Abstract
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is an autosomal dominant autoinflammatory disease linked to chromosome 12p13 and, more specifically, with mutations within the tumor necrosis factor receptor superfamily, member 1A gene (TNFRSF1A gene). It is characterized by the presence of fever, abdominal pain, myalgia, arthralgia or arthritis, and skin rash.
In this report, we describe the case of a patient with tumor necrosis factor receptor-associated periodic syndrome (TRAPS) treated successfully with the anti-interleukin-6 (anti-IL-6) receptor monoclonal antibody tocilizumab, while treatment with anti-TNF α etanercept and infliximab had both failed.
Keywords: TRAPS, anti-TNF α, tocilizumab, biologicals, treatment
Introduction
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is a rare autosomal, dominantly inherited autoinflammatory disease, previously known as Hibernian fever and renamed TRAPS following the elucidation of TNFRSF1A as the susceptibility gene with mutations occurring generally on chromosome 12p13 (1).
Clinically, TRAPS is characterized by recurrent attacks of fever, abdominal pain, periorbital edema, migratory rush, myalgia, arthralgia, arthritis, and serositis (2).
The inflammatory episode is due to the production of cytokines [interleukin-6 (IL-6), IL-1 β, and tumor necrosis factor α: (TNFα)] and acute-phase proteins, such as C-reactive protein (CRP) and serum amyloid A (3). Recently, high serum IL-6 levels have been reported in these patients, and this is therefore believed to play a pivotal part in the pathogenesis of TRAPS. Consistent with these findings, IL-6 blockade with tocilizumab could be an interesting therapeutic in TRAPS syndrome.
To the best of our knowledge, the current report is the second case in the literature, which describes a patient who suffered from TRAPS syndrome in whom treatment with tocilizumab was successfully applied, while treatment with infliximab and etanercept had both failed.
Case Presentation
A 49-year-old man, with a medical history of hypertension and chronic obstructive pulmonary disease, was admitted with symmetric polyarthritis of the hands, increased inflammatory markers, and the absence of rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA). A diagnosis of seronegative rheumatoid arthritis was made, and the patient was treated with methotrexate 15 mg per week, salazopyrin 2 g/d, and hydroxychloroquine 400 mg/d. Three months later, the patient still had high disease activity, with DAS 28 and ESR 5.18. The patient was then treated with infliximab at an induction regimen of 3 mg/kg at 0, 2, and 6 weeks and maintenance therapy every 8 weeks associated with methotrexate, without any significant improvement in disease activity scores and health assessment questionnaire (HAQ). Six months later, the patient developed a fever (37.5–39°) lasting 3–4 days with monthly periodicity, associated with recurrent annular erythematous plaques in the trunk and abdomen and conjunctivitis. Biological assessment showed increased erythrocyte sedimentation rate (ESR) 29 mm/h; normal values <20 mm/h) and CRP of 1.5 mg/dL; normal <0.5 mg/dL). An underlying infectious disease was ruled out, while antinuclear antibodies, anti-neutrophil cytoplasmic antibodies, anti-DNA antibodies, and C3 and C4 were negative. These findings evoked the diagnosis of TRAPS syndrome. A molecular diagnosis of TRAPS was confirmed with a p.Arg121Gln-type mutation (R92Q mutation) identified on the chromosome of the TNFRSF1A gene.
The patient received etanercept 50 mg per week for 6 months without significant clinical and biological improvement. The patient still had polyarthritis of the hands, fever, rash, and a high level of C-reactive protein (12.6 mg/dL) (Figure 1).
Figure 1.
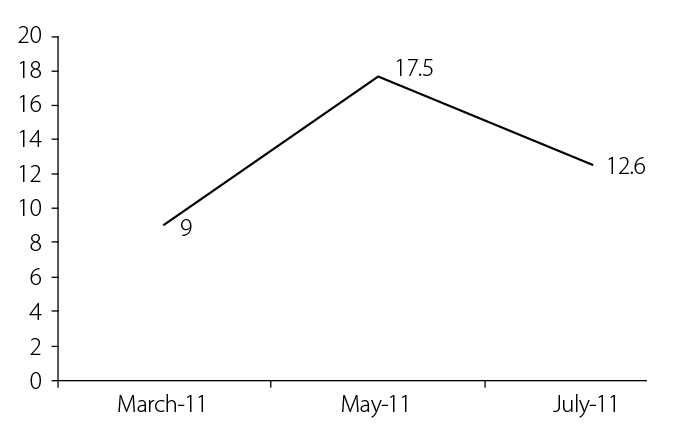
Changes in the level of C-reactive protein (in mg/dL) during etanercept treatment
Since IL-6 levels are elevated in TRAPS, we hypothesized that tocilizumab might be effective. So, our patient was treated with tocilizumab, administered 8 mg/kg every 4 weeks. The patient responded promptly with improved physical condition; reduction of fever, rash, and polyarthritis; and improvement of quality of life. Markers of inflammation ESR and CRP were within the normal range-2 mm/h and 0.05 mg/dL, respectively (Figure 2). During treatment with tocilizumab, no new attacks occurred.
