡ | 414.97 | 10,658.18 | 40,000.00 | −5.93 |
| 103675/117690 | GH5, endo-1,4-β-glucanase (Cel5B)‡ | 567.05 | 14,037.16 | 27,715.90 | −5.20 |
| 113670/90657 | GH10, endo-1,4-β-xylanase‡ (Xyn10A-1) | 145.72 | 3,401.12 | 2,565.03 | −4.36 |
| 55907/none | Feruloyl esterase-like protein‡ | 15.15 | 292.26 | 295.84 | −4.28 |
| 112732/59600 | GH53, arabinogalactan endo-β-galactosidase | 67.14 | 1,161.40 | 845.39 | −3.90 |
| 115648/108962 | GH5, endo-1,4-β-glucanase (Cel5A)‡ | 1,281.83 | 18,023.56 | 18,075.02 | −3.82 |
| 105534/none | GH10, endo-1,4-β-xylanase‡ | 191.33 | 2,595.68 | 2,490.97 | −3.73 |
| 126692/111332 | GH79, endo-β-glucuronidase‡ | 280.47 | 3,693.17 | 3,375.15 | −3.66 |
| 55267/62952 | Feruloyl esterase-like protein | 6.50 | 78.31 | 67.22 | −3.48 |
| 95568/none | GH5, endo-1,4-β-mannosidase‡ | 336.24 | 3,709.42 | 3,670.98 | −3.46 |
| 57321/121831 | GH5, endo-1,4-β-mannosidase‡ (Man5A) | 768.00 | 9,072.98 | 7,533.55 | −3.43 |
| 108959/110682 | CE16 | 680.29 | 7,037.75 | 5,530.26 | −3.21 |
| 125801/none | CE16‡ | 898.50 | 8,878.10 | 7,453.55 | −3.18 |
| 127469/51213 | GH3, exo-1,4-β-xylosidase‡ | 179.48 | 1,862.10 | 1,345.82 | −3.16 |
| 130413/none | GH115‡ | 228.66 | 2,141.71 | 1,561.95 | −3.02 |
| 57564/56576 | GH2, β-mannosidase‡ | 491.70 | 4,424.31 | 3,441.54 | −3.00 |
| 128500/128225 | GH3, β-glucosidase‡ | 89.14 | 651.60 | 764.68 | −2.99 |
| 128150/98662 | GH27, α-galactosidase‡ | 527.60 | 4,444.57 | 3,462.98 | −2.91 |
| 110809/none | GH43, α-l-arabinofuranosidase‡ | 271.14 | 2,325.25 | 1,300.73 | −2.74 |
| 107557/none | GH3, β-glucosidase‡ | 611.85 | 3,777.99 | 3,909.44 | −2.65 |
| 46915/95677 | GH3, β-glucosidase‡ | 37.06 | 264.56 | 158.74 | −2.51 |
| 100745/54928 | CE8 | 68.00 | 470.28 | 193.50 | −2.29 |
| 120395/none | GH27, α-galactosidase‡ | 232.84 | 1,332.89 | 887.59 | −2.25 |
| 62300/none | GH16, putative laminarinase | 172.25 | 941.88 | 640.80 | −2.20 |
| 112941/61089 | GH16, putative laminarinase‡ | 352.10 | 1,941.99 | 1,050.66 | −2.09 |
| 127993/128101 | GH35, β-galactosidase‡ | 229.84 | 1,123.50 | 814.75 | −2.08 |
| 128334§ | GH16, putative laminarinase | 135.34 | 511.84 | 593.66 | −2.03 |
| 128269§ | GH5, β-mannosidase | 15.93 | 73.89 | 54.45 | −2.01 |
| 116992§ | GH92, α-1,2-mannosidase‡ | 23.10 | 108.61 | 70.45 | −1.95 |
| 113112§ | GH15-CBM20, glucoamylase‡ | 54.66 | 229.57 | 182.17 | −1.91 |
| 52805/112669 | GH12, endo-1,4-β-glucanase | 176.46 | 852.44 | 258.96 | −1.65 |
| 54936§ | CE8, Pectin esterase precursor | 22.86 | 93.07 | 36.07 | −1.50 |
Differentially expressed CAZy genes that have predicted secretion signal peptides are shown. Early expression (0–5 mm) is compared with late expression (15–20 mm or 30–35 mm). Criteria for inclusion are fold change in expression greater than fourfold (FDR < 0.05) and FPKM > 5. Data are mean values from three bioreplicates. CBM, cellulose-binding module; CE, carbohydrate esterase.
