Abstract
Objective
Cognitive remediation is emerging as an effective psychosocial intervention for addressing untreated cognitive and functional impairments in persons with schizophrenia, and might achieve its benefits through neuroplastic changes in brain connectivity. This study seeks to examine the effects of cognitive enhancement therapy (CET) on fronto-temporal brain connectivity in a randomized controlled trial with individuals in the early course of schizophrenia.
Method
Stabilized, early course outpatients with schizophrenia or schizoaffective disorder (N = 41) were randomly assigned to CET (n = 25) or an active enriched supportive therapy (EST) control (n = 16) and treated for 2 years. Functional MRI data were collected annually, and pseudo resting-state functional connectivity analysis was used to examine differential changes in fronto-temporal connectivity between those treated with CET compared with EST.
Results
Individuals receiving CET evidenced significantly less functional connectivity loss between the resting-state network and the left dorsolateral prefrontal cortex as well as significantly increased connectivity with the right insular cortex compared to EST (all corrected p < .01). These neural networks are involved in emotion processing and problem-solving. Increased connectivity with the right insula significantly mediated CET effects on improved emotion perception (z′ = −1.96, p = .021), and increased connectivity with the left dorsolateral prefrontal cortex mediated CET-related improvements in emotion regulation (z′ = −1.71, p = .052).
Conclusions
These findings provide preliminary evidence that CET, a psychosocial cognitive remediation intervention, may enhance connectivity between frontal and temporal brain regions implicated in problem-solving and emotion processing in service of cognitive enhancement in schizophrenia.
Keywords: cognitive remediation, functional magnetic resonance imaging, schizophrenia, social cognition
Schizophrenia is a severe and persistent psychiatric condition characterized by significant functional disability. The condition generally begins in early adulthood and, for most individuals, it is a life-long illness requiring ongoing treatment and management. Schizophrenia affects many spheres of a person’s life, including the ability to work, to develop and maintain fulfilling interpersonal relationships, and to be able to live independently. Some of the strongest contributors to community outcomes in this condition (Fett et al., 2011) include neurocognitive impairments in attention, memory, and problem-solving (Heinrichs & Zakzanis, 1998); deficits in social cognition (Savla, Vella, Armstrong, Penn, & Twamley, 2013); and the ability to process and interpret socio-emotional information in others (Newman, 2001). These cognitive challenges begin early in the course of schizophrenia (Eack, Dworakowski, et al., 2010), are persistent (Braw et al., 2008; Green et al., 2012), and are unresponsive to current antipsychotic treatments (Keefe et al., 2007). The importance of treating cognitive impairments in those with schizophrenia has led to several initiatives to support the development of cognitive enhancing interventions (Marder & Fenton, 2004; Carter & Barch, 2007). Social workers have pioneered the development, testing, and implementation of many of the psychosocial treatments for schizophrenia, and recent advances have highlighted cognitive remediation as one of the most effective psychosocial interventions for improving cognition and functional outcomes for persons with schizophrenia (Hogarty et al., 2004; Wykes, Huddy, Cellard, McGurk, & Czobor, 2011).
Cognitive remediation is a diverse set of approaches designed to improve information processing abilities through the progressive and strategic practice of targeted cognitive exercises. Treatment is often, but not always, computer based. The most effective approaches focus on broad neurocognitive and social-cognitive targets within the context of psychosocial rehabilitation (see Eack, 2012 for a review). Meta-analytic reviews have indicated that cognitive remediation can produce greater improvements in cognition than antipsychotic drug treatment, with significant associated benefits to functional recovery (Kurtz & Richardson, 2012; Wykes et al., 2011). The assumption underlying these interventions is that the repeated practice of cognitive tasks will strengthen the neural networks supporting information processing, and thereby result in improved cognition.
