Abstract
Objective
The routine measurement of IgA anticardiolipin (aCL) and IgA anti-β2 glycoprotein I (anti-β2 GPI) antibodies remain controversial despite several studies demonstrating an association with thromboembolic disease in patients with systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS). This controversy may be a contributing factor for the current under use of IgA antiphospholipid antibodies. We aimed to investigate the nature of discrepant IgA anti-β2 GPI reactivity to help define the diagnostic value of IgA antiphospholipid antibodies.
Material and Methods
Four sera selected from SLE/APS patients and positive for antiphospholipid antibodies but having discrepant IgA anti-β2 GPI reactivity on two commercial assays were studied. IgA antibodies were affinity purified to investigate anti-β2 GPI reactivity. Column wash through and eluent fractions were tested on both IgA anti-β2 GPI assays. Results were normalized to total protein. Assay conjugates and standards from the discrepant assays were interchanged.
Results
The diseased samples were strongly positive in one assay [144–388 IgA antiphospholipid (APL) units] and negative or weakly positive in another assay (9.9–53 APL units). IgA eluents from IgA anti-β2 GPI positive samples reacted 10 times stronger on the reactive assay. When normalized to protein content, the eluents showed no cross-reactivity for IgG or IgM anti-β2 GPI antibodies, confirming IgA isotype specificity. Conjugate interchange confirmed that both assays bound IgA anti-β2 GPI antibodies, but the anti-IgA conjugate from the reactive assay was 4 times stronger, suggesting that its ability to detect IgA anti-β2 GPI antibodies was partially dependent on the anti-IgA conjugate and calibration.
Conclusion
These results confirm not only the presence of IgA anti-β2 GPI antibodies in the selected patient samples but also highlight an IgA conjugate issue for the unreactive assay, causing an underestimation of IgA anti-β2 GPI. This finding may assist in the ongoing standardization efforts of APS antibody testing. In addition, conclusions from published clinical studies may need to be revised as some assays may understate IgA significance.
Keywords: Systemic lupus erythematosus, antiphospholipid syndrome, IgA anti-β2 glycoprotein I, antiphospholipid antibodies
Introduction
Elevated plasma levels of antiphospholipid antibodies have been associated with an increased risk of thromboembolic complications in patients with systemic autoimmune diseases such as systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS) (1, 2). In these patients, most of the pro-thrombotic antiphospholipid antibodies are directed to the lipid-binding plasma protein β2 glycoprotein I (β2 GPI) or to β2 GPI/phospholipid complexes, rather than to phospholipids alone (3, 4). The primary serological criteria to classify APS includes the demonstration of IgG or IgM β2 GPI-dependent anti-cardiolipin (aCL) and/or IgG or IgM anti-β2 GPI antibodies detected by solid-phase immunoassays (5). IgA antiphospholipid antibody determination was not included in the above classification criteria but is to be considered only in certain situations. However, the association of IgA antiphospholipid antibodies in autoimmune patients with thromboembolic events had been confirmed by several groups (6, 7). The diagnostic value of IgA aCL and anti-β2 GPI antibodies has been largely ignored because this antibody isotype is commonly present with IgG and IgM antibodies. Murthy et al. (8) recently reported the presence of isolated IgA anti-β2 GPI antibodies and concluded that this isotype may identify additional patients with the clinical features of APS as well as recommended the testing for IgA antibodies when other antiphospholipid antibodies are absent and APS is suspected.
Despite the fact that IgA anti-β2 GPI antibodies are thrombogenic and associated with clinical manifestations of APS, their use in the clinical laboratory for the assessment of autoimmune-mediated thrombotic risk remains limited. In addition, antiphospholipid antibody proficiency testing had revealed widely discrepant results among laboratories and commonly used commercial IgA anti-β2 GPI enzyme-linked immunoassay (ELISA) tests when testing SLE and/or APS serum samples. Our group speculated that these discrepancies may have contributed to the exclusion of IgA aCL and anti-β2 GPI antibodies from the current classification criteria for APS (5). We investigated the nature of the IgA anti-β2 GPI antibody discrepancy on selected clinically and serologically well-characterized SLE and/or APS samples by isolating IgA antibodies to analyze the reactivity of the fractions on two discrepant assays. One hypothesis to explain the discrepancy was that coated β2 GPI of one assay displayed the open (reactive) β2 GPI configuration, while the other assay had the closed (non-reactive) configuration (9).
