Significance
Plant endogenous molecules, such as the Arabidopsis thaliana elicitor peptides (AtPeps), activate defense responses by means of cell surface-located receptors that serve as an excellent model to study the implications of endomembrane trafficking in plant immunity. Here we used fluorescently labeled and bioactive pep1 to probe in vivo the intracellular dynamics and the fate of the active receptor-ligand complexes in the Arabidopsis root meristem. We show that AtPep1 internalization depends on its receptors and that clathrin-mediated endocytosis is essential for AtPep1-induced responses.
Keywords: Arabidopsis, clathrin, endogenous peptides, PEPR, endocytosis
Abstract
The Arabidopsis thaliana endogenous elicitor peptides (AtPeps) are released into the apoplast after cellular damage caused by pathogens or wounding to induce innate immunity by direct binding to the membrane-localized leucine-rich repeat receptor kinases, PEP RECEPTOR1 (PEPR1) and PEPR2. Although the PEPR-mediated signaling components and responses have been studied extensively, the contributions of the subcellular localization and dynamics of the active PEPRs remain largely unknown. We used live-cell imaging of the fluorescently labeled and bioactive pep1 to visualize the intracellular behavior of the PEPRs in the Arabidopsis root meristem. We found that AtPep1 decorated the plasma membrane (PM) in a receptor-dependent manner and cointernalized with PEPRs. Trafficking of the AtPep1-PEPR1 complexes to the vacuole required neither the trans-Golgi network/early endosome (TGN/EE)-localized vacuolar H+-ATPase activity nor the function of the brefeldin A-sensitive ADP-ribosylation factor-guanine exchange factors (ARF-GEFs). In addition, AtPep1 and different TGN/EE markers colocalized only rarely, implying that the intracellular route of this receptor–ligand pair is largely independent of the TGN/EE. Inducible overexpression of the Arabidopsis clathrin coat disassembly factor, Auxilin2, which inhibits clathrin-mediated endocytosis (CME), impaired the AtPep1-PEPR1 internalization and compromised AtPep1-mediated responses. Our results show that clathrin function at the PM is required to induce plant defense responses, likely through CME of cell surface-located signaling components.
Danger- or damage-associated molecular patterns (DAMPs) are diverse endogenous molecules that originate from the host to activate immune responses following perception (1). In Arabidopsis, the endogenous elicitor peptides, AtPeps, are characterized as DAMPs because they are able to trigger defense responses reminiscent of those induced in pattern-triggered immunity (2, 3). AtPeps form a family of eight members that mature from their precursor proteins PROPEP1 to PROPEP8. Because AtPeps are induced by pathogen-associated molecular patterns and can induce their own transcription, they are also considered defense response amplifiers (4). All AtPeps are perceived by two homologous leucine-rich repeat (LRR) pattern recognition receptors, PEP RECEPTOR1 (PEPR1) and PEPR2, but early studies implicated PEPR1 as the primary receptor for AtPep1 (2, 4, 5). AtPep1 is a 23-aa peptide derived from the C terminus of a 92-aa precursor protein, AtPROPEP1 (3, 6). The 10 C-terminal amino acids of AtPep1 bind the PEPR1-LRR domain and trigger interaction between PEPR1 and its coreceptor, BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 (BAK1) (5). Despite the ever-growing number of plant signaling peptides, their intracellular dynamics have not been reported to date. PEPRs are structurally similar to the plasma membrane (PM)-localized receptor kinases FLAGELLIN SENSING2 (FLS2) and BRASSINOSTEROID INSENSITIVE1 (BRI1), which undergo endocytosis both independently and dependently of their ligands, the bacterial peptide flagellin 22 (flg22) and the brassinosteroid (BR) hormone, respectively (7, 8).
Endocytosis is proposed to function as a spatial and temporal modulator of the outcome of receptor-mediated responses by regulating the amounts of the respective receptors and ligands at the PM and their localization into the cell (9). In plants, clathrin-mediated endocytosis (CME) is the major internalization route (10). Functional studies of mutants in the clathrin machinery support the conserved mechanism of CME in plants and its importance for growth, development, and plant defense responses (11, 12), although, in general, the role of CME in plant immunity has not been addressed completely.
Here we used live-cell imaging of the fluorescently labeled pep1 (TAMRA-pep1) to study the contributions of the subcellular localization and dynamics of the active PEPRs to AtPep1 responses. We found that AtPep1-PEPR1 complexes are internalized via CME and transported to the vacuole via a trans-Golgi network/early endosome (TGN/EE)-independent pathway. We show that impaired clathrin function compromised the AtPep1 responses, indicating that clathrin plays a positive role in danger-associated peptide signaling in Arabidopsis.
Results
AtPep1 Labeled the PM of Arabidopsis Root Meristem Cells in a Receptor-Dependent Manner.
To simultaneously monitor the localization of the AtPep1-bound and presumably active PEPRs in living cells, we synthesized a fluorescently labeled AtPep1, designated TAMRA-pep1. When applied to plants, TAMRA-pep1 was stable (Fig. S1) and biologically active at nanomolar concentrations, as evaluated by different readouts for AtPep1 responses (Fig. S2). The localization of the labeled peptide was studied in epidermal cells of the root meristem of 5-d-old wild type (WT) Arabidopsis seedlings incubated with 100 nM TAMRA-pep1 for different time points and visualized immediately after three washouts (Fig. S3A). Incubation with TAMRA-pep1 for 60 s was sufficient to label all cells in the root meristem (Fig. 1A); however, previous promoter-β-glucoronidase (GUS) reporter studies of the PEPR1 and PEPR2 genes in Arabidopsis have shown that the two receptors are expressed in leaves and in the root differentiation zone, but not in the root meristem (6), in agreement with our results (Fig. S4A).
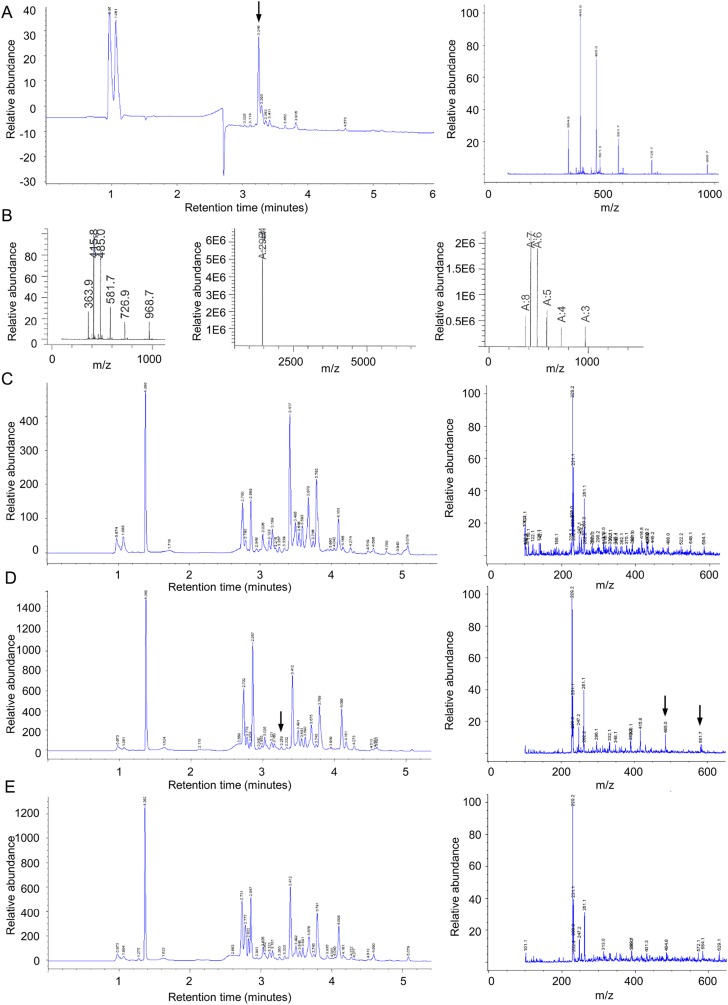
Fig. S1.
Identification of TAMRA-pep1 by LC-MS in plant tissues. (A) TAMRA-pep1 was first identified in the medium at a final concentration of 50 µM. (Left) Chromatogram at 254 nm showing the tagged peptide eluting at 3.25 min. (Right) LC-MS peak chromatogram of the fraction at 3.26 min. (B) The correct expected molecular weight of the TAMRA-pep1 (2,904) was assessed by a deconvolution procedure. (C–E) Chromatogram at 254 nm (Left) and LC-MS peak chromatogram at 3.26 min (Right) of pRPS5A::PEPR1-GFP–expressing root extracts not incubated with TAMRA-pep1 (mock control) (C) or incubated with the peptide (50 µM) for 1 h (D) or 2 h (E). Note that TAMRA-pep1 was not detected after 2 h. Arrows indicate TAMRA-pep1–specific peaks.
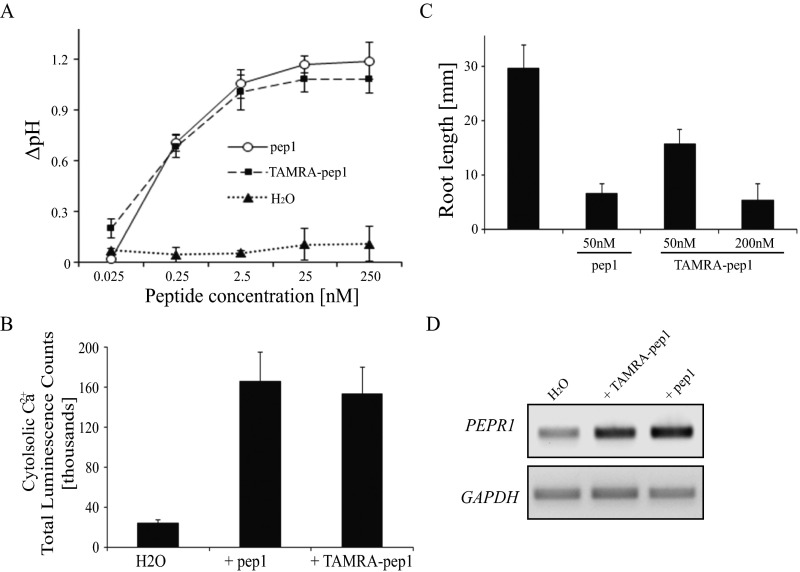
Fig. S2.
Biologically active TAMRA-pep1. (A) Measurement of the pH of Arabidopsis cell suspensions after 20 min of exposure to different concentrations of pep1 or TAMRA-pep1 (n = 9). (B) Total luminescence of aquorin-expressing Arabidopsis plants subjected to the aquaporin Ca2+ assay and treated with 10 nM pep1 or TAMRA-pep1 (n = 9). (C) Root growth inhibition of Col-0 in the presence of pep1 or TAMRA-pep1 (n = 10). (D) RT-PCR analysis of PEPR1 gene in 6-d-old Arabidopsis seedlings treated for 60 min with 100 nM pep1 or TAMRA-pep1. GAPDH (At1g13440) and water served as loading control and control, respectively. These experiments were repeated at least twice, with similar results. Error bars indicate SD. n, number of wells in A and number of seedlings in B and C.
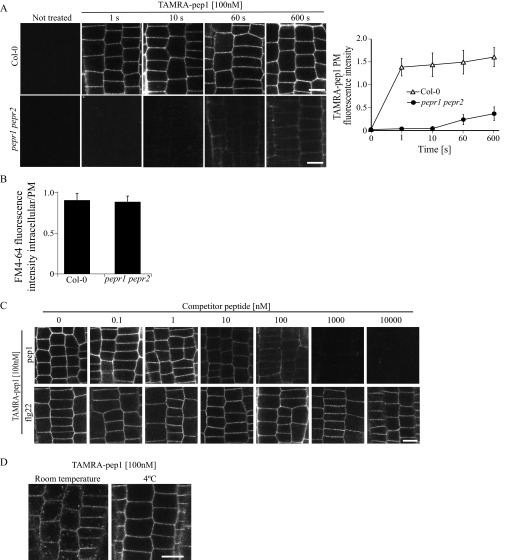
Fig. S3.
