Abstract
Takayasu’s arteritis (TAK) is a rare, chronic large-vessel vasculitis (LVV) that predominantly affects the aorta, its major branches, and the pulmonary arteries. Recent advances in the diagnosis, clinical course, disease assessment with biomarkers/imaging and new clinical tools, patient-reported outcomes, and new treatment options of TAK are discussed in this review. Conventional angiography, the gold standard method for initial diagnosis, appears to have been replaced with new imaging modalities such as magnetic resonance angiography (MRA) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in recent years. MRA and FDG-PET are also promising for the assessment of disease activity. New tools for disease assessment such as Indian Takayasu’s Arteritis Score 2010 (ITAS2010) and color Doppler ultrasound (CDUS) aim to better characterize and quantify disease activity; however, different imaging modalities in routine follow-up are not incorporated sufficiently in these approaches. Prognosis is possibly getting better, with lower mortality in recent years; however, it is difficult to assess the widely different vascular intervention rates among the clinical series. Leflunomide, tumor necrosis factor (TNF)-α antagonists, and tocilizumab are new options for patients resistant to conventional therapies. There is a clear need to develop a validated set of outcome measures for use in clinical trials of TAK. The Outcome Measures in Rheumatology (OMERACT) Vasculitis Working Group has taken on this task, finished a Delphi exercise with experts, and aims to develop a core set of outcomes for LVV in accordance with OMERACT Filter 2.0.
Keywords: Takayasu’s arteritis, disease assessment, outcome
Introduction
Takayasu’s arteritis (TAK) is a rare, chronic large-vessel arteritis that predominantly affects the aorta, its major branches, and the pulmonary arteries. Segmental stenosis, occlusion, dilatation, or aneurysm formation may occur in the vessel wall during the course of the disease (1–4). All large arteries can be affected, although the ascending/descending aorta, subclavian arteries, and extracranial arteries such as carotids are most frequently involved (60%–90%). Various signs and symptoms such as constitutional features (fever, malaise, anorexia, and weight loss), extremity pain, claudication, lightheadedness, bruits, absent or diminished pulses, and reduced blood pressure can be present according to the vessel involved (5). The disease generally has a prolonged indolent course. Acute events such as visual loss or stroke are not very frequent in TAK and are observed at variable rates in different populations. In this review, we will summarize the recent developments in the diagnosis, clinical course, disease assessment with biomarkers/imaging and new clinical tools, patient-reported outcomes, and new treatment options of TAK.
Diagnosis
A set of classification criteria for TAK was established by the American College of Rheumatology (ACR) in 1990 (6). Although this criteria have not been criticized much as other criteria sets with over 90% sensitivity and specificity, the control group formed of mainly small-vessel vasculitides (used for similar ACR classification criteria sets) which have limited common clinical features with TAK. Usefulness of these criteria is limited in real-life settings because of the lack of a control group with atherosclerotic or congenital aortic vessel disease, particularly in the middle-aged population. Recent studies demonstrating the overlap between giant cell arteritis (GCA) and TAK and new entities such as IgG4-related diseases involving the aorta make the discrimination among large vessel vasculitis (LVV) more difficult (7, 8). To overcome these problems, a global project, Diagnostic and Classification in Vasculitis Study (DCVAS), is underway to develop new classification criteria for all vasculitides.
Prevalance and ethnicity
According to a nationwide Japanese registry, there were at least 5881 patients with TAK in Japan in 2011, with the prevalence believed to be over 0.004% (9). The estimated annual incidence is 0.4/million in Denmark. No mortality was observed in this Danish series in 11.5 years of follow-up (10). A systematic review evaluated 197 patients from 7 Arab countries with a population of approximately 80 million, where TAK is believed to have a low prevalence (11). The demographic and clinical findings of TAK in Arabs was reported to be similar to those in other parts of the world and the overall mortality was low over a follow-up period of 5.4 years, with the course of the disease “quite stable” in approximately 50% of patients.
A comparative study from France investigated TAK among white, North African, and black patients (12). The median age at diagnosis was 39.3 years in white, 28.4 years in North African, and 28.0 years in black patients. North African patients had more frequent occurrence of ischemic stroke and poorer survival than white patients. The 5-year and 10-year survival rates were 100% and 95.0%, respectively, in whites, 100% at both 5 years and 10 years in blacks, and only 67.4% at both 5 years and 10 years in North African patients, suggesting major differences in prognosis according to ethnicity.
Clinical course
Various new clinical series published recently have better characterized the natural course of TAK. Grayson et al. demonstrated that among 6 different vasculitides, TAK has the highest rate of new, severe manifestation (ischemic, vascular) (incidence: 44%) (13). The clinical features of “vascular symmetry” in TAK were investigated in 2 separate studies from United States and France. Cluster analysis revealed that TAK lesions mostly develop in a symmetric manner in paired vascular territories and disease extension is contiguous in the aorta (14). A similar finding was also observed in the case of GCA (15). Computer-derived classification models distinguished TAK from GCA in 2 subgroups, defining 26% and 18% of the study sample, respectively; however, 56% of patients were classified into a subgroup that did not strongly differentiate between TAK and GCA.
A series from Portugal reported a high incidence of stroke development concomitant with diagnosis among patients with TAK (16). In another cohort, male gender represented an independent risk factor for the occurrence of abdominal pain and ascending aortic aneurysms (17).
A pediatric TAK series with a median age of onset of 12.5 years has been published from India (18). The most common presenting features were hypertension, headache, and fever. The majority of patients were active with increased acute-phase response (APR) and high activity scores. Although short-term remission was observed in most patients, only 29% showed sustained remission in 5 years. With aggressive medical and surgical intervention, the disease is stabilized in most patients. Another pediatric series of 71 patients has been reported from Brazil with 50% children (<10 years old). Although the clinical and angiographic data were similar between children and adolescents (10–20 years old), anemia and thrombocytosis were more common among children (19).
Most large series have been published from East Asian countries. In a series of 204 patients, active patients had a higher incidence of significant aortic valve regurgitation and pulmonary hypertension and higher levels of NT-pro-brain natriuretic peptide (20). Active TAK patients also had more frequent involvement of the ascending aorta and the aortic arch and its main branches than the inactive group. In another large series from China, coronary artery involvement was 7.7% among 587 patients, and 8 patients died during the follow-up period of 5.8 years (21). Among 180 patients from Korea, the standardized incidence ratio (SIR) of cancer in patients with TAK (1.3) was comparable to that in the general population, although the risk of myelodysplastic syndrome was significantly increased (SIR: 51.3) (22).
