Significance
Much of human behavior results from a desire for social status. From an evolutionary perspective, answering the question of why we pursue status must consider how status affects reproduction, especially in nonindustrial societies with natural fertility. In a metaanalysis of 288 results from 33 nonindustrial populations, we find that status is significantly associated with men’s reproductive success, consistent with an evolved basis for status pursuit. Status hierarchies have changed dramatically throughout human history, yet we find that the association between status and reproductive success does not depend on subsistence category (foraging, horticulture, pastoralism, agriculture) or how status is measured. These findings suggest no significant increase in selection on status-enhancing traits with the domestication of plants and animals.
Keywords: status, hierarchy, reproduction, egalitarianism, evolution
Abstract
Social status motivates much of human behavior. However, status may have been a relatively weak target of selection for much of human evolution if ancestral foragers tended to be more egalitarian. We test the “egalitarianism hypothesis” that status has a significantly smaller effect on reproductive success (RS) in foragers compared with nonforagers. We also test between alternative male reproductive strategies, in particular whether reproductive benefits of status are due to lower offspring mortality (parental investment) or increased fertility (mating effort). We performed a phylogenetic multilevel metaanalysis of 288 statistical associations between measures of male status (physical formidability, hunting ability, material wealth, political influence) and RS (mating success, wife quality, fertility, offspring mortality, and number of surviving offspring) from 46 studies in 33 nonindustrial societies. We found a significant overall effect of status on RS (r = 0.19), though this effect was significantly lower than for nonhuman primates (r = 0.80). There was substantial variation due to marriage system and measure of RS, in particular status associated with offspring mortality only in polygynous societies (r = −0.08), and with wife quality only in monogamous societies (r = 0.15). However, the effects of status on RS did not differ significantly by status measure or subsistence type: foraging, horticulture, pastoralism, and agriculture. These results suggest that traits that facilitate status acquisition were not subject to substantially greater selection with domestication of plants and animals, and are part of reproductive strategies that enhance fertility more than offspring well-being.
Social status is a fundamental human motive (1). Social status can be defined as relative access to contested resources within a group, particularly the deference or admiration of group members (2, 3). To gain status, individuals attempt to influence perceptions of their dominance (i.e., ability to inflict costs on others) or prestige (i.e., ability to confer benefits on others) (3, 4). Dominance and prestige can be difficult to disentangle because dominance can be a source of prestige and vice versa (5). The particular traits that underlie dominance and prestige may vary across groups, but dominance and prestige are often based on conspicuous, interindividual differences in body size (6); intelligence and skill (7); consumption (8); and generosity (9, 10). Relative to the status hierarchies of other primates, prestige contributes heavily to human status, because of our interdependence in production and reproduction (11, 12), and thus the value of social partners and mates who are strong, skilled, wealthy, or generous (3, 13). Furthermore, humans frequently cooperate against dominant individuals who act coercively (14, 15).
Traits that facilitate status acquisition, including a desire for status, can have positive fitness consequences. Group members relinquish or offer reproductive opportunities to high-status individuals, to avoid costs from competition or gain reciprocal benefits. When mating is not monogamous, high-status males are able to translate status into large reproductive gains, leading to a strong correlation between dominance rank and mating success across male primates (16). In humans, the reproductive benefits of status reached their peak in premodern states and empires, where sultans, kings, and emperors could control access to a large number of women (17). Studies of the Y chromosome suggest a large increase in male reproductive skew with the rise and spread of agriculture 10,000 y ago (18), and common Y haplotypes can be traced to the lineages of high-status rulers such as Genghis Khan (19, 20). In modern industrial societies with monogamy and low fertility, several studies find that male fertility associates modestly with wealth, largely due to higher childlessness among poorer men (21–24).
Most of human history transpired in small-scale societies, who relied on foraging for subsistence. Observation and archaeology of foragers reveals tremendous variation in status hierarchy (25). In low-density relatively nomadic forager societies, decision-making is typically consensus based (at least among adult men), and status inequality is limited by fluid group membership, coalitional checks on would-be dominants, and cooperative production and interdependence (14, 25–27). Leadership tends to arise occasionally to meet situational demands and typically involves little or no material benefit relative to followers (28, 29). Variance in male reproduction can be small and not appreciably greater than variance in female reproduction (17). Nonetheless, men’s hunting among foragers has been attributed in part to status-seeking, because the most successful male hunters attract more sharing partners, more allies in the context of male–male competition, and more fertile, productive wives and extramarital partners (30–33). Some Australian foragers were gerontocratic and maintained high levels of polygyny, even at relatively low population density (34). A minority of known forager societies were sedentary and lived at relatively high population density, including groups from the west coast of North America, who recognized chiefs and who were more polygynous (35, 36). At least in foragers from western North America, polygyny was associated with the privatization of resource extraction sites, such as riverine fish runs (37). In aggregate, however, foragers from the ethnographic and archaeological record may lack the inequality in material resources that contributes to status hierarchy and reproductive inequality in pastoral or agricultural societies (38–40). Furthermore, foragers often lack the population density and associated collective action problems that can cause the rank and file to prefer more institutionalized, even coercive, political leadership (41–44).
The egalitarianism described of many if not most foragers in the ethnographic and archaeological record suggests that status may have been a relatively weak target of selection throughout much of the evolutionary history of modern humans. Positive selection on status may have increased significantly with domestication of plants and animals, due to greater material wealth inequality, institutionalized leadership, and male reproductive skew (17, 18, 39). This “egalitarianism hypothesis” can be tested with quantitative estimates of the relationship between male status and reproductive success (RS) in contemporary foragers compared with nonforagers.
How status translates into RS bears on debates about the evolution of men’s reproductive strategies, particularly men’s parenting vs. mating effort (45, 46). Men’s status pursuit has been framed in terms of mating effort (30), but the deference and admiration of group members can include aid for men’s families that functions as indirect parental investment (13). Positive associations of status with fertility are consistent with mating effort, whereas negative associations with offspring mortality are consistent with parenting effort. If status associates with fertility but not with offspring mortality, this does not necessarily mean that men’s status goals do not concern parenting effort, because there are many extrinsic reasons for offspring mortality. Rather, such results would suggest that by acquiring status, men maximize mating effort more than parenting effort—the “mating effort hypothesis.”
