Abstract
Compared to tropical corals, much less is known about deep-sea coral biology and ecology. Although the microbial communities of some deep-sea corals have been described, this is the first study to characterize the bacterial community associated with the deep-sea octocoral, Paramuricea placomus. Samples from five colonies of P. placomus were collected from Baltimore Canyon (379–382 m depth) in the Atlantic Ocean off the east coast of the United States of America. DNA was extracted from the coral samples and 16S rRNA gene amplicons were pyrosequenced using V4-V5 primers. Three samples sequenced deeply (>4,000 sequences each) and were further analyzed. The dominant microbial phylum was Proteobacteria, but other major phyla included Firmicutes and Planctomycetes. A conserved community of bacterial taxa held in common across the three P. placomus colonies was identified, comprising 68–90% of the total bacterial community depending on the coral individual. The bacterial community of P. placomus does not appear to include the genus Endozoicomonas, which has been found previously to be the dominant bacterial associate in several temperate and tropical gorgonians. Inferred functionality suggests the possibility of nitrogen cycling by the core bacterial community.
Keywords: Cold-water coral, Bacteria, Gorgonian, Submarine canyon, Microbiome
Introduction
Cold-water corals provide critical three-dimensional habitat, creating biodiversity hot spots in the deep ocean (Buhl-Mortensen & Mortensen, 2005; Miller et al., 2012; Roberts et al., 2009). In addition to creating habitat for fishes and invertebrates, these corals are themselves landscapes for microbial associates (Penn et al., 2006; Yakimov et al., 2006). Studies on human gut microbes are revealing connections between internal microbiota and host-organism health and immunity (Turnbaugh et al., 2007); likewise, coral-associated microbiota are connected to the health and resilience of their hosts (Pantos et al., 2003; Ritchie, 2006). Bacterial pathogens or dysbiosis (microbial imbalance) have been linked to coral disease outbreaks on tropical reefs (e.g., Bythell, Pantos & Richardson, 2004; Mouchka, Hewson & Harvell, 2010) and mass die-offs of temperate corals (mainly gorgonians) (Bally & Garrabou, 2007; Hall-Spencer, Pike & Munn, 2007; Vezzulli et al., 2010). These studies have resulted in considerable attention and research into coral-associated microbes, particularly since the application of molecular tools and DNA sequencing in 2001 (Rohwer et al., 2001).
While much of the focus has remained on reef-building stony corals, increasing attention is being paid to gorgonians which also host diverse bacterial communities. Regardless of tropical, temperate, or deep-sea, most gorgonian bacterial microbiomes are dominated by Proteobacteria (Bayer et al., 2013; Brück et al., 2007; Correa et al., 2013; Duque-Alarcón, Santiago-Vázquez & Kerr, 2012; La Rivière, Garrabou & Bally, 2015; La Rivière et al., 2013; Penn et al., 2006; Ransome et al., 2014; Robertson et al., 2016; Sunagawa, Woodley & Medina, 2010; Vezzulli et al., 2013). However, Tenericutes (specifically Mycoplasmas) (Gray et al., 2011; Holm & Heidelberg, 2016) and recently Spirochaetes (Holm & Heidelberg, 2016; Lawler et al., 2016; Van de Water et al., 2016) have also been shown to be dominant or co-dominant in a few species. In gammaproteobacterial-dominated gorgonians, often the dominant genus was Endozoicomonas (Bayer et al., 2013; Correa et al., 2013; La Rivière et al., 2013; Ransome et al., 2014; Robertson et al., 2016; Vezzulli et al., 2013), while a common alphaproteobacterial genus was Stenotrophomonas (Bayer et al., 2013; Brück et al., 2007; Correa et al., 2013; Robertson et al., 2016). Comparable research on deep-sea corals has been slower, due to the high cost and difficulty of obtaining samples. Most of the focus has been on the deep-sea stony coral, Lophelia pertusa (Galkiewicz et al., 2011; Hansson et al., 2009; Kellogg, Lisle & Galkiewicz, 2009; Neulinger et al., 2008; Schöttner et al., 2009; Yakimov et al., 2006). However, microbial studies have been conducted on a few deep-sea octocorals (Gray et al., 2011; Lawler et al., 2016; Penn et al., 2006).
Paramuricea placomus (Linnaeus, 1758) is a plexaurid gorgonian coral endemic to the North Atlantic Ocean, occurring on both sides of the Atlantic basin (Buhl-Mortensen & Buhl-Mortensen, 2014; Simpson, Eckelbarger & Watling, 2005). In the western Atlantic, it has been documented on seamounts and deep reef areas along the east coast of the United States and into the Gulf of Mexico (Lumsden et al., 2007). Although commonly encountered as by-catch in fishing nets, little is known about P. placomus (Simpson, Eckelbarger & Watling, 2005). A temperate congener (P. clavata) in the Mediterranean was the focus of microbiological studies (La Rivière, Garrabou & Bally, 2010; La Rivière et al., 2013; Vezzulli et al., 2013; Vezzulli et al., 2010).
During a 2012 research expedition in and near Baltimore Canyon off the mid-Atlantic coast of the United States, we collected samples of P. placomus in order to describe the bacterial associates of a deep-sea Paramuricea species. Based on reports that have shown tropical (Littman et al., 2009; Rohwer et al., 2002), temperate (Van de Water et al., 2016), and cold-water (Kellogg, Lisle & Galkiewicz, 2009; Lawler et al., 2016) corals to have conserved bacterial communities associated with them, we hypothesized that P. placomus would also have a specific bacterial community with an identifiable core component shared by all individuals.
Materials and Methods
Sample site and collections
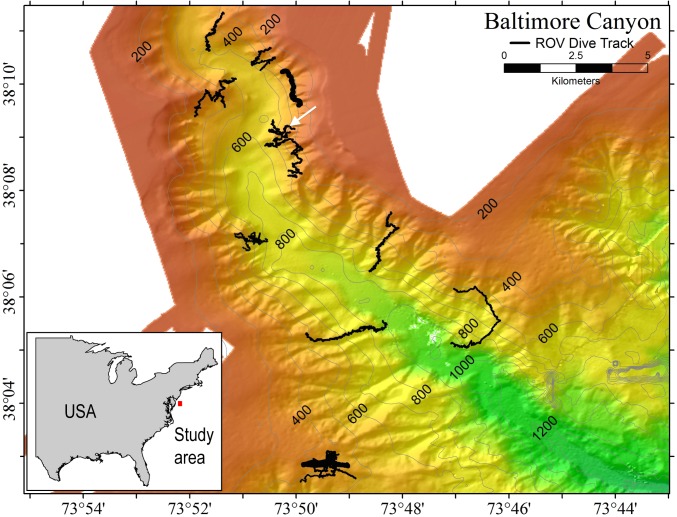
Samples of P. placomus were collected from a single area in Baltimore Canyon (38°09.08′N, 73°50.26′W; white arrow, Fig. 1) on 12 September 2012, during a research cruise using the NOAA ship Nancy Foster. In spite of a number of other dives in this canyon (Fig. 1), this coral species was only observed in this one relatively small area. Collections were made using the remotely-operated vehicle (ROV) Kraken II (Univ. of Connecticut) during dive number ROV-2012-NF-19 between 10:30–11:30 Eastern Daylight Time (14:30–15:30 Coordinated Universal Time (UTC)). The sample site was a flat plateau with a depth range of 379–382 m, water temperature of 5.8–6.0 °C and salinity of 35.0. Samples NF12.19Q2, NF12.19Q5, NF12.19Q6 and NF12.19Q7 were all within one meter of each other and were collected without repositioning the ROV. This was done both to save time on a dive with multiple other objectives (repositioning the ROV can be time consuming and stirs up the substrate reducing visibility) and because the collections were being shared with a coral genetics group that was interested in the relationships of the close colonies. The ROV was moved a short distance away (2 to 3 m) to collect NF12.19Q1, at which point all the quivers dedicated to microbiology samples were filled. All coral colonies had adult galatheid squat lobster (Eumunida picta) associates, except NF12.19Q2, which hosted several galatheids that were too small to identify. Specimen NF12.19Q1 was a larger colony, with a dark purple stalk and mainly yellow polyps, in contrast to the other four specimens which had much paler, lavender stalks, and more variegated yellow and lavender polyps (Figs. 2B and 2C).
Figure 1. Multibeam sonar map of Baltimore Canyon showing P. placomus sample collection location (white arrow).
Locations of all ROV dives made during the 2012 cruise are indicated by black lines. Depth contours are in meters.