Material and Methods
Samples
Four disease-state serum samples were selected from patients with the diagnosis of SLE and/or APS and a history of thromboembolic disease. The diagnosis of SLE and/or APS was established by the attending physicians, according to current criteria (5). These samples exhibited multiple positive antiphospholipid antibody titers (IgG and IgM anti-cardiolipin, anti-phosphatidylserine, and/or anti-β2 GPI). Regarding IgA anti-β2 GPI antibodies, the selected samples had strongly positive titers on one commonly used ELISA test, while negative or low positive titers on other similar test. One negative serum sample from a healthy donor was also included as control. Samples were obtained from Access Biologicals (Vista, California, United States) under their ethics committee and informed consent protocols.
IgA anti-β2 GPI ELISAs
The discrepant IgA anti-β2 GPI results were confirmed on two commercial IgA anti-β2 GPI antibody ELISA tests, according to the manufacturer’s instructions. Assay A, REAADS IgA anti-β2 GPI (Corgenix, Inc.; Broomfield, CO, USA) and assay B, Quanta Lite β2 GPI IgA ELISA (Inova, Inc.; San Diego, CA, USA).
IgA affinity purification
Five milliliters (mL) of serum from each of the selected sample for IgA antibody purification were dialyzed overnight in 100 volumes of equilibration/wash buffer (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.2) using Slide-A-Lyser Cassettes (Thermo Scientific; Rockford, IL, USA) and then filtered through 0.2-μm filter. Dialyzed serum was then loaded onto 2-mL PeptideM/Agarose gel-pdm-2 (Invivogen, San Diego, California, United States) packed into a 4-mL column that had been equilibrated with 20 mL of equilibration/wash buffer. After loading, the column was washed with 40 mL of equilibration/wash buffer, collected, and referred to as the “wash through” fraction. After washing, the material retained in the column was eluted with 0.1 M Glycine, pH 2.5 at 0.5 mL/minute, thereby collecting 1-mL fractions. Pooled (7.5 mL) fractions containing the major protein peak determined at A280 nm were collected and immediately neutralized using 400 μL of 1 M TRIS (pH 8.0) referred to as the “eluent” fraction. Total protein of the fractions was determined using Pierce BCA Protein Assay Kit (Thermo Scientific; Rockford, IL, USA), according to manufacturer specifications.
Results
Table 1 summarizes the antiphospholipid antibody profile of the four selected diseased samples and the negative control. All diseased samples showed strong positive reactions for IgA anti-β2 GPI antibodies in assay A (144–388 APL units) and negative or weak positive reactions in assay B (9.9–53 APL units). The normal cut-off value was <20 APL units for both assays. The diseased samples were also positive for IgG anti-β2 GPI antibodies in both assays.
Table 1.
IgG, IgM, and IgA antiphospholipid antibody titers of selected SLE/APS samples determined on two enzyme-linked immunoassay (ELISA) tests
| Sample | IgA anti-β2 GPI* | IgG anti-β2 GPI* | IgM anti-β2 GPI* | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| SLE/APS | Assay A | Assay B | Assay A | Assay B | Assay A |
| 1 | 252 | 19 | 159 | 78 | 50 |
| 2 | 168 | 9.9 | 163 | 192 | 4.7 |
| 3 | 144 | 10 | 237 | 204 | 6.4 |
| 4 | 388 | 53 | 143 | 41 | 16 |
| Negative | 2.9 | 3.1 | 1.8 | 1.5 | 2.5 |
Results expressed in IgG (GPL), IgM (MPL), and IgA (APL) antiphospholipid units.