We test the egalitarianism and mating effort hypotheses via a phylogenetic multilevel metaanalysis of 288 statistical associations between male status and RS, from 46 studies in 33 nonindustrial societies. We focused on nonindustrial societies to limit effects of widespread contraception, modern medicine, and low fertility norms associated with the demographic transition. We coded all societies by their main mode of subsistence: foraging (subsistence on undomesticated plants and animals), horticulture (cultivation of domesticated plants in garden plots, based on simple tools), pastoralism (heavy reliance on herding domesticated animals), and agriculture (cultivation of domesticated plants using technologies such as plows and traction animals). Different types of status measures were categorized as formidability (e.g., height, weight, strength, warriorship), hunting skill (e.g., return rate, skill ranking), material wealth (e.g., total value of owned goods, income, livestock, or land ownership), and political influence (e.g., headman, influence ranking). Of the status measures, political influence is closest to our definition of status. The other measures better represent traits that tend to confer status, based on their contribution to group-wide judgments of who is dominant or prestigious. Finally, measures of RS were classified as surviving offspring (number of offspring surviving to a certain age), fertility (total offspring born), offspring mortality (e.g., offspring mortality rate, proportion of offspring dying), mating success (e.g., number of wives or affairs, age at marriage or probability of marriage), and wife quality (e.g., wife’s age or interbirth interval, wife’s productivity). All societies were coded by presence or absence of polygyny. The complete dataset is reported in Dataset S1.
Results
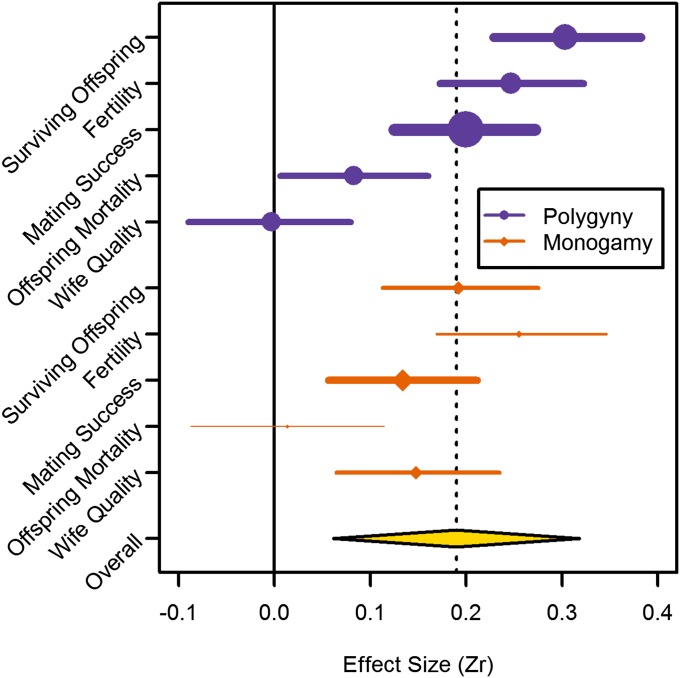
We begin with an intercept-only model to estimate the weighted overall effect size for the association between male status and RS. This effect size was significantly greater than zero [Zr = 0.19, 95% confidence interval (CI) = 0.09–0.31, k = 288; Fig. 1]. Adding a dummy variable for whether the original analysis controlled for age did not change these results. To best explain variation in the effect of status on RS as a function of different covariates, we applied model comparison based on the deviance information criterion (DIC; Methods). Table 1 lists the six top-ranked models, with all others receiving DIC weights <0.001. The top two models, together receiving ∼90% of DIC weight, contain polygyny and measures of RS and their interaction, but not subsistence type or status type, which only appear in models with low DIC weight. Table 2 presents a summary model for which coefficients were averaged according to DIC weight. In this averaged model, effect sizes vary only minimally as a function of subsistence type or status type, lending no support to the egalitarianism hypothesis. However, there is considerable variation according to RS measure and marriage system (polygyny vs. monogamy), as shown in Fig. 1. In particular, surviving offspring, fertility, and mating success tended to be more strongly associated with status than wife quality and offspring mortality, especially in polygynous groups, thus supporting the mating effort hypothesis. Additionally, the effects of status on wife quality and offspring mortality varied between polygynous and monogamous societies, with wife quality being significantly associated in the latter but not the former, whereas the opposite was true of offspring mortality.
Fig. 1.
Variation in the weighted effect of male status on RS, as a function of RS measure and marriage system (polygyny vs. monogamy) based on averaged coefficients (Table 2). Overall effect size based on intercept-only model. Before metaanalysis, all effect sizes were coded such that positive signs indicate positive contributions to RS (e.g., a negative effect of status on offspring mortality was coded as positive). Point size and line width are proportional to the number of results contributing to each weighted effect size.
Table 1.
Model comparison showing six top-ranked models explaining variation in the association between male status and reproductive success
 |
Cells in gray indicate that a covariate was included in a given model. Polygyny (P), RS type (RS), and their interaction were included in all top-ranked models, whereas status type (St) and subsistence type (Su) only appeared in models with low DIC weight (<0.05). The interaction between subsistence type and RS type did not appear in any model.
Table 2.
Averaged model coefficients based on DIC weights (Table 1)
| Coefficient | Posterior mean | Lower 95% CI | Upper 95% CI |
| Baseline | 0.303 | 0.230 | 0.375 |
| RS type = fertility | 0.247 | 0.180 | 0.321 |
| RS type = mating success | 0.200 | 0.128 | 0.266 |
| RS type = offspring mortality | 0.084 | 0.010 | 0.158 |
| RS type = wife quality | −0.003 | −0.086 | 0.076 |
| Polygyny = absent | 0.190 | 0.111 | 0.264 |
| RS type = fertility, polygyny = absent | 0.254 | 0.169 | 0.342 |
| RS type = mating success, polygyny = absent | 0.133 | 0.063 | 0.208 |
| RS type = offspring mortality, polygyny = absent | 0.012 | −0.088 | 0.105 |
| RS type = wife quality, polygyny = absent | 0.146 | 0.067 | 0.227 |
| Age control = no | 0.302 | 0.229 | 0.378 |
| Subsistence = horticulture | 0.301 | 0.230 | 0.375 |
| Subsistence = pastoralism | 0.308 | 0.234 | 0.380 |
| Subsistence = agriculture | 0.305 | 0.235 | 0.380 |
| Status = hunting ability | 0.304 | 0.232 | 0.377 |
| Status = political influence | 0.304 | 0.232 | 0.377 |
| Status = wealth | 0.304 | 0.233 | 0.378 |
The baseline level refers to RS type = surviving offspring, subsistence = foraging, status = formidability, polygyny = present, age control = yes, and other coefficients represent deviations from this baseline as indicated. All effect sizes were coded such that positive signs indicate positive contributions to RS (e.g., a negative effect of status on offspring mortality was coded as positive). Though there is substantial variation according to RS type and polygyny (Fig. 1), effect sizes are largely the same for different subsistence types or different status types.