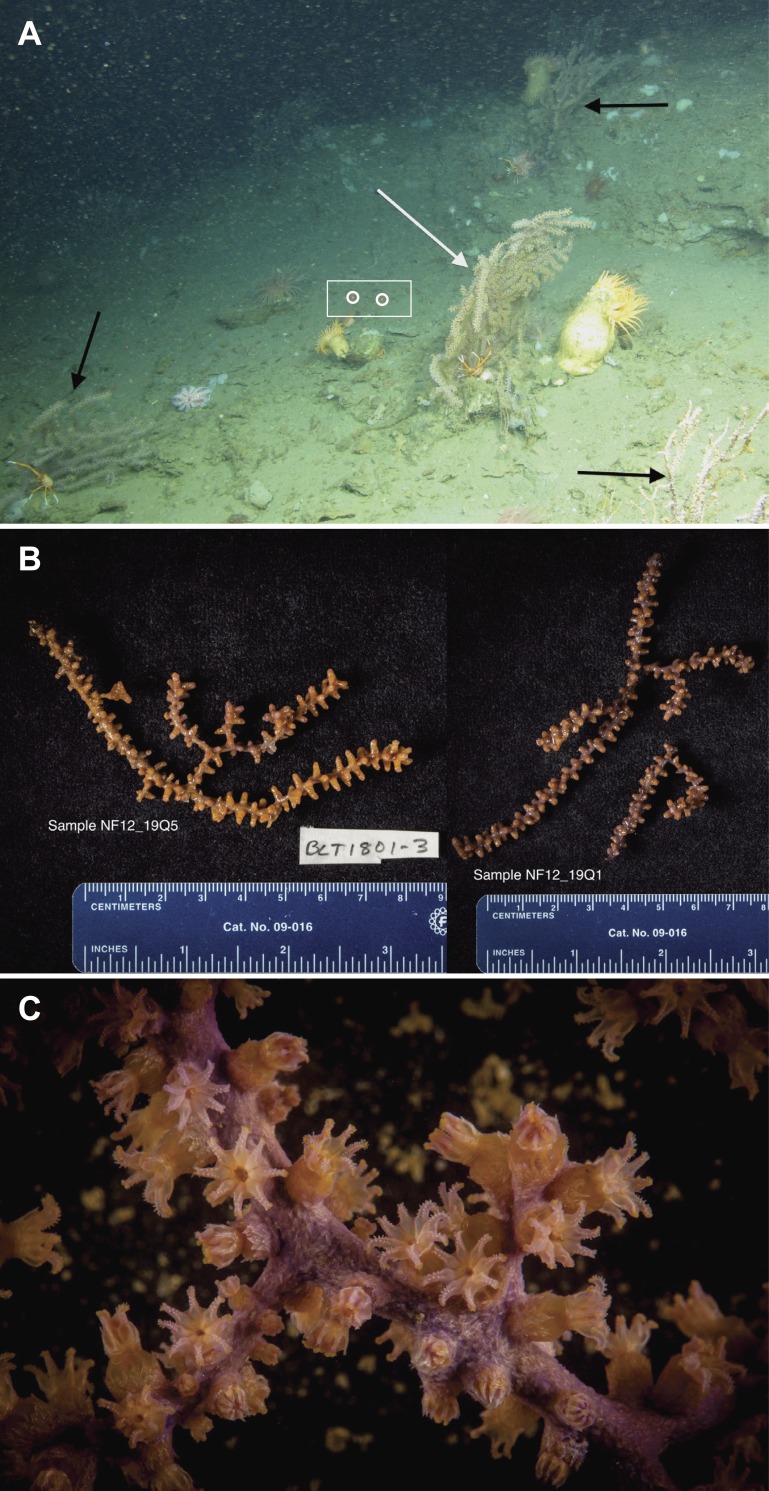
Figure 2. Images of P. placomus.
(A) In situ photo of NF12.19Q1 (white arrow) showing general site rugosity and proximity (>1 m) of other P. placomus colonies (black arrows). For scale, red laser dots (circled in white and surrounded by a white box for clarity) in the center of the image are 10 cm apart; (B) Specimen photos of NF12.19Q5 and NF12.19Q1 showing differences in color pattern; (C) Close up image of P. placomus showing color variation between the stalk and the polyps. Image credit for (A): Deepwater Canyons 2012-Pathways to the Abyss, BOEM/NOAA-OER/USGS. Image credit for (B) and (C): Art Howard, Deepwater Canyons 2012–Pathways to the Abyss, BOEM/NOAA-OER/USGS.
Small pieces of each coral colony (ca. 5–15 cm) were removed using the ROV’s manipulator arm and placed into individual polyvinyl chloride (PVC) quivers that had been washed, ethanol sterilized, filled with freshwater, and sealed with a rubber stopper while the ROV was on deck. The freshwater evacuated at depth when the quiver was opened to receive the coral sample, so that only seawater local to the coral samples was entrained during collection. Each coral sample was placed in a separate quiver and sealed at depth to prevent microbial contamination from other corals or different water masses during ascent. All collections occurred during 1 h. Upon recovery of the ROV (7 h after collection), the samples were removed from the quivers using ethanol-sterilized forceps, trimmed if necessary with ethanol-sterilized shears (to select a part of the coral sample that was not in contact with the ROV collection claw), and placed into individual, sterile 50 mL tubes. The tubes were filled with RNAlater solution (Life Technologies, Grand Island, NY, USA) to preserve the samples, placed at 4 °C overnight to allow the fixative to infiltrate the samples, and then transferred to −20 °C until ready for processing. If sufficient biomass remained, specimen photos were taken of the samples (Fig. 2B) and tissue was shared with a research group working on octocoral phylogenies.
Nucleic acid extraction
Microbial community DNA was extracted from P. placomus following the protocol described in Sunagawa, Woodley & Medina (2010). Rather than grinding the sample, the protocol was modified by clipping a small piece (one polyp and its attached piece of central skeleton) from each octocoral sample using sterile forceps and shears and then placing it into a bead tube supplied with the PowerPlant DNA extraction kit (MO BIO, Carlsbad, CA, USA). Polyps were always collected from the part of the coral sample furthest from the end grasped during collection to limit any contamination from the ROV claw. The Sunagawa protocol was further modified by increasing the proteinase K (Ambion, Grand Island, NY, USA) incubation to 90 min from the protocol’s stated 60 min. Extracted DNA was quantified using the PicoGreen DNA quantification kit (Invitrogen, Grand Island, NY, USA) and the presence of bacterial DNA was confirmed by PCR amplification of 16S rRNA genes, using primers Eco8F (Edwards et al., 1989) and 1492R (Stackebrandt & Liesack, 1993), AmpliTaq Gold polymerase, and thermal cycled as follows: 1 cycle of 95 °C for 15 min; 30 cycles of 95 °C for 1 min, 54 °C for 1 min, and 72 °C for 2 min; and a final extension of 72 °C for 10 min.
16S rRNA gene pyrosequencing
The DNA extractions were sent to Selah Genomics (Greenville, SC) for sequencing. Each of the five P. placomus samples was amplified using barcoded primers (Integrated DNA Technologies, Inc., Coralville, IA, USA) targeting the V4-V5 hypervariable region of the 16S rRNA gene (563F/926R); V4-forward: 5′-AYTGGGYDTAAAGNG and V5-reverse: 5′-CCGTCAATTYYTTTRAGTTT (Claesson et al., 2010) Amplification was done using Roche’s FSHF (High Fidelity) polymerase and the following cycling parameters: 1 cycle of 95 °C for 2 min; 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min; and a final extension of 72 °C for 4 min. The amplicons then underwent 454 pyrosequencing using Titanium FLX chemistry. Sequence data from all five samples were deposited in the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA297333.
Bioinformatics
A fully commented workflow describing the analysis conducted, as well as all the resulting output files, is available as a USGS data release at http://dx.doi.org/10.5066/F7HQ3WZZ (Kellogg, 2015). The software QIIME 1.9 (Caporaso et al., 2010b) was used to process and analyze the sequence data, and specific python scripts listed below were run within QIIME. The libraries were split and then quality checked using the following parameters: length between 200 and 700, quality score of 25 with a 50 bp running quality window, one primer mismatch allowed, and a maximum homopolymer run of 6 (Kunin et al., 2010). The data were denoised (Kunin et al., 2010; Quince et al., 2009) and then operational taxonomic units (OTUs) were picked using the pick_open_reference_otus.py script (Rideout et al., 2014) which combines a closed OTU picking against the reference database (Greengenes release 13_8; DeSantis et al., 2006) with a de novo method so as not to lose novel OTUs. We employed the usearch61 OTU-picking method (Edgar, 2010) in this script since it incorporates chimera-checking. Alignment was performed with PyNAST (version 1.2.2) (Caporaso et al., 2010a) and taxonomy was assigned with uclust (Edgar, 2010). Absolute singletons (OTUs that occur only once in the dataset) were removed from the OTU table as a default in this method. Any non-bacterial sequences (i.e., archaeal, eukaryotic, chloroplast, or mitochondrial) were removed in a post-OTU picking step. Two of the libraries sequenced poorly (Table 1), so the remaining three libraries were rarefied to 4,300 sequences and rarefaction curves were generated (Fig. 3). Diversity calculations were accomplished using alpha_diversity.py and beta_diversity.py. Effective number of species (the number of equally abundant species needed to obtain the same mean proportional species abundance as that observed in the data) was calculated by taking the inverse of the natural logarithm of the Shannon diversity value. Taxa relative abundance summaries were generated using summarize_taxa_through_plots.py, with the resulting data passed to Excel and then R-Studio (R Core Development Team, 2015) using the vegan (Oksanen et al., 2016) and ggplot2 (Wickham, 2009) packages to produce taxa summary plots. The core microbiome was analyzed with compute_core_microbiome.py with the minimum fraction of samples set at 100%. Comparisons of bacterial community dissimilarity between coral individuals were calculated using Similarity Percentages (SIMPER) from PRIMER (Clarke, 1993). SIMPER was run using abundance data with no transformation, using the sample names (NF12.19Q1, NF12.19Q2, NF12.19Q5) as factors.
Table 1. Summary statistics for 454 sequencing of 16S rRNA genes from P. placomus.
| Sample | Raw reads | Filtered reads | No. reads subsampled | Observed OTUs | Effective # of species | Shannon index | Chao1 richness | ACE richness | Simpson evenness |
|---|---|---|---|---|---|---|---|---|---|
| NF12.19Q1 | 4,393 | 4,300 | 4,300 | 105 | 54 | 3.98 | 107.80 | 108.20 | 0.0574 |
| NF12.19Q2 | 7,577 | 7,268 | 4,300 | 107 | 36 | 3.58 | 117.91 | 115.17 | 0.0468 |
| NF12.19Q5 | 6,197 | 5,635 | 4,300 | 168 | 97 | 4.57 | 170.36 | 173.47 | 0.0305 |
| NF12.19Q6 | 404 | 262 | – | – | – | – | – | – | – |
| NF12.19Q7 | 428 | 389 | – | – | – | – | – | – | – |
Figure 3. Rarefaction curves for bacterial diversity in three individuals of P. placomus collected from Baltimore Canyon.