Significantly higher maternal complications, including pregnancy-induced hypertension, preeclampsia, postpartum hemorrhage and preterm labor, were demonstrated among 29 pregnancies in India (23). In terms of neonatal outcomes, there was an increased incidence of intrauterine growth restriction and neonates requiring neonatal intensive care unit admissions. A better prognosis has been published from Japan among 26 pregnancies, with only 2 cases with hypertension, 5 abortions, and only 2 growth restrictions among newborns (24).
Prognosis
Long-term prognosis has been investigated in a limited number of series of TAK. Among the different series, mortality appears to range between 3%–15%. In one of the largest series with a long follow-up period from Mayo Clinic, overall survival was much better than that in earlier series, with 97% at 10 and 86% at 15 years. (25) However, mortality was still increased compared with the general population [standardized mortality ratio (SMR): 3.0]. Disease phenotype and severity of disease expression due to ethnicity, differences in medical therapy (e.g., less frequent use of glucocorticoids and cytotoxic agents), and variations in access to surgical therapy may result in different mortality rates. Similarly, the rate of surgical procedures is widely discrepant among series. Arterial reconstruction and bypass grafting may be necessary in up to 70% of patients with TAK to reverse some of the complications of the disease, e.g., renovascular hypertension (26). The rate of surgical therapy has been reported to vary between 12% and 50% in different cohorts (2, 25, 27). The restenosis rate after bypass procedures is also widely variable between 5% and 31%. Percutaneous transluminal angioplasty/stenting has been reported to have a higher restenosis rate in the Indian cohort compared with the other reports (12%–71.4%) (26, 28). The restenosis rate was reduced when surgical treatment was performed during the inactive stage of the disease and under treatment with both glucocorticoids and immunosuppressive (IS) agents (29–31). These data suggest that early immunosuppression and choice of treatment may be influencing the different rates of outcome in the literature. Finally, Ohigashi et al. first showed a decrease in the mortality of TAK, with a mortality rate of 2.8% during the follow-up period of 2000–2010 (32).
Disease assessment
Physical examination
Physical examination for new vascular signs is a simple first approach for disease assessment in TAK. However, the limitations of physical examination were recently shown in a study comparing physical signs with imaging data (15). When bruits, absent pulses, or blood pressure differences are evaluated as physical signs, the presence of any single item has a sensitivity of 52%–71% and a specificity of 59%–86%. Although specificity is higher if 2 abnormal examination findings are present together (88%–100%), the sensitivity of pairs of examination findings is low (6%–30%). In contrast, presence of ischemic symptoms or even signs may also not always indicate active inflammation of the vessel wall in TAK. However, practical issues such as “contrast load,” which may be problematic in the long-term, and the possible unclear nature of new vessel wall changes, which may be due to “shear stress” or early atherosclerosis and not necessarily require a change in medical therapy or endovascular intervention, make routine follow-up with imaging difficult even in the most experienced tertiary centers. Further prospective studies are also required to clarify the exact role of routine follow-up with imaging vs. clinical features to demonstrate the feasibility of this approach.
Laboratory search for a biomarker
As in other inflammatory disorders, search for a convenient, reliable, and validated biomarker for TAK still continues. APR evaluation [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels] is frequently advocated for disease assessment in TAK, despite being shown to be neither sensitive nor specific enough to monitor disease activity (3). In one study, active disease was present in 23% of patients with TAK who showed normal laboratory parameters. Similarly, ESR was elevated in only 72% of patients considered to have an active disease and was still high in 44% of patients considered to be clinically inactive. Serum autoantibodies such as anti-endothelial antibodies (33) and serum biomarkers such as IL-6, IL-8, IL-18, and matrix metalloproteinase-9 (34, 35) have been suggested as candidate biomarkers. We also recently demonstrated that IL-6, IL-8, and IL-18 are elevated in the sera of patients with TAK; however, the association with disease activity was not consistent and sufficient to use as a biomarker (Alibaz-Oner F, unpublished observation).
Recently, pentraxin 3 (PTX3), which is produced by immune and vascular cells in response to proinflammatory signals, was suggested as a biomarker for disease activity in patients with TAK (36–38). In a single-center study from Italy, the levels of PTX3 were higher in patients with active TAK (median: >2.14 ng/mL) than in patients with inactive TAK (0.63 ng/mL), patients with infections (0.26 ng/mL), and healthy controls (0.11 ng/mL) (35). In another study from Japan, among the 28 patients with active TAK, 71% were positive for hsCRP and 82% for PTX3. However, these data require confirmatory studies to show whether PTX3 is superior to CRP (37).
Imaging in TAK: recent developments
The earliest detectable abnormality is usually the thickening of the vessel wall by inflammation in TAK. Magnetic resonance angiography (MRA), ultrasound (US), and to a lesser degree, computed tomography (CT) can detect vessel wall thickening. Conventional digital subtraction angiography is the “gold standard” for detecting stenosis, occlusions, and aneurysms that characterize the latter stages of TAK; however, it is the least sensitive method for visualizing wall thickness.
CT/MRA
Contrast-enhanced MRA or CT angiography (CTA) allows non-invasive imaging of the aorta and its major branches. Recently, MRA has become popular for the diagnosis of TAK (Figure 1). Compared with invasive angiography, three-dimensional MRA can effectively show vessel wall thickening. Contrast-enhanced MRA allows better soft-tissue differentiation and can depict other signs of inflammation, including mural edema and increased mural vascularity. Another advantage of MRA is the lack of iodinated contrast material (14, 39, 40). MRA has been extensively used in the current literature for evaluating vascular inflammation and has increasingly replaced invasive angiography. Although MRA appears to be highly accurate, sensitive, and safe compared with invasive angiography in the diagnosis of TAK (41), 2% of stenotic arteries were wrongly portrayed as occluded in a previous study.
Figure 1.

Three-dimensional reconstruction of computed tomography angiography (CTA) data demonstrating high-grade stenosis in the left subclavian artery
Some recent studies have suggested that MRA technology also has the potential to assess disease activity and response to treatment. Andrews et al. (42) and Choe at al. (39) detected edema and enhancement of the vascular wall, as well as reduction of the mural diameter on MR images, associated with disease activity. Furthermore, these studies suggested that there is a close correlation between wall thickness and/or edema of the vessel, enhancement of the wall detected by MR imaging, and APRs. In another recent study analyzing the imaging manifestations of contrast-enhanced MRA, the scores were moderately correlated to CRP levels, platelet counts, and fibrinogen levels (p<0.05), suggesting that an MRA scoring system for lumen stenosis, wall thickness, and wall enhancement could be a non-invasive approach to facilitate assessment of TAK activity (43). However, in a study by Tso et al. (44), although the MRA scans of 94% of the patients revealed vessel wall edema during periods of unequivocally active disease, 56% also showed edema during apparent clinical remission. This study highlights some of the problems of vessel wall assessment according to imaging alone and suggests that imaging and clinical assessment should still be complementary.