The term neuroplasticity refers to the capacity of the human brain to adapt to new environmental demands and experiences (see Keshavan, Mehta, Padmanabhan, & Shah, 2015 for review). Growing evidence has indicated the psychosocial interventions used by social work practitioners can provide the types of new environmental experiences needed to positively shape individual brain function and development (Garland & Howard, 2009). Landmark studies have shown that the learning process is accompanied by fundamental growth and reorganization of neural networks and pathways. For example, when individuals learn complex finger sequences such as those pianists must master, an accompanying expansion of neural activity in the brain’s motor cortex is observed (Karni et al., 1995). Numerous studies have shown that cognitive remediation interventions can increase prefrontal brain function in individuals with schizophrenia (Subramaniam et al., 2012; Wexler, Anderson, Fulbright, & Gore, 2000; Wykes et al., 2002). Further, we have previously observed that cognitive enhancement therapy (CET; Hogarty & Greenwald, 2006) can protect against gray matter loss when applied early in the course of the schizophrenia (Eack, Hogarty, et al., 2010). This neuroprotection has been shown to mediate the effects of CET on measures of social cognition and emotion processing (Eack et al., 2009).
Although these results highlight the potential neuroplasticity effects of cognitive remediation in people living with schizophrenia (Ramsay & MacDonald, 2015), the brain is an extensive complex communications network, and effects on specific brain regions are not likely to exist in isolation. For example, protecting against gray matter loss in the brain’s hippocampal region (associated with memory) is likely to have effects on both the region’s afferent (incoming) connections with the prefrontal cortex and efferent (outgoing) communications with medial temporal structures. This property of intercommunication between brain structures is commonly referred to as connectivity (Rubinov & Sporns, 2010), and neuroscientists are increasingly recognizing the importance of connectivity in understanding the complex processes that contribute to brain disorders (Just, Cherkassky, Keller, & Minshew, 2004).
One of the most common ways to characterize brain connectivity is to examine inter-regional covariance patterns of neural activity while individuals are at rest (i.e., lying down, awake, and not performing a specific cognitive task), known as resting-state or default mode functional connectivity (Greicius, Krasnow, Reiss, & Menon, 2003). “Pseudo” resting-state data can be obtained by aggregating rest blocks of a given task, which provides comparable data to those collected continuously outside of a task context (Fair et al., 2007), and this is the approach used for the present study. Measures of resting-state functional connectivity have been widely used in the field of mental health to understand changes in brain communication-associated disability across many psychiatric conditions, including Alzheimer’s disease, depression, and schizophrenia (Greicius, 2008). Such measures provide an opportunity to characterize the basal connectivity profile of the neural system, which has been repeatedly shown to be reduced in schizophrenia (Garrity et al., 2007; Liang et al., 2006), particularly long-term fronto-temporal connections (Zhou et al., 2007). These reductions in connectivity contribute to, at least in part, the observed impairments in neurocognitive and social-cognitive functions that many cognitive remediation interventions aim to treat (Sui et al., 2015). However, to date, no study has examined the impact of cognitive remediation on resting-state functional connectivity. Thus, the extent to which psychosocial treatment can enhance neural connectivity in persons with schizophrenia or other conditions remains largely unknown.
This study sought to explore the 2-year effects of CET, a comprehensive cognitive remediation intervention, on pseudo resting-state functional connectivity in young people in the early course of schizophrenia. Understanding the impact of cognitive remediation on functional connectivity is important for identifying the neural mechanisms by which cognition can be improved in those with schizophrenia, as well as characterizing the ability of psychosocial treatment to alter connectivity parameters in those with the disorder. We hypothesized that individuals treated with CET would demonstrate increased pseudo resting-state connectivity with prefrontal and temporal brain networks, which in turn, would contribute to improved social-cognitive outcomes in those with schizophrenia, as assessed using behavioral measures of emotion processing.