PM labeling of TAMRA-pep1. (A) PM of Arabidopsis WT (Col-0) and pepr1perp2 double-mutant root meristem epidermal cells labeled within seconds after application by TAMRA-pep1. The 5-d-old seedlings were treated with TAMRA-pep1 (100 nM) for different time points, washed three times, and then imaged. (Right) Quantification of fluorescence intensity in the PM (n = 48). Error bars indicate SD. (B) Quantification of the FM4-64 signal intensity in Col-0 and pepr1perp2 double mutant. Values represent the intracellular/PM signal intensity ratio in root meristem epidermal cells of 5-d-old seedlings treated with FM4-64 (4 µM, 5 min, three washouts) and kept in the presence of 100 nM pep1 for another 90 min (n >32). Error bars indicate SEM. No statistically significant difference (*P ≤ 0.01, Student’s t test) was found. (C) pep1, but not flg22, is able to compete with TAMRA-pep1. Five-day-old Arabidopsis seedlings were incubated with various concentrations of pep1 or flg22 for 5 min, then treated with TAMRA-pep1 for 10 s, washed, and imaged. The fluorescence intensity of the PM is quantified in Fig. 1D. (D) Temperature-dependent internalization of TAMRA-pep1. Five-day-old Arabidopsis seedlings were incubated at 4 °C for 2 h before treatment with TAMRA-pep1 (100 nM, 10 s), washed, and imaged after incubation for 40 min at 4 °C. The same TAMRA-pep1 treatment was done with seedlings at room temperature. n, number of cells analyzed. (Scale bars: 10 µM.)
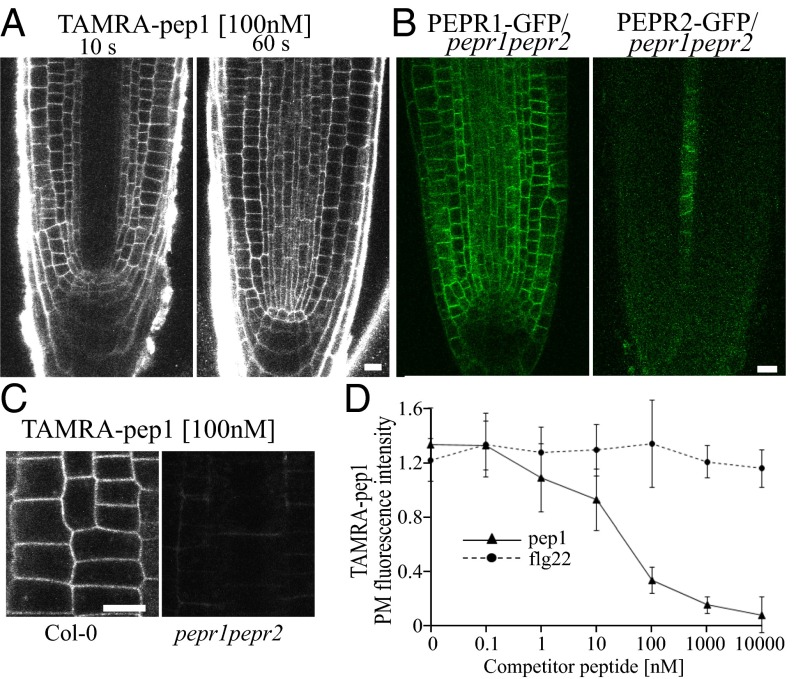
Fig. 1.
PEPR-dependent PM labeling of TAMRA-pep1. (A) Root tips of Arabidopsis Col-0 seedlings treated with TAMRA-pep1 (100 nM, for 10 s and 60 s) and imaged after three washouts. (B) Localization of PEPR1-GFP and PEPR2-GFP expressed under endogenous promoters and complementing the pepr1pepr2 double mutant. (C) Unlabeled PM of the pepr1perp2 double mutant by TAMRA-pep1. Root epidermal cells were treated with TAMRA-pep1 for 60 s and imaged as in A. (D) Quantification of TAMRA-pep1 PM fluorescence intensity in root epidermal cells (n = 48) incubated with different concentrations of pep1 or flg22 for 5 min, then treated with TAMRA-pep1 (100 nM, 10 s) and imaged after three washouts (Fig. S3C). All experiments were performed using 5-d-old seedlings. Error bars indicate SD. n, number of cells. (Scale bars: 10 µm.)
Fig. S4.
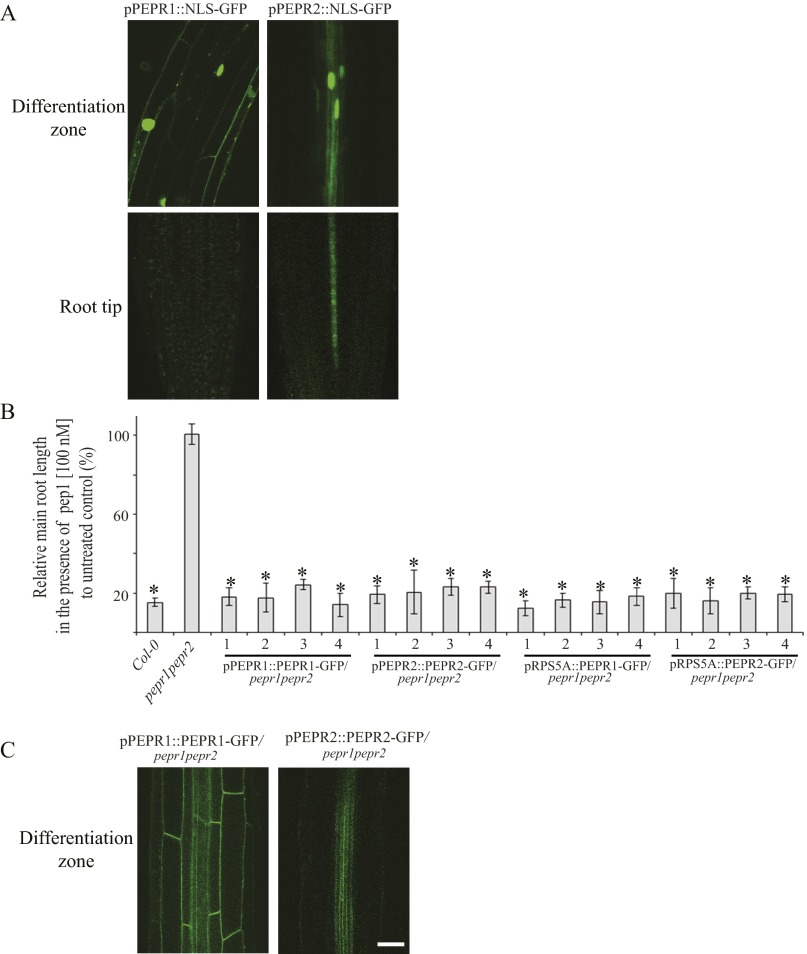
Expression patterns and subcellular localization of PEPR1 and PEPR2 in the Arabidopsis roots. (A) pPEPR1::NLS-GFP and pPEPR2::NLS-GFP expression in the differentiation zone (Top) and meristem (Bottom) of the roots of 5-d-old Arabidopsis seedlings. (B) PEPR1 and PEPR2 fused to GFP and expressed under their endogenous promoters or under the RPS5A promoter complemented with the pepr1pepr2 double mutant. Root growth inhibition analysis of Arabidopsis Col-0, pepr1pepr2, pPEPR1::PEPR1-GFP/pepr1pepr2, pPEPR2::PEPR2-GFP/pepr1pepr2, pRPS5A::PEPR1-GFP/pepr1pepr2, and pRPS5A::PEPR2-GFP/pepr1pepr2 seedlings (n = 10). Seedlings were germinated on 1/2MS medium and then transferred for 4 d to 1/2MS medium supplemented with pep1 (100 nM). Root growth is presented relative to the untreated control. Four independent transgenic lines were analyzed for each construct. n, number of seedlings analyzed. Error bars indicate SD. *P < 0.001 (Student’s t test) relative to the untreated control. (C) Localization of PEPR1-GFP and PEPR2-GFP expressed under their endogenous promoter in the pepr1pepr2 mutant in the differentiation zone of the roots of 5-d-old Arabidopsis seedlings. (Scale bars: 30 µm.)
Because TAMRA-pep1 efficiently labeled the PM of root meristem cells, we wanted to clarify this discrepancy in localization patterns. Therefore, we expressed the genomic sequences of PEPR1 and PEPR2 fused to GFP under their native promoters into the pepr1pepr2 double mutant and evaluated their expression patterns in the root meristem. The two chimeric proteins, PEPR1-GFP and PEPR2-GFP, were functional, because they complemented the pepr1pepr2 double mutant (Fig. S4B). Whereas the expression pattern of the PEPR2-GFP protein was similar to that of the pPEPR2::NLS-GFP fusion, the PEPR1-GFP signal was detected in root cells of the differentiation zone and also in the root meristem, in correlation with the TAMRA-pep1 localization (Fig. 1B and Fig. S4C).
The PM labeling by TAMRA-pep1 in the root meristem depended on the receptors, as demonstrated by the finding that root cells of the pepr1pepr2 double mutant lacked fluorescent labeling despite functional endocytosis (Fig. 1C and Fig. S3 A and B). This observation was further supported by a competition experiment in which pretreatment with a 10-fold excess of unlabeled pep1 prevented PM labeling by TAMRA-pep1, whereas labeling was unaffected by pretreatment with flg22, known to bind and activate the LRR receptor kinase FLS2 (7) (Fig. 1D and Fig. S3C).
Receptor-Mediated Endocytosis of AtPep1.
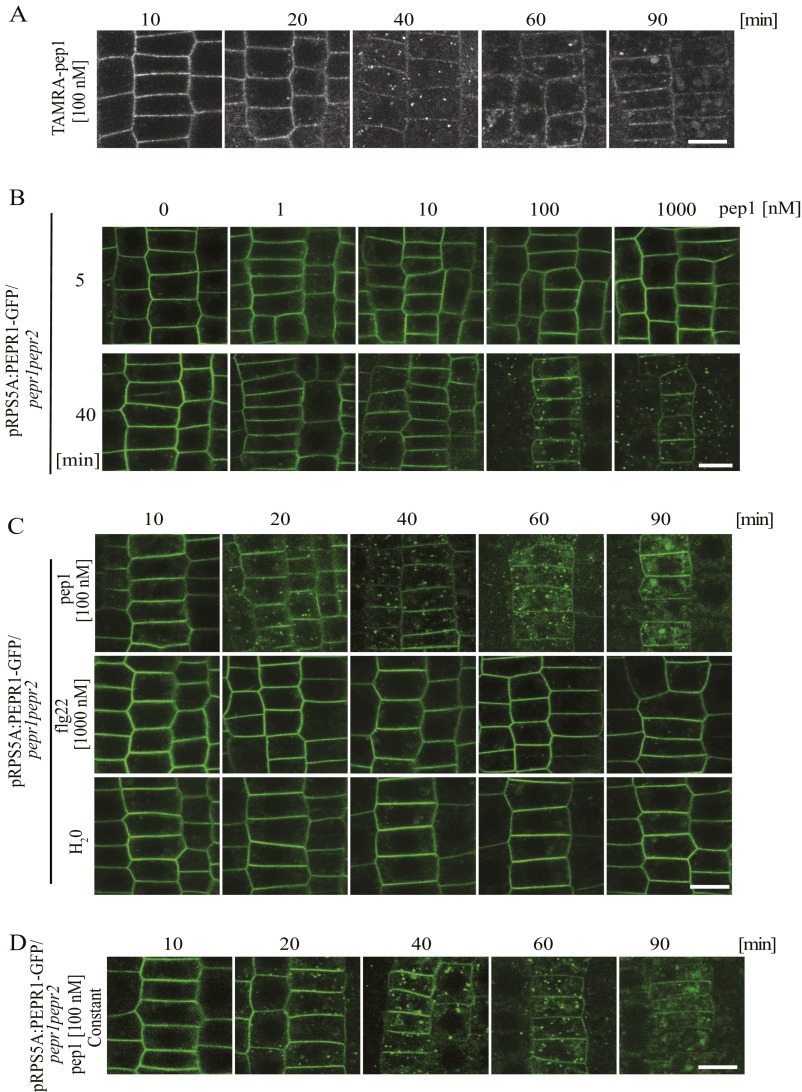
To monitor the behavior of the bioactive fluorescently labeled pep1 over time, Arabidopsis seedlings were pulsed with TAMRA-pep1 (100 nM, 10 s), washed, and imaged at different time points (referred to as chases) (Fig. S5A). After a 10-min chase, most of the TAMRA fluorescence was associated with the PM. Some puncta were visible inside the cell after a 20-min chase and became more evident after a 40-min chase. Later, after a 90-min chase, TAMRA-pep1 was concentrated into structures that resembled vacuoles likely undergoing degradation (Fig. S5A). The TAMRA-pep1 internalization depended on the temperature (Fig. S3D).