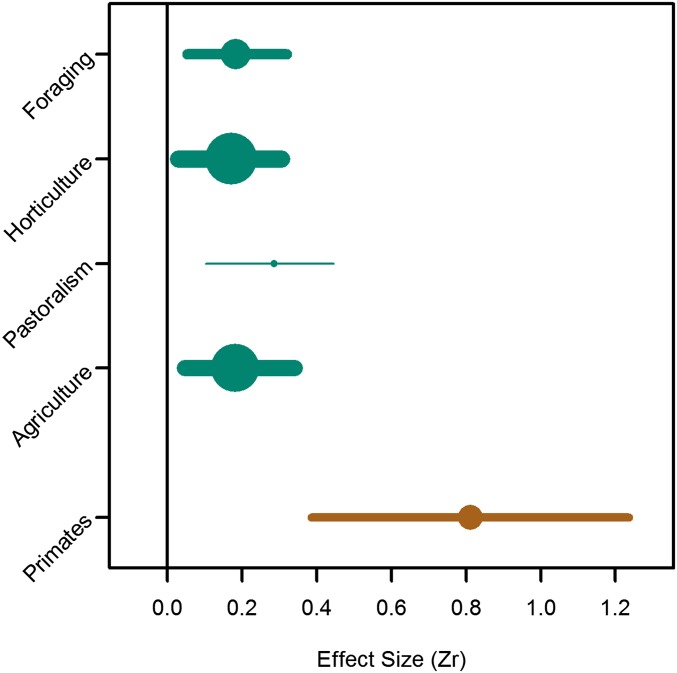
Because polygyny is somewhat confounded with subsistence type, being more common among pastoralists and horticulturalists, the strong polygyny effect found in the model comparison could eclipse any effect of subsistence type. To provide another more direct test of the egalitarianism hypothesis, we therefore fit a model with subsistence type as the only covariate (DIC = −221), which showed no significant differences in the effects of status on RS across foragers (Zr = 0.18, 95% CI = 0.05–0.32, k = 64), horticulturalists (Zr = 0.17, 95% CI = 0.03–0.31, k = 108), agriculturalists (Zr = 0.18, 95% CI = 0.05–0.34, k = 101), and pastoralists (Zr = 0.29, 95% CI = 0.10–0.44, k = 15), though it tended to be slightly higher among the latter (Fig. 2).
Fig. 2.
Comparing weighted effect sizes of men’s status on measures of RS, from the model with subsistence as the only covariate, with effects of male dominance rank on mating success in nonhuman primates (16). Minimal variation was found across subsistence types, yet as a group, humans have significantly lower effects of male status on reproduction compared with nonhuman primates. Point size and line width are proportional to the number of results contributing to each weighted effect size.
To put these results into a larger comparative context, we conducted a phylogenetic multilevel metaanalysis of the association between male rank and mating success among nonhuman primates, focusing on just one dataset (16), which resulted in an overall weighted effect size that was significantly higher than the one for humans (Zr = 0.80, 95% CI = 0.43–1.19, k = 53; Fig. 2). These results did not change qualitatively when excluding adolescent males from the dataset (16).
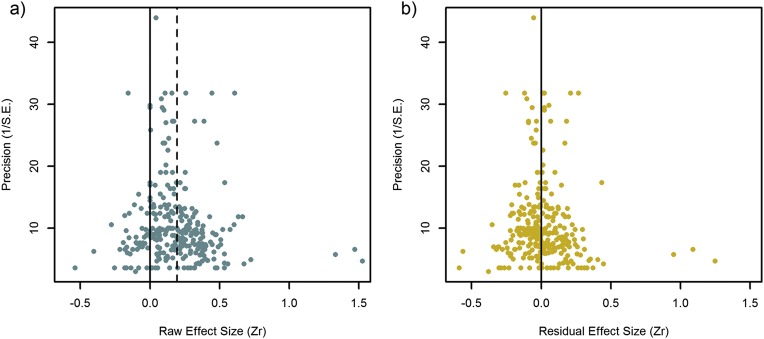
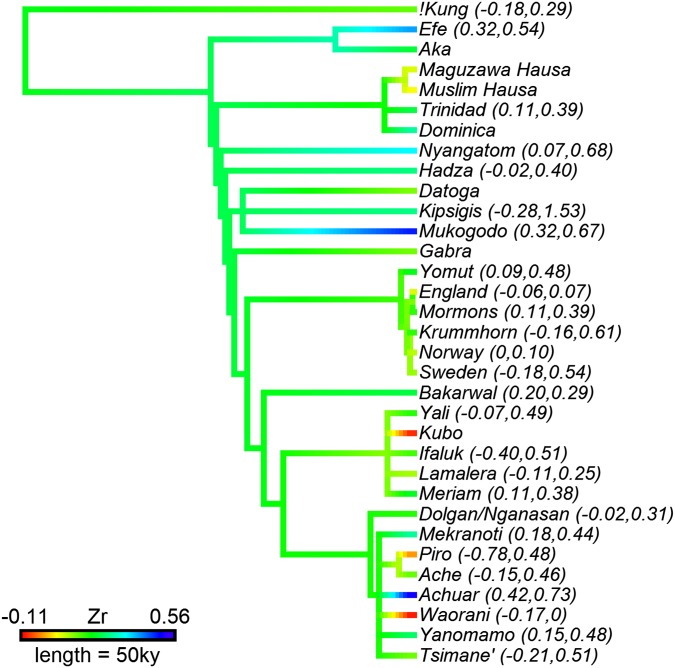
No significant publication bias was detected in any model (Methods and Fig. S1), and variation explained at the level of studies and populations, as well as by phylogeny, was minimal throughout (<1% of total variance; Fig. S2).
Fig. S1.
Funnel plots on (A) the raw effect sizes and (B) the metaregression residuals, taken from the averaged model (Table 2). Solid vertical line indicates zero, and dashed vertical line indicates overall weighted effect size from the intercept-only model. Asymmetry in the funnel plot, specifically missing null or negative effects at low precision (lower left corner of the funnel), would indicate publication bias. Even though there seem to be a few outliers with large effects at low precision, Egger’s test did not reveal significant asymmetry for any model, hence publication bias does not seem to be a concern for this field.
Fig. S2.
Phylogenetic tree of the study populations with average effect sizes color coded and ranges indicated in parentheses for populations with multiple measures. Values for ancestral branches represent maximum-likelihood estimates (100), and the length of the legend bar indicates 50,000 y. Consistent with the low phylogenetic signals found in the models, variation within populations can exceed total variation in average effect sizes (e.g., Krummhorn, Piro, and Tsimane), average effect sizes do not visibly cluster, and all ancestral branches are reconstructed at similar values; this suggests that the association between male status and fitness depends on current socioecological conditions and not (vertical) cultural transmission.