CAZy genes were assigned according to www.cazy.org (58). Genes were annotated with Blast2GO v3.2, and then were further verified by BlastP with the UnitProt database via the NCBI (www.ncbi.nlm.nih.gov/) and JGI (genome.jgi.doe.gov/Pospl1/Pospl1.home.html) databases.
Genes are ranked according to the ratios of early expression (0–5 mm) to late expression (average of the expression levels at 15–20 mm and 30–35 mm).
A second allele is present in these cases but is excluded because its fold change in expression was less than fourfold or its FPKM was <5.
Some CAZys and relevant sugar transporters were up-regulated at the hyphal front relative to older hyphae (Table S2 and Dataset S3), including four GHs and two expansin-like genes. Notably, a polygalacturonase (111730/43189, pectinase) was up-regulated by about 15-fold during early decay, with a maximum FPKM approaching 1,800 at the hyphal front.
Enzyme Activities and Colocalization.
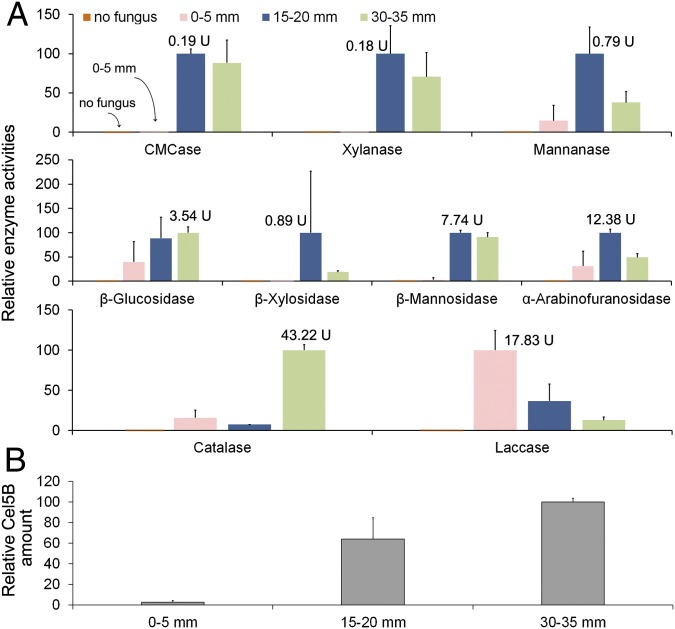
Endoglucanase (carboxymethylcellulase)-, xylanase-, and mannanase-specific activities (units per milligram of fungal biomass) were low at the hyphal front and increased distally. Similar trends were shown for β-glucosidase, β-xylosidase, β-mannosidase, and α-arabinofuranosidase activities (Fig. 3A). In line with these results, Western blot analysis of one endoglucanase, Cel5B (103675/117690), showed that this protein was nearly undetectable at the hyphal front (0–5 mm) but substantially increased distally at 15–20 and 30–35 mm (Fig. 3B). The above results are in line with the RNA-seq results for Cel5B and other GH genes.
Fig. 3.
Presence and activity of P. placenta GHs and oxidoreductases in aspen wafers. (A) Relative activities of relevant water-extracted enzymes with likely lignocellulolytic roles (SI Materials and Methods). Activities were measured as units per milligram of total fungal protein and were normalized for each enzyme by setting the highest activity (shown beside the column) at 100. (B) Relative amounts of secreted endoglucanase Cel5B (protein ID 103675/117690) via Western blotting. Error bars represent the SD of two bioreplicates, with 12 wafers’ sections pooled for each replicate.
Among the LOX enzymes, relatively few are predicted to be secreted, but we were able to perform several assays. Catalase activity increased by nearly sixfold at 30–35 mm compared with the hyphal front (Fig. 3A), in line with the up-regulated expression patterns of catalase genes in older hyphae. For alcohol oxidase and glucose oxidase assays, no activities were detected, perhaps due to proteins being intracellular or periplasmic, or because the catalase activity may have consumed the H2O2 whose production is the basis for the oxidase assays. We also assayed laccase activities, and although these activity levels were low, colorimetric observations of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) oxidation in the extracts revealed a gradient opposite to the gradient of the GH activities, with the highest activity located at the hyphal front. This result is in line with the RNA-seq results for one candidate laccase (47589), which was up-regulated by 1.8-fold at the hyphal front (P < 0.05) (Dataset S1).