Fig. S5.
AtPep1 and PEPR1 internalize with similar dynamics. (A) Internalization of TAMRA-pep1 in root epidermis of 5-d-old Arabidopsis seedlings after incubation with TAMRA-pep1 (100 nM, 10 s), three washouts, and imaging at the indicated time points. Root meristem epidermal cells are shown. (B) PEPR1-GFP internalization was triggered by pep1 in a dose-dependent manner. Seedlings were treated with different concentrations of pep1 for 10 s, washed three times, and imaged after chases of 5 min and 40 min. (C) Internalization of PEPR1-GFP after treatment with pep1 (100 nM, 10 s) as in A. Application of flg22 and mock (water) did not change the PEPR1-GFP localization. (D) PEPR1-GFP internalization on constant elicitation with pep1. The 5-d-old pepr1pepr2 seedlings complemented with PEPR1-GFP expressed from the RPS5A promoter were used in B, C, and D. (Scale bars: 10 µm.)
To investigate whether PEPR1 and PEPR2 are also internalized after stimulation with pep1, we used pepr1pepr2 Arabidopsis plants complemented with pRPS5A::PEPR1-GFP or pRPS5A::PEPR2-GFP to allow robust expression of both receptors in the root meristem cells (Fig. S4B). The subcellular localization of PEPR1-GFP was evaluated after treatment with different pep1 concentrations after 5-min and 40-min chases (Fig. S5B). Although some PEPR1-GFP–labeled intracellular puncta were detected even without pep1 treatment, their presence was induced by pep1 in a time- and dose-dependent manner and was largely colocalized with the endocytic tracer FM4-64 (10) (Fig. S6A).
Fig. S6.
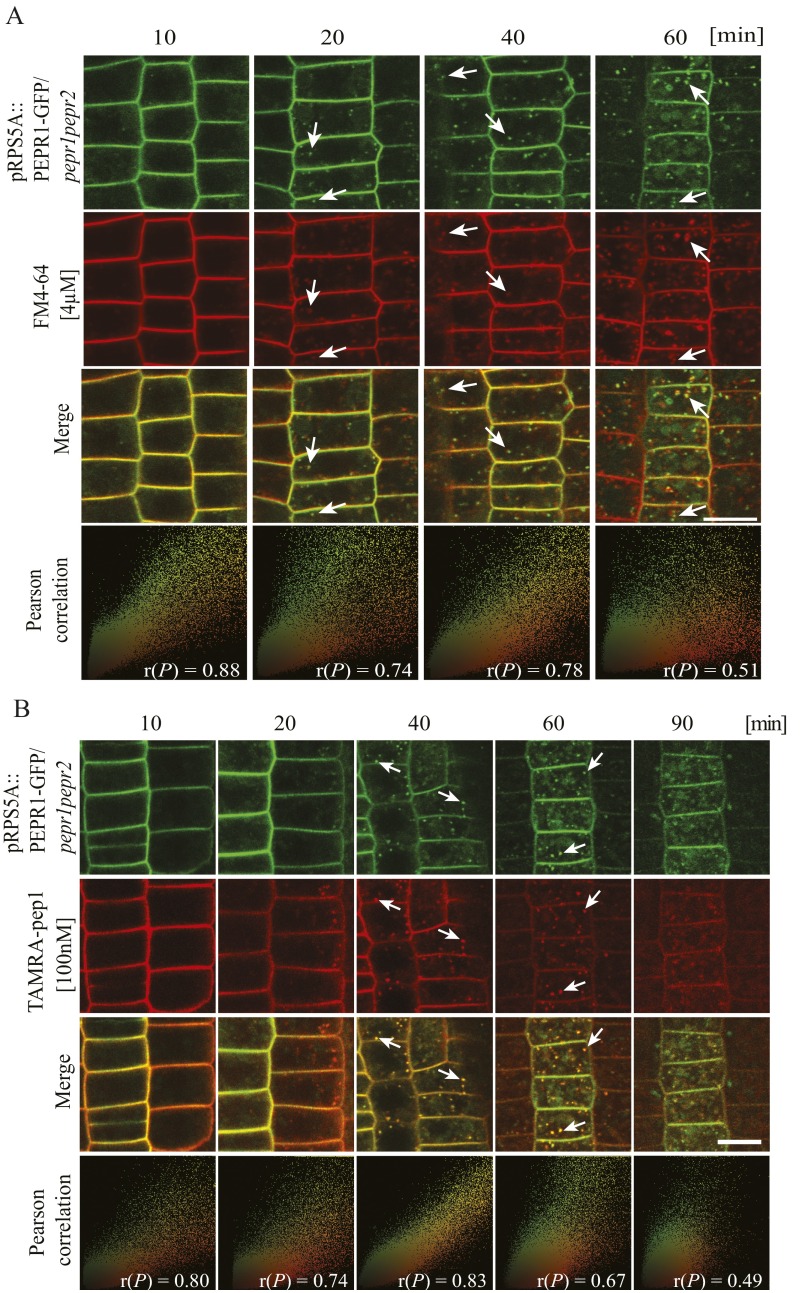
(A) Cointernalization of PEPR1-GFP and FM4-64 after elicitation with pep1. Here 5-d-old pepr1pepr2 seedlings complemented with pRPS5A::PEPR1-GFP construct were stained with FM4-64 (4 µM, 5 min, three washouts), maintained in the presence of pep1 (100 nM), and imaged at the indicated times. (B) Cointernalization of AtPep1 and its receptor PEPR2. pRPS5A::PEPR2-GFP/pepr1pepr2 seedlings as in A were treated with TAMRA-pep1 (100 nM, 10 s), washed three times, and imaged at the indicated times. As a colocalization indicator, the Pearson correlation, r(P), was calculated for merged images. Arrows point to colocalized endosomes. (Scale bar: 10 µm.)
To determine whether the PEPR1 internalization followed similar TAMRA-pep1 dynamics, pRPS5A::PEPR1-GFP/pepr1pepr2 seedlings were pulsed with pep1 (100 nM, 10 s) and imaged over time. Comparable to TAMRA-pep1, the PEPR1-GFP signal was first associated with the PM (10-min chase); later (20-min chase), punctate vesicle-like structures began to appear and became more abundant after a 40-min chase. At later time points (90-min chase), PEPR1-GFP accumulated in the vacuole (Fig. S5C). In contrast, PEPR1-GFP remained localized in the PM after treatment with flg22 (Fig. S5B), suggesting that the PEPR1 internalization is specifically induced by its ligand. The temporal dynamics of the PEPR1-GFP internalization after continuous application of pep1 remained the same as after the 10-s pulse (Fig. S5D).
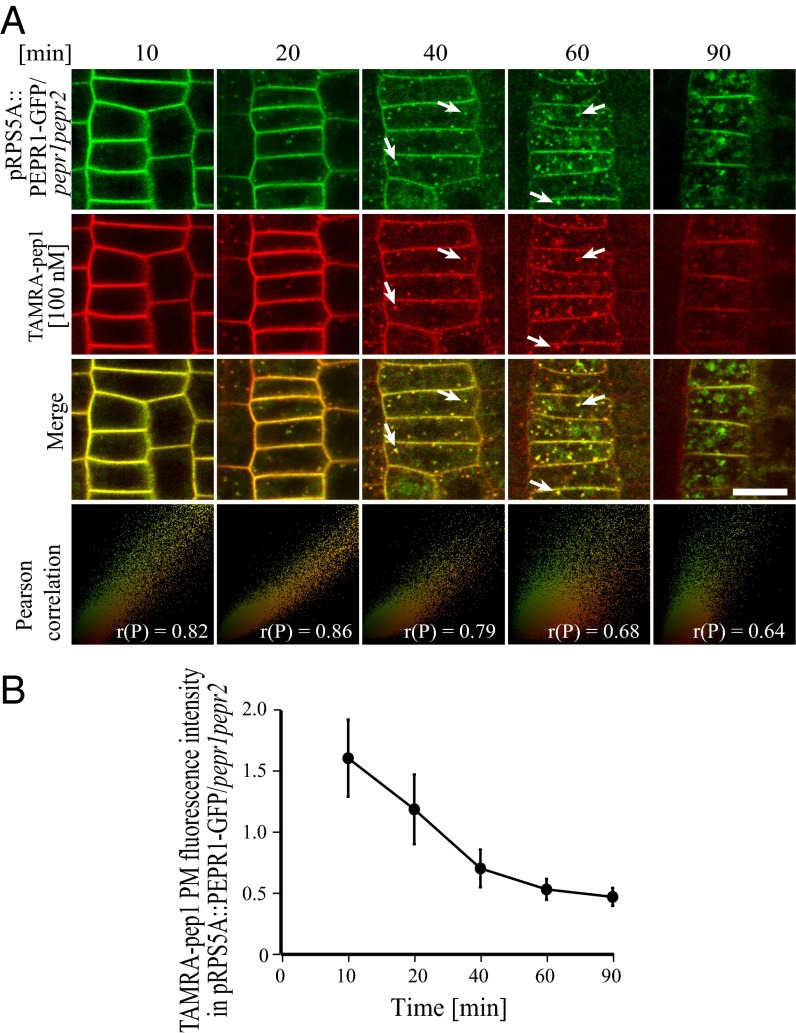
To study the subcellular dynamics of AtPep1-PEPRs, ligand-receptor complexes in root meristem epidermal cells of pRPS5A::PEPR1-GFP/pepr1pepr2 and pRPS5A::PEPR2-GFP/pepr1pepr2 seedlings were pulsed with TAMRA-pep1 (100 nM, 10 s) and imaged after washout. TAMRA-pep1 and PEPR1-GFP and PEPR2-GFP cointernalized with the same temporal dynamics (Fig. 2 and Fig. S6B) as previously shown individually for TAMRA-pep1 and PEPR1-GFP. High Pearson’s correlation coefficient values were obtained when TAMRA-pep1 colocalized with either PEPR1-GFP or PEPR2-GFP; thus, our observations are consistent with the view of a receptor-mediated AtPep1 internalization.
Fig. 2.
Internalization of AtPep1 and its receptor PEPR1. (A) Here 5-d-old pepr1pepr2 seedlings complemented with the pRPS5A::PEPR1-GFP construct were treated with TAMRA-pep1 for 10 s, washed with medium three times, and imaged at the indicated times. As a colocalization indicator, the Pearson correlation (P) was calculated for merged images. White arrows point to colocalized structures. (Scale bars: 10 µm.) (B) Quantification of PEPR1-GFP PM fluorescence intensity in A (n = 27). n, number of cells analyzed. Error bars indicate SD.
The AtPep1-PEPR Trafficking Is Largely Independent of V-ATPase Activity at the TGN/EE.