Normal cut-off value of <20
SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome
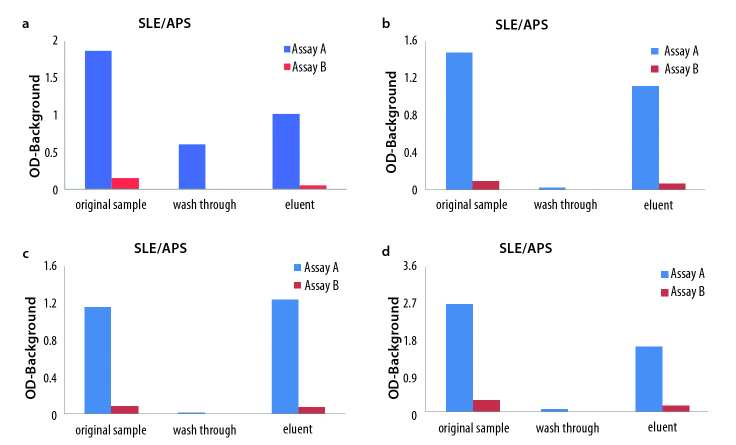
The reactivity of the original samples (before purification and after column separation; wash through and eluent fractions, respectively) tested on both IgA anti-β2 GPI assays is summarized in Figure 1 a–d. IgA anti-β2 GPI antibody reactivity was detected in all four eluents only when tested on assay A. Comparing optical density (OD), the eluents of the IgA aβ2 GPI positive samples reacted 10 times stronger on assay A compared to assay B. The ODs of assay B were within the negative range of the assay (OD<0.200). The negative sample did not show reactivity for IgA anti-β2 GPI antibodies in both assays (not shown). These results clearly demonstrated that IgA anti-β2 GPI antibodies were present in the original samples but not detected in assay B.
Figure 1.
a–d. The original sample, wash through, and eluent fractions from the four selected positive SLE/APS samples were tested on Assay A (blue) and Assay B (red). IgA anti-β2 GPI reactivity is expressed as optical density (OD) minus background. Eluent fractions containing purified IgA from all the four samples reacted only on IgA anti-β2 GPI assay A (blue).
Affinity purification of IgA antibodies: SLE/APS (a), SLE/APS (b), SLE/APS (c), SLE/APS (d)
SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome
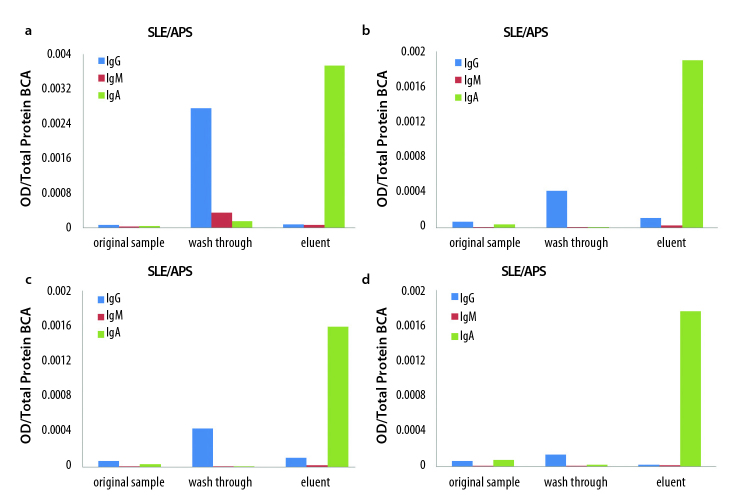
To rule out the possibility of cross-reactivity, column wash through and eluent fractions were tested for IgG, IgM, and IgA anti-β2 GPI antibodies on assay A, and the OD results were normalized against total protein content. Strong IgA anti-β2 GPI antibody reactivity was observed in the eluent but not in the wash-through fractions, indicating that high titers of IgA anti-β2 GPI antibodies were present in the samples, and that IgA was successfully isolated in the eluent fractions (Figure 2 a–d). These results also confirmed the IgA antibody isotype specificity because IgG and IgM anti-β2 GPI antibodies present in the samples did not cross-react on the IgA anti-β2 GPI assay A.