Discussion
Our results support a two-step oxidative-hydrolytic mechanism for the brown rot fungus P. placenta, which entails a brief oxidative pretreatment that has been obscured in earlier studies. We arrived at this conclusion by growing the fungus directionally, thus extending over space the temporal progression of decay, after which we performed both transcript analyses and enzyme assays. The results of the two approaches (transcriptomics and enzyme assays) agree with each other. The shift from oxidoreductase-dominated to GH-dominated expression by P. placenta generally occurred at a short distance behind the hyphal front, confining key oxidative dynamics to a narrow zone of mycelial growth. As an example, all three of P. placenta’s endoglucanases (Cel5A, Cel5B, and Cel12A) were up-regulated in the 15- to 20-mm aspen sections by 14-, 25-, and 26-fold, respectively, relative to values at the hyphal front (0- to 5-mm sections) (Table S2). By contrast, there was a reverse pattern in quinone reductase expression, as shown by a 10-fold decrease, in 15- to 20-mm sections vs. 0- to 5-mm sections (Table S1). In colonized spruce wood, for which immediately adjacent sections were analyzed at 5-mm intervals, quantitative PCR analysis showed that this interval of oxidoreductive gene up-regulation persisted for only 48 h, given the growth rates we observed (Fig. S2). This brief window of differential gene regulation has been missed in earlier studies of brown rot that used homogenized whole-wood specimens, likely because that approach inevitably mixes hyphae acting asynchronously at different stages of biodegradation (7, 9, 21, 26, 29).
Given that we observed both hyphal tips and branching hyphae in all wood sections yet were able to discern differential gene expression across the sections, our results raise the question of how uniformly P. placenta controls gene expression. A unified mechanism could apply locally to all cell types, but it is also possible that differential expression could be limited to a particular subpopulation of cells. Although apical hyphae are often presumed responsible for the bulk of fungal protein secretion (30, 31), expression patterns that are locally heterogeneous can arise due to nonuniform gene expression within the mycelium (32) and to protein secretion from subapical hyphal regions (33). Heterogeneous secretion of lignin peroxidases has, for example, been shown in the wood-degrading fungus Phanerochaete chrysosporium (34). In our case, we cannot discern the relative contributions of hyphal tips and subapical hyphae, but the well-defined gene up-regulation patterns we observed on a fine spatial scale are striking, given the heterogeneous hyphal populations.
Many of the up-regulated LOX genes in the frontal zone (0–5 mm) of the advancing P. placenta mycelium likely have roles in biodegradative Fenton chemistry (9, 35, 36). These processes include oxalate production for iron chelation, production of methoxyhydroquinones that reduce Fe3+ and O2, enzymatic iron transport and reduction, and enzymatic H2O2 generation (Table S1). Some of these and other up-regulated LOX genes near the hyphal front of P. placenta have been classified as encoding auxiliary enzymes that support lignin degradation by white rot fungi (25). This category includes genes for the quinone reductase and the ∆12 fatty acid desaturase, both highly up-regulated in our case. The latter enzyme is linked to linoleic acid production and lignin degradation via lipid peroxidation in white rot fungi such as Ceriporiopsis subvermispora (37, 38). Accordingly, it is possible that the functions of some up-regulated LOX genes in P. placenta have been adapted from white rot ancestors to enable a staggered oxidative-hydrolytic mechanism during brown rot. Retention or recruitment of some white rot LOX genes could contribute to the partial ligninolysis that brown rot fungi are able to achieve and could facilitate subsequent access by GHs that hydrolyze structural polysaccharides (19, 20, 39).