Discussion
Male social status associates with RS across nonindustrial human societies (Fig. 1). Contra the egalitarianism hypothesis, the effect of status on men’s RS does not differ significantly by subsistence type (Fig. 2), despite subsistence-associated variation in political egalitarianism. These results suggest positive selection for traits that facilitate status acquisition (including men’s motivation to seek it) did not increase substantially when foragers began domesticating plants and animals. In fact, the association between status and reproduction during the Pleistocene and Early Holocene may have been greater than we estimate for modern foragers, if modern foragers are more egalitarian due to confinement to marginal habitat (but see ref. 47). Nonetheless, selection for status-enhancing traits throughout human evolution does not entail that the lineages of particular high-status men consistently experienced greater RS (48). The genotypes of high-status men may at times represent fitness peaks, which mutation and sexual recombination break down in successive generations. Status acquisition may depend on adaptations that condition behavior to uncorrelated genetic variation, such as genetic variation that is associated with intelligence or strength and maintained from generation to generation by mutation–selection balance (49).
Why do we not find a stronger effect of status in nonforaging societies? First, effects of status vary considerably within subsistence categories. Yanomamö warriors but not Waorani warriors produced more surviving offspring, despite similar political ecologies and horticultural subsistence (50, 51). In studies of rural communities in 19th-century Finland and Sweden, wealth disparity was low and not a strong predictor of selection in the former (52), but land ownership associated with number of surviving offspring in the latter (53). Even within the same population, the effect of status can vary significantly (Fig. S2). Among the Tsimane’ of Amazonian Bolivia, villages differ in political inequality (44) and whether political influence associates with RS (Dataset S1), in proportion to their distance from the market town. There may be more variation within than between subsistence categories in the socioecological factors that favor inequality, including constraints on migration (54), access to and inheritance of monopolizable material wealth (37–40, 55), and collective action problems that catalyze more centralized or coercive leadership (41–44). Future studies of status and reproduction within and across societies should apply more direct metrics of these factors.
Second, the effect of status on men’s RS does not vary by status measure, whether status is represented by physical formidability, hunting skill, material wealth, or political influence; this suggests that different, population-specific means of gaining status can be equally effective when it comes to men’s RS. William Irons, one of the first researchers to test the status–reproduction relationship in a small-scale society, made a similar claim: “In most human societies cultural success consists in accomplishing those things which make biological success… probable” (56). If we had measures of status that aligned more closely with dominance or prestige, we may have found greater variation in their effects on reproduction, though dominance and prestige are difficult to disentangle. Even physical formidability combines dominance and prestige, because larger men are often preferred as leaders, or provide other benefits as coalition members (28). Also, our measures of status likely vary in how much they represent dominance or prestige depending on the population, e.g., political influence may involve more coercion in Oceanic horticulturalists, who recognize chiefs, relative to Amazonian horticulturalists. In Oceania, land is more of a limiting factor in men’s production, which can increase conflict, material wealth inequality, and political inequality (39).
Third, access to more monopolizable material wealth can create tradeoffs between status and fertility. Investments in status motivate reduced fertility where offspring quality depends heavily on inherited material wealth (57). Those at the very top of the hierarchy may not face such tradeoffs, which could explain why Y chromosome studies find large increases in reproductive skew associated with the rise of agriculture (18), yet we find no greater, linear effect of status in nonforaging populations.
We find support for the mating effort hypothesis. The smaller effect of status on offspring mortality relative to mating success and fertility suggests traits related to male status evolved to enhance mating effort more than parenting effort. At least, status is more effective in enhancing mating success and fertility, perhaps due to unavoidable extrinsic causes of offspring mortality. Nevertheless, the pursuit of status can represent multiple reproductive strategies, within and across individuals. A study of the Tsimane’ found that high-status men have more extramarital affairs, but they also have higher fertility and lower offspring mortality within their marriages (13). Also, reproductive strategies change over the life course. In a separate study of Tsimane’ men, extramarital affairs declined as men’s number of dependents increased (58). A study of Hadza foragers found that men increase their production of less-widely shared foods when they have newborns in camp (59). In many societies, fatherhood is associated with a decrease in testosterone level (60). The change in testosterone may facilitate a shift not away from status seeking, but rather away from aspects of status seeking that focus on mate access and toward aspects of status seeking that focus on social network building (61). Status may increasingly serve an insurance function as men age, enabling preferential access to resources during periods of famine, illness, or conflict (62). In various nonindustrial societies, status is synonymous with number of allies (13), who are sources of aid during conflict (63), illness (33, 64), and resource shortage (12, 65). Even if these social connections do not lower offspring mortality, they can be instrumental to adult offspring’s own status acquisition (66).
The effect of status on number of surviving offspring, the RS measure that most closely corresponds to biological fitness, is roughly a third smaller in monogamous populations (Fig. 1 and Table 2). Monogamy cross-cuts subsistence mode: foragers (37%), horticulturalists (22%), pastoralists (0%), and agriculturalists (55%). The monogamy effect is due in part to lower variance across men in mating success, including lower variance in number of mates and in years married. In foragers, lower frequency of polygyny associates with greater parity in the time men spend married, because more men are marrying at earlier ages (67). In 19th-century Utah, transitions to monogamy reduced the strength of sexual selection among men, due equally to greater parity in the time men spend married and in their reproductive rate (68).
High-status men in our sample of monogamous but not polygynous societies are married to higher-quality wives (e.g., more productive, higher body mass index, lower interbirth interval), which may reflect an effect of monogamy on the market value of high-status men. Dowry is more prevalent in monogamous, economically stratified societies, arguably for the same reason (69). Despite the association of status with wife quality in monogamous societies, the children of high-status men in our sample of polygynous but not monogamous societies are less likely to die before reproductive age. Though polygyny can positively associate with child health within communities (70), our offspring mortality effect is not necessarily concentrated in polygynous households. Only two of the nine populations with negative associations between status and offspring mortality show high levels of polygyny, whereas polygyny in the other seven populations is relatively infrequent and typically sororal.
Why societies are monogamous or have a lower frequency of polygyny may depend on socioecological factors, which are partly independent of broad subsistence categories and which increase the competitiveness of lower-status men, increase the costs to high-status men of having multiple mating partners, or increase female choice. These factors include low variance in men’s mate value (71, 72), higher percent contribution by men to household diet (73), dilution of wealth transmission across generations (74), and a male-biased operational sex ratio (75, 76). In populations where operational sex ratios are male biased, men may derive more value from status as a means of maintaining an existing mate than as a means of acquiring new mates (75), or at least more so than in populations where operational sex ratios are female biased. In the latter, men suffer fewer costs from pursuing a less-committed sexual strategy because men are in greater demand (76). An alternative evolutionary explanation of marriage practices emphasizes cultural group selection, whereby monogamy has spread, at least in agricultural societies, by reducing intragroup sexual competition and thus increasing group productivity and competitiveness (77). The near absence of any phylogenetic signal in our data speaks against a strong influence of vertical cultural transmission, though it does not preclude horizontal cultural transmission. Monogamy among the foragers and horticulturalists in our sample is partly a consequence of contact with Christianity. However, cultural evolution models alone do not easily explain why high-status men would forego reproductive opportunities.