Figure 2.
a–d. The original sample, wash through, and eluent fractions from the selected positive SLE/APS samples were tested on Assay A for IgG (blue), IgM (red), and IgA (green) anti-β2 GPI antibodies. Antibody reactivity was normalized to total protein and expressed as optical density (OD) over total protein. Only purified IgA antibodies (green) reacted on the IgA anti-β2 GPI assay A.
IgA specificity of affinity-purified antibodies. SLE/APS (a), SLE/APS (b), SLE/APS (c), SLE/APS (d)
SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome
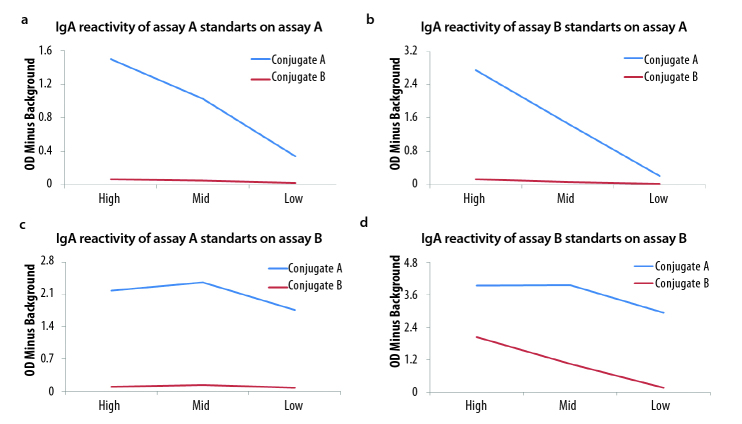
To further explore the nature of the low IgA anti-β2 GPI antibody reactivity observed with assay B, IgA anti-human conjugate from assay A and assay B were interchanged and tested on plates from both assay. The results are summarized in Figure 3 a–d. Conjugate from assay A reacted with reagents from both assays. However, conjugate from assay B reacted only with assay B reagents (Figure 3d). This finding confirms that β2 GPI-coated plates of assay B bound IgA anti-β2 GPI antibodies, thereby questioning the hypothesis that the reason for discrepant results was due to the non-reactive, closed β2 GPI conformation on the assay B plate. When the reactivity of both conjugates tested on assay B were compared (Figure 3d), the conjugate from assay A was 4 times more reactive, suggesting that the ability of assay A to detect IgA anti-β2 GPI antibodies is partially dependent on the anti-IgA conjugate and calibration.
Figure 3.
a–d. Tests standards (high, medium, and low) were tested on each IgA anti-β2 GPI assay, and anti-human IgA conjugates were interchanged (blue from assay A and red from assay B).
There was a 4 times stronger reactivity of conjugate A when tested on assay B than conjugate B, suggesting that IgA detection in assay B is partially dependent on the IgA conjugate and calibration.
Standards A on assay A (a), standards B on assay B (b), standards A on assay B (c), standards B on assay B (d), and conjugate B did not elicit IgA anti-β2 GPI antibody reactivity when tested on assay A (a–c) but only when tested on assay B (d)
Discussion
Several studies have unambiguously demonstrated an association between IgA anti-β2 GPI antibodies with an increased risk of thromboembolic vascular disease in patients with systemic autoimmune diseases such as SLE and APS (10–12). Purified IgA antibodies with aCL and anti-β2 GPI activity from APS patients caused venous thrombus formation and the upregulation of tissue factor (TF) when injected intravenously into a mouse experimental model, confirming their pathogenic role in coagulation (13). However, the routine measurement of IgA antiphospholipid antibodies in clinical laboratories remains controversial. Moreover, IgA anti-β2 GPI antibodies have now been associated with atherosclerotic disease (14, 15), with macrovascular disease in scleroderma patients (16), and with autoimmune liver disease (17). These reports have provided additional support for IgA anti-β2 GPI antibody testing not only for APS diagnosis but also for the serological risk assessment of atherothrombotic disease. The observed inter-laboratory variability together with the unresolved standardization of antiphospholipid antibody testing may continue to hamper the routine determination of IgA anti-β2 GPI antibodies.