In distal sections of the P. placenta mycelium, the delayed and marked up-regulation of CAZys, specifically GHs, implies that cellulases and hemicellulases have a significant role in wood decay, contrary to the classical view that these enzymes cannot infiltrate the wood cell wall during brown rot (40). These CAZys include not only several previously characterized endoglucanases (29) but also numerous hemicellulases and carbohydrate esterases, many of which remain poorly characterized (e.g., feruloyl esterases, GH53 arabinogalactan endo-β-galactosidase). Some of these enzymes may be responsible for the early losses in hemicellulose side chains that characterize brown rot (41). Our observation of increased gene transcription and enzyme activity for α-l-arabinofuranosidase behind the hyphal front offers a representative example. This α-l-arabinofuranosidase is an enzyme within a family (GH43) that contains a great deal of subtle variation, not fully captured by assays that use simple synthetic substrates (42). These GH43s are expanded in many wood-degrading fungi, including Schizophyllum commune (43). Up-regulation of hemicellulases and carbohydrate esterases implies that enzymatic hemicellulose hydrolysis is integral to brown rot, even though hemicellulase genes [including GH43s (44)] are less numerous and less diverse in brown rot fungi than in white rot fungi (11).
The LOX genes up-regulated late, alongside the general up-regulation of GHs, include genes that may have a protective role rather than a direct role in lignocellulose deconstruction. For example, those genes encoding catalases may have a role in attenuating Fenton chemistry to prevent oxidative damage to GHs that are secreted during the hydrolytic stage of biodegradation (Table S1). In addition, aryl alcohol oxidases and glutathione S-transferases may be expressed as a detoxification response after oxidative pretreatments release potentially toxic fragments from lignocellulose (45, 46). This result also suggests that aryl alcohol oxidases, which are thought to participate in H2O2 generation to support lignin degradation by white rot fungi (47, 48), may have a different function during brown rot, relegating H2O2 generation to another pathway.
Conversely, the CAZys up-regulated early, where LOX up-regulation dominated, may synergize with LOXs during decay initiation. These CAZys include a GH28 pectinase and two expansin-like proteins (Table S2). Expansins have been implicated in binding and swelling cellulose to improve accessibility (49), and expansin-like genes have been reported in the white rot fungus Bjerkandrea adusta (termed “loosenin”) (50) and in S. commune (43). Their contribution might be complemented by the GH28 polygalacturonase (pectin depolymerase) of P. placenta (39% identity to the GH28 polygalacturonase in Aspergillus niger; GenBank database accession no. CAA38085.1), which may target pectin as an accessible, easily hydrolyzed polymer to uncouple lignocellulosic constituents as decay is initiated. This complementary coordination of gene regulation may also involve genes of unknown function, particularly when gene products include secretion signal peptides. These potential early roles for selected CAZys are also complicated by the fact that the proteins would be susceptible to damage by the extracellular reactive oxygen species-generating system, so additional work focused on these CAZys is in order. However, it is notable that synergy between polygalacturonases and oxalic acid is harnessed by bacteria to degrade plant cell walls (e.g., ref. 51), and was proposed by Green et al. (52) to initiate loosening of plant cell walls and facilitate access by brown rot fungi.
The expression patterns we report here support a two-step biodegradative mechanism in P. placenta, and may explain why brown rot is so efficient despite losses of many lignocellulolytic genes. Some of the components involved may show promise in biotechnological or other applied uses. Our results also underscore that it is important to consider how biodegradative components may be spatially segregated during the deconstruction of a complex, recalcitrant, solid substrate such as lignocellulose. The spatially resolved system we used here helps address this problem, and may offer a way to colocalize fungal expression patterns with physical modifications in wood (e.g., increases in porosity) that enable secreted proteins to access their substrates.
Materials and Methods
Colonization of Wood Wafers with P. placenta.
P. placenta dikaryotic strain MAD 698-R (American Type Culture Collection 44394) was maintained on 1.5% (wt/vol) malt extract agar for routine culturing and to inoculate modified American Society for Testing and Materials soil-block microcosms (5). Vertically oriented wood wafers were colonized directionally from a predeveloped P. placenta hyphal mat. At harvest, wafers were sectioned to segregate the temporal progression of wood decay spatially, an approach modified from Schilling et al. (53) (Fig. 1A). In brief, a 1:1:1 mixture of soil, peat, and vermiculite hydrated to 40–45% (wt/vol) moisture content was packed into pint glass jars to one-third full. Feeder strips on the media surface were inoculated with 1-cm diameter agar plugs from P. placenta cultures and allowed to form a confluent mycelial lawn over 2–3 wk. Wood wafers were cut into 60 × 25 × 2.5-mm sections, with the largest face being the cross-section and with the tangential plane in contact with the mycelium when propped in jars. Cultures were incubated at room temperature for 3–4 wk until the visible hyphal front had advanced ∼50 mm up the wafers. Wafers with flat hyphal fronts (horizontal lines) were then harvested, scraped free of surface hyphae, and sectioned into 5-mm sections using a razor blade sterilized with each cut, with the first cut along the hyphal front. Distance from the hyphal front was used to delineate sections on wafers (Fig. 1A) for analyses per section in triplicate.