The reduction in reproductive gains for high-status men in monogamous societies, at least when measured as surviving offspring, is echoed by the overall lower effects of status on reproduction in humans compared with nonhuman primates (Fig. 2). This reduction in reproductive payoffs to high status could reflect increased interdependence in food production, greater ability to form leveling coalitions, increased payoffs to mate-guarding due to changes in sex ratio, or increased opportunities for female choice (75, 78). Arranged marriage has likely been common since at least the early migrations of humans out of Africa (79), but even when male kin control marriage arrangements, females may exert significant mate choice via surreptitious extrapair copulation (80).
Why women pursue status may differ from men. Some have argued that sexual selection theory predicts men are more driven to compete for privileged positions within community-wide coalitions, whereas women are more motivated by a strong dyadic support network (81, 82). Women also face constraints on community-wide networking due to the sexual division of labor. However, women in nonindustrial societies often have important roles in community decision-making (81, 83) and conflict resolution (84, 85), particularly where inheritance is matrilineal (81). Better understanding of how women’s status is shaped by socioecological variation may be a boon for policies aimed at increasing the representation of women in leadership positions, and remains an open field of investigation for evolutionary anthropologists.
Methods
A multifaceted search produced the 46 studies that comprise the metaanalysis. Journals that focus on anthropology (e.g., Current Anthropology) and the behavioral sciences (e.g., Evolution and Human Behavior) as well as more general science journals (e.g., Science) were repeatedly searched for terms related to social status, including status, hunting, leadership, and wealth. The reference list of each relevant study was scoured for additional studies. Criteria for inclusion were a nonindustrial sample, at least one measure of male social status, and at least one quantitative association of male social status with a measure of RS. We excluded two studies that restricted their analysis to status grades within the nobility rather than across the entire population (86, 87). Status measures could include direct measures of status (e.g., political leadership) or more indirect measures of status (e.g., wealth) that the study authors associated with status in their sample. Measures of RS could include number of offspring surviving to a certain age, fertility, offspring mortality rate, and their proximate determinants, including number of mates, mates’ interbirth interval, the health of mates and offspring, mates’ productivity, and age of spouses at marriage.
Effect sizes were calculated from the published information on statistical tests (e.g., r, d, or F statistics, sample size n, and/or associated P values), and all effect sizes were transformed into Fisher’s Z (Zr) and its associated variance following standard metaanalytic procedures (88). All effect sizes were coded such that positive signs indicate positive contributions to RS (e.g., a negative effect of status on offspring mortality or age of marriage was coded as positive). Where incomplete or insufficient information was published [e.g., P < 0.05, not significant (n.s.)], we solicited complete information or raw data from the first author for studies published in the past 5 y, and otherwise conservatively used the upper boundary of the published interval (P < 0.05) or assumed an effect size of zero (n.s.). A dummy variable was included to indicate whether the analysis controlled for men’s age, either by focusing only on completed fertility, by including age as a covariate, by analyzing different age cohorts separately, or by age-matching high- and low-status men. The collection of all analyzed data was approved by the universities affiliated with the respective studies.
We used Bayesian phylogenetic multilevel metaregression (89, 90) to estimate an overall weighted effect size and to model the influence of different covariates. We controlled for cultural and phylogenetic history by including a phylogenetic tree (Fig. S2) based on a recent supertree of human cultures that combines linguistic and genetic information (91). See SI Methods for details. Each effect size was weighted by its SE. To account for repeated measures, variation at the level of studies and populations was modeled using random effects. For our model comparison, the full model considered was (see model 11 in ref. 89):
All possible combinations of these covariates were fit and ranked based on their DIC weight using the MuMIn package (92). Coefficients were then averaged according to DIC weight to produce an average model, setting coefficients of parameters not included in a model to zero (93). In all analyses the proportion of total variance explained by the study-level and population-level random effects as well as the phylogenetic signal (90) was <0.01, indicating that behavioral variation within levels far outweighed variation between levels (Fig. S2). We tested for publication bias using Egger’s regression on the metaregression residuals (90); no analysis revealed significant publication bias (Fig. S1), consistent with negative results in this field being published in high-impact journals (51). For the analysis of the nonhuman primate sample, a consensus tree was downloaded from the 10kTrees website (94) and intraspecific variation was modeled using a species-level random effect (90).
All analyses were run in R 3.0.2 (95) using the MCMCglmm package (89) with standard inverse gamma priors for random effects, a burn-in period of 20,000 iterations, and 100,000 iterations in total thinned to 8,000 samples. Model convergence was assessed visually by plotting time series and histograms of the Markov chains (Fig. S3) and by calculating the Gelman–Rubin diagnostic on multiple runs of the same model (<1.1 in all analyses).
Fig. S3.
Examples of MCMCglmm parameter estimates from the subsistence type-only model. (Left) Time series of the parameter as the Markov chain iterates. (Right) Posterior density estimate of the parameter (89). The fact that there is no trend in the time series indicates that the model has converged, yielding nice posterior distributions from which to calculate means and confidence intervals.
SI Methods: Constructing the Phylogeny
To build a phylogenetic tree of all study populations, we based the branching structure on a recent supertree of human cultures (91), adding or matching our study populations (if they were not already included on the supertree) with populations on the supertree as follows: Achuar, unresolved polytomy within South America; Bakarwal, Kalash (based on geography); Datoga, unresolved polytomy of East African pastoralists placed on Masaai branch; Dolgan/Nganasan, Yakut (based on geography); Dominica, West African polytomy placed on Fulani branch; Efe, Mbuti; Gabra, Oromo; Ifaluk, Kosrean (based on geography); Kipsigis, unresolved polytomy of East African pastoralists placed on Masaai branch; Krummhorn, unresolved polytomy placed on German branch; Kubo, Goroka (based on geography); Lamalera, Lembata; Mekranoti, Kaingang; Meriam, Kambera; Mormons, sister taxon to England; Mukogodo, unresolved polytomy of East African pastoralists placed on Masaai branch; Norway, unresolved polytomy placed on German branch; Nyangatom, Anuahk (based on language family); Piro, Curripaco (based on language family); Sweden, unresolved polytomy placed on German branch; Trinidad, West African polytomy placed on Fulani branch; Tsimane, unresolved polytomy within South America; Waorani, unresolved polytomy within South America; Yanomamo, unresolved polytomy within South America; Yomut, Turk.