Although there were five samples collected, only three were successfully sequenced. Operational taxonomic units (calculated using a 97% sequence similarity cutoff) identified by 454 pyrosequencing of 16S rRNA genes.
Results and Discussion
During this cruise 18 ROV dives were conducted from the head to the mouth of Baltimore Canyon, over sections of hardbottom (usually compacted mud) along both walls (Fig. 1). A depth range of 234–1,001 m was covered, with the bulk of the dives at 300–600 m. While other octocoral species, such as Paragorgia arborea and Primnoa resedaeformis were encountered at multiple locations in this canyon, P. placomus was only found at one location in Baltimore Canyon (Fig. 1). During a subsequent cruise in 2013, P. placomus was also sighted at a single location in Norfolk Canyon (37 02.96 N, 74 37.03 W; 448 m); unfortunately, the coral was identified from video review after the cruise, so no samples could be collected for comparison. In both cases, the P. placomus habitat was a flat rocky terrace on top of a steep wall. In Baltimore Canyon, the patch of P. placomus consisted of similarly-sized colonies (<1 m tall), with each colony typically less than one meter from another, possibly suggesting a single recruitment event (Fig. 2A). In Norfolk Canyon, the larger colonies were of similar size to those in Baltimore Canyon, but other smaller colonies were also observed. Like most corals, successful colonization of Paramuricea sp. requires hard substrata (Doughty, Quattrini & Cordes, 2013). Most of the octocoral species in these canyons were observed on underhangs or steep walls and boulders, presumably where sediment deposition was minimized. Colonies of P. placomus, however, were only found on relatively flat terrace-type habitat that had a veneer of sediment. Many flat terrace areas were observed in both canyons but distribution of this species was very limited, suggesting that other factors influence successful recruitment and colonization. These may include larval delivery (controlled by water currents), a lack of appropriate settlement cues, and sub-optimal water conditions (e.g., particle load) (Doughty, Quattrini & Cordes, 2013; Mienis et al., 2012). Also, sister species like P. clavata are surface brooders, which often results in short dispersal distances and was suggested as a reason for clumped distributions of the genus in the Gulf of Mexico (Doughty, Quattrini & Cordes, 2013).
Bacterial diversity associated with P. placomus in Baltimore Canyon
All five of the Baltimore Canyon Paramuricea colonies share the same DNA sequence for the mtMutS gene (SC France & RW Clostio, pers. comm., 2015), which is consistent with their identification as P. placomus (Thoma, 2013). Because of the colonies close proximity to each other (<1 meter in most cases), they may not be genetically distinct. Due to the small number of successful sequence reads in two of the samples (NF12.19Q6 and NF12.19Q7), only sequences from the other three samples were further analyzed (Table 1, Figs. 3 and 4).
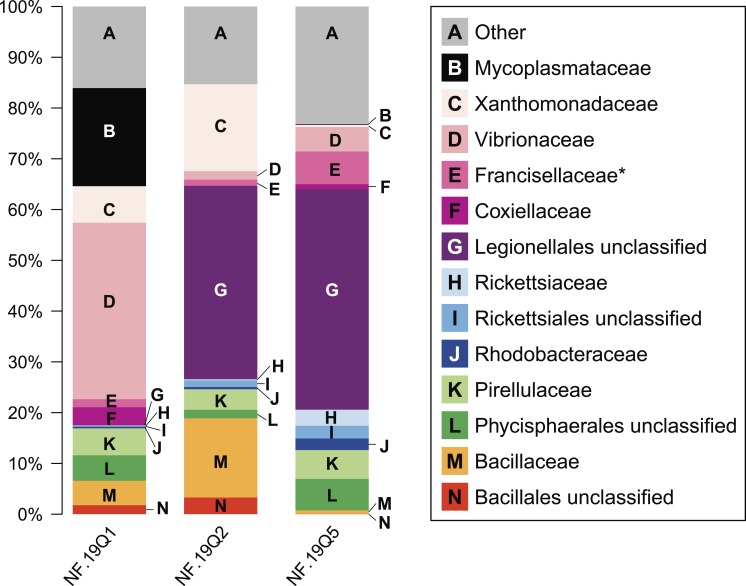
Figure 4. Relative abundance of common taxonomic groups in P. placomus samples.
Families (or the nearest identifiable phylogenetic level) that represent ≥1% of the total taxa are shown. All remaining taxa are summarized as “Other” [A]. B = Tenericutes. Gammaproteobacterial groups are C–G, alphaproteobacterial groups are H–J, Planctomycetes are K–L, and Firmicutes are M–N. *See note in reference to Francisellaceae in Table 2.
We chose to use an open OTU picking method so as to capture maximum diversity, including novel environmental sequences that do not have close matches in reference databases. The P. placomus Shannon index values (range = 3.58–4.57; Table 1) were similar to those seen for tropical stony corals like Orbicella annularis (Barott et al., 2011), but were higher than those seen for unimpacted P. clavata (range = 0.8–1.3) in the Mediterranean (Vezzulli et al., 2013), suggesting higher bacterial species richness compared to the temperate sister species (with the obvious caveat that the three studies did not use identical methodologies). Converting the Shannon index values to effective number of species (Hill, 1973), also known as true diversities (Table 1), allows a linear comparison of the magnitude of true diversity differences between two communities. The diversity of healthy P. clavata bacterial communities with Shannon index values of 0.8–1.3 (Vezzulli et al., 2013) is equivalent to that of communities with 2–4 equally-common species, compared to a range of 36–97 equally-common species (i.e., effective number of species) in P. placomus (Table 1). Using this metric, P. placomus bacterial communities are roughly 9 to 50 times more diverse than those associated with P. clavata.
As seen in most octocorals (Brück et al., 2007; Duque-Alarcón, Santiago-Vázquez & Kerr, 2012; Gray et al., 2011; Sunagawa, Woodley & Medina, 2010; Webster & Bourne, 2007), the bacterial community was dominated by proteobacterial sequences (>50% relative abundance). Other major phyla included the Firmicutes (10%) and Planctomycetes (10%). Tenericutes were 20% of sample NF12.19Q1, and less than 1% in the other two samples. Present at or below 1% relative abundance were Acidobacteria, Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Cyanobacteria, Fusobacteria, Gemmatimonadetes, Lentisphaerae, and Verrucomicrobia.
At the microbial family level, pronounced differences occurred between sample NF12.19Q1 and samples NF12.19Q2 and NF12.19Q5; most notably, reductions in unclassified Legionellales and alphaproteobacterial groups, and increases in Vibrionaceae, and Mycoplasmataceae (Fig. 4). Similarity percentages (SIMPER) analysis (Clarke, 1993) showed the average dissimilarity between NF12.19Q1 and NF12.19Q2 or NF12.19Q5 was 50.63–51.03, and the groups responsible for more than 5% of that dissimilarity were Legionellales (11.9%), Vibrionaceae (7.1–9.4%), and Mycoplasmataceae (7.7–9.0%). The average dissimilarity between samples NF12.19Q2 and NF12.19Q5 was 44.04 and was driven by Xanthomonadaceae (7.5%) and Bacillaceae (6.8%).
Mycoplasma sp. sequences were previously found associated with other deep-sea corals, including the scleractinian L. pertusa (Kellogg, Lisle & Galkiewicz, 2009; Neulinger et al., 2009; Neulinger et al., 2008), and octocorals including a bamboo coral (Penn et al., 2006), Cryogorgia koolsae (Gray et al., 2011) and Plumarella superba (Gray et al., 2011). A phylogenetic comparison revealed two ‘coral’ Mycoplasma clades: the deep-sea octocoral clones clustered together with sequences from the tropical coral Muricea elongata, and the sequences from L. pertusa formed a separate cluster (Gray et al., 2011). We aligned the 329 bp Mycoplasma 16S rRNA sequence found in P. placomus against V4-V5 regions derived from nearly full-length 16S rRNA sequences available from L. pertusa (GenBank Accession number AM911412.1), bamboo coral (DQ395563.1), and M. elongata (DQ917875.1, DQ917898.1), to determine if it clustered in either of the two deep-sea coral clades. Note that we were unable to include other octocoral sequences because the clones were too short and did not include enough of the V4-V5 variable region for alignment. The Mycoplasma from P. placomus did not cluster with any of the other coral mycoplasmal sequences.
All three P. placomus samples showed higher relative abundance of gammaproteobacterial families (Fig. 4, letters C–G) compared to alphaproteobacterial families (Fig. 4, letters H–J). The bacterial community of temperate sister-species P. clavata, is heavily dominated by Gammaproteobacteria (>90%), whereas other corals from the family Plexauridae (Cryogorgia koolsae (Gray et al., 2011). Eunicea fusca (Duque-Alarcón, Santiago-Vázquez & Kerr, 2012) Muricea elongata (Ranzer, Restrepo & Kerr, 2006), and Swiftia exertia (Brück et al., 2007)) tend to have a roughly equal distribution of Alpha- and Gammaproteobacteria, but at much lower relative abundances (ca. 15–37% each).