RNA Isolation and RNA-Seq Analysis.
Total RNA for P. placenta was isolated from each wood section with TRIzol (Life Technologies) and then cleaned up with a Qiagen RNeasy Mini Kit (Qiagen, Inc.) to remove organic inhibitors before downstream RNA-seq or quantitative RT-PCR (qRT-PCR). For RNA-seq, TruSeq RNA v2 libraries were prepared and sequenced on a HiSeq 2500 System (Illumina, Inc.) by using the standard protocols from Illumina. Sequenced reads were mapped against the genome of P. placenta MAD 698-R (v1.0) with Tophat (v2.0.13) in the Galaxy platform (Table S3). By using the reference transcript models in the JGI database, expression levels (FPKM) and significances of differences were estimated with Cuffdiff (Galaxy Tool Version 2.2.1.3) using geometric normalization. The FDR was set as <0.05. The profiling data for whole-genome transcripts and DEGs are available in Dataset S1. Hierarchical clustering and GO enrichment of the DEGs were analyzed by HCE (v3.0) and Blast2Go (v3.2.7), respectively.
The expression levels of relevant LOX and GH genes were also analyzed via qRT-PCR along the advancing hyphal front in spruce wafers (n = 3) (Table S4). More detail is available in SI Materials and Methods about RNA extraction, RNA-seq, and qRT-PCR.
Protein Extraction, Enzyme Activity Assays, and Western Blotting.
Sections at set distances from the hyphal front were pooled from wafers for protein extraction in 0.05 M citrate buffer (pH 5.0) for 24 h with stirring at 4 °C. The extracts were filtered through miracloth and 0.2-μm filters, and protein concentration was then determined using the Bio-Rad Protein Assay Kit (Bio-Rad). Activities of endoglucanase, xylanase, and mannanase were measured with dinitrosalicylic acid reagent using, respectively, three model substrates: 0.5% carboxymethyl cellulose, 0.5% birchwood xylan, and 0.25% locust bean gum (glucomannan), according to Ghose (54). β-Glucosidase, β-xylosidase, β-mannosidase, and β-arabinofuranosidase activities were measured by using 10 mM 4-nitrophenyl-β-d-glucopyranoside, 5 mM 4-nitrophenyl-β-d-xylopyranoside, 10 mM 4-nitrophenyl-β-d-mannopyranoside, and 1 mg⋅mL−1 4-nitrophenyl-α-l-arabinofuranoside, respectively. Laccase activities of the crude extracts were measured with 0.5 mM ABTS as substrate (55). According to Aro et al. (56), fungal biomass in wood wafers was approximated by determining the 0.05 M NaOH-extractable proteins in different sections that had been ground in liquid N2. The physical presence of endoglucanase Cel5B, a well-characterized GH of P. placenta, was also measured in the protein extracts using Western blotting (details are provided in SI Materials and Methods).
Supplementary Material
Acknowledgments
This work was supported by the US Department of Energy Office of Science [Early Career Grant DE-SC0004012 from the Office of Biological and Ecological Research (BER) to J.S.S.; BER Grant DE-SC0012742 to J.S.S., K.E.H., M.F., and J.Z.]. Confocal microscopy was funded by User Facility Grant 48607 at the Environmental Molecular Sciences Laboratory of Pacific Northwest National Laboratory (to J.S.S., J.Z., and G.N.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE84529).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608454113/-/DCSupplemental.
References
- 1.Mester T, Varela E, Tien M. 2004. Wood degradation by brown-rot and white-rot fungi. The Mycota II, Genetics and Biotechnology (Springer-Verlag Berlin Heidelberg, New York), ed Kück U, 2nd Ed, pp 355–368.
- 2.Goodell B, Qian Y, Jellison J. 2008. Fungal decay of wood: Soft rot-brown rot-white rot. Development of Commercial Wood Preservatives, ACS Symposium Series, eds Schultz T, et al. (American Chemical Society, Washington, DC), pp 9–31.