Figure 2.
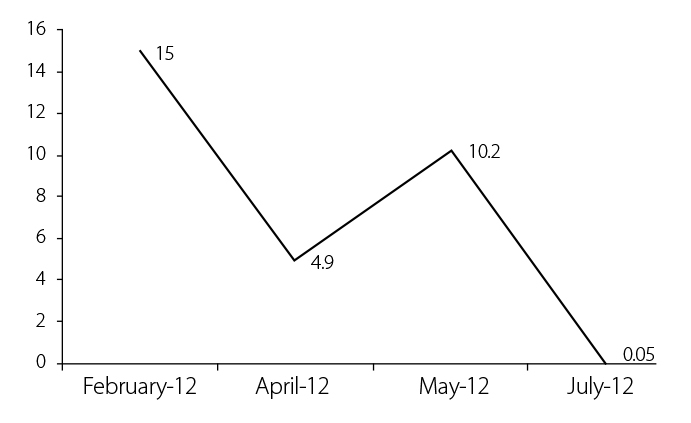
Changes in the level of C-reactive protein (in mg/dL) during tocilizumab treatment
Discussion
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is an autosomal dominant inherited periodic fever related to mutations in soluble TNF receptor superfamily 1A. Patients with TRAPS experience recurrent attacks of fever and pain in the joints, abdomen, muscles, skin, and eyes. TRAPS is caused by mutations in the gene encoding TNF receptor superfamily 1A (TNFRSF1A) on chromosome 12p13 (1). The prevailing hypothesis for TRAPS is defective TNFRSF1A shedding from cell membranes in response to a stimulus. However, a consequence of most TRAPS mutations is the activation of the transcription factor NF-κB (nuclear factor κB) and the MAPK (mitogen-activated protein kinase) families of transcriptional activators, which are able to up-regulate expression of a number of genes, including pro-inflammatory cytokines (4).
Patients suffering from TRAPS are mainly treated with nonsteroidal anti-inflammatory drugs or corticosteroids. The pathogenic hypothesis involving defective TNFRSF1A shedding is consistent with a therapeutic effect of anti-TNF α agents. Etanercept could be very interesting therapeutic; it diminishes the effects of TNF by binding to soluble and membrane-bound TNF receptors. It may be useful in the treatment of TRAPS attacks as a glucocorticoid-sparing agent (5). An anti-TNFα therapy could significantly improve the symptoms and reduce the frequency of attacks. But, there are some cases with a lack of efficacy of anti-TNFα, possibly due to other cytokines that become more important, like IL-1 β and IL-6. This is consistent with observations of clinical improvement in TRAPS syndrome after treatment with anakinra or tocilizumab in patients with disease refractory to anti-TNF α agent. Recently, tocilizumab, an anti-interleukin 6 agent, was successfully developed as a therapeutic agent for the treatment of rheumatoid arthritis. The notable effectiveness of this agent and consideration of its mode of action have motivated new initiatives in applying this treatment to a broad range of inflammatory and autoimmune conditions, including TRAPS syndrome (6). In clinical practice, IL-6 is a proinflammatory cytokine; it induces fever and many of the clinical attributes associated with systemic inflammation (7, 8). Tocilizumab could be an interesting treatment in TRAPS syndrome and other inflammatory diseases via its action on the liver by reducing the production of a wide spectrum of acute phase proteins, such as C-reactive protein (CRP), serum amyloid protein A (SAA), haptoglobin, fibrinogen, and hepcidin. It has also the ability to inhibit B-cell differentiation into antibody-producing B cells and T differentiation of CD4-positive T-helper cells to IL-17-producing T-helper cells (Th17), which amplify the inflammatory response and produce other proinflammatory cytokines (9).
The effects of tocilizumab observed in our patient indicate that IL-6 plays a prominent role in stimulating many of the clinical features of the attacks of inflammation in TRAPS.