The dominance of Gammaproteobacteria in P. clavata was due to the presence of a single bacterial genus, Endozoicomonas, as shown by both clone libraries (La Rivière et al., 2013)) and 16S rRNA amplicon pyrosequencing (Vezzulli et al., 2013). We did not detect this genus or its family (Hahellaceae) in our P. placomus samples. Rarefaction curves (Fig. 3) level off, indicating that the absence of Endozoicomonas in these samples is unlikely to be due to under sampling of the P. placomus bacterial communities. The genus Endozoicomonas dominated two other species of temperate gorgonians (Bayer et al., 2013; Ransome et al., 2014), and a recent study has suggested a coadaptation between clades of bacteria within Hahellaceae and several temperate gorgonian species (La Rivière, Garrabou & Bally, 2015). A small number of Endozoicomonas sequences were detected in the bacterial communities associated with the deep-sea coral L. pertusa (Kellogg, Lisle & Galkiewicz, 2009; Van Bleijswijk et al., 2015), and in two Anthothela grandiflora samples from Norfolk Canyon (Lawler et al., 2016). This would suggest that this bacterial group’s absence in P. placomus was not driven by a cold temperature or other depth-related limitation. Two studies of shallow-water scleractinian corals found that the corals located in their preferred habitats were dominated by Endozoicomonas, but had more diverse microbiomes in other habitats (Pantos et al., 2015; Roder et al., 2015). This raises questions of whether the absence of this bacterial group in P. placomus is driven by biogeography (due to the isolated nature of this particular coral population) or whether this could be a diagnostic feature of this species. In comparing P. placomus core taxa (Table 2) to the 16S rRNA amplicon pyrosequencing data from P. clavata (Vezzulli et al., 2013), the shared bacterial taxa for the coral genus Paramuricea are Actinobacteria, Rhodobacterales, Burkholderiales, and Vibrionales. Congeneric tropical acroporid corals harbored similar bacterial communities (Littman et al., 2009) and two deep-sea Anthothela species shared statistically indistinguishable bacterial communities (Lawler et al., 2016), so the lack of overlap between the bacterial communities of P. placomus and P. clavata was unexpected. However, perhaps it should not be surprising given (a) the differences in the studies’ methodologies and (b) the great differences between these corals’ environments: depth, pressure, temperature, and available food sources.
Table 2. Core bacterial taxa shared by three P. placomus samples and their relative abundance in each sample, as a percentage of the total taxa.
| Core taxa | # OTUs | NF12.19Q1 | NF12.19Q2 | NF12.19Q5 | ||||
|---|---|---|---|---|---|---|---|---|
| Phylum | Class | Order | Family | Genus | ||||
| Actinobacteria | Actinobacteria | Actinomycetales | Microbacteriaceae | Curtobacterium | 1 | 0.0 | 0.1 | 0.1 |
| Actinobacteria | Actinobacteria | Actinomycetales | Propionibacteriaceae | Propionibacterium | 1 | 0.3 | 0.6 | 0.9 |
| Bacteroidetes | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | 1 | 1.0 | 0.0 | 0.2 | |
| Firmicutes | Bacilli | Bacillales | 1 | 1.8 | 3.3 | 0.1 | ||
| Firmicutes | Bacilli | Bacillales | Alicyclobacillaceae | Alicyclobacillus | 1 | 0.4 | 0.1 | 0.0 |
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | 1 | 4.8 | 15.6 | 0.7 |
| Planctomycetes | Phycisphaerae | Phycisphaerales | 9 | 5.0 | 1.7 | 6.2 | ||
| Planctomycetes | Planctomycetia | Pirellulales | Pirellulaceae | 6 | 5.3 | 4.0 | 5.7 | |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Bradyrhizobiaceae | Bradyrhizobium | 1 | 0.9 | 0.4 | 0.0 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Phyllobacteriaceae | 1 | 0.6 | 0.1 | 0.0 | |
| Proteobacteria | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | 2 | 0.3 | 0.4 | 2.0 | |
| Proteobacteria | Alphaproteobacteria | Rhodospirillales | Rhodospirillaceae | Magnetospirillum | 1 | 0.2 | 0.3 | 0.1 |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Alcaligenaceae | 1 | 0.0 | 0.2 | 0.1 | |
| Proteobacteria | Betaproteobacteria | Burkholderiales | Comamonadaceae | 2 | 0.3 | 1.3 | 0.7 | |
| Proteobacteria | Deltaproteobacteria | NB1-j | JTB38 | 1 | 0.7 | 0.2 | 0.9 | |
| Proteobacteria | Epsilonproteobacteria | Campylobacterales | 1 | 0.3 | 0.6 | 0.8 | ||
| Proteobacteria | Gammaproteobacteria | Alteromonadales | 2 | 0.2 | 1.7 | 0.5 | ||
| Proteobacteria | Gammaproteobacteria | Alteromonadales | OM60 | 1 | 0.3 | 0.0 | 0.2 | |
| Proteobacteria | Gammaproteobacteria | Enterobacteriales | Enterobacteriaceae | 1 | 0.3 | 0.5 | 0.2 | |
| Proteobacteria | Gammaproteobacteria | Legionellales | 2 | 0.1 | 38.1 | 43.5 | ||
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | Acinetobacter | 1 | 0.4 | 0.5 | 0.2 |
| Proteobacteria | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Pseudomonas | 2 | 1.3 | 0.0 | 0.7 |
| Proteobacteria | Gammaproteobacteria | Thiotrichales | Francisellaceae* | Caedibacter | 3 | 1.6 | 1.2 | 6.4 |
| Proteobacteria | Gammaproteobacteria | Vibrionales | Vibrionaceae | 1 | 34.3 | 0.7 | 1.3 | |
| Proteobacteria | Gammaproteobacteria | Vibrionales | Vibrionaceae | Enterovibrio | 1 | 0.1 | 0.9 | 3.3 |
| Proteobacteria | Gammaproteobacteria | Vibrionales | Vibrionaceae | Photobacterium | 1 | 0.4 | 0.0 | 0.2 |
| Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | 1 | 0.3 | 0.1 | 0.2 | |
| Proteobacteria | Gammaproteobacteria | Xanthomonadales | Xanthomonadaceae | Lysobacter | 1 | 6.9 | 17.0 | 0.1 |
| TOTALS | 68.1 | 89.6 | 75.3 | |||||
Notes.
Caedibacter are actually incertae sedis, being closely related to both Legionella and Francisella, but placed in Francisellaceae by Greengenes database.
A value of 0.0 means <0.1%.
Composition of conserved core bacterial community
Sequences shared by all three coral samples were examined to identify core bacterial taxa (characterized to the lowest possible taxon, down to genus, Table 2). It is recognized that this population of P. placomus is both isolated and likely highly clonal, and therefore may not reflect all the bacterial diversity present across the large geographic range of this coral species, or could contain regionally-specific taxa. While other studies have included bacterial groups present in 30–50% of the coral samples as part of the core (Ainsworth et al., 2015), given the small sample size of this study we have opted for the most conservative approach; requiring the OTU be present in 100% of the samples. For each sample, the relative abundance of each taxon is shown in relation to the total taxa (Table 2). For samples NF12.19Q5 and NF12.19Q2 the core taxa make up 75 to nearly 90% of the total community, suggesting a strongly species-specific bacterial community. The core taxa constitute 68% of sample NF12.19Q1, in spite of its visibly different appearance at the family level (Fig. 4). This can be contrasted against the microbiome of the temperate gorgonian Eunicella cavolini, where only 7 of 2067 OTUs (less than 1%) were shared across 9 samples (Bayer et al., 2013).
A member of the P. placomus core (Table 2), Propionibacterium, was recently identified as a conserved member of the core microbiome across a number of tropical and mesophotic corals (Ainsworth et al., 2015). The authors used fluorescently-labeled probes to localize the Propionibacterium to the corals’ endosymbiotic algae (zooxanthellae) and speculated these bacteria had a role in facilitating the success of the dinoflagellate-coral symbiosis, and/or meeting the coral host’s energy requirements. Interestingly, Propionibacterium spp. have been cultivated or detected by molecular techniques from both azooxanthellate (Lawler et al., 2016; Santiago-Vázquez et al., 2007) and zooxathellate corals (Bayer et al., 2013; De Castro et al., 2010; Nithyanand, Manju & Pandian, 2011). This raises an interesting question as to whether these conserved bacteria have multiple roles, or play a different role in azooxanthellate corals.
The phylum Firmicutes is present in the P. placomus core, has been identified as a core component conserved across multiple tropical coral holobionts (Ainsworth et al., 2015), and appears to be a minor component in most corals, although more are being detected by pyrosequencing than were previously by clone libraries (e.g., Ainsworth et al., 2015; Lawler et al., 2016; Sunagawa, Woodley & Medina, 2010; Van de Water et al., 2015). However, we may still be underestimating the contribution of Firmicutes to coral microbiota due to spore-formers’ relative resistance to DNA extraction (Filippidou et al., 2015). Culture-based work revealed that bacterial communities associated with several tropical stony corals and mesophotic azooxanthellate corals include a number of Bacillus species (Beleneva, Dautova & Zhukova, 2005; Brück et al., 2007; Nithyanand & Pandian, 2009). Culture-independent work, including microarrays (Kellogg et al., 2014; Kellogg et al., 2013) and pyrosequencing (Morrow et al., 2012), also revealed the Bacillaceae family to be a common associate of tropical corals. While abilities vary based on species, Bacillus spp. are known to produce siderophores to acquire iron and are assumed to have important roles in carbon and nitrogen cycling (Logan & De Vos, 2009).
The P. placomus core bacterial community includes Alpha-, Beta-, Delta-, Epsilon- and Gammaproteobacteria (Table 2). Legionellales sequences have not been commonly detected in association with corals. Single clones of Legionellales were obtained from the skeletons of Mediterranean corals Cladocora caespitosa and Balanophyllia europaea (Meron et al., 2012) and less than 10 clones similar to Legionella feeleii were associated with diseased colonies of temperate gorgonian Eunicella verrucosa (Ransome et al., 2014). A recent study of three deep-sea coral species within the family Anthothelidae detected this order as a minor component of their bacterial communities (Lawler et al., 2016). This bacterial order is defined as comprising facultative and obligate intracellular parasites, many of which are associated with free-living protozoa. It therefore remains to be determined if Legionellales are direct associates of the gorgonian, or infect amoebae that are part of the holobiont microbiome (Garrity, Bell & Lilburn, 2005; Rowbotham, 1986).