We then time-calibrated our tree using the chronos function in the ape package (96), using the following dates to constrain the calibration: root, 134–188 ky (97); divergence of pygmies and Bantu, 70 ky (98); divergence Western and Eastern pygmies, 27 ky (98); divergence between Africans and non-Africans, 62–95 ky (97); divergence of Amerindians, 15 ky (99); deepest node within South America, 12–14 ky (99); divergence of Papuans/Australians, 45 ky (99); divergence between Muslim and Maguzawa Hausa, 3–0.1 ky; and divergence between England and Mormons, 0.5–0.1 ky.
Supplementary Material
Acknowledgments
We thank Michael Gurven, Paul Hooper, and three anonymous reviewers who provided comments on earlier drafts that led to substantial improvements; Shinichi Nakagawa for sharing his R code to run Egger’s regression; and Eric Alden Smith, John Ziker, and Luke Glowacki who generously provided analyses or data associated with their published studies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10739.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1606800113/-/DCSupplemental.
References
- 1.Anderson C, Hildreth JA, Howland L. Is the desire for status a fundamental human motive? A review of the empirical literature. Psychol Bull. 2015;141(3):574–601. doi: 10.1037/a0038781. [DOI] [PubMed] [Google Scholar]
- 2.Barkow J. Prestige and culture: A biosocial interpretation. Curr Anthropol. 1975;16(4):553–572. [Google Scholar]
- 3.Henrich J, Gil-White FJ. The evolution of prestige: Freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol Hum Behav. 2001;22(3):165–196. doi: 10.1016/s1090-5138(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JT, Tracy JL, Foulsham T, Kingstone A, Henrich J. Two ways to the top: Evidence that dominance and prestige are distinct yet viable avenues to social rank and influence. J Pers Soc Psychol. 2013;104(1):103–125. doi: 10.1037/a0030398. [DOI] [PubMed] [Google Scholar]
- 5.Chapais B. Competence and the evolutionary origins of status and power in humans. Hum Nat. 2015;26(2):161–183. doi: 10.1007/s12110-015-9227-6. [DOI] [PubMed] [Google Scholar]
- 6.Lukaszewski AW, Simmons ZL, Anderson C, Roney JR. The role of physical formidability in human social status allocation. J Pers Soc Psychol. 2016;110(3):385–406. doi: 10.1037/pspi0000042. [DOI] [PubMed] [Google Scholar]
- 7.Miller G. The Mating Mind: How Sexual Choice Shaped the Evolution of Human Nature. Anchor Books; New York: 2001. [Google Scholar]
- 8.Frank R. Luxury Fever: Why Money Fails to Satisfy in an Era of Excess. Free Press; New York: 1999. [Google Scholar]
- 9.Harbaugh W. The prestige motive for making charitable transfers. Am Econ Rev. 1998;88(2):277–282. [Google Scholar]
- 10.Barclay P, Willer R. Partner choice creates competitive altruism in humans. Proc Biol Sci. 2007;274(1610):749–753. doi: 10.1098/rspb.2006.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hrdy S. Mother Nature: Maternal Instincts and How They Shape the Human Species. Ballantine; New York: 1999. [Google Scholar]
- 12.Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: The role of transfers in shaping the evolved human life history. Exp Gerontol. 2012;47(10):807–813. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Rueden C, Gurven M, Kaplan H. Why do men seek status? Fitness payoffs to dominance and prestige. Proc Roy Soc B. 2011;278(1715):2223–2232. doi: 10.1098/rspb.2010.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehm C. Hierarchy in the Forest: The Evolution of Egalitarian Behavior. Harvard Univ Press; Cambridge, MA: 1999. [Google Scholar]
- 15.Gavrilets S. On the evolutionary origins of the egalitarian syndrome. Proc Natl Acad Sci USA. 2012;109(35):14069–14074. doi: 10.1073/pnas.1201718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowlishaw G, Dunbar R. Dominance rank and mating success in male primates. Anim Behav. 1991;41(6):1045–1056. [Google Scholar]
- 17.Betzig L. Means, variances, and ranges in reproductive success: Comparative evidence. Evol Hum Behav. 2012;33(4):309–317. [Google Scholar]
- 18.Karmin M, et al. A recent bottleneck of Y chromosome diversity coincides with a global change in culture. Genome Res. 2015;25(4):459–466. doi: 10.1101/gr.186684.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balaresque P, et al. Y-chromosome descent clusters and male differential reproductive success: Young lineage expansions dominate Asian pastoral nomadic populations. Eur J Hum Genet. 2015;23(10):1413–1422. doi: 10.1038/ejhg.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poznik GD, et al. 1000 Genomes Project Consortium Punctuated bursts in human male demography inferred from 1,244 worldwide Y-chromosome sequences. Nat Genet. 2016;48(6):593–599. doi: 10.1038/ng.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopcroft R. Sex, status, and reproductive success in the contemporary United States. Evol Hum Behav. 2006;27(2):104–120. [Google Scholar]
- 22.Fieder M, Huber S. The effects of sex and childlessness on the association between status and reproductive output in modern society. Evol Hum Behav. 2007;28(6):392–398. [Google Scholar]
- 23.Nettle D, Pollet TV. Natural selection on male wealth in humans. Am Nat. 2008;172(5):658–666. doi: 10.1086/591690. [DOI] [PubMed] [Google Scholar]
- 24.Barthold J, Myrskyla M, Jones O. Childlessness drives the sex difference in the association between income and reproductive success of modern Europeans. Evol Hum Behav. 2012;33(6):628–638. [Google Scholar]
- 25.Kelly R. The Foraging Spectrum: Diversity in Hunter-Gatherer Lifeways. Smithsonian Institution Press; Washington, DC: 1995. [Google Scholar]
- 26.Wiessner P. In: Food and the Status Quest: An Interdisciplinary Perspective. Wiessner P, Schiefehovel W, editors. Berghahn Books; Providence, RI: 1996. pp. 171–192. [Google Scholar]
- 27.Jaeggi AV, Boose KF, White FJ, Gurven M. Obstacles and catalysts of cooperation in humans, bonobos, and chimpanzees: Behavioural reaction norms can help explain variation in sex roles, inequality, war and peace. Behaviour. 2016 doi: 10.1163/1568539X-00003347. [DOI] [Google Scholar]
- 28.von Rueden C, Gurven M, Kaplan H, Stieglitz J. Leadership in an egalitarian society. Hum Nat. 2014;25(4):538–566. doi: 10.1007/s12110-014-9213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JE, et al. Leadership in mammalian societies: Emergence, distribution, power, and payoff. Trends Ecol Evol. 2016;31(1):54–66. doi: 10.1016/j.tree.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Hawkes K, Bliege Bird R. Showing off, handicap signaling, and the evolution of men’s work. Evol Anthr. 2002;11(1):58–67. [Google Scholar]