US
As a non-invasive modality, US has recently been extensively studied in TAK, particularly to investigate the changes in carotid arteries. Doppler US can detect stenosis in carotid arteries with high sensitivity (90%) and specificity (91%) (45). Contrast-enhanced US may allow the identification of inflammation-driven hyperemia and neovascularization, a potential marker of disease activity. Contrast-enhanced US has recently been reported to show vessel wall thickness in TAK and GCA and increased carotid intima-media thickness (CIMT) in TAK (46, 47). A scoring system for TAK assessment with color Doppler US (CDUS.K) has also been recently presented (48). This score examines 19 vascular regions in a standardized manner, scoring each for both stenosis and flow pattern. The correlation with the angiography score was good; however, intrathoracic vessels, such as the commonly involved subclavian arteries, were particularly difficult to visualize and produced the lowest kappa values in this study. CDUS.K also scores each vessel dichotomously (as 0 and 1), thus limiting the assessment of further changes in the vessel lumen. A good correlation between CDUS.K and the clinical activity score was also present, but it is not known how much that reflects a stenosis score versus a score of more diffuse alterations in flow pattern. Although non-invasiveness and lack of ionizing radiation increase the feasibility of US, further studies are warranted to confirm the potential of US for monitoring disease activity and treatment in TAK (49).
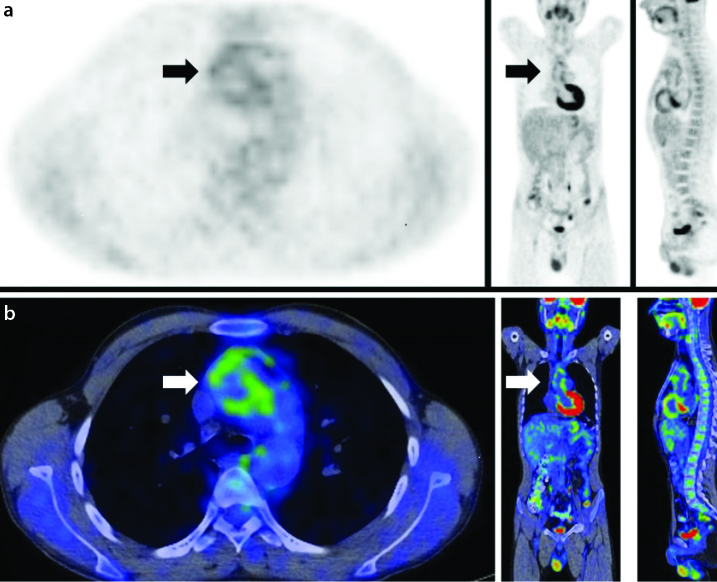
18F-fluorodeoxyglucose positron emission tomography (FDG-PET)
FDG-PET, a modality based on the regional distribution of the glucose analogue 18F-FDG, is an operator-independent, non-invasive metabolic imaging method (Figure 2). FDG-PET is a sensitive and specific imaging tool for LVV (42). The method of assessing 18F-FDG uptake varies according to the study. Some studies quantify 18F-FDG uptake using a standard uptake value (SUV), whereas others use a semi-quantitative method comparing the 18F-FDG uptake of a vascular region of interest (ROI) with that of the liver using a 0–3 grading system [0=no uptake present; 3=high-grade uptake (uptake higher than that by the liver uptake)] (41, 50, 51). Measuring the mean SUV in the center of the inferior vena cava and calculating the target/background ratio as max SUV in the arterial wall/mean SUV in the inferior vena cava is also suggested to differentiate active and inactive TAK (52) with a max SUV cutoff of 2.1. Kobayashi et al. (50) first established a cutoff for max SUV (strong accumulation: SUV of >2; weak accumulation: SUV of 1.2–2.3) with a sensitivity of 90.9% and a specificity of 88.8%. In another study containing the biggest sample size in the literature with 38 patients, 18F-FDG uptake was associated with clinical disease activity and markers of inflammation, and FDG-PET reflected changes in clinical disease activity (53).
Figure 2.
a, b. Axial, coronal, and sagittal positron emission tomography (PET) (a) and PET/computed tomography (CT) fusion (b) images of the same patient show fluorine-18-fluorodeoxyglucose (18F-FDG) uptake at the level of the ascending aorta and the aortic arch (arrows) consistent with activated disease
In contrast to these studies with high sensitivity and specificity, Arnaud et al. (54) showed a lack of correlation between uptake of 18-FDG, clinical disease activity, and levels of the markers of inflammation. They pointed out that previous studies used various invalid criteria for active TAK and may therefore be biased. They depended exclusively on clinical symptoms without considering the markers of inflammation when assessing clinical disease activity and reported that the FDG-PET scan had a sensitivity of 69.2% and a specificity of 33.3% for clinically active TAK. In a recent study, Karapolat et al. found out that 18-FDG PET findings were mostly consistent with clinical disease activity, having sensitivity and specificity values of 100% and 88.9%, respectively (55). In another study, SUVmax values were shown to change during the initial untreated phase of TAK compared to follow-up studies and immunosuppression was shown to decrease the positivity of PET to 32% in active patients (56). In a meta-analysis of the literature, the pooled sensitivity and specificity of 18-FDG-PET were found to be 70.1% and 77.2%, respectively. The positive and negative likelihood ratios were 2.31 and 0.34 and the authors concluded that 18-FDG-PET had moderate diagnostic value in assessing TAK activity (57).