In addition to the dominant phylotypes of Legionellales, another major component of the core gammaproteobacterial taxa, particularly for sample NF12.19Q1, is the Vibrionaceae, including the genera Enterovibrio and Photobacterium (Table 2). Vibrionaceae are a common component of coral microbiota, having been found in healthy tropical (Bourne & Munn, 2005; Chimetto et al., 2008; Daniels et al., 2011; Lampert et al., 2006) and cold-water corals (Galkiewicz et al., 2011; Gray et al., 2011). While this bacterial group has been linked to a number of diseases in tropical (e.g., Arotsker et al., 2009; Ben-Haim & Rosenberg, 2002; Cervino et al., 2004) and temperate (Bally & Garrabou, 2007; Hall-Spencer, Pike & Munn, 2007) corals, pathogenicity seems to be driven by water temperatures greater than 20 °C.
While other members of the order Xanthomonadales have been found in association with corals (Brück et al., 2007; Cárdenas et al., 2012; Rohwer et al., 2002), Lysobacter was only known from freshwater and soil environments (Christensen, 2005). However, this genus’s ability to degrade chitin (Christensen, 2005) would be useful for a coral host that feeds on zooplankton. Lysobacter are named for their antimicrobial activity, active against not only bacteria, but also yeasts, filamentous fungi, and nematodes (Christensen, 2005), suggesting this genus has a potential role in protecting and maintaining the microbial balance of the coral.
A component of the core microbiome of P. placomus was identified as Francisellaceae (Table 2 and Fig. 4). However, when those sequences were run through RDP Classifier (Wang et al., 2007) they were identified as Thiotrichales incertae sedis, genus Caedibacter. Further evaluation using BLAST (Altschul et al., 1990) showed that the closest matches in GenBank included uncultured Caedibacter clones derived from the coral Orbicella faveolata (FJ425613, FJ425621). The genus Caedibacter consists of endosymbionts of Paramecium and has been shown to be polyphyletic (Beier et al., 2002), including both Alphaproteobacteria similar to Rickettsiales and Gammaproteobacteria similar to both Legionella and Francisella (as seen here). As with Legionellales, the presence of this bacterial group hints at the presence of eukaryotic members (i.e., protist hosts) in these coral microbiomes.
Core: Nitrogen cycling?
A recent characterization of the core microbiome of the deep-sea octocoral Anthothela grandiflora revealed the possibility of a nearly complete nitrogen cycle, based on previously described abilities of particular bacteria present in the coral (Lawler et al., 2016). With this in mind, we examined the core bacterial community associated with the three P. placomus colonies (Table 2) and determined that a similar possibility exists for this coral species (Fig. 5).
Figure 5. Core bacterial groups potential roles within the nitrogen cycle.
A number of the bacterial groups present within the core microbiome of these three P. placomus samples were previously recognized for their roles within the nitrogen cycle. This diagram illustrates a simplified overview of the bacterial groups with their possible functions. This figure was adapted from one presented in Wegley et al. (2007).
The alphaproteobacterial genus Bradyrhizobium is typically an intracellular nitrogen-fixing symbiont in the root nodules of plants; however, some free-living strains of Bradyrhizobium have been observed to fix nitrogen with particular carbon sources and under low oxygen conditions (Kuykendall, 2005). Bacterial nitrogen-fixation has been documented in shallow-water corals (Cardini et al., 2015; Lesser et al., 2007; Shashar et al., 1994; Williams, Viner & Broughton, 1987), and nifH genes from Bradyrhizobium and other rhizobial species have been detected in three tropical coral species (Lema, Willis & Bourne, 2012). Bradyrhizobium spp. also have been identified previously in association with the deep-sea octocoral Plumarella superba (Gray et al., 2011), as well as in the tropical corals Orbicella annularis (Kellogg et al., 2013) and Siderastrea siderea (Kellogg et al., 2014). Magnetospirillum spp. are capable of fixing atmospheric nitrogen (Bazylinski et al., 2000) and can also use nitrate and ammonium as nitrogen sources (Schüler & Schleifer, 2005). Pirellulaceae are ammonia-oxidizing bacteria found in sponges (Gade et al., 2004; Mohamed et al., 2010) and the deep-sea octocoral Alcyonium grandiflorum (Lawler et al., 2016), and may be conducting nitrification. Propionibacterium (Allison & Macfarlane, 1989) and Photobacterium (Thyssen & Ollevier, 2005) have been shown to reduce nitrate to nitrite. Bacillus spp. have been shown to carry out denitrification (Verbaendert et al., 2011) and also nitrate/nitrite ammonification (Hoffmann et al., 1998). Lastly, members of the Campylobacterales are known to reduce nitrate and nitrite to ammonium (De Vries et al., 1980). Nitrogen cycling has recently been confirmed in the deep-sea coral Lophelia pertusa (Middelburg et al., 2015), and given that P. placomus is likely to have an inconsistent diet of nano-zooplankton and detrital particulates (Ribes, Coma & Gili, 1999), the ability to recycle and retain nitrogen would be beneficial (Fig. 5). Further work with metagenomics and transcriptomics remains to be conducted to confirm this hypothesis.
Conclusions
Based on these three samples from the Baltimore Canyon population, the deep-sea plexaurid octocoral P. placomus has a species-specific bacterial community that shows very little overlap with previously characterized temperate gorgonians, including sister-species P. clavata. The bacterial community of this species is dominated by Proteobacteria but has similar diversity to that of tropical stony corals. Conserved core bacterial taxa comprise 68–90% of the total community, leaving roughly 10–30% individual variability between coral colonies. Additional sampling from other locations is required to confirm the consistency of these findings across the coral’s geographic range. The composition of the core suggests that nitrogen cycling may be carried out by the bacterial associates. This study is the first description of the bacterial microbiota of a deep-sea paramuricid species and provides a baseline for comparison by future studies to address questions regarding biogeography, ecology, and resilience of these corals to anthropogenic impacts and changing climate.
Acknowledgments
Thanks are extended to the captain and crew of the NOAA ship Nancy Foster and to the Kraken II ROV team. The authors also thank Ashley Shade (Michigan State Univ.) and the Explorations in Data Analysis for Metagenomic Advances in Microbial Ecology (EDAMAME) Workshop for critical lessons in workflow organization and bioinformatics tools.
Michael Rhode (UNC-Wilmington) produced the multibeam map of Baltimore Canyon used in Fig. 1 and provided assistance at sea. Dawn Goldsmith (USGS) formatted Fig. 4 using R. Betsy Boynton (USGS) drafted the inset map used in Fig. 1 and prepared final versions of all the figures. Scott France and Rachel Clostio (University of Louisiana at Lafayette) provided the coral genetic results confirming host species identity.
Any use of trade names is for descriptive purposes only and does not imply endorsement by the US government.
Funding Statement
Funding for this project was provided by the US Geological Survey’s Ecosystems Mission Area, Environments Program through the Outer Continental Shelf study on Mid-Atlantic Canyons. Additional funding was sponsored by the National Oceanographic Partnership Program and supplied by the Bureau of Ocean Energy Management (BOEM) contract number M10PC00100 (contracted to CSA Ocean Sciences, Inc.). The Nancy Foster and Kraken II were provided by the NOAA Office of Ocean Exploration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Christina A. Kellogg conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Steve W. Ross contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
Sandra D. Brooke contributed reagents/materials/analysis tools, reviewed drafts of the paper.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
NCBI Sequence Read Archive (SRA) under BioProject number PRJNA297333.
Data Availability
The following information was supplied regarding data availability:
Kellogg, 2015. Cold-water coral microbiomes (Paramuricea placomus) from Baltimore Canyon: raw and processed data. US Geological Survey data release: http://dx.doi.org/10.5066/F7HQ3WZZ.