In an attempt to provide insights into these pending issues, we column purified IgA antibodies from selected diseased serum samples that exhibit discrepant IgA anti-β2 GPI results. The column fractions containing IgA were analyzed for anti-β2 GPI reactivity on the discrepant assays. Our results not only confirmed the presence of IgA anti-β2 GPI antibodies in the selected samples (Figure 1 a–d) but also highlighted the lack of reactivity of purified IgA antibodies in one of the commercial assays. We also demonstrated that the selected samples contained significant amounts of IgA anti-β2 GPI antibodies when results were normalized for total protein content (Figure 2 a–d).
One hypothesis to explain the discrepancy was that microplates from assay B were coated with human β2 GPI in the closed (un-reactive) conformation, causing the lack of IgA reactivity. Because the microplate from assay B was capable of reacting with the IgA calibrators (Figure 3 a–d), the non-reactive or closed configuration hypothesis was disregarded. However, there was a four times stronger IgA conjugate reactivity for assay A than conjugate B. This may explain a 10 times underestimation of IgA anti-β2 GPI antibody reactivity observed in assay B likely because of IgA conjugate strength and calibration of the assay.
These findings may assist in the ongoing standardization efforts of APS antibody testing. An intriguing idea is to conduct standardization studies using a common conjugate as a key reagent rather than a standard antibody preparation. In addition, conclusions from previously published clinical studies may need to be revised as some assays may understate IgA presence and significance. Nonetheless, the diagnostic value of IgA anti-β2 GPI antibodies has been confirmed by several groups and should be incorporated in APS in the future. The “Non-criteria” Antiphospholipid Task Force convened at the 2010 International Congress on Antiphospholipid Antibodies concluded that IgA anti-β2 GPI antibodies should be incorporated when testing SLE and APS patients, particularly when other antiphospholipid tests are negative or inconclusive (18).
Footnotes
Ethics Committee Approval: Ethics Committee approval was received for this study from Access Biologicals.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: All authors contributed equally during the preparation of this manuscript.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The author declared that this study has received no financial support.
References
- 1.Ginsburg KS, Liang MH, Newcomer L, Goldhaber SZ, Schur PH, Hennekens CH, et al. Anticardiolipin antibodies and the risk for ischemic stroke and venous thrombosis. Ann Intern Med. 1992;117:997–1002. doi: 10.7326/0003-4819-117-12-997. http://dx.doi.org/10.7326/0003-4819-117-12-997. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–27. doi: 10.1002/art.10187. http://dx.doi.org/10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 3.de Groot PG, Urbanus RT. The significance of autoantibodies against β2-glycoprotein I. Blood. 2012;120:266–74. doi: 10.1182/blood-2012-03-378646. http://dx.doi.org/10.1182/blood-2012-03-378646. [DOI] [PubMed] [Google Scholar]
- 4.Lakos G, Kiss E, Regëczy N, Tarján P, Soltész P, Zeher M, et al. Isotype distribution and clinical relevance of anti-β2-glycoprotein I (β2-GPI) antibodies: importance of IgA isotype. Clin Exp Immunol. 1999;117:574–9. doi: 10.1046/j.1365-2249.1999.01007.x. http://dx.doi.org/10.1046/j.1365-2249.1999.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. http://dx.doi.org/10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopez LR, Santos ME, Espinoza LR, La Rosa FG. Clinical significance of immunoglobulin A versus immunoglobulins G and M anti-cardiolipin antibodies in patients with systemic lupus erythematosus. Correlation with thrombosis, thrombocytopenia, and recurrent abortion. Am J Clin Pathol. 1992;98:449–54. doi: 10.1093/ajcp/98.4.449. [DOI] [PubMed] [Google Scholar]