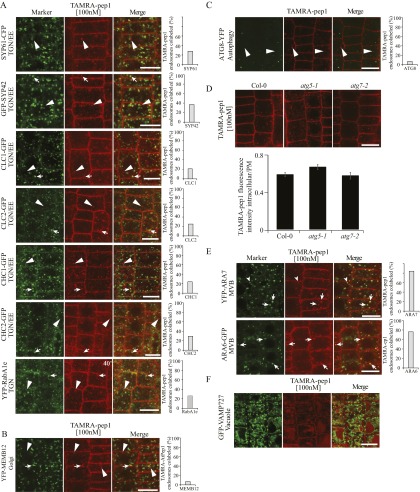
To better understand the endocytic route of PEPR with its ligand, we incubated various fluorescence-tagged endomembrane markers with TAMRA-pep1 and assessed their colocalization after a 40-min chase by counting as positive only the vesicles with colocalization values >0.5 calculated by Pearson’s correlation coefficient. TAMRA-pep1 colocalized only partially (18–37%) with all tested TGN markers, including VHA-a1, SYP61, SYP42, clathrin, and YFP-RabA1e (13–17) (Fig. S7A). TAMRA-pep1 colabeled only 5% of the Golgi SNARE, MEMB12 (17) and 3.4% of the autophagy marker ATG8 (Fig. S7 B and C). In addition, TAMRA-pep1 uptake was not affected in different autophagy mutants (Fig. S7D). In contrast, colocalization increased (76–84%) between TAMRA-pep1 and the multivesicular body (MVB) markers ARA7 and ARA6 (17, 18) (Fig. S7E). Finally, TAMRA-pep1 accumulated in the vacuoles marked by VAMP727 (17) (Fig. S7F). Taken together, the live-cell imaging analyses revealed that TAMRA-pep1 and, presumably, its bound receptors follow an endocytic trafficking route from the PM to the vacuole, passing through late endosomal compartments and, unexpectedly, only partially via the TGN/EEs or endomembranes neighboring it. This observation is in contrast to the BR receptor and its ligand that colabeled >80% of the TGN/EE compartments marked by the VHA-a1-RFP, as shown previously (8).
Fig. S7.
Endocytic route of TAMRA-pep1 in Arabidopsis root epidermal meristem cells. (A) Only partial colocalization of TAMRA-pep1–labeled vesicles with the TGN markers VHA-a1-GFP, SYP61-CFP, GFP-SYP42, CLC1-GFP, CLC2-GFP, CHC1-GFP, CHC2-GFP, and YFP-RabA1e. (B) Lack of colocalization of TAMRA-pep1–labeled vesicles with the Golgi compartments marked by YFP-MEMB12 and with the autophagy compartments marked by ATG8-YFP (C). (D) TAMRA-pep1 internalization was not impaired in the autophagy mutants atg5-1 and atg7-2. (Top) Representative root epidermis images of seedlings treated with TAMRA-pep1 (100 nM, 10 s, three washouts, 40-min chase). (Bottom) Quantification of TAMRA-pep1 uptake. The graph illustrates the intracellular/PM fluorescence intensity ratio (n >45). n, number of analyzed cells. Error bars indicate SEM. (E) Labeling of most TAMRA-pep1 vesicles with the MVB markers YFP-ARA7 and ARA6-GFP. (F) Accumulation of TAMRA-pep1 fluorescence in the vacuoles, delimited by the tonoplast marker GFP-VAMP727. The 5-d-old seedlings were treated with TAMRA-pep1 (100 nM, 10 s) and washed three times with medium. Root tip epidermal cells were imaged after chases of 40 min (A–E) and 90 min (F). The graphs in A, B, C, and E show the percentage of TAMRA-pep1–positive vesicles labeled by the markers (n >300). n, TAMRA-pep1–positive vesicles. Only the TAMRA-pep1–positive vesicles with a colocalization value >0.5 as calculated with the Pearson’s correlation coefficient were considered colocalized. Arrows and arrowheads point colocalized and uncolocalized endosomes, respectively. (Scale bars: 10 µm.)
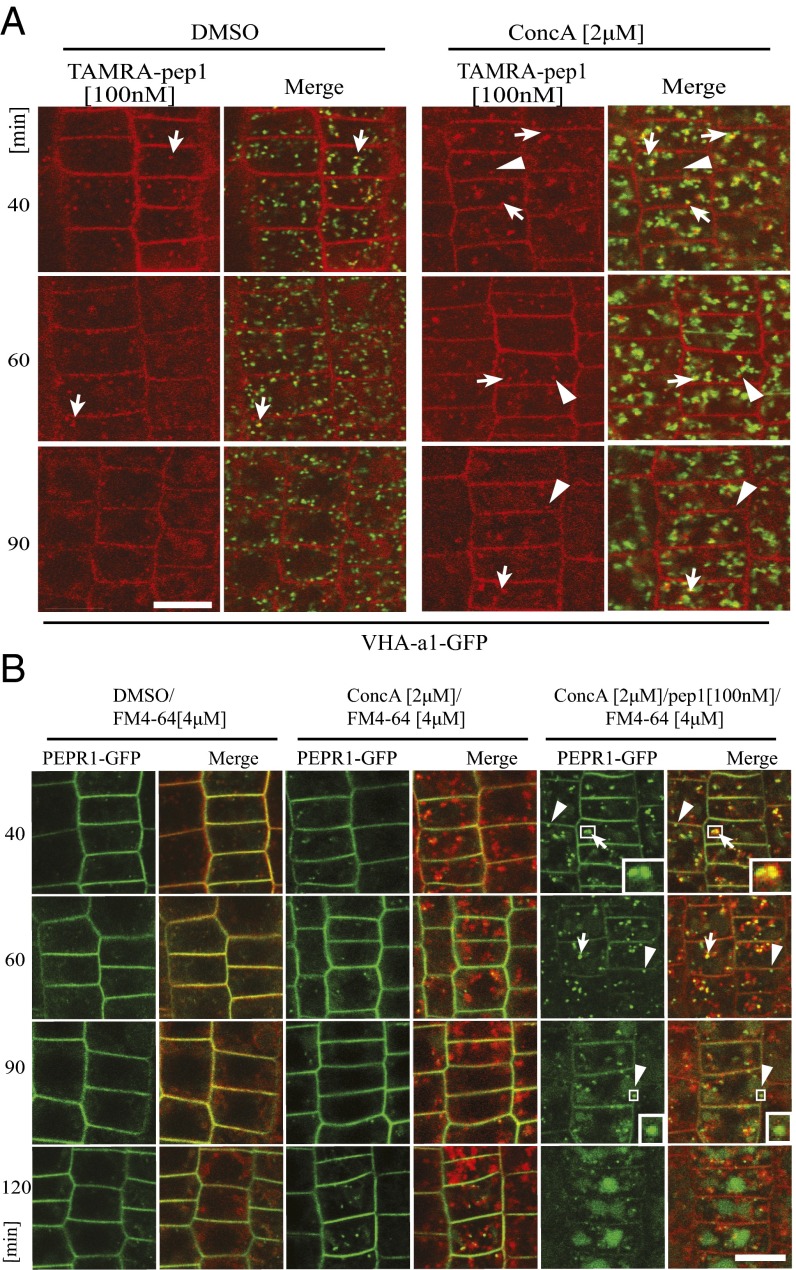
To support the colocalization studies, we investigated whether the trafficking route of the AtPep1-PEPR complexes requires the function of the vacuolar H+-ATPase (V-ATPase), which is present in the TGN/EE compartments to acidify them (13). We examined the subcellular dynamics and localization of TAMRA-pep1 in transgenic lines expressing VHA-a1-GFP in the presence of the specific V-ATPase inhibitor concanamycin A (ConcA) (13) (Fig. 3A). Surprisingly, after a 40-min chase, the fluorescent probe did not accumulate in typical ConcA bodies, as seen for the VHA-a1-GFP, despite some minor colocalization. After a 90-min chase, the TAMRA-pep1 signal was visible in the vacuole, indicating that its trafficking, although somewhat delayed (Fig. S8A), was not blocked by the inhibition of the V-ATPase activity. These findings are supported by the subcellular dynamics of PEPR1-GFP in the presence of ConcA and the endosomal tracer FM4-64, previously shown to accumulate into the TGN/EE-positive ConcA bodies (13) (Fig. 3B). For this experiment, pRPS5A::PEPR1-GFP/pepr1pepr2 seedlings were pretreated with ConcA (2 µM, 30 min), then pulsed with pep1 or with water (mock control), in the presence of FM4-64 and ConcA. Seedlings stained with FM4-64 pretreated and treated with DMSO were used as a control for the ConcA treatment. Without pep1 elicitation, PEPR1-GFP localization was not significantly affected by ConcA, although at later points some GFP-positive compartments were seen either in the proximity of or colocalizing with the FM4-64–positive ConcA bodies (Fig. 3B). After pretreatment with ConcA and pep1 application in the presence of the inhibitor, a considerable portion of PEPR1-GFP–marked vesicles clearly colocalized with FM4-64 into the ConcA bodies and were still better visualized after 40- and 60-min chases (Fig. 3B), but, as seen for TAMRA-pep1 (Fig. 3A), the PEPR1-GFP trafficking to the vacuoles was not blocked. Based on the low colocalization between TAMRA-pep1 and VHA-a1-GFP and the ineffectiveness of ConcA, we concluded that trafficking of the AtPep1-PEPR complexes to the vacuole does not strictly require the V-ATPase activity associated with TGN/EE.
Fig. 3.
The trafficking of TAMRA-pep1 and PEPR1-GFP to the vacuole did not require V-ATPase activity. (A) Subcellular localization of TAMRA-pep1 in Arabidopsis root meristem epidermis of transgenic seedlings expressing the TGN/EE marker VHA-a1-GFP in the presence of ConcA and DMSO (mock control) over time. Seedlings were pretreated with ConcA (2 μM, 30 min) and then with TAMRA-pep1 (100 nM, 10-s pulse, three washouts) in the presence of ConcA (2 μM) for 40, 60, and 90 min before imaging. Arrows and arrowheads point to colocalized and uncolocalized vesicles marked with TAMRA-pep1 and VHA-a1-GFP, respectively. (B) Subcellular localization of PEPR1-GFP in Arabidopsis root epidermal meristem cells in the presence of DMSO, ConcA (2 µM), and ConcA (2 µM)/pep1 (100 nM for 10 s) over time. pepr1pepr2 seedlings complemented with the pRPS5A::PEPR1-GFP construct were pretreated with ConcA (2 µM, 30 min) and then exposed or not to pep1, stained with FM4-64 (4 µM, 5 min, three washouts), and kept in the presence of ConcA (2 µM) for the indicated times until imaging. Arrows and arrowheads point to PEPR1-GFP vesicles colocalized and uncolocalized with FM4-64, respectively. (Insets) 3× magnification showing details of the PEPR1-GFP vesicles. In all experiments, 5-d-old seedlings were used. (Scale bars: 10 µm.)
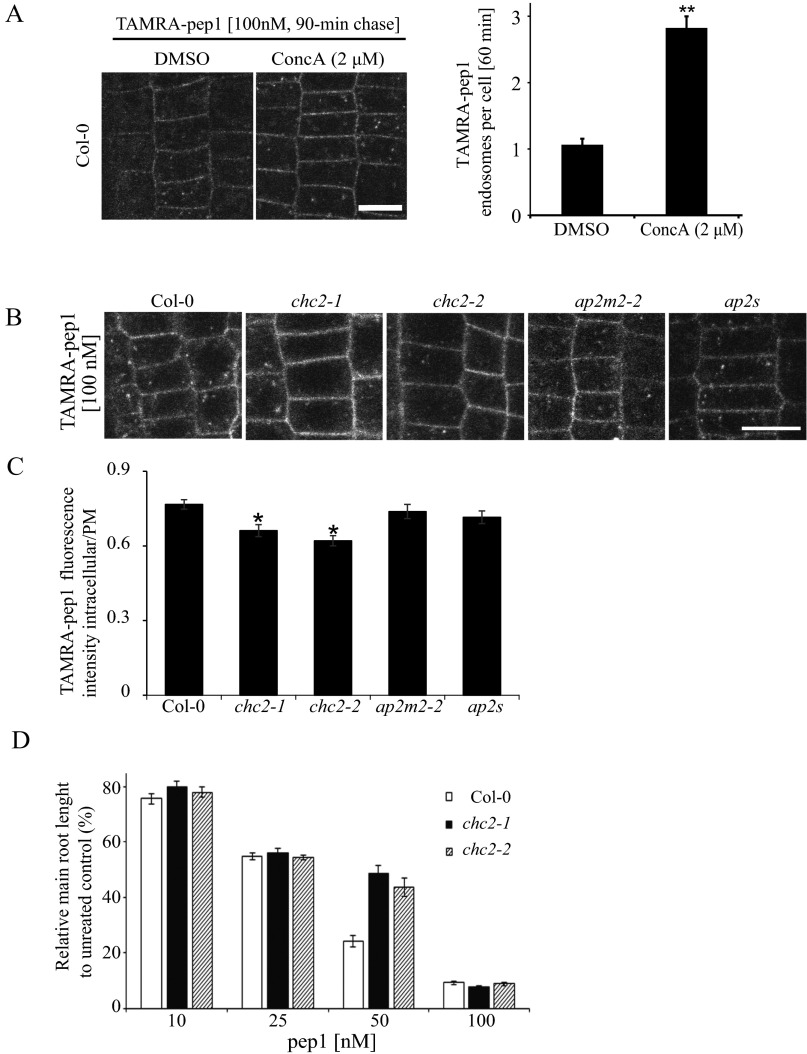
Fig. S8.