To our knowledge, this is the second case in the literature of the successful treatment of TRAPS with tocilizumab. Vaitla et al. (10) reported tocilizumab outcomes in a patient with TRAPS. In their case, the evolving acute attack was aborted, and further attacks of TRAPS were prevented. Our patient did not require corticosteroids and showed significant clinical improvement in scores for pain, stiffness, and well-being.
In conclusion, we considered tocilizumab an effective treatment option for a patient with TRAPS, whose disease had been treated unsuccessfully with an anti-TNF agent. These preliminary findings need to be confirmed with long-term follow-up.
Footnotes
Informed consent: Written informed consent was obtained from patients who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - N.A.; Design - N.A.; Supervision - M.S.S.; Materials - N.A.; Data Collection and/or Processing - N.A.; Analysis and/or Interpretation - N.A.; Literature Review - N.A.; Writer - N.A.; Critical Review - M.S.S.
Conflict of Interest: The authors declared no conflict of interest.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.McDermott EM, Smillie DM, Powell RJ. Clinical spectrum of familial Hibernian fever: a 14-year follow-up study of the index case and extended family. Mayo Clin Proc. 1997;72:806–17. doi: 10.4065/72.9.806. http://dx.doi.org/10.4065/72.9.806. [DOI] [PubMed] [Google Scholar]
- 2.Omenetti A, Federici S, Gattorno M. Inherited autoinflammatory diseases: a critical digest of the recent literature. Clin Exp Rheumatol. 2013;31:118–26. [PubMed] [Google Scholar]
- 3.Nowlan ML, Drewe E, Bulsara H, Esposito N, Robins RA, Tighe PJ, et al. Systemic cytokine levels and the effects of etanercept in TNF receptor-associated periodic syndrome (TRAPS) involving a C33Y mutation in TNFRSF1A. Rheumatology (Oxford) 2006;45:31–7. doi: 10.1093/rheumatology/kei090. http://dx.doi.org/10.1093/rheumatology/kei090. [DOI] [PubMed] [Google Scholar]
- 4.Turner MD, Chaudhry A, Nedjai B. Tumour necrosis factor receptor trafficking dysfunction opens the TRAPS door to pro-inflammatory cytokine secretion. Biosci Rep. 2012;32:105–12. doi: 10.1042/BSR20110089. http://dx.doi.org/10.1042/BSR20110089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drewe E, Mc Dermott EM, Powell PT, Isaacs JD, Powell RJ. Prospective study of anti-tumour necrosis factor receptor superfamily 1B fusion protein, and case study of anti-tumour necrosis factor receptor superfamily 1A fusion protein, in tumour necrosis factor receptor associated periodic syndrome (TRAPS): clinical and laboratory findings in a series of seven patients. Rheumatology (Oxford) 2003;42:235–9. doi: 10.1093/rheumatology/keg070. http://dx.doi.org/10.1093/rheumatology/keg070. [DOI] [PubMed] [Google Scholar]
- 6.Kaly L, Rosner I. Tocilizumab. A novel therapy for non-organ-specific autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26:157–65. doi: 10.1016/j.berh.2012.01.001. http://dx.doi.org/10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Kimura A, Kishimoto T. IL6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5. doi: 10.1002/eji.201040391. http://dx.doi.org/10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 8.Cantarini L, Lucherini OM, Muscari I, Frediani B, Galeazzi M, Brizi MG, et al. Tumour necrosis factor receptor-associated periodic syndrome (TRAPS): state of the art and future perspectives. Autoimmun Rev. 2012;12:38–43. doi: 10.1016/j.autrev.2012.07.020. http://dx.doi.org/10.4065/72.9.806. [DOI] [PubMed] [Google Scholar]
- 9.Murakami M, Nishimoto N. The value of blocking Il-6 outside of rheumatoid arthritis: current perspective. Current Opinion in Rheumatology. 2011;23:273–7. doi: 10.1097/BOR.0b013e3283456797. http://dx.doi.org/10.1093/rheumatology/kei090. [DOI] [PubMed] [Google Scholar]
- 10.Vaitla PM, Radford PM, Tighe PJ, Powell RJ, McDermott EM, Todd I, et al. Role of interleukin-6 in a patient with TNF receptor-associated periodic syndrome: assessment of outcomes following treatment with the antiinterleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum. 2011;63:1151–5. doi: 10.1002/art.30215. http://dx.doi.org/10.1042/BSR20110089. [DOI] [PubMed] [Google Scholar]