References
- Ainsworth et al. (2015).Ainsworth TD, Krause L, Bridge T, Torda G, Raina J-B, Zakrzewski M, Gates RD, Padilla-Gamiño JL, Spalding HL, Smith C, Woolsey ES, Bourne DG, Bongaerts P, Hoegh-Guldberg O, Leggat W. The coral core microbiome identifies rare bacterial taxa as ubiquitous endosymbionts. The ISME Journal. 2015;9:2261–2274. doi: 10.1038/ismej.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison & Macfarlane (1989).Allison C, Macfarlane GT. Dissimilatory nitrate reduction by Propionibacterium acnes. Applied and Environmental Microbiology. 1989;55:2899–2903. doi: 10.1128/aem.55.11.2899-2903.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul et al. (1990).Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arotsker et al. (2009).Arotsker L, Siboni N, Ben-Dov E, Kramarsky-Winter E, Loya Y, Kushmaro A. Vibrio sp. as a potentially important member of the Black Band Disease (BBD) consortium in Favia sp. corals. FEMS Microbiology Ecology. 2009;70:515–524. doi: 10.1111/j.1574-6941.2009.00770.x. [DOI] [PubMed] [Google Scholar]
- Bally & Garrabou (2007).Bally M, Garrabou J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Global Change Biology. 2007;13:2078–2088. doi: 10.1111/j.1365-2486.2007.01423.x. [DOI] [Google Scholar]
- Barott et al. (2011).Barott KL, Rodriguez-Brito B, Janouškovec J, Marhaver K, Smith JE, Keeling P, Rohwer FL. Microbial diversity associated with four functional groups of benthic reef algae and the reef-building coral Montastraea annularis. Environmental Microbiology. 2011;13:1192–1204. doi: 10.1111/j.1462-2920.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- Bayer et al. (2013).Bayer T, Arif C, Ferrier-Pagès C, Zoccola D, Aranda M, Voolstra CR. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Marine Ecology Progress Series. 2013;479:75–84. doi: 10.3354/meps10197. [DOI] [Google Scholar]
- Bazylinski et al. (2000).Bazylinski DA, Dean AJ, Schüler D, Phillips EJP, Lovley DR. N2-dependent growth and nitrogenase activity in the metal-metabolizing bacteria, Geobacter and Magnetospirillum species. Environmental Microbiology. 2000;2:266–273. doi: 10.1046/j.1462-2920.2000.00096.x. [DOI] [PubMed] [Google Scholar]
- Beier et al. (2002).Beier CL, Horn M, Michel R, Schweikert M, Görtz H-D, Wagner M. The genus Caedibacter comprises endosymbionts of Paramecium spp. related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria) Applied and Environmental Microbiology. 2002;68:6043–6050. doi: 10.1128/AEM.68.12.6043-6050.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beleneva, Dautova & Zhukova (2005).Beleneva IA, Dautova TI, Zhukova NV. Characterization of communities of heterotrophic bacteria associated with healthy and diseased corals in Nha Trang Bay (Vietnam) Microbiology. 2005;74:579–587. doi: 10.1007/s11021-005-0106-8. [DOI] [PubMed] [Google Scholar]
- Ben-Haim & Rosenberg (2002).Ben-Haim Y, Rosenberg E. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Marine Biology. 2002;141:47–55. doi: 10.1007/s00227-002-0797-6. [DOI] [Google Scholar]
- Van Bleijswijk et al. (2015).Van Bleijswijk JDL, Whalen C, Duineveld GCA, Lavaleye MSS, Witte HJ, Mienis F. Microbial assemblages on a cold-water coral mound at the SE Rockall Bank (NE Atlantic): interactions with hydrography and topography. Biogeosciences Discussions. 2015;12:1509–1542. doi: 10.5194/bgd-12-1509-2015. [DOI] [Google Scholar]
- Bourne & Munn (2005).Bourne DG, Munn CB. Diversity of bacteria associated with the coral Pocillopora damicornis from the Great Barrier Reef. Environmental Microbiology. 2005;7:1162–1174. doi: 10.1111/j.1462-2920.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- Brück et al. (2007).Brück TB, Brück WM, Santiago-Vázquez LZ, McCarthy PJ, Kerr RG. Diversity of the bacterial communities associated with the azooxanthellate deep water octocorals Leptogorgia minimata, Iciligorgia schrammi, and Swiftia exertia. Marine Biotechnology. 2007;9:561–576. doi: 10.1007/s10126-007-9009-1. [DOI] [PubMed] [Google Scholar]
- Buhl-Mortensen & Buhl-Mortensen (2014).Buhl-Mortensen P, Buhl-Mortensen L. Diverse and vulnerable deep-water biotopes in the Hardangerfjord. Marine Biology Research. 2014;10:253–267. doi: 10.1080/17451000.2013.810759. [DOI] [Google Scholar]
- Buhl-Mortensen & Mortensen (2005).Buhl-Mortensen L, Mortensen PB. Distribution and diversity of species associated with deep-sea gorgonian corals off Atlantic Canada. In: Freiwald A, Roberts JM, editors. Cold-water corals and ecosystems. Springer-Verlag; Berlin: 2005. pp. 849–879. [Google Scholar]
- Bythell, Pantos & Richardson (2004).Bythell JC, Pantos O, Richardson LL. White plague, white band, and other “white” diseases. In: Rosenberg E, Loya Y, editors. Coral health and disease. Springer-Verlag; Berlin: 2004. pp. 351–365. [Google Scholar]
- Caporaso et al. (2010a).Caporaso JG, Bittinger K, Bushman FD, Desantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010a;26:266–267. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso et al. (2010b).Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Gonzalez Peña A, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010b;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas et al. (2012).Cárdenas A, Rodriguez-R LM, Pizarro V, Cadavid LF, Arévalo-Ferro C. Shifts in bacterial communities of two caribbean reef-building coral species affected by white plague disease. The ISME Journal. 2012;6:502–512. doi: 10.1038/ismej.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini et al. (2015).Cardini U, Bednarz VN, Naumann MS, Van Hoytema N, Rix L, Foster RA, Al-Rshaidat MMD, Wild C. Functional significance of dinitrogen fixation in sustaining coral productivity under oligotrophic conditions. Proceedings of the Royal Society of London Series B-Biological Sciences. 2015;282:20152257. doi: 10.1098/rspb.2015.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervino et al. (2004).Cervino JM, Hayes RL, Polson SW, Polson SC, Goreau TJ, Martinez RJ, Smith GW. Relationship of Vibrio species infection and elevated temperatures to yellow blotch/band disease in Caribbean corals. Applied and Environmental Microbiology. 2004;70:6855–6864. doi: 10.1128/AEM.70.11.6855-6864.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimetto et al. (2008).Chimetto LA, Brocchi M, Thompson CC, Martins RCR, Ramos HR, Thompson FL. Vibrios dominate as culturable nitrogen-fixing bacteria of the Brazilian coral Mussismilia hispida. Systematic and Applied Microbiology. 2008;31:312–319. doi: 10.1016/j.syapm.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Christensen (2005).Christensen P. Family I. Xanthomonadaceae, genus IV. Lysobacter. Bergey’s Manual of Systematic Bacteriology. 2005;2:95–101. [Google Scholar]
- Claesson et al. (2010).Claesson MJ, Wang Q, O’Sullivan O, Greene-Diniz R, Cole JR, Ross RP, O’Toole PW. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Research. 2010;38:e2529. doi: 10.1093/nar/gkq873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke (1993).Clarke KR. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology. 1993;18:117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- Correa et al. (2013).Correa H, Haltli B, Duque C, Kerr R. Bacterial communities of the gorgonian octocoral Pseudopterogorgia elisabethae. Microbial Ecology. 2013;66:972–985. doi: 10.1007/s00248-013-0267-3. [DOI] [PubMed] [Google Scholar]
- Daniels et al. (2011).Daniels CA, Zeifman A, Heym K, Ritchie KB, Watson CA, Berzins I, Breitbart M. Spatial heterogeneity of bacterial communities in the mucus of Montastraea annularis. Marine Ecology Progress Series. 2011;426:29–40. doi: 10.3354/meps09024. [DOI] [Google Scholar]
- De Castro et al. (2010).De Castro AP, Dias Araújo S, Jr, Reis AMM, Moura RL, Francini-Filho RB, Pappas G, Jr, Rodrigues TB, Thompson FL, Krüger RH. Bacterial community associated with healthy and diseased reef coral Mussismilia hispida from Eastern Brazil. Microbial Ecology. 2010;59:658–667. doi: 10.1007/s00248-010-9646-1. [DOI] [PubMed] [Google Scholar]
- De Vries et al. (1980).De Vries W, Niekus HGD, Boellaard M, Stouthamer AH. Growth yields and energy generation by Campylobacter sputorum subspecies bubulus during growth in continuous culture with different hydrogen acceptors. Archives of Microbiology. 1980;124:221–227. doi: 10.1007/BF00427730. [DOI] [PubMed] [Google Scholar]
- DeSantis et al. (2006).DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughty, Quattrini & Cordes (2013).Doughty CL, Quattrini AM, Cordes EE. Insights into the population dynamics of the deep-sea coral genus Paramuricea in the Gulf of Mexico. Deep-Sea Research Part II-Topical Studies in Oceanography. 2013;99:71–82. doi: 10.1016/j.dsr2.2013.05.023. [DOI] [Google Scholar]
- Duque-Alarcón, Santiago-Vázquez & Kerr (2012).Duque-Alarcón A, Santiago-Vázquez LZ, Kerr RG. A microbial community analysis of the octocoral Eunicea fusca. Electronic Journal of Biotechnology. 2012;15:1–9. [Google Scholar]
- Edgar (2010).Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Edwards et al. (1989).Edwards U, Rogall T, Blocker H, Emde M, Bottger EC. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Research. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippidou et al. (2015).Filippidou S, Junier T, Wunderlin T, Lo C-C, Li P-E, Chain PS, Junier P. Under-detection of endospore-forming Firmicutes in metagenomic data. Computational and Structural Biotechnology Journal. 2015;13:299–306. doi: 10.1016/j.csbj.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade et al. (2004).Gade D, Schlesner H, Glöckner FO, Amann R, Pfeiffer S, Thomm M. Identification of Plantomycetes with order-, genus-, and strain-specific 16S rRNA-targeted probes. Microbial Ecology. 2004;47:243–251. doi: 10.1007/s00248-003-1016-9. [DOI] [PubMed] [Google Scholar]