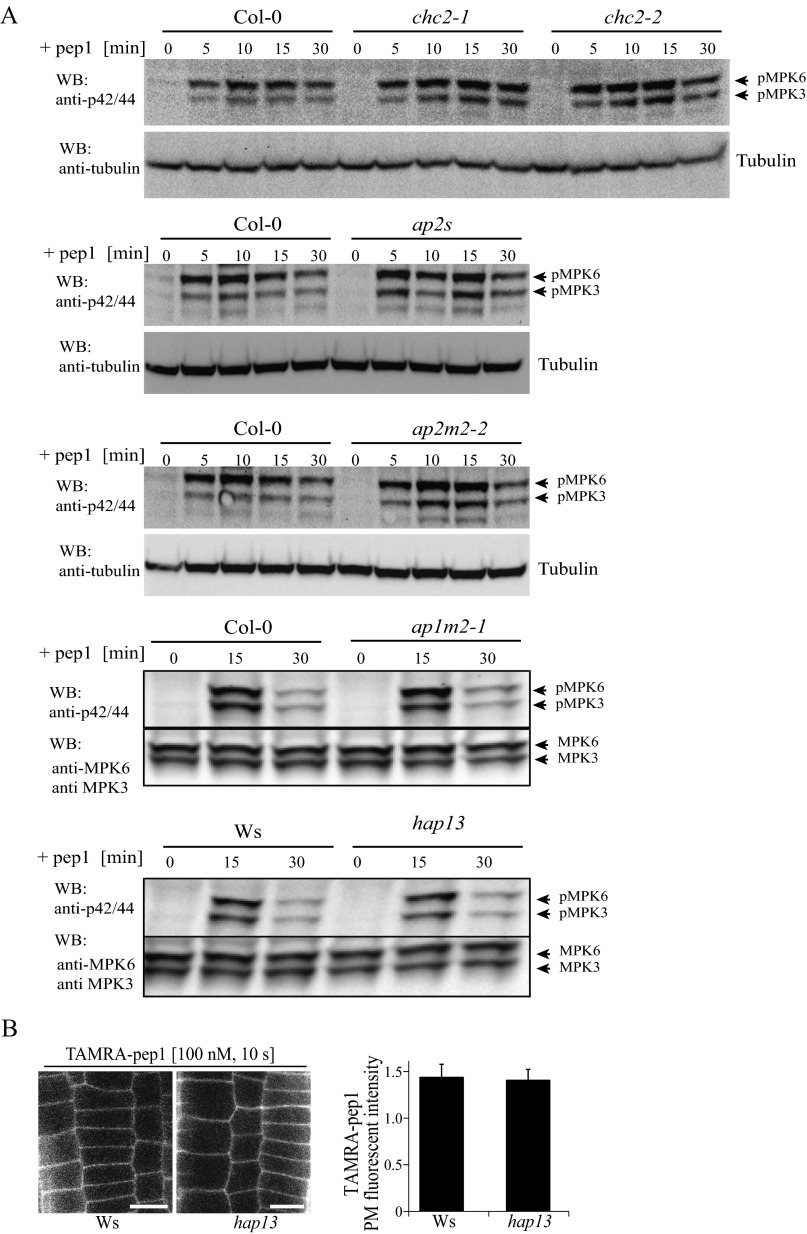
Clathrin-dependent TAMRA-pep1 internalization and AtPep1-mediated responses. (A) Impaired activity of vacuolar H+-ATPase by the presence of ConcA slightly delaying the pass of TAMRA-pep1 to the vacuole. Quantification of the number of TAMRA-pep1 endosomes per cell (n = 36). n, number of cells analyzed. Error bars indicate SEM. **P ≤ 0.001 (Student’s t test) relative to Col-0. The 5-d-old seedlings were pretreated with ConcA (2 µM, 30 min), then treated with TAMRA-pep1 (100 nM, 10 s), washed three times, and kept in the presence of ConcA (2 μM) until imaging (60-min chase). DMSO served as a control. (Scale bars: 10 μm.) (B) TAMRA-pep1 uptake in WT Arabidopsis (Col-0; control) and chc2-1, chc2-2, ap2m-2, and ap2s homozygous mutants. The 5-d-old seedlings were treated with TAMRA-pep1 (100 nM, 10 s), washed three times, and imaged after a 40-min chase. (C) Quantification of the TAMRA-pep1 uptake in B. Graphs illustrate the intracellular/PM fluorescence intensity ratio (n >45). n, number of analyzed cells. Error bars indicate SEM. *P ≤ 0.01 (Student’s t test) relative to Col-0. (Scale bar: 10 µm.) (D) Root growth analysis of Col-0, chc2-1, and chc2-2 seedlings grown in the presence of various concentrations of pep1. Data are the sum of two biological repeats, and the main root length is presented as relative to the mock control. Error bars indicate SEM.
PEPR1 Secretion, but Not Its Endocytosis, Depends on ARF-GEF.
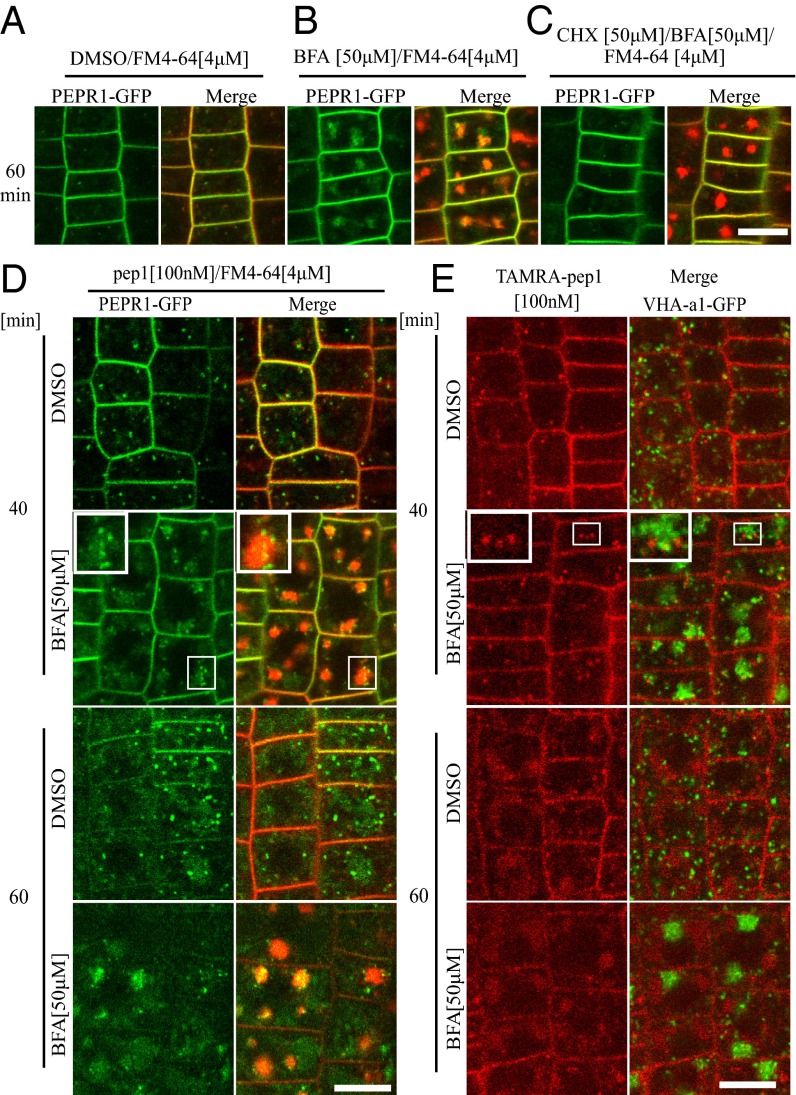
Most of the plant PM proteins, including receptors, constitutively cycle between PM and endosomal compartments, a mechanism that modulates their abundance and activity (19). Therefore, to investigate whether PEPR1 also undergoes recycling, we applied the fungal inhibitor brefeldin A (BFA), which is also routinely used to block recycling in plants through inhibition of the BFA-sensitive ADP-ribosylation factor-guanine exchange factors (ARF-GEFs) (20). Following treatment with BFA (50 µM, 60 min), PEPR1-GFP fluorescence clearly accumulated in BFA bodies that were also costained with the endocytic tracer FM4-64 (Fig. 4 A and B). Because PEPR-GFP did not accumulate into BFA bodies in the presence of BFA and the protein synthesis inhibitor cycloheximide (CHX) (Fig. 4C), we concluded that the majority of the PEPR1-fluorescent signals that accumulated in the BFA bodies without CHX belonged to newly synthetized, but not recycled, PEPR1 proteins.
Fig. 4.
PEPR1 secretion, but not its endocytosis, depends on ARF-GEF. (A–C) Subcellular localization of PEPR1-GFP after treatment for 60 min with DMSO (A), BFA (50 µM) (B), and CHX (50 µM) + BFA (50 µM) (C). (D) Experiments performed after pretreatment with CHX for 1 h. Subcellular localization of PEPR1-GFP after 40 and 60 min of pep1 elicitation (100 nM, 10-s pulse) in the presence or absence of BFA (50 µM). pepr1pepr2 seedlings complemented with the pRPS5A::PEPR1-GFP construct were stained with FM4-64 (4 µM, 5 min, three washouts). (E) Subcellular localization of TAMRA-pep1 in the presence or absence of BFA. Seedlings expressing VHA-a1-GFP were treated with TAMRA-pep1 for 10 s, washed, kept in the presence or absence of BFA (50 µM), and imaged after 40 and 60 min. (Insets) Details of BFA bodies at 2.5× magnification. In all experiments, 5-d-old seedlings were used. (Scale bars: 10 µm.)
Next, we visualized PEPR1-GFP fluorescence after 40- and 60-min chases following AtPep1 elicitation in the presence of BFA (50 µM) (Fig. 4D). After a 40-min chase, the PEPR1-GFP fluorescence accumulated into BFA bodies (likely derived from secretion) and into a population of vesicles arranged around the BFA bodies, some of which seemed to colocalize with the BFA bodies (Fig. 4D, Inset). After a 60-min chase, the PEPR1-GFP fluorescent signal was also seen in the vacuole, indicating that the ligand-induced internalization of PEPR1 was not blocked. To confirm this observation, we evaluated the internalization of TAMRA-pep1 in the presence of BFA (Fig. 4E). The treatment was performed in transgenic plants expressing the endosomal marker VHA-a1-GFP, which has been detected in the core of BFA bodies (13). As expected, the localization pattern and temporal dynamics of TAMRA-pep1 in the presence of BFA were similar to those of PEPR1-GFP after activation (Fig. 4E). These observations suggest that the endocytic trafficking of AtPep1-PEPR complexes is not compromised in the presence of BFA, as previously reported for FLS2 in Arabidopsis leaves (7).
Clathrin Is Required for AtPep1-PEPR1 Complex Internalization and AtPep1 Responses.