Outcome measures in TAK: disease activity
The simple definition of “active disease,” which was first reported in a study from the National Institute of Health (NIH) by Kerr et al. (presence of constitutional symptoms, new bruits, APR, or new angiographic features), is commonly applied in studies of TAK (58). The Birmingham Vasculitis Activity Score (BVAS) documents evidence of active vasculitis with a 1-page form (59). Although designed to apply to all vasculitides, BVAS is mostly used in therapeutic trials of ANCA-associated vasculitis and is validated for use in small- and medium-vessel vasculitis. However, most of the 11 organ systems in BVAS are not involved in TAK. The “disease extent index for Takayasu’s arteritis (DEI.Tak)” is another recently developed assessment tool in which items corresponding to large arterial disease carry greater weights than general items of the disease, and changes in physical examination in the previous 3 months are the basis of evaluation (60). In a study from Turkey, most patients with TAK with slow disease progression demonstrated no change in the DEI.Tak score (61). Among the DEI.Tak(-) group, 31% were considered to have “active/persistent” disease according to physician’s global assessment (PGA), whereas 18% of patients with a DEI.Tak score of ≥1 were considered to be inactive by PGA. Recently, a new version of DEI.Tak, the Indian Takayasu’s Arteritis Score 2010 (ITAS2010) was introduced (62). ITAS2010 has only 6 systems and scoring is weighted for vascular items (0–2). ITAS2010 appears to have good comprehensiveness and the interrater agreement is better than that obtained with PGA (0.97 vs 0.82). However, convergent validity, when assessed in comparison with PGA, is quite low at initial evaluation but improves at subsequent study visits (r=0.51, 0.64, and 0.72). Although CRP and ESR had weak correlations with ITAS2010, the authors made a further attempt to incorporate APR to the score (ITAS2010-A) by adding extra 1–3 points for elevated ESR or CRP levels. This change resulted in higher ITAS2010-A scores both in active and inactive patients. The fact that items on the ITAS2010 are still present even during apparent remission is problematic and illustrates the substantial difficulty in differentiating activity from damage due to non-vasculitis related problems in this disease (63). The suggestion to have a cut-off of 4 points to separate active and inactive disease states does not satisfactorily address the underlying limitation of the ITAS2010 assessment method. The low correlation of ITAS2010 with PGA suggests that some physicians may accept “active” patients as only those with increased APR or new abnormalities on vascular imaging studies (such as new vessel wall enhancement or thickening seen by MRI or PET).
ITAS has recently been used in a clinical trial of tocilizumab in TAK (64) and in a study investigating the markers of endothelial injury and repair, circulating endothelial cell (CEC), circulating endothelial progenitor cell (CEPC), and vascular endothelial growth factor (VEGF) (65).
A consensus definition of refractory disease for TAK has been proposed by the Turkish Takayasu Group: “angiographic or clinical progression despite treatment” or any of the following characteristics: corticosteroid dose of >7.5 mg/day after 6 months of treatment despite the administration of conventional ISs (methotrexate, azathioprine, leflunomide, or cyclophosphamide), new surgery on account of persistent disease activity, frequent attacks (>3/year), or mortality associated with disease activity (66).
Measuring health-related quality of life in TAK
Disease-related damage and treatment toxicity can severely impact patients’ quality of life and functional status, and patient-reported outcomes (PROs) are accepted to be major components of disease assessment in systemic vasculitides (67). Thus, it is important to measure the health-related quality of life (HRQoL) in patients with TAK and determine the effect of treatments on this domain of illness. It has been shown that patients with vasculitis judge the importance of various disease manifestations differently from how physicians rate the same problems (68). Two previous studies have used the Short-Form 36 (SF-36) questionnaire to measure HRQoL in TAK (69, 70). In another study from Turkey, when patient-reported outcomes such as SF-36, Health Assessment Questionnaire (HAQ), and hospital anxiety and depression scale (HADS) were investigated together, all SF-36 subscores were lower and HAQ and HADS scores were higher in patients with TAK, associated with active disease. Patients having anxiety and depression or with high HAQ scores also reported worse SF-36 scores (71). In another study, fibromyalgia (FM), defined according to new ACR FM criteria (without tender point scores), was found at a higher prevalence in active patients with TAK. New FM criteria subscales (WPI and SSS) were also correlated with scales such as the SF-36, HDAS, and HAQ in patients with TAK, suggesting that in a minority of patients with FM and TAK, PROs may be affected by the presence of FM (72).
Treatment
There are no controlled, clinical therapeutic trials for TAK (73). However, recent uncontrolled data of leflunomide, tumor necrosis factor (TNF)-α antagonists, and tocilizumab in refractory TAK appear promising (74). Leflunomide was shown to be effective in a short-term study of 14 patients with active TAK despite therapy with corticosteroids and IS agents. In this study, activity decreased with leflunomide (from 93% to 20%), mean daily dose of prednisone (34.2 to 13.9 mg), and the median values of ESR and CRP fell. Among biological agents, tocilizumab is currently the mostly popular and has been studied in 9 case series and some individual cases in the last 5 years (75–82). A recent literature review summarized 44 cases in 2013, with a mean follow-up period of 9 months (83). At the last visit, tocilizumab was continued in 53% and was discontinued in the 15 remaining patients because of remission (n=5), relapse (n=3), persistent radiological activity (n=3), cutaneous rash (n=2), and severe infection (n=1). As sustained remission could only be achieved with long-term treatment in most patients, different biological agents may be required during long-term follow-up. Whether these biological agents should be considered earlier in the treatment algorithm of these complicated patients remains an area of interest (72).
In conclusion, conventional angiography, the gold standard method for the diagnosis of TAK, seems to have been replaced with new imaging modalities such as MRA and FDG-PET in recent years. The data reported by recent studies support that MRA and FDG-PET are also promising for the assessment of disease activity. New tools for disease assessment such as Indian Takayasu’s Arteritis Score 2010 (ITAS2010) and CDUS aim to better characterize and quantify disease activity; however, different imaging modalities in routine follow-up are not incorporated sufficiently in these approaches. Leflunomide, TNF-α antagonists, and tocilizumab are new options for patients resistant to conventional therapies.
There is a clear need to develop a validated set of outcome measures for use in clinical trials of TAK. The OMERACT Vasculitis Working Group has taken on this task and first reviewed current evidence. The Working Group than advanced a research agenda that includes parallel projects to understand the perspectives and insight into outcomes of importance in LVV by expert and experienced physicians and investigators as well as patients. A Delphi exercise has been completed to determine experts’ consensus opinions on the disease domains and subdomains of importance to study in LVV and to determine a preliminary set of outcomes and outcome instruments to capture data on the domains (65). Further studies will explore the definitions of flare, remission, and response and aim to improve disease assessment tools with expert opinion and patient data.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - H.D.; Design - F.A.O., H.D., S.Z.A.; Supervision - H.D.; Materials - F.A.O.; Data Collection and/or Processing - F.A.O.; Analysis and/or Interpretation - F.A.O., S.Z.A., H.D.; Literature Review - F.A.O., H.D.; Writer - F.A.O., S.Z.A., H.D.; Critical Review - H.D.