- 7.Sweiss NJ, Bo R, Kapadia R, Manst D, Mahmood F, Adhikari T, et al. IgA anti-β2-glycoprotein I autoantibodies are associated with an increased risk of thromboembolic events in patients with systemic lupus erythematosus. PLoS One. 2010;5:e12280. doi: 10.1371/journal.pone.0012280. http://dx.doi.org/10.1371/journal.pone.0012280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy V, Willis R, Romay-Penabad Z, Ruiz-Limón P, Martínez-Martínez LA, Jatwani S, et al. Value of isolated IgA anti-β2-glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum. 2013;65:3186–93. doi: 10.1002/art.38131. http://dx.doi.org/10.1002/art.38131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agar C, van Os GM, Mörgelin M, Sprenger RR, Marquart JA, Urbanus RT, et al. Beta2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–43. doi: 10.1182/blood-2009-12-260976. http://dx.doi.org/10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 10.Fanopoulos D, Teodorescu MR, Varga J, Teodorescu M. High frequency of abnormal levels of IgA anti-beta2-glycoprotein I antibodies in patients with systemic lupus erythematosus: relationship with antiphospholipid syndrome. J Rheumatol. 1998;25:675–80. [PubMed] [Google Scholar]
- 11.Shen YM, Lee R, Frenkel E, Sarode R. IgA antiphospholipid antibodies are an independent risk factor for thromboses. Lupus. 2008;17:996–1003. doi: 10.1177/0961203308093460. http://dx.doi.org/10.1177/0961203308093460. [DOI] [PubMed] [Google Scholar]
- 12.Meijide H, Sciascia S, Sanna G, Khamashta MA, Bertolaccini ML. The clinical relevance of IgA anticardiolipin and IgA anti-β2-glycoprotein I antiphospholipid antibodies: a systematic review. Autoimmun Rev. 2013;12:421–5. doi: 10.1016/j.autrev.2012.08.002. http://dx.doi.org/10.1016/j.autrev.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Pierangeli SS, Liu SW, Anderson G, Barker JH, Harris EN. Thrombogenic properties of murine anti-cardiolipin antibodies induced by beta2-glycoprotein 1 and human immunoglobulin G antiphospholipid antibodies. Circulation. 1996;94:1746–51. doi: 10.1161/01.cir.94.7.1746. http://dx.doi.org/10.1161/01.CIR.94.7.1746. [DOI] [PubMed] [Google Scholar]
- 14.Lopez LR, Dier KJ, Lopez D, Merrill JT, Fink CA. Anti-beta2-glycoprotein I and antiphosphatidylserine antibodies are predictors of arterial thrombosis in patients with antiphospholipid syndrome. Am J Clin Pathol. 2004;121:142–9. doi: 10.1309/YVQ6-PX76-XMYM-3J29. http://dx.doi.org/10.1309/YVQ6PX76XMYM3J29. [DOI] [PubMed] [Google Scholar]
- 15.Staub HL, Franck M, Ranzolin A, Norman GL, Iverson GM, von Mühlen CA. IgA antibodies to beta2-glycoprotein I and atherosclerosis. Autoimmun Rev. 2006;6:104–6. doi: 10.1016/j.autrev.2006.06.014. http://dx.doi.org/10.1016/j.autrev.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Boin F, Franchini S, Colantuoni E, Rosen A, Wigley FM, Casciola-Rosen L. Independent association of anti-beta(2)-glycoprotein I antibodies with macrovascular disease and mortality in scleroderma patients. Arthritis Rheum. 2009;60:2480–9. doi: 10.1002/art.24684. http://dx.doi.org/10.1002/art.24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabeta S, Norman GL, Gatselis N, Liaskos C, Papamichalis PA, Garagounis A, et al. IgA anti-beta2GPI antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501–11. doi: 10.1007/s10875-008-9211-6. http://dx.doi.org/10.1007/s10875-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 18.Bertolaccini ML, Amengual O, Atsumi T, Binder WL, de Laat B, Forastiero R, et al. Lupus; ‘Non-criteria’ aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies; Galveston, TX, USA. April 2010; 2011. pp. 191–205. http://dx.doi.org/10.1177/0961203310397082. [DOI] [PubMed] [Google Scholar]