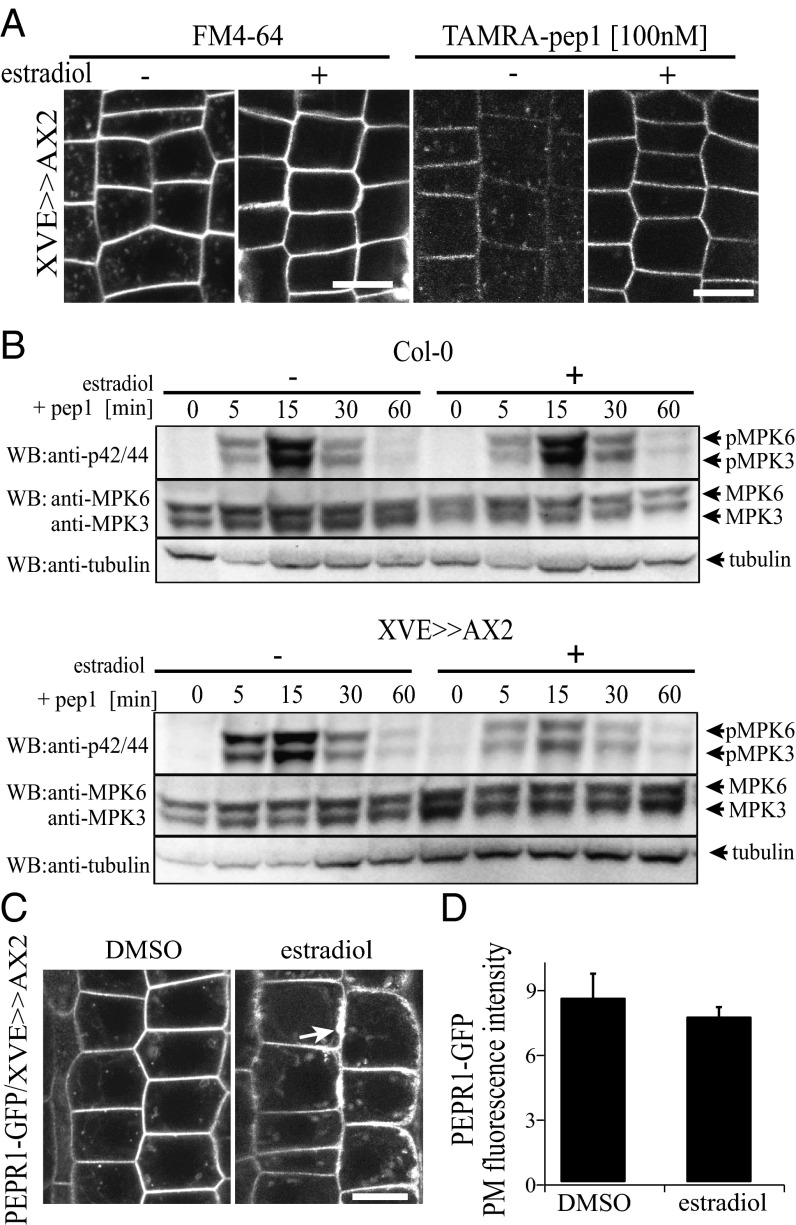
In plants, CME is the major internalization route of plant PM proteins (10). To gain further insight into the internalization mechanism of AtPep1-PEPR complexes, we characterized TAMRA-pep1 endocytosis in the clathrin heavy chain 2 (chc2) knockout mutants (chc2-1 and chc2-2) (11). In both chc2 alleles, the intracellular accumulation of TAMRA-pep1 was slightly, but significantly, reduced, implying its impaired internalization (Fig. S8 B and C). To corroborate these observations, we took advantage of the inducible line expressing the clathrin-interacting protein Auxilin2 (AX2) (XVE>>AX2), the overexpression of which blocked CME in Arabidopsis (Fig. 5A). When seedlings were induced with 5 µM estradiol, monitored as root growth arrest, both the internalization of TAMRA-pep1 and FM4-64 were completely blocked, suggesting that the AtPep1-PEPR complexes undergo CME.
Fig. 5.
Clathrin dependence of AtPep1-PEPR internalization and responses. (A) The internalization of FM4-64 (Left; 4 µM, 5 min, three washouts, 30-min chase) and TAMRA-pep1 (Right; 100 nM, 10-s pulse, three washouts, 40-min chase) was blocked after induction of Auxilin2 (AX2) expression in 5-d-old XVE>>AX2 seedlings with 5 µM estradiol for 24 h. (B) Pep1-induced (20 nM) MAPK activation was impaired after the XVE>>AX2 line had been treated with 5 µM estradiol for 24 h. Phosphorylation of MPK6 and MPK3 was detected with anti–phospho-p44/p42-MPK antibody. The blot was reprobed with anti-MPK6, ant-MPK3, and anti-tubulin to show protein levels. Individual MPKs were identified by molecular mass and are indicated by arrows. These experiments were repeated five times. (C) Subcellular localization of PEPR1-GFP in root epidermal cells of pRPS5A::PEPR1-GFP/XVE>>AX2-expressing seedlings before (DMSO) and after induction with 5 µM estradiol for 24 h. (D) Quantification of PEPR1-GFP PM fluorescence intensity in C (n = 36). n, number of cells analyzed. Error bars indicate SEM. No statistically significant difference (*P ≤ 0.01, Student’s t test) was found. (Scale bars: 10 µm.)
To investigate whether the internalization of AtPep1-PEPR requires the known CME adaptor AP-2, we also carried out TAMRA-pep1 uptake experiments in root epidermal cells of ap2m-2 (21) and ap2s (22) mutants defective in the medium and small subunits of the Arabidopsis AP-2, respectively (Fig. S8 B and C). Notably, the uptake of TAMRA-pep1 was not significantly compromised in any of these lines, in contrast to the previously reported, partially inhibited FM4-64 uptake (22, 23).
We next tested whether CME impairment would affect early and late AtPep1 responses, including mitogen-activated protein kinase (MAPK) activation and root growth inhibition, respectively (12). Although the MAPK phosphorylation caused by pep1 application was not affected in any of the tested chc2, ap2m, and ap2s alleles (Fig. S9), it was strongly reduced when XVE>>AX2 seedlings were induced for 24 h with estradiol and treated with pep1 (Fig. 5B). Interestingly, the root sensitivity of the chc2 mutants to pep1 application was also altered, but only at one pep1 concentration (Fig. S8D). To rule out the possibility that the reduced MAPK activation was not due to impaired secretion, we checked the amount of PEPR1-GFP receptors and the TAMRA-pep1 available at the PM by measuring the fluorescence intensity before and after estradiol induction in XVE>>AX2 seedlings. We observed a PM-localized receptor and, in some cases, even an excessive amount of membranous material that accumulated at the periphery of epidermal cells, suggesting that secretion was not blocked (Fig. 5 C and D). Complementary to these results, MAPK activation after pep1 application was not compromised in mutants in the AP1M subunit of the TGN/EE-localized AP-1 complex, which is essential for post-Golgi trafficking (24) (Fig. S9 A and B). Based on the foregoing findings, we conclude that clathrin is required for AtPep1-PEPR–mediated responses through CME regulation.
Fig. S9.
MAPK activation on pep1 application. (A) WT (Col-0 or Ws) Arabidopsis plants and chc1-1, chc2-1, chc2-2, ap2m-2, ap2s, ap1m2-1, and hap13 homozygous mutants treated with pep1 (20 nM) for the indicated times. Phosphorylation of MPK6 and MPK3 was detected with anti–phospho-p44/p42-MPK antibody. The immunoblot was reprobed with anti-MPK6, anti-MPK3, and anti-tubulin antibodies for protein quantification. Individual MPKs were identified by molecular mass and are indicated by arrows. (B) PM labeling of TAMRA-pep1 in WT (Ws) and hap13 mutant root meristem epidermal cells. Seedlings were treated with TAMRA-pep1 (100 nM) for 10 s, washed three times, and imaged. (Right) Quantification of PM fluorescence intensity (n = 32). n, number of analyzed cells. Error bars indicate SEM. No statistically significant difference was found (*P ≤ 0.01, Student’s t test). WB, Western blot.
Discussion
Here we used live-cell imaging of fluorescently labeled and biologically active pep1 (TAMRA-pep1) to assess the importance of the subcellular localization and dynamics of the AtPep-PEPR complexes for signaling in the root meristem. TAMRA-pep1 associated rapidly and in a receptor-dependent manner with the PM, in agreement with previous biochemical work showing that receptor-ligand complexes are formed within seconds (5). The TAMRA-pep1 localization correlated with the expression pattern of the PEPR1-GFP protein. TAMRA-pep1 and PEPR-GFP were simultaneously internalized and transported to the vacuole, most probably as a mechanism to desensitize cells after the AtPep1 stimulation, as reported for other ligands (8, 25). Interestingly, the temporal dynamics of the AtPep1-PEPR complexes differed from those of BR-BRI1, which internalize very rapidly (8), but were similar to those of the FLS2 receptor (7). Thus, diverse signaling responses require endocytosis with different dynamics and, notably, pattern recognition receptors follow ligand-induced endosomal trafficking with similar dynamics.
Because TGN/EE is the first compartment that gathers endocytosed cargos (26), high colabeling was expected between the TGN/EE markers and TAMRA-pep1, as previously observed for the BRI1 receptor-ligand complexes (8). Surprisingly, and similar to FLS2 (7, 27), the colocalization of TAMRA-pep1 with all tested TGN/EE markers, including clathrin (28) and VHA-a1, was low, in contrast to the MVB markers, for which high colocalization values were obtained. Possibly, the AtPep1-PEPR complexes either are internalized directly to MVBs bypassing the TGN/EE or are transported to the MVBs via a V-ATPase–negative subpopulation of the TGN. In agreement, the transport of TAMRA-pep1 to the vacuole, although delayed, was not blocked by the inhibitor of the V-ATPase activity, ConcA (13), similarly to the boron transporter BOR1 at high boron concentrations (29) and the FLS2 receptor (7, 27). Intriguingly, ARA7 colocalized with the VHA-a1 in the TGN/EE, thus marking the TGN/EE subdomains that will mature into MVBs (30). Therefore, PEPRs might associate with TGN/EE subpopulations that are partially excluded from the V-ATPase activity preceding the switch to ARA7-dependent TGN/EE maturation into MVBs. The identity of this TGN subpopulation remains to be determined, however. The fungal toxin BFA induced the accumulation of the inactive PEPR1-GFP into BFA bodies, but these bodies were not detected after protein synthesis inhibition, implying that the signal detected in BFA bodies derives from secreted, instead of recycled, receptors.
Although we cannot rule out a BFA-insensitive recycling pathway or a very slow recycling rate, our results suggest that the abundance of the inactive receptors at the PM is regulated mainly by secretion. Because PEPRs function as innate immunity amplifiers and their expression is also induced by AtPeps (4), we can predict that after elicitation the internalized PEPRs are replaced by newly synthetized proteins. Accordingly, BFA did not affect the AtPep1-PEPR1 trafficking to the vacuole, as previously reported for the activated FLS2 receptor and the fluorescent BR analog (7, 8).
Direct evidence for the role of CME in plant endogenous peptide-mediated responses has not yet been reported. We have shown that the endocytosis of AtPep1-PEPR1 depends on clathrin, because TAMRA-pep1 internalization was slightly reduced in chc2 alleles and completely blocked after overexpression of the clathrin-interacting protein AX2 that seemingly prevents clathrin recruitment to the nascent endocytic pits. The inhibition of TAMRA-pep1 internalization after overexpression of AX2 correlated with severely reduced activation of MAPKs after pep1 application. In agreement, in response to pep1, the root growth insensitivity of the two chc2 alleles was increased but, surprisingly, at only one pep1 concentration. Taken together, our results reveal that AtPep1-mediated responses are compromised and that the severity of the phenotypes, at least related to early MAPK responses, correlates with the extent of the CME inhibition at the PM.
In plants, clathrin-coated vesicles are also essential for sorting cargos for recycling and possibly secretion at the TGN/EE (28). Given that the amounts of PEPR1-GFP and TAMRA-pep1 at the PM were not reduced after AX2 induction, we conclude that the secretion of PEPR1 is not significantly affected. In addition, the AtPep1-mediated MAPK activation was not altered by mutations in the medium subunit of the TGN/EE-localized AP-1, which is essential for post-Golgi trafficking (24). Despite their involvement in CME, the mutant alleles of AP-2, ap2m-2 and ap2s (21, 22), did not affect TAMRA-pep1 uptake or AtPep1-mediated MAPK activation, raising the question of whether PEPR endocytosis is mediated by AP-2.
Finally, in contrast to the BR signaling, clathrin is required for AtPep1-mediated responses. Whether CME is necessary for the delivery of signaling complexes to as-yet undefined endosomal compartments or for the removal of unknown negative AtPep1 signaling components from the PM remains to be determined.
Materials and Methods
The experimental procedures followed for this study are described in detail in SI Materials and Methods.