Conflict of Interest: The authors declared no conflict of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, et al. Takayasu arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. http://dx.doi.org/10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bıcakcıgil M, Aksu K, Kamali S, Ozbalkan Z, Ates A, Karadag O, et al. Takayasu’s arteritis in Turkey - clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27:S59–64. [PubMed] [Google Scholar]
- 3.Mason JC. Takayasu arteritis-advances in diagnosis and management. Nat Rev Rheumatol. 2010;6:406–15. doi: 10.1038/nrrheum.2010.82. http://dx.doi.org/10.1038/nrrheum.2010.82. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. http://dx.doi.org/10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 5.Vanoli M, Daina E, Salvarani C, Sabbadini MG, Rossi C, Bacchiani G, et al. Takayasu’s arteritis: A study of 104 Italian patients. Arthritis Rheum. 2005;53:100–7. doi: 10.1002/art.20922. http://dx.doi.org/10.1002/art.20922. [DOI] [PubMed] [Google Scholar]
- 6.Arent WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 7.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore) 2009;88:221–6. doi: 10.1097/MD.0b013e3181af70c1. http://dx.doi.org/10.1097/MD.0b013e3181af70c1. [DOI] [PubMed] [Google Scholar]
- 8.Kim YJ, Park YS, Koo BS, So MW, Kim YG, Lee CK, et al. Immunoglobulin G4-related disease with lymphoplasmacytic aortitis mimicking Takayasu arteritis. J Clin Rheumatol. 2011;17:451–2. doi: 10.1097/RHU.0b013e31823ac028. http://dx.doi.org/10.1097/RHU.0b013e31823ac028. [DOI] [PubMed] [Google Scholar]
- 9.Terao C, Yoshifuji H, Mimori T. Recent advances in Takayasu arteritis. Int J Rheum Dis. 2014;17:238–47. doi: 10.1111/1756-185X.12309. http://dx.doi.org/10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer L, Faurschou M, Baslund B. A population-based study of Takayasu’s arteritis in eastern Denmark. Clin Exp Rheumatol. 2011;29:S40–2. [PubMed] [Google Scholar]
- 11.Mustafa KN. Takayasu’s arteritis in Arabs. Clin Rheumatol. 2014;33:1777–83. doi: 10.1007/s10067-014-2633-z. http://dx.doi.org/10.1038/nrrheum.2010.82. [DOI] [PubMed] [Google Scholar]
- 12.Arnaud L, Haroche J, Limal N, Toledano D, Gambotti L, Costedoat Chalumeau N, et al. Takayasu arteritis in France: a single-center retrospective study of 82 cases comparing white, North African, and black patients. Medicine (Baltimore) 2010;89:1–17. doi: 10.1097/MD.0b013e3181cba0a3. http://dx.doi.org/10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 13.Grayson PC, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, et al. Vasculitis Clinical Research Consortium. New features of disease after diagnosis in 6 forms of systemic vasculitis. J Rheumatol. 2013;40:1905–12. doi: 10.3899/jrheum.121473. http://dx.doi.org/10.1002/art.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev. 2011;11:61–7. doi: 10.1016/j.autrev.2011.08.001. http://dx.doi.org/10.1097/MD.0b013e3181af70c1. [DOI] [PubMed] [Google Scholar]
- 15.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Vasculitis Clinical Research Consortium. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheum Dis. 2012;71:1329–34. doi: 10.1136/annrheumdis-2011-200795. http://dx.doi.org/10.1097/RHU.0b013e31823ac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Paula LE, Alverne AR, Shinjo SK. Clinical and vascular features of Takayasu arteritis at the time of ischemic stroke. Acta Reumatol Port. 2013;38:248–51. [PubMed] [Google Scholar]
- 17.Mont’Alverne AR, Paula LE, Shinjo SK. Features of the onset of Takayasu’s arteritis according to gender. Arq Bras Cardiol. 2013;101:359–63. doi: 10.5935/abc.20130180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goel R, Kumar TS, Danda D, Joseph G, Jeyaseelan V, Surin AK, Bacon P. Childhood-onset Takayasu Arteritis - Experience from a Tertiary Care Center in South India. J Rheumatol. 2014;41:1183–9. doi: 10.3899/jrheum.131117. http://dx.doi.org/10.1111/1756-185X.12309. [DOI] [PubMed] [Google Scholar]
- 19.Clemente G, Hilario MO, Lederman H, Silva CA, Sallum AM, Campos LM, et al. Takayasu arteritis in a Brazilian multicenter study: children with a longer diagnosis delay than adolescents. Clin Exp Rheumatol. 2014;32:S128–33. [PubMed] [Google Scholar]
- 20.Lee GY, Jang SY, Ko SM, Kim EK, Lee SH, Han H, et al. Cardiovascular manifestations of Takayasu arteritis and their relationship to the disease activity: analysis of 204 Korean patients at a single center. Int J Cardiol. 2012;159:14–20. doi: 10.1016/j.ijcard.2011.01.094. http://dx.doi.org/10.1007/s10067-014-2633-z. [DOI] [PubMed] [Google Scholar]
- 21.Sun T, Zhang H, Ma W, Yang L, Jiang X, Wu H, et al. Coronary artery involvemet in takayasu arteritis in 45 Chinese patients. J Rheumatol. 2013;40:493–7. doi: 10.3899/jrheum.120813. http://dx.doi.org/10.1097/MD.0b013e3181cba0a3. [DOI] [PubMed] [Google Scholar]
- 22.Park JK, Choi IA, Lee EY, Song YW, Lee EB. Incidence of malignancy in Takayasu arteritis in Korea. Rheumatol Int. 2014;34:517–21. doi: 10.1007/s00296-013-2887-9. http://dx.doi.org/10.3899/jrheum.121473. [DOI] [PubMed] [Google Scholar]