- 3.Schilling JS, et al. Lignocellulose modifications by brown rot fungi and their effects, as pretreatments, on cellulolysis. Bioresour Technol. 2012;116:147–154. doi: 10.1016/j.biortech.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Cowling EB. 1961. Comparative Biochemistry of the Decay of Sweetgum Sapwood by White-Rot and Brown-Rot Fungi, Technical Bulletin No 1258 (US Department of Agriculture, Washington, DC)
- 5.ASTM International . ASTM Standard D1413-07e1. Standard Test Method for Wood Preservatives by Laboratory Soil-Block Cultures. ASTM International; West Conshohocken, PA: 2007. [Google Scholar]
- 6.Kleman-Leyer K, Agosin E, Conner AH, Kirk TK. Changes in the molecular size distribution of cellulose during attack by white-rot and brown-rot fungi. Appl Environ Microbiol. 1992;58(4):1266–1270. doi: 10.1128/aem.58.4.1266-1270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol. 2006;8(12):2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- 8.Schilling JS, Kaffenberger JT, Liew FJ, Song Z. Signature wood modifications reveal decomposer community history. PLoS One. 2015;10(3):e0120679. doi: 10.1371/journal.pone.0120679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez D, et al. Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci USA. 2009;106(6):1954–1959. doi: 10.1073/pnas.0809575106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floudas D, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336(6089):1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 11.Hori C, et al. Genomewide analysis of polysaccharides degrading enzymes in 11 white- and brown-rot Polyporales provides insight into mechanisms of wood decay. Mycologia. 2013;105(6):1412–1427. doi: 10.3852/13-072. [DOI] [PubMed] [Google Scholar]
- 12.Riley R, et al. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc Natl Acad Sci USA. 2014;111(27):9923–9928. doi: 10.1073/pnas.1400592111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammel KE, Kapich AN, Jensen KA, Ryan ZC. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol. 2002;30(4):446–453. [Google Scholar]
- 14.Koenigs JW. Hydrogen peroxide and iron: A proposed system for decomposition of wood by brown-rot basidiomycetes. Wood Fiber. 1974;6(1):66–79. [Google Scholar]
- 15.Kerem Z, Bao W, Hammel KE. Rapid polyether cleavage via extracellular one-electron oxidation by a brown-rot basidiomycete. Proc Natl Acad Sci USA. 1998;95(18):10373–10377. doi: 10.1073/pnas.95.18.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen KA, Jr, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular Fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol. 2001;67(6):2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer C, Kreisel G, Fahr K, Kässbohrer J, Schlosser D. Degradation of 2-fluorophenol by the brown-rot fungus Gloeophyllum striatum: Evidence for the involvement of extracellular Fenton chemistry. Appl Microbiol Biotechnol. 2004;64(3):387–395. doi: 10.1007/s00253-003-1445-x. [DOI] [PubMed] [Google Scholar]
- 18.Rätto M, Ritschkoff AC, Viikari L. The effect of oxidative pretreatment on cellulose degradation by Poria placenta and Trichoderma reesei cellulases. Appl Microbiol Biotechnol. 1997;48(1):53–57. [Google Scholar]
- 19.Filley TR, et al. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown-rot fungi. Org Geochem. 2002;33(2):111–124. [Google Scholar]
- 20.Yelle DJ, Wei D, Ralph J, Hammel KE. Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia placenta. Environ Microbiol. 2011;13(4):1091–1100. doi: 10.1111/j.1462-2920.2010.02417.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaffenberger JT, Schilling JS. Comparing lignocellulose physiochemistry after decomposition by brown rot fungi with distinct evolutionary origins. Environ Microbiol. 2015;17(12):4885–4897. doi: 10.1111/1462-2920.12615. [DOI] [PubMed] [Google Scholar]
- 22.Hyde SM, Wood PM. A mechanism for production of hydroxyl radicals by the brown-rot fungus Coniophora puteana: Fe(III) reduction by cellobiose dehydrogenase and Fe(II) oxidation at a distance from the hyphae. Microbiology. 1997;143(1):259–266. doi: 10.1099/00221287-143-1-259. [DOI] [PubMed] [Google Scholar]
- 23.Hunt CG, et al. Spatial mapping of extracellular oxidant production by a white rot basidiomycete on wood reveals details of ligninolytic mechanism. Environ Microbiol. 2013;15(3):956–966. doi: 10.1111/1462-2920.12039. [DOI] [PubMed] [Google Scholar]