Plant Material and Growth Conditions.
Arabidopsis thaliana (L.) Heynh. (Columbia accession, Col-0), pepr1pepr2 (2), chc2-1 and chc2-2 (11), ap1m2-1 and hap13 (Ws) (24), ap2m-2 (21), ap2s (22), atg5-1 (31), and atg7-2 (32) plants were used. Generation of constructs, used primers (Table S1), transgenic lines, media, and growth conditions are described in SI Materials and Methods.
Table S1.
Primers used for cloning and RT-PCR analysis
| DNA region | Primer | Sequence 5′–3′ |
| Cloning | ||
| Genomic PEPR1 | Forward | GGGGACAAGTTTGTACAAAAAAGCAGGCTCAATGAAGAATCTTGGGGGGTTGTTC |
| Reverse | GGGGACCACTTTGTACAAGAAAGCTGGGTACCGAACTGAATCAGAGGAGCA | |
| Genomic PEPR2 | Forward | GGGGACAAGTTTGTACAAAAAAGCAGGCTCAATGAGGAATCTTGGGTTACTCG |
| Reverse | GGGGACCACTTTGTACAAGAAAGCTGGGTAGTGAACTGAACCCGAAGTGCTTCT | |
| Promoter PEPR1 | Forward | GGGGACAACTTTGTATAGAAAAGTTGCTTCACTGATCTGTTTGTTGCAAAC |
| Reverse | GGGGACTGCTTTTTTGTACAAACTTGCCTGAGTTTAAAGATCGAGAAACATG | |
| Promoter PEPR2 | Forward | GGGGACAACTTTGTATAGAAAAGTTGCTATTAGGGTGGTCTATCGGTCAG |
| Reverse | GGGGACTGCTTTTTTGTACAAACTTGCATTAGAGCTCAAGAGACTGAAATATG | |
| CDS Auxilin2 | Forward | CACCATGGATGATTTCACAGGATTGTT |
| Reverse | TCAAAAGAGTTCCTCTGAGTTGAAT | |
| RT-PCR | ||
| GAPDH | Forward | TTGGTGACAACAGGTCAAGCA |
| Reverse | AAACTTGTCGCTCAATGCAA | |
| PEPR1 | Forward | GTTTTGGCTGAGGAAAGACG |
| Reverse | ACATTGTACCGTGCAGACCA | |
Peptides.
pep1 (ATKVKAKQRGKEKVSSGRPGQHN) and pep1 labeled with 5′-carboxytetramethylrhodamine (TAMRA-pep1) were purchased from Life Technologies. flg22 was acquired from Genscript. The peptides were dissolved in water to obtain peptide stocks of 100 µM. Details are provided in SI Materials and Methods.
Chemical Treatments.
BFA (50 mM), ConcA (2 mM), and CHX (50 mM) were purchased from Sigma-Aldrich. FM4-64 (2 mM) was acquired from Molecular Probes. Here 5-d-old seedlings were incubated for the indicated times into 1 mL of growth medium containing 2 µM ConcA, 50 µM BFA, 50 µM CHX, or a combination of BFA and CHX. Details are provided in SI Materials and Methods.
Confocal Microscopy.
Arabidopsis seedlings were imaged with an Olympus FluoView 1000 inverted confocal microscope or a Zeiss LSM 880 confocal laser scanning microscope. Details are provided in SI Materials and Methods.
SI Materials and Methods
Plant Material and Growth Conditions.
All mutants and transgenic lines used were in the background of the Arabidopsis thaliana (L.) Heynh. accession Columbia 0 (Col-0). Seeds were sterilized, maintained for 2 d at 4 °C in the dark, and germinated on vertical half-strength Murashige and Skoog (1/2 MS) medium [1% (wt/vol) sucrose] agar plates, pH 5.8, at 22 °C in a 16-h/8-h light–dark cycle for 5 d with a light intensity of 120 µE m−2 s−1 (E, Einstein; 1E = 1 mol of photons). The following mutant and transgenic Arabidopsis lines have been described previously: pepr1pepr2 (2); chc2-1 and chc-2 (11); ap1m2-1 and hapless (hap13) (24); ap2m-2 (21); ap2s (22); autophagy5-1 (atg5-1) (31) and atg7-2 (32); GFP-VAMP727 (33); VHA-a1-GFP (13); YFP-ARA7, YFP-RabA1e and YFP-MEMB12 (17); ARA6-GFP (18); and CLC1-GFP, CLC2-GFP, CHC1-GFP, and CHC2-GFP (16). The pUBQ10::ATG8-YFP/Col-0 line was a kind gift of M. Nowack and M. Fendrych, VIB–Ghent University, Ghent, Belgium. The hap13 mutant was isolated in Wassilewskija (Ws) ecotype.
Generation of Constructs.
The PEPR1 and PEPR2 promoters and coding sequences were amplified by PCR with KaPaHIFI polymerase (Sopachem) from genomic DNA (accession Col-0) with specific primers (Table S1). The fragments thus obtained were introduced into pDONR entry vectors (pDONRP4-P1R promoter sequences and pDONR221 coding sequences) by means of the Gateway system-compatible attB sites (Invitrogen). To create transcriptional reporter vectors of PEPR1 and PEPR2, the entry clones pDONRP4-P1R-pPEPR1/PEPR2 were introduced into the destination vector pMK7S*NFm14GW with the Gateway-based cloning (Invitrogen) to yield pMK7S*NFm14GW-pPEPR1/2::NLS-GFP. The entry vectors pDONRP4-P1R-pPEPR1/PEPR2 and pDONR221-PEPR1/PEPR2 were used in a triple LR reaction (MultiSite-Gateway; Invitrogen), combining pDONRP2-P3R-GFP and pB7m34GW to yield pB7m34GW-pPEPR1/PEPR2::PEPR1/PEPR2-GFP. To express PEPR1/PEPR2 under the RPS5A promoter, the entry vector pDONRP4-P1R-pPEPR1/PEPR2 was replaced by pDONRP4-P1R-proRPS5A, producing the pB7m34GW-pRPS5A::PEPR1/PEPR2-GFP construct. The XVE>>AX2 line was generated using the coding sequence of the Arabidopsis Auxilin2 (At4g12770) gene, which was introduced into the estradiol-inducible vector pMDC7B(UBQ10) via pENTR/d-TOPO Gateway (Invitrogen), according to the manufacturer’s instructions.
Generation of Arabidopsis Transgenic Lines.
Arabidopsis plants were transformed with Agrobacterium tumefaciens by means of the floral dip method. Plants expressing NLS-GFP under the endogenous promoters of PEPR1 and PEPR2 were transformed into a Col-0 background. Primary transformants were selected on 1/2MS medium containing kanamycin (50 mg/L). The pRPS5A::PEPR1/PEPR2-GFP constructs were dipped into the pepr1pepr2 or XVE>>AX2 background, and transformants were selected on 1/2MS medium containing 10 mg/L phosphinothricin. For imaging line XVE>>AX2, seedlings were germinated as described previously for 4 d and then transferred to 1/2MS plates containing 5 µM β-estradiol (Sigma-Aldrich) for 24 h. DMSO instead of β-estradiol served as a control.
Peptides.
The peptide pep1 (ATKVKAKQRGKEKVSSGRPGQHN) with an HPLC purity of 95.16% and molecular weight of 2,491.78, and the peptide pep1 labeled with 5′-carboxytetramethylrhodamine at the N-terminal (TAMRA-pep1) with a HPLC purity of 97.07% and molecular weight of 2,905.75, were purchased from Life Technologies. The peptide flg22 (QRLSTGSRINSAKDDAAGLQIA), with an HPLC purity of 95% and a molecular weight of 2,272.50, was acquired from Genscript (catalog no. RP19986). The peptides were dissolved in water to obtain peptide stocks of 100 µM. Further dilutions were done with 1/2MS medium.
TAMRA-pep1 Analyses by LC-MS.
Here 5-d-old pRPS5A:PEPR1-GFP–expressing pepr1pepr2 seedlings were dipped into 1 mL of 50 µM TAMRA-pep1 dissolved in 1/2MS medium, pulsed for 10 s, washed three times with 1/2MS liquid medium, and transferred to agar plates. Shoots and roots were separated at the indicated time points, and roots were ground in liquid nitrogen to a fine powder. Then 100 μL of extraction buffer (20% CH3CN containing 20 mM Tris⋅HCl, pH 6.8) was added to 100 mg of powder. After incubation for 30 min in a shaker, the mixture was centrifuged (13,000 × g for 30 min at 4 °C), and the supernatant was filtered through a 0.2-μm filter (Corning Costar Spin-X cellulose acetate centrifuge tube filter) and analyzed by LC-MS. LC-MS data were collected on an Agilent 1100 Series instrument with a Phenomenex Kinetex C18 100Å column (150 × 4.6 mm, 5 μm at 35 °C) connected to an ES-MSD type VL mass detector (quadrupole ion trap mass spectrometer) with a flow rate of 1.5 mL/min with the following solvent systems: (A) 0.1% HCOOH in H2O and (B) CH3CN. The column was flushed with 100% A for 2 min, then a gradient from 0 to 100% B over 6 min was used, followed by 2 min of flushing with 100% B. MS spectra were collected in positive mode. A deconvolution procedure was applied to deliver the total molecular weight of the TAMRA-pep1 as calculated from the different signals for the multiply charged species.
Imaging.
Arabidopsis seedlings were imaged on an Olympus FluoView 1000 inverted confocal microscope equipped with a water-corrected 60× objective (NA 1.2) at digital zoom 3 or on a Zeiss LSM 880 equipped with a water-corrected 63× objective (C-Apochromat). For the evaluation of the TAMRA-pep1 PM labeling on different cell layers of the root meristem and of the root expression patterns of the AtPep1 receptors, digital zoom 1 was used. The excitation wavelength was 488 nm for GFP and YFP, 458 nm for CFP, and 559 nm for TAMRA and FM4-64. Emission was detected at 500–530 nm for GFP and YFP, 460–500 nm for CFP, and 570–670 nm for TAMRA and FM4-64. For TAMRA-pep1, the intensity was manipulated with the Olympus software.
TAMRA-pep1 Application and Competition Assays.
For the PM-labeling assay, 5-d-old seedlings were dipped into 500 µL of 100 nM TAMRA-pep1 dissolved in 1/2MS medium, pulsed for different times as indicated, washed three times with 1/2MS liquid medium, and transferred to coverslips for visualization of meristem epidermal cells under the confocal microscope. For the competition assay, seedlings were pretreated with 0, 0.1, 1, 10, 100, 1,000, and 10,000 nM of pep1 or flg22 for 5 min and then washed three times with 1/2MS medium before being dipped into TAMRA-pep1 for 10 s.
Quantification of PM Fluorescent Intensity.
For quantification, images were converted to 8-bit images, and fixed regions of interest were selected to measure the fluorescent intensity of the PM and background into the intracellular space with ImageJ. Then the relative PM fluorescence was calculated by dividing the PM intensity by the background intensity. Six epidermal cells from eight plants were quantified.
TAMRA-pep1 Internalization Assay.
The 5-d-old seedlings were dipped into 500 µL of 100 nM TAMRA-pep1 dissolved in 1/2MS medium for 10 s, washed three times with 1/2MS liquid medium, kept in 500 µL of 1/2MS medium over a piece of parafilm placed in a Petri dish, and then imaged for the indicated time points. To compare TAMRA-pep1 uptake between the clathrin mutants and the Col-0 WT, images were quantified with ImageJ. To this end, images were first converted to 8-bit images, and then the entire PM and intracellular space were selected with a brush tool size of 5 pixels and the polygon selection tool, respectively. Then the average intensity of the top 50 highest pixels for both the PM and the intracellular space was used to obtain a ratio between intracellular and PM fluorescence. Six epidermal cells from eight plants were quantified.
Colocalization Analysis.