- 23.Mandal D, Mandal S, Dattaray C, Banerjee D, Ghosh P, Ghosh A, et al. Takayasu arteritis in pregnancy: an analysis from eastern India. Arch Gynecol Obstet. 2012;285:567–71. doi: 10.1007/s00404-011-1998-3. http://dx.doi.org/10.1016/j.autrev.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Hidaka N, Yamanaka Y, Fujita Y, Fukushima K, Wake N. Clinical manifestations of pregnancy in patients with Takayasu arteritis: experience from a single tertiary center. Arch Gynecol Obstet. 2012;285:377–85. doi: 10.1007/s00404-011-1992-9. http://dx.doi.org/10.1136/annrheumdis-2011-200795. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt J, Kermani TA, Bacani AK, Crowson CS, Cooper LT, Matteson EL, et al. Diagnostic features, treatment, and outcomes of Takayasu arteritis in a US cohort of 126 patients. Mayo Clin Proc. 2013;88:822–30. doi: 10.1016/j.mayocp.2013.04.025. http://dx.doi.org/10.3899/jrheum.131117. [DOI] [PubMed] [Google Scholar]
- 26.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Limitations of therapy and a guarded prognosis in an American cohort of Takayasu arteritis patients. Arthritis Rheum. 2007;56:1000–9. doi: 10.1002/art.22404. http://dx.doi.org/10.1016/j.ijcard.2011.01.094. [DOI] [PubMed] [Google Scholar]
- 27.Fields CE, Bower TC, Cooper LT, Hoskin T, Noel AA, Panneton JM, et al. Takayasu’s arteritis: Operative results and influence of disease activity. J Vasc Surg. 2006;43:64–71. doi: 10.1016/j.jvs.2005.10.010. http://dx.doi.org/10.3899/jrheum.120813. [DOI] [PubMed] [Google Scholar]
- 28.Perera AH, Youngstein T, Gibbs RG, Jackson JE, Wolfe JH, Mason JC. Optimizing the outcome of vascular intervention for Takayasu arteritis. Br J Surg. 2014;101:43–50. doi: 10.1002/bjs.9372. http://dx.doi.org/10.1007/s00296-013-2887-9. [DOI] [PubMed] [Google Scholar]
- 29.Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, et al. Retrospective analysis of surgery versus endovascular intervention in Takayasuarteritis: a multicenter experience. Circulation. 2012;125:813–9. doi: 10.1161/CIRCULATIONAHA.111.058032. http://dx.doi.org/10.1007/s00404-011-1998-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim YW, Kim DI, Park YJ, Yang SS, Lee GY, Kim DK, et al. Surgical bypass vs endovascular treatment for patients with supra-aortic arterial occlusive disease due to Takayasu arteritis. J Vasc Surg. 2012;55:693–700. doi: 10.1016/j.jvs.2011.09.051. http://dx.doi.org/10.1007/s00404-011-1992-9. [DOI] [PubMed] [Google Scholar]
- 31.Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim WH. Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu’s arteritis. Rheumatology (Oxford) 2006;45:600–5. doi: 10.1093/rheumatology/kei245. http://dx.doi.org/10.1016/j.mayocp.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Ohigashi H, Haraguchi G, Konishi M, Tezuka D, Kamiishi T, Ishihara T, et al. Improved prognosis of takayasu arteritis over the past decade-comprehensive analysis of 106 patients. Circ J. 2012;76:1004–11. doi: 10.1253/circj.cj-11-1108. http://dx.doi.org/10.1002/art.22404. [DOI] [PubMed] [Google Scholar]
- 33.Lee SK. Anti-endothelial cell antibodies and antiphospholipid antibodies in Takayasu’s arteritis: correlations of their titers and isotype distributions with disease activity. Clin Exp Rheumatol. 2006;24:S10–16. [PubMed] [Google Scholar]
- 34.Noris M, Daina E, Gamba S, Bonazzola S, Remuzzi G. Interleukin-6 and RANTES in Takayasu Arteritis: A Guide for Therapeutic Decisions? Circulation. 1999;100:55–60. doi: 10.1161/01.cir.100.1.55. http://dx.doi.org/10.1016/j.jvs.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Tripathy NK, Sinha N, Nityanand S. Interleukin-8 in Takayasu’s arteritis: plasma levels and relationship with disease activity. Clin Exp Rheumatol. 2004;22:S27–30. [PubMed] [Google Scholar]
- 36.Dagna L, Salvo F, Tiraboschi M, Bozzolo EP, Franchini S, Doglioni C, Manfredi AA, et al. Pentraxin-3 as a marker of disease activity in Takayasu arteritis. Ann Intern Med. 2011;155:425–33. doi: 10.7326/0003-4819-155-7-201110040-00005. http://dx.doi.org/10.1002/bjs.9372. [DOI] [PubMed] [Google Scholar]
- 37.Ishihara T, Haraguchi G, Kamiishi T, Tezuka D, Inagaki H, Isobe M. Sensitive assessment of activity of Takayasu’s arteritis by pentraxin3, a new biomarker. J Am Coll Cardiol. 2011;57:1712–3. doi: 10.1016/j.jacc.2010.10.058. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.058032. [DOI] [PubMed] [Google Scholar]
- 38.Ishihara T, Haraguchi G, Tezuka D, Kamiishi T, Inagaki H, Isobe M. Diagnosis and assessment of Takayasu arteritis by multiple biomarkers. Circ J. 2013;77:477–83. doi: 10.1253/circj.cj-12-0131. Epub 2012 Oct 26. http://dx.doi.org/10.1016/j.jvs.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 39.Maffei S, Di Renzo M, Bova G, Auteri A, Pasqui AL. Takayasu’s arteritis: a review of the literature. Intern Emerg Med. 2006;1:105–12. doi: 10.1007/BF02936534. http://dx.doi.org/10.1093/rheumatology/kei245. [DOI] [PubMed] [Google Scholar]
- 40.Choe YH, Han BK, Koh EM, Do YS, Lee WR. Takayasu’s arteritis: assessment of disease activity with contrast enhanced MRA imaging. Am J Roentgenol. 2000;175:505–11. doi: 10.2214/ajr.175.2.1750505. http://dx.doi.org/10.1253/circj.CJ-11-1108. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Li D, Yan F, Dai X, Li Y, Ma L. Evaluation of Takayasu arteritis activity by delayed contrast-enhanced magnetic resonance imaging. Int J Cardiol. 2012;155:262–7. doi: 10.1016/j.ijcard.2010.10.002. http://dx.doi.org/10.1161/01.CIR.100.1.55. [DOI] [PubMed] [Google Scholar]