- 31.Smith EA. Why do good hunters have higher reproductive success? Hum Nat. 2004;15(4):343–364. doi: 10.1007/s12110-004-1013-9. [DOI] [PubMed] [Google Scholar]
- 32.Gurven M, von Rueden C. Hunting, social status and biological fitness. Soc Biol. 2006;53(1-2):81–99. doi: 10.1080/19485565.2006.9989118. [DOI] [PubMed] [Google Scholar]
- 33.Gurven M, Allen-Arave W, Hill K, Hurtado M. “It’s a Wonderful Life”. Signaling generosity among the Ache of Paraguay. Evol Hum Behav. 2000;21(4):263–282. doi: 10.1016/s1090-5138(00)00032-5. [DOI] [PubMed] [Google Scholar]
- 34.Hiatt L. Arguments about Aborigines: Australia and the Evolution of Social Anthropology. Cambridge Univ Press; Cambridge, UK: 1996. [Google Scholar]
- 35.Ames KM. The Northwest Coast. Evol Anthr. 2003;12(1):19–33. [Google Scholar]
- 36.Arnold J. In: The Evolution of Leadership: Transitions in Decision Making from Small-Scale to Middle-Range Societies. Vaughn KJ, Eerkens JW, Kantner J, editors. SAR; Santa Fe, NM: 2010. pp. 121–146. [Google Scholar]
- 37.Sellen D, Hruschka D. Extracted food resource defense polygyny in native western North American societies at contact. Curr Anthropol. 2004;45(5):707–714. [Google Scholar]
- 38.Cashdan EA. Egalitarianism among hunters and gatherers. Am Anthropol. 1980;82(1):116–120. [Google Scholar]
- 39.Kaplan HS, Hooper PL, Gurven M. The evolutionary and ecological roots of human social organization. Philos Trans R Soc Lond B Biol Sci. 2009;364(1533):3289–3299. doi: 10.1098/rstb.2009.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borgerhoff Mulder M, et al. Intergenerational wealth transmission and the dynamics of inequality in small-scale societies. Science. 2009;326(5953):682–688. doi: 10.1126/science.1178336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Service E. Origins of the State and Civilization. Norton; New York: 1975. [Google Scholar]
- 42.Carneiro RL. The transition from quantity to quality: A neglected causal mechanism in accounting for social evolution. Proc Natl Acad Sci USA. 2000;97(23):12926–12931. doi: 10.1073/pnas.240462397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper PL, Kaplan HS, Boone JL. A theory of leadership in human cooperative groups. J Theor Biol. 2010;265(4):633–646. doi: 10.1016/j.jtbi.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 44.Glowacki L, von Rueden C. Leadership solves collective action problems in small-scale societies. Phil Trans Roy Soc B. 2015;370(1683):20150010. doi: 10.1098/rstb.2015.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkes K. In: Kinship and Behavior in Primates. Chapais B, Berman CM, editors. Oxford Univ Press; Oxford: 2004. pp. 443–473. [Google Scholar]
- 46.Winking J, Gurven M, Kaplan H, Stieglitz J. The goals of direct paternal care among a South Amerindian population. Am J Phys Anthropol. 2009;139(3):295–304. doi: 10.1002/ajpa.20981. [DOI] [PubMed] [Google Scholar]
- 47.Marlowe FW. Hunter-gatherers and human evolution. Evol Anthr. 2005;14(2):54–67. doi: 10.1016/j.jhevol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Lansing JS, et al. Male dominance rarely skews the frequency distribution of Y chromosome haplotypes in human populations. Proc Natl Acad Sci USA. 2008;105(33):11645–11650. doi: 10.1073/pnas.0710158105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Rueden C, Lukaszewski A, Gurven M. Adaptive personality calibration in a human society: Effects of embodied capital on prosocial traits. Behav Ecol. 2015;26(4):1071–1082. [Google Scholar]
- 50.Chagnon NA. Life histories, blood revenge, and warfare in a tribal population. Science. 1988;239(4843):985–992. doi: 10.1126/science.239.4843.985. [DOI] [PubMed] [Google Scholar]
- 51.Beckerman S, et al. Life histories, blood revenge, and reproductive success among the Waorani of Ecuador. Proc Natl Acad Sci USA. 2009;106(20):8134–8139. doi: 10.1073/pnas.0901431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Courtiol A, Pettay JE, Jokela M, Rotkirch A, Lummaa V. Natural and sexual selection in a monogamous historical human population. Proc Natl Acad Sci USA. 2012;109(21):8044–8049. doi: 10.1073/pnas.1118174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low B. Occupational status, land ownership, and reproductive behavior in 19th century Sweden: Tuna parish. Am Anthropol. 1990;92(2):457–468. [Google Scholar]
- 54.Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Anim Behav. 1983;31:667–682. [Google Scholar]
- 55.Boone JL. In: Evolutionary Ecology and Human Behavior. Smith EA, Winterhalder B, editors. de Gruyter; New York: 1992. pp. 301–337. [Google Scholar]
- 56.Irons W. In: Evolutionary Biology and Human Social Behavior: An Anthropological Perspective. Chagnon NA, Irons W, editors. Duxbury; North Scituate, MA: 1979. pp. 284–302. [Google Scholar]
- 57.Shenk MK, Kaplan H, Hooper PL. Status competition, inequality, and fertility: Implications for the demographic transition. Phil Trans R Soc B. 2016;371(1692):20150150. doi: 10.1098/rstb.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winking J, Kaplan H, Gurven M, Rucas S. Why do men marry and why do they stray? Proc Biol Sci. 2007;274(1618):1643–1649. doi: 10.1098/rspb.2006.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marlowe F. A critical period for provisioning by Hadza men. Evol Hum Behav. 2003;24(3):217–229. [Google Scholar]
- 60.Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc Natl Acad Sci USA. 2011;108(39):16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gettler LT. Testosterone, fatherhood, and social networks. In: Trevathan W, Rosenberg KR, editors. Costly and Cute: Helpless Infants and Human Evolution. Univ of New Mexico Press; Albuquerque, NM: 2016. pp. 149–176. in press. [Google Scholar]
- 62.Boone JL. The evolution of magnanimity: When is it better to give than to receive? Hum Nat. 1998;9(1):1–21. doi: 10.1007/s12110-998-1009-y. [DOI] [PubMed] [Google Scholar]
- 63.Patton JQ. Meat sharing for coalitional support. Evol Hum Behav. 2005;26(2):137–157. [Google Scholar]