For the colocalization analysis of confocal images acquired by confocal laser microscopy, ImageJ was used with the plugin PSC colocalization to obtain the Pearson correlation coefficients as colocalization readouts, as well as a pixel distribution scatterplot in which the pixels in the green image served as the y-coordinates and the intensity of the corresponding pixels in the red image served as the x-coordinates. For colocalization between TAMRA-pep1 and PEPR1-GFP and PEPR2-GFP, the regions of interest were selected, and colocalization analysis was carried out with a threshold of 10. To determine the percentage of TAMRA-pep1 vesicles colocalized with different endomembrane markers, each individual TAMRA-pep1 spot was selected as an individual region of interest and colocalization was analyzed with a threshold of 10, considering the spots for which the Person correlation value was >0.5 as positively labeled endosomes. A threshold of 10 was used to avoid noise. Calculations were done by measuring the background gray values present in the analyzed images.
Chemical Treatments.
The inhibitors BFA (50 mM DMSO stock), ConcA (2 mM DMSO stock), and CHX (50 mM DMSO stock) were purchased from Sigma-Aldrich. FM4-64 was acquired from Molecular Probes (2 mM water stock). Five-day-old seedlings were incubated for the indicated times into 1 mL of liquid 1/2MS medium containing 2 µM ConcA, 50 µM BFA, 50 µM CHX, or a combination of BFA and CHX. For seedlings expressing PEPR1-GFP, inhibitor treatments were performed together with the endosomal marker FM4-64 at room temperature. For FM4-64 staining, seedlings were incubated for 5 min in 1 mL of 1/2MS liquid medium containing 4 µM of the dye plus the respective inhibitor treatment. Control treatments were done with equal amounts of DMSO. All washing steps and imaging were carried out in the presence of the respective inhibitors.
MAPK Activation Assay.
chc2-1, chc2-2, ap2s, and ap2m2 seedlings were germinated and grown on 1/2MS medium containing agar for 5 d. ap1m-2 and hap13 seedlings were grown for 10 d to identify homozygous mutants. Seedlings were transferred to multiwell plates (∼10 seeds per well) containing 1 mL per well of 1/2MS medium supplemented with 0.5% (wt/vol) sucrose and treated with 20 nM pep1 for the indicated times. At each time point, 20 seedlings were snap-frozen in liquid nitrogen. Proteins were extracted with a buffer containing 50 mM Tris, pH 7.5, 200 mM NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, 0.1% (vol/vol) Tween 20, 1 mM phenylmethylsulfonyl fluoride, 1 mM DTT, 1× phosphatase inhibitor mixture 2 (P5726; Sigma-Aldrich) and 1× protease inhibitor mixture (P9599; Sigma-Aldrich). Equal amounts of proteins (30 μg) were resolved on 7.5% (wt/vol) polyacrylamide gels and transferred onto a PVDF membrane (Bio-Rad). Primary antibodies against MPK3 (1:2,500; Sigma-Aldrich) and MPK6 (1:8,000; Sigma-Aldrich), against phospho-p44/42 MAP kinase (1:2,500; Cell Signaling Technology), and against tubulin (1:15,000; Sigma-Aldrich) were used with horseradish peroxidase-conjugated anti-rabbit as a secondary antibody (1:8,000; GE Healthcare). Signal detection was performed using Western Lightning Plus ECL (PerkinElmer). For induction of the β-estradiol–inducible line XVE>>AX2, seedlings were germinated and grown as described above for 5 d. β-estradiol was added to the medium at a final concentration of 5 μM for 24 h before the pep1 treatments. DMSO served as a control.
Alkalinization Assay.
For this assay, 1 mL of cell suspension (5 d after subculture) was transferred into each well of a 24-well plate (Corning) and allowed to equilibrate on an orbital shaker at 120 rpm for 1 h. The pH of the medium was measured at the time points of the indicated peptide concentrations with a EA940 pH meter (Orion) with a semimicro pH electrode (Orion).
RT-PCR.
All gene expression experiments were conducted using 6-d-old seedlings. Total RNA was extracted with TriZol reagent (Life Technologies) according to the manufacturer’s instructions, followed by DNase I treatment (Life Technologies) to remove any residue of genomic DNA, and quantified with a Nanodrop spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized from 1 μg of total RNA with Improm-II Reverse Transcriptase (Promega).
The AtPep1-induced gene PEPR1 was analyzed. The GAPDH gene served as a control. The primers used are listed in Table S1. For the PCR reactions, 1 μL of cDNA was used. The reactions were placed in the thermocycler under the following conditions: 94 °C for 2 min and appropriate numbers of cycles of 94 °C for 20 s, 60 °C for 30 s, and 72 °C for 45 s. These experiments were repeated at least three times.
Ca2+ Mobilization Assay.
The induced Ca2+ signaling was evaluated by monitoring the level of cytosolic Ca2+ in Arabidopsis seedlings. Seedlings of the Arabidopsis line homozygous for a single insertion of a transgene encoding a cytoplasmically expressed aquorin-driven by the cauliflower mosaic virus 35S promoter were studied. Seeds were sterilized as described previously and plated on 1/2MS medium without vitamins. The plates were kept under constant light at 24 °C (150 μE m−2 s−1) for 4 d. A single seedling was transferred into each well of a 96-well white microplate (Thermo Labsystems) containing 200 mL of 1/2MS medium supplemented with 2.5 mM coelenterazine cp (Biotium) and incubated in the dark at 24 °C for 16 h. In each plate well containing a single seedling, TAMRA-pep1 or pep1 was added at a final concentration of 10 nM. The resulting luminescence emission was monitored with a microplate reader (Biotec ELx 800) for 24 time points over ∼360 s after addition of the respective peptide. Water served as a control.
Root Growth Assay.
Seeds were sown on 1/2MS solid medium, stratified for 2 d at 4 °C in the dark, and placed vertically in the light. At 10 d (Fig. S2) or 3 d (Fig. S8) after germination, seedlings were transferred to square transparent Petri dishes with solid 1/2MS medium supplemented with or without the indicated amounts of peptides and incubated for another 4 d, after which the plates were scanned and root growth was measured. For measurements, scanned images were processed and evaluated with ImageJ.
Statistical Analysis.
P values were calculated with the two-tailed Student’s t test using Microsoft Excel.
Acknowledgments
We thank D. Van Damme, E. Mylle, M. Castro Silva-Filho, and J. Goeman for providing useful advice and technical assistance; I. Hara-Nishimura, J. Lin, G. Jürgens, M. A. Johnson, and P. Bozhkov for sharing published materials; and M. Nowack and M. Fendrych for kindly donating the pUBQ10::ATG8-YFP-expressing marker line. F.A.O.-M. was supported by special research funding from the Flemish Government for a joint doctorate fellowship at Ghent University, and funding from the Student Program–Graduate Studies Plan Program from the Coordination for the Improvement of Higher Education Personnel, Brazil, for a doctorate fellowship at the University of São Paulo. X.Z. and Q.L. are indebted to the China Science Council and G.P.d.O. to the “Ciência sem Fronteiras” for predoctoral fellowships. R.K. and Y.L. have received postdoctoral fellowships from the Belgian Science Policy Office. This research was supported by Flanders Research Foundation Grant G008416N (to E.R.) and by the São Paulo Research Foundation and the National Council for Scientific and Technological Development (CNPq) (D.S.d.M.). D.S.d.M. is a research fellow of CNPq.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10745.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1605588113/-/DCSupplemental.
References
- 1.Endo S, Betsuyaku S, Fukuda H. Endogenous peptide ligand-receptor systems for diverse signaling networks in plants. Curr Opin Plant Biol. 2014;21:140–146. doi: 10.1016/j.pbi.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Krol E, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem. 2010;285(18):13471–13479. doi: 10.1074/jbc.M109.097394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffaker A, Pearce G, Ryan CA. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA. 2006;103(26):10098–10103. doi: 10.1073/pnas.0603727103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, Huffaker A, Bryan AC, Tax FE, Ryan CA. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell. 2010;22(2):508–522. doi: 10.1105/tpc.109.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J, et al. Structural basis for recognition of an endogenous peptide by the plant receptor kinase PEPR1. Cell Res. 2015;25(1):110–120. doi: 10.1038/cr.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels S, et al. The family of Peps and their precursors in Arabidopsis: Differential expression and localization but similar induction of pattern-triggered immune responses. J Exp Bot. 2013;64(17):5309–5321. doi: 10.1093/jxb/ert330. [DOI] [PubMed] [Google Scholar]
- 7.Beck M, Zhou J, Faulkner C, MacLean D, Robatzek S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012;24(10):4205–4219. doi: 10.1105/tpc.112.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani NG, et al. Fluorescent castasterone reveals BRI1 signaling from the plasma membrane. Nat Chem Biol. 2012;8(6):583–589. doi: 10.1038/nchembio.958. [DOI] [PubMed] [Google Scholar]
- 9.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhonukshe P, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17(6):520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 11.Kitakura S, et al. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell. 2011;23(5):1920–1931. doi: 10.1105/tpc.111.083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JM, et al. Loss of Arabidopsis thaliana Dynamin-Related Protein 2B reveals separation of innate immune signaling pathways. PLoS Pathog. 2014;10(12):e1004578. doi: 10.1371/journal.ppat.1004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dettmer J, Hong-Hermesdorf A, Stierhof Y-D, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18(3):715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robert S, et al. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc Natl Acad Sci USA. 2008;105(24):8464–8469. doi: 10.1073/pnas.0711650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uemura T, Suda Y, Ueda T, Nakano A. Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 2014;55(4):694–703. doi: 10.1093/pcp/pcu010. [DOI] [PubMed] [Google Scholar]
- 16.Dejonghe W, et al. Mitochondrial uncouplers inhibit clathrin-mediated endocytosis largely through cytoplasmic acidification. Nat Commun. 2016;7:11710. doi: 10.1038/ncomms11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geldner N, et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 2009;59(1):169–178. doi: 10.1111/j.1365-313X.2009.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goh T, et al. VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell. 2007;19(11):3504–3515. doi: 10.1105/tpc.107.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleine-Vehn J, Friml J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol. 2008;24:447–473. doi: 10.1146/annurev.cellbio.24.110707.175254. [DOI] [PubMed] [Google Scholar]
- 20.Naramoto S, et al. Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell. 2014;26(7):3062–3076. doi: 10.1105/tpc.114.125880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka S, et al. Identification and dynamics of Arabidopsis adaptor protein-2 complex and its involvement in floral organ development. Plant Cell. 2013;25(8):2958–2969. doi: 10.1105/tpc.113.114082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan L, et al. Dynamic analysis of Arabidopsis AP2 σ subunit reveals a key role in clathrin-mediated endocytosis and plant development. Development. 2013;140(18):3826–3837. doi: 10.1242/dev.095711. [DOI] [PubMed] [Google Scholar]
- 23.Bashline L, Li S, Anderson CT, Lei L, Gu Y. The endocytosis of cellulose synthase in Arabidopsis is dependent on μ2, a clathrin-mediated endocytosis adaptin. Plant Physiol. 2013;163(1):150–160. doi: 10.1104/pp.113.221234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park M, et al. Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc Natl Acad Sci USA. 2013;110(25):10318–10323. doi: 10.1073/pnas.1300460110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JM, Salamango DJ, Leslie ME, Collins CA, Heese A. Sensitivity to Flg22 is modulated by ligand-induced degradation and de novo synthesis of the endogenous flagellin-receptor FLAGELLIN-SENSING2. Plant Physiol. 2014;164(1):440–454. doi: 10.1104/pp.113.229179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viotti C, et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell. 2010;22(4):1344–1357. doi: 10.1105/tpc.109.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi S-W, et al. RABA members act in distinct steps of subcellular trafficking of the FLAGELLIN SENSING2 receptor. Plant Cell. 2013;25(3):1174–1187. doi: 10.1105/tpc.112.108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito E, et al. Dynamic behavior of clathrin in Arabidopsis thaliana unveiled by live imaging. Plant J. 2012;69(2):204–216. doi: 10.1111/j.1365-313X.2011.04782.x. [DOI] [PubMed] [Google Scholar]
- 29.Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc Natl Acad Sci USA. 2005;102(34):12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh MK, et al. Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr Biol. 2014;24(12):1383–1389. doi: 10.1016/j.cub.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiol. 2005;138(4):2097–2110. doi: 10.1104/pp.105.060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofius D, et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell. 2009;137(4):773–783. doi: 10.1016/j.cell.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 33.Ebine K, et al. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell. 2008;20(11):3006–3021. doi: 10.1105/tpc.107.057711. [DOI] [PMC free article] [PubMed] [Google Scholar]