- 42.Andrews J, Al-Nahhas A, Pennell DJ, Hossain MS, Davies KA, Haskard DO, et al. Non-invasive imaging in the diagnosis and management of Takayasu’s arteritis. Ann Rheum Dis. 2004;63:995–1000. doi: 10.1136/ard.2003.015701. http://dx.doi.org/10.7326/0003-4819-155-7-201110040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs M, Briel M, Daikeler T, Walker UA, Rasch H, Berg S, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging. 2012;39:344–53. doi: 10.1007/s00259-011-1967-x. http://dx.doi.org/10.1016/j.jacc.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 44.Tso E, Flamm SD, White RD, Schvartzman PR, Mascha E, Hoffman GS. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46:1634–42. doi: 10.1002/art.10251. http://dx.doi.org/10.1253/circj.CJ-12-0131. [DOI] [PubMed] [Google Scholar]
- 45.Raninen RO, Kupari MM, Pamilo MS, Taavitsainen MJ, Poutanen VP, Pajari RI, et al. Ultrasonography in the quantification of arterial involvement in Takayasu’s arteritis. Scand J Rheumatol. 2000;29:56–61. doi: 10.1080/030097400750001815. http://dx.doi.org/10.1007/BF02936534. [DOI] [PubMed] [Google Scholar]
- 46.Schinkel AF, van den Oord SC, van der Steen AF, van Laar JA, Sijbrands EJ. Utility of contrast-enhanced ultrasound for the assessment of the carotid artery wall in patients with Takayasu or giant cell arteritis. Eur Heart J Cardiovasc Imaging. 2014;155:541–6. doi: 10.1093/ehjci/jet243. http://dx.doi.org/10.2214/ajr.175.2.1750505. [DOI] [PubMed] [Google Scholar]
- 47.Alibaz-Oner F, Yurdakul S, Aytekin S, Direskeneli H. Impaired endothelial function in patients with Takayasu’s arteritis. Acta Cardiol. 2014;69:45–9. doi: 10.1080/ac.69.1.3011344. [DOI] [PubMed] [Google Scholar]
- 48.Sinha D, Mondal S, Nag A, Ghosh A. Development of a colour Doppler ultrasound scoring system in patients of Takayasu’s arteritis and its correlation with clinical activity score (ITAS 2010) Rheumatology (Oxford) 2013;52:2196–202. doi: 10.1093/rheumatology/ket289. http://dx.doi.org/10.1016/j.ijcard.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Bacon P, Direskeneli H. Quantifying disease involvement in Takayasu’s arteritis. Rheumatology (Oxford) 2014;53:1535–6. doi: 10.1093/rheumatology/ket467. http://dx.doi.org/10.1136/ard.2003.015701. [DOI] [PubMed] [Google Scholar]
- 50.De Leeuw K, Bijl M, Jager PL. Additional value of positron emission tomography in diagnosis and follow-up of patients with large vessel vasculitides. Clin Exp Rheumatol. 2004;22:S21–6. [PubMed] [Google Scholar]
- 51.Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46:917–22. [PubMed] [Google Scholar]
- 52.Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, et al. Role of FDG PET-CT in Takayasu Arteritis: Sensitive Detection of Recurrences. JACC Cardiovasc Imaging. 2012;5:422–9. doi: 10.1016/j.jcmg.2012.01.013. http://dx.doi.org/10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 53.Lee KH, Cho A, Choi YJ, Lee SW, Ha YJ, Jung SJ, et al. The role of (18) F-fluorodeoxyglucose-positron emission tomography in the assessment of disease activity in patients with takayasu arteritis. Arthritis Rheum. 2012;64:866–75. doi: 10.1002/art.33413. http://dx.doi.org/10.1002/art.10251. [DOI] [PubMed] [Google Scholar]
- 54.Arnaud L, Haroche J, Malek Z, Archambaud F, Gambotti L, Grimon G, et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum. 2009;60:1193–200. doi: 10.1002/art.24416. http://dx.doi.org/10.1080/030097400750001815. [DOI] [PubMed] [Google Scholar]
- 55.Karapolat I, Kalfa M, Keser G, Yalçin M, Inal V, Kumanlioğlu K, et al. Comparison of F18-FDG PET/CT findings with current clinical disease status in patients with Takayasu’s arteritis. Clin Exp Rheumatol. 2013;31:S15–21. [PubMed] [Google Scholar]
- 56.Santhosh S, Mittal BR, Gayana S, Bhattacharya A, Sharma A, Jain S. F-18 FDG PET/CT in the evaluation of Takayasu arteritis: An experience from the tropics. J Nucl Cardiol. 2014;21:993–1000. doi: 10.1007/s12350-014-9910-8. http://dx.doi.org/10.1093/ehjci/jet243. [DOI] [PubMed] [Google Scholar]
- 57.Cheng Y, Lv N, Wang Z, Chen B, Dang A. 18-FDG-PET in assessing disease activity in Takayasu arteritis: a meta-analysis. Clin Exp Rheumatol. 2013;31:S22–7. [PubMed] [Google Scholar]
- 58.Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS. Takayasu arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. http://dx.doi.org/10.1093/rheumatology/ket289. [DOI] [PubMed] [Google Scholar]
- 59.Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3) Ann Rheum Dis. 2009;68:1827–32. doi: 10.1136/ard.2008.101279. http://dx.doi.org/10.1093/rheumatology/ket467. [DOI] [PubMed] [Google Scholar]
- 60.Sivakumar MRM, Bacon PA. The Indian perspective of Takayasu arteritis and development of a disease extent index (DEI.Tak) to assess Takayasu arteritis. Rheumatology. 2005;44:iii6–7. http://dx.doi.org/10.1016/j.jcmg.2012.01.013. [Google Scholar]
- 61.Aydin SZ, Yilmaz N, Akar S, Aksu K, Kamali S, Yucel E, et al. Assessment of disease activity and progression in Takayasu’s arteritis with Disease Extent Index-Takayasu. Rheumatology (Oxford) 2010;49:1889–93. doi: 10.1093/rheumatology/keq171. http://dx.doi.org/10.1002/art.33478. [DOI] [PubMed] [Google Scholar]
- 62.Misra R, Danda D, Rajappa SM, Ghosh A, Gupta R, Mahendranath KM, et al. Indian Rheumatology Vasculitis (IRAVAS) group. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010) Rheumatology (Oxford) 2013;52:1795–801. doi: 10.1093/rheumatology/ket128. http://dx.doi.org/10.1002/art.24416. [DOI] [PubMed] [Google Scholar]
- 63.Direskeneli H, Aydin SZ, Merkel PA. Disease assessment in Takayasu’s arteritis. Rheumatology (Oxford) 2013;52:1735–6. doi: 10.1093/rheumatology/ket274. http://dx.doi.org/10.1007/s12350-014-9910-8. [DOI] [PubMed] [Google Scholar]