- 24.Wei D, et al. Laccase and its role in production of extracellular reactive oxygen species during wood decay by the brown rot basidiomycete Postia placenta. Appl Environ Microbiol. 2010;76(7):2091–2097. doi: 10.1128/AEM.02929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korripally P, Timokhin VI, Houtman CJ, Mozuch MD, Hammel KE. Evidence from Serpula lacrymans that 2,5-dimethoxyhydroquinone is a lignocellulolytic agent of divergent brown rot basidiomycetes. Appl Environ Microbiol. 2013;79(7):2377–2383. doi: 10.1128/AEM.03880-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanden Wymelenberg A, et al. Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl Environ Microbiol. 2010;76(11):3599–3610. doi: 10.1128/AEM.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Highley TL. Influence of carbon source on cellulase activity of white-rot and brown-rot fungi. Wood Fiber. 1973;5(1):50–58. [Google Scholar]
- 28.Ide M, Ichinose H, Wariishi H. Molecular identification and functional characterization of cytochrome P450 monooxygenases from the brown-rot basidiomycete Postia placenta. Arch Microbiol. 2012;194(4):243–253. doi: 10.1007/s00203-011-0753-2. [DOI] [PubMed] [Google Scholar]
- 29.Ryu JS, et al. Proteomic and functional analysis of the cellulase system expressed by Postia placenta during brown rot of solid wood. Appl Environ Microbiol. 2011;77(22):7933–7941. doi: 10.1128/AEM.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wösten HAB, Moukha SM, Sietsma JH, Wessels JGH. Localization of growth and secretion of proteins in Aspergillus niger. J Gen Microbiol. 1991;137(8):2017–2023. doi: 10.1099/00221287-137-8-2017. [DOI] [PubMed] [Google Scholar]
- 31.Wessels JGH. Wall growth, protein excretion, and morphogenesis in fungi. New Phytol. 1993;123(3):397–413. doi: 10.1111/j.1469-8137.1993.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 32.de Bekker C, Bruning O, Jonker MJ, Breit TM, Wösten HA. Single cell transcriptomics of neighboring hyphae of Aspergillus niger. Genome Biol. 2011;12(8):R71. doi: 10.1186/gb-2011-12-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa Y, Ishikawa E, Shoji JY, Nakano H, Kitamoto K. Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol Microbiol. 2011;81(1):40–55. doi: 10.1111/j.1365-2958.2011.07700.x. [DOI] [PubMed] [Google Scholar]
- 34.Moukha SM, Wösten HAB, Asther M, Wessels JG. In situ localization of the secretion of lignin peroxidases in colonies of Phanerochaete chrysosporium using a sandwiched mode of culture. J Gen Microbiol. 1993;139(5):969–978. doi: 10.1099/00221287-139-5-969. [DOI] [PubMed] [Google Scholar]
- 35.Baldrian P, Valásková V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev. 2008;32(3):501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 36.Korripally P, et al. Regulation of gene expression during the onset of ligninolytic oxidation by Phanerochaete chrysosporium on spruce wood. Appl Environ Microbiol. 2015;81(22):7802–7812. doi: 10.1128/AEM.02064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe T, Tsuda S, Nishimura H, Honda Y, Watanabe T. Characterization of a Δ12-fatty acid desaturase gene from Ceriporiopsis subvermispora, a selective lignin-degrading fungus. Appl Microbiol Biotechnol. 2010;87(1):215–224. doi: 10.1007/s00253-010-2438-1. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Fueyo E, et al. Comparative genomics of Ceriporiopsis subvermispora and Phanerochaete chrysosporium provide insight into selective ligninolysis. Proc Natl Acad Sci USA. 2012;109(14):5458–5463. doi: 10.1073/pnas.1119912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yelle DJ, Ralph J, Lu F, Hammel KE. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol. 2008;10(7):1844–1849. doi: 10.1111/j.1462-2920.2008.01605.x. [DOI] [PubMed] [Google Scholar]
- 40.Flournoy DS, Kirk TK, Highley TL. Wood decay by brown-rot fungi: Changes in pore structure and cell wall volume. Holzforschung. 1991;45(5):383–388. [Google Scholar]
- 41.Curling SF, Clausen CA, Winandy JE. Relationships between mechanical properties, weight loss, and chemical composition of wood during incipient brown-rot decay. Forest Products Journal. 2002;52(7):34–39. [Google Scholar]