- Galkiewicz et al. (2011).Galkiewicz JP, Pratte ZA, Gray MA, Kellogg CA. Characterization of culturable bacteria isolated from the cold-water coral Lophelia pertusa. FEMS Microbiology Ecology. 2011;77:333–346. doi: 10.1111/j.1574-6941.2011.01115.x. [DOI] [PubMed] [Google Scholar]
- Garrity, Bell & Lilburn (2005).Garrity GM, Bell JA, Lilburn T. Order VI: Legionellales. ord. nov. Bergey’s Manual of Systematic Bacteriology. 2005;2:210. [Google Scholar]
- Gray et al. (2011).Gray MA, Stone RP, McLaughlin MR, Kellogg CA. Microbial consortia of gorgonian corals from the Aleutian islands. FEMS Microbiology Ecology. 2011;76:109–120. doi: 10.1111/j.1574-6941.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- Hall-Spencer, Pike & Munn (2007).Hall-Spencer JM, Pike J, Munn CB. Diseases affect cold-water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. Diseases of Aquatic Organisms. 2007;76:87–97. doi: 10.3354/dao076087. [DOI] [PubMed] [Google Scholar]
- Hansson et al. (2009).Hansson L, Agis M, Maier C, Weinbauer MG. Community composition of bacteria associated with cold-water coral Madrepora oculata: within and between colony variability. Marine Ecology Progress Series. 2009;397:89–102. doi: 10.3354/meps08429. [DOI] [Google Scholar]
- Hill (1973).Hill MO. Diversity and eveness: a unifying notation and its consequences. Ecology. 1973;54:427–432. doi: 10.2307/1934352. [DOI] [Google Scholar]
- Hoffmann et al. (1998).Hoffmann T, Frankenberg N, Marino M, Jahn D. Ammonification in Bacillus subtilis utilizing dissimilatory nitrite reductase is dependent on resDE. Journal of Bacteriology. 1998;180:186–189. doi: 10.1128/jb.180.1.186-189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm & Heidelberg (2016).Holm JB, Heidelberg KB. Microbiomes of Muricea californica and M. fruticosa: comparative analyses of two co-occurring eastern Pacific octocorals. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00917. Article 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg (2015).Kellogg CA. Cold-water coral microbiomes (Paramuricea placomus) from Baltimore Canyon: raw and processed data: US Geological Survey Data release. 2015 doi: 10.5066/F7HQ3WZZ. [DOI] [Google Scholar]
- Kellogg, Lisle & Galkiewicz (2009).Kellogg CA, Lisle JT, Galkiewicz JP. Culture-independent characterization of bacterial communities associated with the cold-water coral Lophelia pertusa in the northeastern Gulf of Mexico. Applied and Environmental Microbiology. 2009;75:2294–2303. doi: 10.1128/AEM.02357-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg et al. (2014).Kellogg CA, Piceno YM, Tom LM, DeSantis TZ, Gray MA, Andersen GL. Comparing bacterial community composition of healthy and dark spot-affected Siderastrea siderea in Florida and the Caribbean. PLoS ONE. 2014;9:e2529. doi: 10.1371/journal.pone.0108767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg et al. (2013).Kellogg CA, Piceno YM, Tom LM, DeSantis TZ, Gray MA, Zawada DG, Andersen GL. Comparing bacterial community composition between healthy and white plague-like disease states in Orbicella annularis using PhyloChip™G3 microarrays. PLoS ONE. 2013;8:e2529. doi: 10.1371/journal.pone.0079801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin et al. (2010).Kunin V, Engelbrektson A, Ochman H, Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environmental Microbiology. 2010;12:118–123. doi: 10.1111/j.1462-2920.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- Kuykendall (2005).Kuykendall LD. Family VII. Bradyrhizobiaceae, genus I. Bradyrhizobium. Bergey’s Manual of Systematic Bacteriology. 2005;2:438–443. [Google Scholar]
- La Rivière, Garrabou & Bally (2010).La Rivière M, Garrabou J, Bally M. Spatial and temporal analysis of bacterial diversity associated with the Mediterranean gorgonian Paramuricea clavata. Rapport Commission International pour l’exploration scientifique de la Mer Mediterranée, Monaco. 2010;39:769. [Google Scholar]
- La Rivière, Garrabou & Bally (2015).La Rivière M, Garrabou J, Bally M. Evidence for host specificity among dominant bacterial symbionts in temperate gorgonian corals. Coral Reefs. 2015;34:1087–1098. doi: 10.1007/s00338-015-1334-7. [DOI] [Google Scholar]
- La Rivière et al. (2013).La Rivière M, Roumagnac M, Garrabou J, Bally M. Transient shifts in bacterial communities associated with the temperate gorgonian Paramuricea clavata in the northwestern Mediterranean Sea. PLoS ONE. 2013;8:e2529. doi: 10.1371/journal.pone.0057385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert et al. (2006).Lampert Y, Kelman D, Dubinsky Z, Nitzan Y, Hill RT. Diversity of culturable bacteria in the mucus of the Red Sea coral Fungia scutaria. FEMS Microbiology Ecology. 2006;58:99–108. doi: 10.1111/j.1574-6941.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- Lawler et al. (2016).Lawler SN, Kellogg CA, France SC, Clostio RW, Brooke SD, Ross SW. Coral-associated bacterial diversity is conserved across two deep-sea Anthothela species. Frontiers in Microbiology. 2016;7 doi: 10.3389/fmicb.2016.00458. Article 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema, Willis & Bourne (2012).Lema KA, Willis BL, Bourne DG. Corals form characteristic associations with symbiotic nitrogen-fixing bacteria. Applied and Environmental Microbiology. 2012;78:3136–3144. doi: 10.1128/AEM.07800-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser et al. (2007).Lesser MP, Falcón LI, Rodríguez-Román A, Enríquez S, Hoegh-Guldberg O, Iglesias-Prieto R. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Marine Ecology Progress Series. 2007;346:143–152. doi: 10.3354/meps07008. [DOI] [Google Scholar]
- Littman et al. (2009).Littman RA, Willis BL, Pfeffer C, Bourne DG. Diversities of coral-associated bacteria differ with location, but not species, for three acroporid corals on the Great Barrier Reef. FEMS Microbiology Ecology. 2009;68:152–163. doi: 10.1111/j.1574-6941.2009.00666.x. [DOI] [PubMed] [Google Scholar]
- Logan & De Vos (2009).Logan NA, De Vos P. Genus I. Bacillus. Bergey’s Manual of Systematic Bacteriology. 2009;3:21–128. [Google Scholar]
- Lumsden et al. (2007).Lumsden SE, Hourigan TF, Bruckner AW, Dorr G. The state of deep coral ecosystems of the United States. Silver Spring, MDNOAA Technical Memorandum CRCP-3 ed. 2007
- Meron et al. (2012).Meron D, Rodolfo-Metalpa R, Cunning R, Baker AC, Fine M, Banin E. Changes in coral microbial communities in response to a natural pH gradient. The ISME Journal. 2012;6:1775–1785. doi: 10.1038/ismej.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelburg et al. (2015).Middelburg JJ, Mueller CE, Veuger B, Larsson AI, Form A, Van Oevelen D. Discovery of symbiotic nitrogen fixation and chemoautotrophy in cold-water corals. Scientific Reports. 2015;5 doi: 10.1038/srep17962. Article 17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienis et al. (2012).Mienis F, Duineveld GCA, Davies AJ, Ross SW, Seim H, Bane J, Van Weering T.CE. The influence of near-bed hydrodynamic conditions on cold-water corals in the Viosca Knoll area, Gulf of Mexico. Deep-Sea Research Part I-Oceanographic Research Papers. 2012;60:32–45. doi: 10.1016/j.dsr.2011.10.007. [DOI] [Google Scholar]
- Miller et al. (2012).Miller RJ, Hocevar J, Stone RP, Fedorov DV. Structure-forming corals and sponges and their use as fish habitat in Bering Sea submarine canyons. PLoS ONE. 2012;7:e2529. doi: 10.1371/journal.pone.0033885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed et al. (2010).Mohamed NM, Saito K, Tal Y, Hill RT. Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. The ISME Journal. 2010;4:38–48. doi: 10.1038/ismej.2009.84. [DOI] [PubMed] [Google Scholar]
- Morrow et al. (2012).Morrow KM, Moss AG, Chadwick NE, Liles MR. Bacterial associates of two Caribbean coral species reveal species-specific distribution and geographic variability. Applied and Environmental Microbiology. 2012;78:6438–6449. doi: 10.1128/AEM.01162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchka, Hewson & Harvell (2010).Mouchka ME, Hewson I, Harvell CD. Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integrative and Comparative Biology. 2010;50:662–674. doi: 10.1093/icb/icq061. [DOI] [PubMed] [Google Scholar]
- Neulinger et al. (2009).Neulinger SC, Gärtner A, Järnegren J, Ludvigsen M, Lochte K, Dullo W-C. Tissue-associated “Candidatus Mycoplasma corallicola” and filamentous bacteria on the cold-water coral Lophelia pertusa (Scleractinia) Applied and Environmental Microbiology. 2009;75:1437–1444. doi: 10.1128/AEM.01781-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neulinger et al. (2008).Neulinger SC, Järnegren J, Ludvigsen M, Lochte K, Dullo W-C. Phenotype-specific bacterial communities in the cold-water coral Lophelia pertusa (Scleractinia) and their implications for the coral’s nutrition, health, and distribution. Applied and Environmental Microbiology. 2008;74:7272–7285. doi: 10.1128/AEM.01777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithyanand, Manju & Pandian (2011).Nithyanand P, Manju S, Pandian SK. Phylogenetic characterization of culturable actinomycetes associated with the mucus of the coral Acropora digitifera from Gulf of Mannar. FEMS Microbiology Letters. 2011;314:112–118. doi: 10.1111/j.1574-6968.2010.02149.x. [DOI] [PubMed] [Google Scholar]
- Nithyanand & Pandian (2009).Nithyanand P, Pandian SK. Phylogenetic characterization of culturable bacterial diversity associated with the mucus and tissue of the coral Acropora digitifera from the Gulf of Mannar. FEMS Microbiology Ecology. 2009;69:384–394. doi: 10.1111/j.1574-6941.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- Oksanen et al. (2016).Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens M.HH, Szoecs E, Wagner H. vegan: Community Ecology Package. R package version 2.4-0https://cran.r-project.org/package=vegan 2016