- 64.Goel R, Danda D, Kumar S, Joseph G. Rapid control of disease activity by tocilizumab in 10 ‘difficult-to-treat’ cases of Takayasu arteritis. Int J Rheum Dis. 2013;16:754–61. doi: 10.1111/1756-185X.12220. http://dx.doi.org/10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 65.Dogan S, Piskin O, Solmaz D, Akar S, Gulcu A, Yuksel F, et al. Markers of endothelial damage and repair in Takayasu arteritis: Are they associated with disease activity? Rheumatol Int. 2014;34:1129–38. doi: 10.1007/s00296-013-2937-3. http://dx.doi.org/10.1136/ard.2008.101279. [DOI] [PubMed] [Google Scholar]
- 66.Sahin Z, Bıcakcıgil M, Aksu K, Kamali S, Akar S, Onen F, et al. Takayasu’s arteritis is associated with HLA-B*52, but not with HLA-B*51, in Turkey. Arthritis Res Ther. 2012;14:R27. doi: 10.1186/ar3730. http://dx.doi.org/10.1093/rheumatology/keh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merkel PA, Aydin SZ, Boers M, Cornell C, Direskeneli H, Gebhart D, et al. Current status of outcome measure development in vasculitis. J Rheumatol. 2014;41:593–8. doi: 10.3899/jrheum.131248. http://dx.doi.org/10.1093/rheumatology/keh739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Exley AR, Bacon PA, Luqmani RA, Kitas GD, Gordon C, Savage CO, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–80. doi: 10.1002/art.1780400222. http://dx.doi.org/10.1093/rheumatology/ket128. [DOI] [PubMed] [Google Scholar]
- 69.Akar S, Can G, Binicier O, Aksu K, Akinci B, Solmaz D, et al. Quality of life in patients with Takayasu’s arteritis is impaired and comparable with rheumatoid arthritis and ankylosing spondylitis patients. Clin Rheumatol. 2008;27:859–65. doi: 10.1007/s10067-007-0813-9. http://dx.doi.org/10.1093/rheumatology/ket274. [DOI] [PubMed] [Google Scholar]
- 70.Abularrage CJ, Slidell MB, Sidawy AN, Kreishman P, Amdur RL, Arora S. Quality of life of patients with Takayasu’s arteritis. J Vasc Surg. 2008;47:131–6. doi: 10.1016/j.jvs.2007.09.044. discussion 136–7. http://dx.doi.org/10.1111/1756-185X.12220. [DOI] [PubMed] [Google Scholar]
- 71.Yilmaz N, Can M, Oner FA, Kalfa M, Emmungil H, Karadag O, et al. Impaired quality of life, disability and mental health in Takayasu’s arteritis. Rheumatology (Oxford) 2013;52:1898–904. doi: 10.1093/rheumatology/ket238. http://dx.doi.org/10.1007/s00296-013-2937-3. [DOI] [PubMed] [Google Scholar]
- 72.Alibaz-Oner F, Can M, İlhan B, Polat Ö, Mumcu G, Direskeneli H. Presence of fibromyalgia in patients with Takayasu’s arteritis. Intern Med. 2013;52:2739–42. doi: 10.2169/internalmedicine.52.0848. http://dx.doi.org/10.1186/ar3730. [DOI] [PubMed] [Google Scholar]
- 73.Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014;53:793–801. doi: 10.1093/rheumatology/ket320. http://dx.doi.org/10.3899/jrheum.131248. [DOI] [PubMed] [Google Scholar]
- 74.Clifford A, Hoffman GS. Recent advances in the medical management of Takayasu arteritis: an update on use of biologic therapies. Curr Opin Rheumatol. 2014;26:7–15. doi: 10.1097/BOR.0000000000000004. http://dx.doi.org/10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 75.Loricera J, Blanco R, Casta-eda S, Humbría A, Ortego-Centeno N, Narváez J, et al. Tocilizumab in refractory aortitis: study on 16 patients and literature review. Clin Exp Rheumatol. 2014;32:S79–89. Epub 2014 May 15. [PubMed] [Google Scholar]
- 76.Ca-as CA, Ca-as F, Izquierdo JH, Echeverri AF, Mejía M, Bonilla-Abadía F, et al. Efficacy and safety of anti-interleukin 6 receptor monoclonal antibody (tocilizumab) in Colombian patients with Takayasu arteritis. J Clin Rheumatol. 2014;20:125–9. doi: 10.1097/RHU.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 77.Goel R, Danda D, Kumar S, Joseph G. Rapid control of disease activity by tocilizumab in 10 ‘difficult-to-treat’ cases of Takayasuarteritis. Int J Rheum Dis. 2013;16:754–61. doi: 10.1111/1756-185X.12220. http://dx.doi.org/10.1007/s10067-007-0813-9. [DOI] [PubMed] [Google Scholar]
- 78.Nakaoka Y, Higuchi K, Arita Y, Otsuki M, Yamamoto K, Hashimoto-Kataoka T, et al. Tocilizumab for the treatment of patients with refractory Takayasu arteritis. Int Heart J. 2013;54:405–11. doi: 10.1536/ihj.54.405. http://dx.doi.org/10.1016/j.jvs.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 79.Tombetti E, Franchini S, Papa M, Sabbadini MG, Baldissera E. Treatment of refractory Takayasu arteritis with tocilizumab: 7 Italian patients from a single referral center. J Rheumatol. 2013;40:2047–51. doi: 10.3899/jrheum.130536. http://dx.doi.org/10.1093/rheumatology/ket238. [DOI] [PubMed] [Google Scholar]
- 80.Youngstein T, Peters JE, Hamdulay SS, Mewar D, Price-Forbes A, Lloyd M, et al. Serial analysis of clinical and imaging indices reveals prolonged efficacy of TNF-α and IL-6 receptor targeted therapies in refractory Takayasu arteritis. Clin Exp Rheumatol. 2014;32:S11–8. Epub 2013 Sep 30. [PubMed] [Google Scholar]
- 81.Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, et al. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken) 2012;64:1720–9. doi: 10.1002/acr.21750. http://dx.doi.org/10.2169/internalmedicine.52.0848. [DOI] [PubMed] [Google Scholar]
- 82.Salvarani C, Magnani L, Catanoso MG, Pipitone N, Versari A, Dardani L, et al. Rescue treatment with tocilizumab for Takayasu arteritis resistant to TNF-α blockers. Clin Exp Rheumatol. 2012;30:S90–3. [PubMed] [Google Scholar]
- 83.Abisror N, Mekinian A, Lavigne C, Vandenhende MA, Soussan M, Fain O, et al. Tocilizumab in refractory Takayasu arteritis: a case series and updated literature review. Autoimmun Rev. 2013;12:1143–9. doi: 10.1016/j.autrev.2013.06.019. http://dx.doi.org/10.1093/rheumatology/ket320. [DOI] [PubMed] [Google Scholar]