- 42.Mewis K, Lenfant N, Lombard V, Henrissat B. Dividing the large glycoside hydrolase family 43 into subfamilies: A motivation for detailed enzyme characterization. Appl Environ Microbiol. 2016;82(6):1686–1692. doi: 10.1128/AEM.03453-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu N, et al. Comparative analysis of the secretomes of Schizophyllum commune and other wood-decay basidiomycetes during solid-state fermentation reveals its unique lignocellulose-degrading enzyme system. Biotechnol Biofuels. 2016;9:42. doi: 10.1186/s13068-016-0461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Floudas D, et al. Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genet Biol. 2015;76:78–92. doi: 10.1016/j.fgb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thuillier A, et al. Transcriptomic responses of Phanerochaete chrysosporium to oak acetonic extracts: Focus on a new glutathione transferase. Appl Environ Microbiol. 2014;80(20):6316–6327. doi: 10.1128/AEM.02103-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman D, Kowbel DJ, Glass NL, Yarden O, Hadar Y. Detoxification of 5-hydroxymethylfurfural by the Pleurotus ostreatus lignolytic enzymes aryl alcohol oxidase and dehydrogenase. Biotechnol Biofuels. 2015;8:63. doi: 10.1186/s13068-015-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutiérrez A, Caramelo L, Prieto A, Martínez MJ, Martínez AT. Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus. Appl Environ Microbiol. 1994;60(6):1783–1788. doi: 10.1128/aem.60.6.1783-1788.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernández-Ortega A, Ferreira P, Martínez AT. Fungal aryl-alcohol oxidase: A peroxide-producing flavoenzyme involved in lignin degradation. Appl Microbiol Biotechnol. 2012;93(4):1395–1410. doi: 10.1007/s00253-011-3836-8. [DOI] [PubMed] [Google Scholar]
- 49.McQueen-Mason S, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quiroz-Castañeda RE, Martínez-Anaya C, Cuervo-Soto LI, Segovia L, Folch-Mallol JL. Loosenin, a novel protein with cellulose-disrupting activity from Bjerkandera adusta. Microb Cell Fact. 2011;10:8. doi: 10.1186/1475-2859-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amadioha AC. A synergism between oxalic acid and polygalacturonases in the depolymerization of potato tuber tissue. World J Microbiol Biotechnol. 1993;9(5):599–600. doi: 10.1007/BF00386304. [DOI] [PubMed] [Google Scholar]
- 52.Green F, 3rd, Clausen CA, Kuster TA, Highley TL. Induction of polygalacturonase and the formation of oxalic acid by pectin in brown-rot fungi. World J Microbiol Biotechnol. 1995;11(5):519–524. doi: 10.1007/BF00286366. [DOI] [PubMed] [Google Scholar]
- 53.Schilling JS, Duncan SM, Presley GN, Jurgens JA, Blanchette RA. Colocalizing incipient reactions in wood degraded by the brown rot fungus Postia placenta. Int Biodeterior Biodegradation. 2013;83:56–62. [Google Scholar]
- 54.Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59(2):257–268. [Google Scholar]
- 55.Zhang J, et al. Improved biomass saccharification by Trichoderma reesei through heterologous expression of lacA gene from Trametes sp. AH28-2. J Biosci Bioeng. 2012;113(6):697–703. doi: 10.1016/j.jbiosc.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Aro N, Ilmén M, Saloheimo A, Penttilä M. ACEI of Trichoderma reesei is a repressor of cellulase and xylanase expression. Appl Environ Microbiol. 2003;69(1):56–65. doi: 10.1128/AEM.69.1.56-65.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels. 2013;6(1):41. doi: 10.1186/1754-6834-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(Database issue) D1:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salame TM, Knop D, Levinson D, Yarden O, Hadar Y. Redundancy among manganese peroxidases in Pleurotus ostreatus. Appl Environ Microbiol. 2013;79(7):2405–2415. doi: 10.1128/AEM.03849-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Renart J, Reiser J, Stark GR. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: A method for studying antibody specificity and antigen structure. Proc Natl Acad Sci USA. 1979;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song ZW, Vailc A, Sadowsky MJ, Schilling JS. Quantitative PCR for measuring biomass of decomposer fungi in planta. Fungal Ecol. 2014;7(1):39–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.