- Pantos et al. (2015).Pantos O, Bongaerts P, Dennis PG, Tyson GW, Hoegh-Guldberg O. Habitat-specific environmental conditions primarily control the microbiomes of the coral Seriatopora hystrix. The ISME Journal. 2015;9:1916–1927. doi: 10.1038/ismej.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos et al. (2003).Pantos O, Cooney RP, Le Tissier MDA, Barer MR, O’Donnell AG, Bythell JC. The bacterial ecology of a plague-like disease affecting the Caribbean coral Montastrea annularis. Environmental Microbiology. 2003;5:370–382. doi: 10.1046/j.1462-2920.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- Penn et al. (2006).Penn K, Wu D, Eisen JA, Ward N. Characterization of bacterial communities associated with deep-sea corals on Gulf of Alaska seamounts. Applied and Environmental Microbiology. 2006;72:1680–1683. doi: 10.1128/AEM.72.2.1680-1683.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quince et al. (2009).Quince C, Lanzén A, Curtis TP, Davenport RJ, Hall N, Head IM, Read LF, Sloan WT. Accurate determination of microbial diversity from 454 pyrosequencing data. Nature Methods. 2009;6:639–641. doi: 10.1038/NMETH.1361. [DOI] [PubMed] [Google Scholar]
- R Core Development Team (2015).R Core Development Team . R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- Ransome et al. (2014).Ransome E, Rowley SJ, Thomas S, Tait K, Munn CB. Disturbance to conserved bacterial communities in the cold-water gorgonian coral Eunicella verrucosa. FEMS Microbiology Ecology. 2014;90:404–416. doi: 10.1111/1574-694.12398. [DOI] [PubMed] [Google Scholar]
- Ranzer, Restrepo & Kerr (2006).Ranzer LK, Restrepo PF, Kerr RG. Microbial community profiles of bleached and wild-type Muricea elongata. 2006. http://www.ncbi.nlm.nih.gov/popset/134140623?report=genbank http://www.ncbi.nlm.nih.gov/popset/134140623?report=genbank
- Ribes, Coma & Gili (1999).Ribes M, Coma R, Gili J-M. Heterogeneous feeding in benthic suspension feeders: the natural diet and grazing rate of the temperate gorgonian Paramuricea clavata (Cnidaria: Octocorallia) over a year cycle. Marine Ecology Progress Series. 1999;183:125–137. doi: 10.3354/meps183125. [DOI] [Google Scholar]
- Rideout et al. (2014).Rideout JR, He Y, Navas-Molina JA, Walters WA, Ursell LK, Gibbons SM, Chase J, McDonald D, Gonzalez A, Robbins-Pianka A, Clemente JC, Gilbert JA, Huse SM, Zhou H-W, Knight R, Caporaso JG. Subsampled open-reference clustering creates consistent, comprehensive OTU definitions and scales to billions of sequences. PeerJ. 2014;2:e2529. doi: 10.7717/peerj.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie (2006).Ritchie KB. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Marine Ecology Progress Series. 2006;322:1–14. doi: 10.3354/meps322001. [DOI] [Google Scholar]
- Roberts et al. (2009).Roberts JM, Wheeler A, Freiwald A, Cairns S. Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- Robertson et al. (2016).Robertson V, Haltli B, McCauley EP, Overy DP, Kerr RG. Highly variable bacterial communities associated with the octocoral Antillogorgia elisabethae. Microorganisms. 2016;4 doi: 10.3390/microorganisms4030023. Article 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder et al. (2015).Roder C, Bayer T, Aranda M, Kruse M, Voolstra CR. Microbiome structure of the fungid coral Ctenactis echinata aligns with environmental differences. Molecular Ecology. 2015;24:3501–3511. doi: 10.1111/mec.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer et al. (2001).Rohwer F, Breitbart M, Jara J, Azam F, Knowlton N. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs. 2001;20:85-91. doi: 10.1007/s003380100138. [DOI] [Google Scholar]
- Rohwer et al. (2002).Rohwer F, Seguritan V, Azam F, Knowlton N. Diversity and distribution of coral-associated bacteria. Marine Ecology Progress Series. 2002;243:1–10. doi: 10.3354/meps243001. [DOI] [Google Scholar]
- Rowbotham (1986).Rowbotham TJ. Current views on the relationships between amoebae, legionellae and man. Israel Journal of Medical Sciences. 1986;22:678–689. [PubMed] [Google Scholar]
- Santiago-Vázquez et al. (2007).Santiago-Vázquez LZ, Brück TB, Brück W, Duque-Alarcón AP, McCarthy PJ, Kerr RG. The diversity of the bacterial communities associated with the azooxanthellate hexacoral Cirrhipathes lutkeni. The ISME Journal. 2007;1:654–659. doi: 10.1038/ismej.2007.77. [DOI] [PubMed] [Google Scholar]
- Schöttner et al. (2009).Schöttner S, Hoffmann F, Wild C, Rapp HT, Boetius A, Ramette A. Inter- and intra-habitat bacterial diversity associated with cold-water corals. The ISME Journal. 2009;3:756–759. doi: 10.1038/ismej.2009.15. [DOI] [PubMed] [Google Scholar]
- Schüler & Schleifer (2005).Schüler D, Schleifer K-H. Family I. Rhodospirillaceae, genus IV. Magnetospirillum 2005 [Google Scholar]
- Shashar et al. (1994).Shashar N, Cohen Y, Loya Y, Sar N. Nitrogen fixation (acetylene reduction) in stony corals: evidence for coral-bacteria interactions. Marine Ecology Progress Series. 1994;111:259–264. doi: 10.3354/meps111259. [DOI] [Google Scholar]
- Simpson, Eckelbarger & Watling (2005).Simpson AW, Eckelbarger J, Watling L. Some aspects of the reproductive biology of Paramuricea placomus (Octocorallia) from the Gulf of Maine . Abstract 1195Integrative and Comparative Biology. 2005;45 [Google Scholar]
- Stackebrandt & Liesack (1993).Stackebrandt E, Liesack W. Nucleic acids and classification. In: Goodfellow M, O’Donnell AG, editors. Handbook of new bacterial systematics. Academic Press; London: 1993. pp. 152–189. [Google Scholar]
- Sunagawa, Woodley & Medina (2010).Sunagawa S, Woodley CM, Medina M. Threatened corals provide underexplored microbial habitats. PLoS ONE. 2010;5:e2529. doi: 10.1371/journal.pone.0009554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma (2013).Thoma JN. PhD dissertation. 2013. Molecular and morphological diversity of sea fans with emphasis on deep-sea octocorals of the order Alcyonacea Lamouroux 1812. [Google Scholar]
- Thyssen & Ollevier (2005).Thyssen A, Ollevier F. Genus II Photobacterium. Bergey’s Manual of Systematic Bacteriology. 2005;2:546–552. [Google Scholar]
- Turnbaugh et al. (2007).Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett C, Knight R, Gordon JI. The human microbiome project: exploring the microbial part of ourselves in a changing world. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water et al. (2015).Van de Water JAJM, Ainsworth TD, Leggat W, Bourne DG, Willis BL, Van Oppen MJH. The coral immune response facilitates protection against microbes during tissue regeneration. Molecular Ecology. 2015;24:3390–3404. doi: 10.1111/mec.13257. [DOI] [PubMed] [Google Scholar]
- Van de Water et al. (2016).Van de Water JAJM, Melkonian R, Junca H, Voolstra CR, Reynaud S, Allemand D, Ferrier-Pagès C. Spirochaetes dominate the microbial community associated with the red coral Corallium rubrum on a broad geographic scale. Scientific Reports. 2016;6:27277. doi: 10.1038/srep27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbaendert et al. (2011).Verbaendert I, Boon N, De Vos P, Heylen K. Denitrification is a common feature among members of the genus Bacillus. Systematic and Applied Microbiology. 2011;34:385–391. doi: 10.1016/j.syapm.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Vezzulli et al. (2013).Vezzulli L, Pezzati E, Huete-Stauffer C, Pruzzo C, Cerrano C. 16SrDNA pyrosequencing of the Mediterranean gorgonian Paramuricea clavata reveals a link among alterations in bacterial holobiont members, anthropogenic influence and disease outbreaks. PLoS ONE. 2013;8:e2529. doi: 10.1371/journal.pone.0067745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzulli et al. (2010).Vezzulli L, Previati M, Pruzzo C, Marchese A, Bourne DG, Cerrano C, Consortium V. Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environmental Microbiology. 2010;12:2007–2019. doi: 10.1111/j.1462-2920.2010.02209.x. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2007).Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster & Bourne (2007).Webster NS, Bourne DG. Bacterial community structure associated with the Antarctic soft coral, Alcyonium antarcticum. FEMS Microbiology Ecology. 2007;59:81–94. doi: 10.1111/j.1574-6941.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Wegley et al. (2007).Wegley L, Edwards R, Rodriguez-Brito B, Liu H, Rohwer F. Metagenomic analysis of the microbial community associated with the coral Porites astreoides. Environmental Microbiology. 2007;9:2707–2719. doi: 10.1111/j.1462-2920.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- Wickham (2009).Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag; New York: 2009. [Google Scholar]
- Williams, Viner & Broughton (1987).Williams WM, Viner AB, Broughton WJ. Nitrogen-fixation (acetylene-reduction) associated with the living coral Acropora variabilis. Marine Biology. 1987;94:531–535. doi: 10.1007/BF00431399. [DOI] [Google Scholar]
- Yakimov et al. (2006).Yakimov MM, Cappello S, Crisafi E, Trusi A, Savini A, Corselli C, Scarfi S, Giuliano L. Phylogenetic survey of metabolically active microbial communities associated with the deep-sea coral Lophelia pertusa from the Apulian plateau, Central Mediterranean Sea. Deep Sea Research. 2006;53:62–75. doi: 10.1016/j.dsr.2005.07.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
Kellogg, 2015. Cold-water coral microbiomes (Paramuricea placomus) from Baltimore Canyon: raw and processed data. US Geological Survey data release: http://dx.doi.org/10.5066/F7HQ3WZZ.