- 64.Lyle HF, 3rd, Smith EA. The reputational and social network benefits of prosociality in an Andean community. Proc Natl Acad Sci USA. 2014;111(13):4820–4825. doi: 10.1073/pnas.1318372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiessner P. Hunting, healing, and hxaro exchange: A long-term perspective on !Kung (Ju/’hoansi) large-game hunting. Evol Hum Behav. 2002;23(6):407–436. [Google Scholar]
- 66.Scelza BA. Fathers’ presence speeds the social and reproductive career of sons. Curr Anthropol. 2010;51(2):295–303. [Google Scholar]
- 67.Marlowe FW, Berbesque JC. The human operational sex ratio: Effects of marriage, concealed ovulation, and menopause on mate competition. J Hum Evol. 2012;63(6):834–842. doi: 10.1016/j.jhevol.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Moorad JA, Promislow D, Smith KR, Wade MJ. Mating system change reduces the strength of sexual selection in an American frontier population of the 19th century. Evol Hum Behav. 2011;32(2):147–155. doi: 10.1016/j.evolhumbehav.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaulin SJ, Boster JS. Dowry as female competition. Am Anthropol. 1990;92(4):994–1005. [Google Scholar]
- 70.Lawson DW, et al. No evidence that polygynous marriage is a harmful cultural practice in northern Tanzania. Proc Natl Acad Sci USA. 2015;112(45):13827–13832. doi: 10.1073/pnas.1507151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Low BS. Marriage systems and pathogen stress in human societies. Am Zool. 1990;30:325–339. [Google Scholar]
- 72.Pollet TV, Nettle D. Market forces affect patterns of polygyny in Uganda. Proc Natl Acad Sci USA. 2009;106(7):2114–2117. doi: 10.1073/pnas.0810016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marlowe F. The mating system of foragers in the standard cross-cultural sample. Cross-Cultural Res. 2003;37:282–306. [Google Scholar]
- 74.Fortunato L, Archetti M. Evolution of monogamous marriage by maximization of inclusive fitness. J Evol Biol. 2010;23(1):149–156. doi: 10.1111/j.1420-9101.2009.01884.x. [DOI] [PubMed] [Google Scholar]
- 75.Coxworth JE, Kim PS, McQueen JS, Hawkes K. Grandmothering life histories and human pair bonding. Proc Natl Acad Sci USA. 2015;112(38):11806–11811. doi: 10.1073/pnas.1599993112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schacht R, Borgerhoff Mulder M. Sex ratio effects on reproductive strategies in humans. R Soc Open Sci. 2015;2(1):140402. doi: 10.1098/rsos.140402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Henrich J, Boyd R, Richerson PJ. The puzzle of monogamous marriage. Philos Trans R Soc Lond B Biol Sci. 2012;367(1589):657–669. doi: 10.1098/rstb.2011.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapais B. Monogamy, strongly bonded groups, and the evolution of human social structure. Evol Anthropol. 2013;22(2):52–65. doi: 10.1002/evan.21345. [DOI] [PubMed] [Google Scholar]
- 79.Walker RS, Hill KR, Flinn MV, Ellsworth RM. Evolutionary history of hunter-gatherer marriage practices. PLoS One. 2011;6(4):e19066. doi: 10.1371/journal.pone.0019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scelza BA. Female choice and extra-pair paternity in a traditional human population. Biol Lett. 2011;7(6):889–891. doi: 10.1098/rsbl.2011.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Low B. Sex, coalitions, and politics in pre-industrial societies. Politics Life Sci. 1992;11(1):63–80. [Google Scholar]
- 82.Benenson J, Markovits H. Warriors and Worriers: The Survival of the Sexes. Oxford Univ Press; New York: 2014. [Google Scholar]
- 83.Lee R. In: Politics and History in Band Societies. Leacock E, Lee R, editors. Cambridge Univ Press; New York: 1982. pp. 37–59. [Google Scholar]
- 84.Wiessner P. Norm enforcement among the Ju/’hoansi Bushmen: A case of strong reciprocity? Hum Nat. 2005;16(2):115–145. doi: 10.1007/s12110-005-1000-9. [DOI] [PubMed] [Google Scholar]
- 85.Bowser B, Patton J. In: The Evolution of Leadership: Transitions in Decision Making from Small-Scale to Middle-Range Societies. Vaughn KJ, Eerkens JW, Kantner J, editors. SAR; Santa Fe, NM: 2010. pp. 51–71. [Google Scholar]
- 86.Boone JL. Parental investment and elite family structure in preindustrial states: A case study of late medieval-early modern Portuguese genealogies. Am Anthropol. 1986;88(4):859–878. [Google Scholar]
- 87.Feng W, Lee J, Campbell C. Marital fertility control among the Qing nobility: Implications for two types of preventive check. Popul Stud (Camb) 1995;49(3):383–400. doi: 10.1080/0032472031000148736. [DOI] [PubMed] [Google Scholar]
- 88.Card NA. Applied Meta-Analysis for Social Science Research. Guilford; New York: 2011. [Google Scholar]
- 89.Hadfield JD, Nakagawa S. General quantitative genetic methods for comparative biology: Phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J Evol Biol. 2010;23(3):494–508. doi: 10.1111/j.1420-9101.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa S, Santos ESA. Methodological issues and advances in biological meta-analysis. Evol Ecol. 2012;26(5):1253–1274. [Google Scholar]
- 91.Duda P, Jan Zrzavý Human population history revealed by a supertree approach. Sci Rep. 2016;6:29890. doi: 10.1038/srep29890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barton K. 2015 MuMIn: Multimodel inference. Version 1.13.4. Available at https://cran.r-project.org/. Accessed June 1, 2016.
- 93.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer; New York: 2002. [Google Scholar]
- 94.Arnold C, Matthews LJ, Nunn CL. The 10kTrees website: A new online resource for primate phylogeny. Evol Anthropol. 2010;19:114–118. [Google Scholar]
- 95.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- 96.Paradis E. Analysis of Phylogenetics and Evolution with R. 2nd Ed Springer; New York: 2012. [Google Scholar]
- 97.Fu Q, et al. A revised timescale for human evolution based on ancient mitochondrial genomes. Curr Biol. 2013;23(7):553–559. doi: 10.1016/j.cub.2013.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batini C, et al. Insights into the demographic history of African Pygmies from complete mitochondrial genomes. Mol Biol Evol. 2011;28(2):1099–1110. doi: 10.1093/molbev/msq294. [DOI] [PubMed] [Google Scholar]
- 99.Henn BM, Cavalli-Sforza LL, Feldman MW. The great human expansion. Proc Natl Acad Sci USA. 2012;109(44):17758–17764. doi: 10.1073/pnas.1212380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Revell LJ. Phytools: An R package for phylogenetic comparative biology (and other things) Methods Ecol Evol. 2012;3(2):217–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.