Method
Participants
Individuals in the early course of schizophrenia (n = 25) or schizoaffective disorder (n = 16) were recruited for a 2-year randomized controlled trial of CET or enriched supportive therapy (EST); a description of the parent trial has been published elsewhere (Eack et al., 2009). Eligible participants included outpatients with schizophrenia, schizoaffective disorder, or schizophreniform disorder (all of whom converted to schizophrenia) verified by the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 2002) who (a) had experienced their first psychotic symptom within the past 8 years, (b) were stabilized on antipsychotic medication, (c) had an IQ of 80 or greater, (d) were not abusing substances within 2 months prior to study enrollment, and (e) demonstrated significant social and cognitive disability on the Cognitive Styles and Social Cognition Eligibility Interview (Hogarty et al., 2004). A total of 58 individuals were randomized and treated in this trial, 41 of whom had available functional neuroimaging data for analysis. Sample characteristics are presented in Table 1, indicating the sample was young, mostly male, and racially diverse (68% White, 17% African American, 12% Asian, 3% other). Although most participants had attended some college, at the study baseline few participants were employed in competitive jobs. No significant differences emerged between treatment groups with regard to demographic or pretreatment clinical and medication characteristics.
Table 1.
Demographic and Clinical Characteristics of Individuals in the Early Course of Schizophrenia Randomized to a Trial of Cognitive Enhancement Therapy (CET) or Enriched Supportive Therapy (EST)
| Cognitive enhancement therapy (n = 25) |
Enriched supportive therapy (n = 16) |
||||
|---|---|---|---|---|---|
| Variable | M / N | SD / % | M / N | SD / % | p a |
| Age | 24.95 | 5.16 | 26.13 | 5.29 | .485 |
| Male | 14 | 56% | 12 | 75% | .368 |
| White | 17 | 68% | 11 | 69% | .500 |
| Completed college | 8 | 32% | 6 | 38% | .980 |
| Employed | 8 | 32% | 6 | 38% | .980 |
| Schizophrenia diagnosis | 16 | 64% | 10 | 62% | 1.00 |
| Illness duration (years) | 3.36 | 2.45 | 2.97 | 1.84 | .584 |
| IQ | 97.56 | 8.29 | 101.00 | 7.38 | .184 |
| BPRS total | 39.08 | 8.93 | 41.00 | 12.23 | .565 |
| Antipsychotic dose (cpz) | 374.67 | 266.28 | 420.10 | 297.47 | .613 |
| Medication adherent | 23 | 92% | 14 | 88% | 1.00 |
Note. BPRS = Brief Psychiatric Rating Scale, cpz = chlorpromazine equivalence
Chi-square test or independent t-test, two-tailed, for significant differences between CET and EST participants.
Functional Neuroimaging Data Collection and Processing
Functional MRI data were collected for most participants on a 3T Signa whole-body scanner and head coil (voxel size = 3.125 × 3.125 × 3.200mm, TR = 1500ms, TE = 18ms, flip angle = 70°, FOV = 200mm, 64 × 64 matrix, 26 slices, slice thickness = 3.2mm). Near the end of the study, the original scanner was replaced and a small number of scans (6%) were collected on a 3T Siemens Tim Trio scanner and head coil (voxel size = 3.125 × 3.125 × 3.200mm, TR = 1500ms, TE = 30ms, flip angle = 70°, FOV = 200mm, 64 × 64 matrix, 26 slices, slice thickness = 3.2mm) with acquisition parameters matched as closely as possible. Pseudo resting-state data (i.e., those collected during rest periods of a task) were collected during rest times of a 1040 s attentional control task asking participants to respond to directional arrows of a stimulus with either their right or left hand (Snitz et al., 2005). Each of the 120 trials of this task contained a 10.5 s rest period (seven volumes, one collected every 1.5 s) after the final stimulus was presented, which asked participants to view a fixation cross (black screen with a single white cross [+] in the center). These data were used to measure brain connectivity at rest, and in accordance with recommendations for the collection of pseudo resting-state data from task data (Fair et al., 2007), our analysis was conservatively restricted to the final two rest volumes (7.5 s and 9 s after the trial ended) to ensure that the hemodynamic response from task stimuli had fully resolved. Previous research has shown that when using this approach, pseudo resting-state data collected during rest blocks of a task are similar to those collected during continuous resting states (Fair et al., 2007). Pseudo resting-state data were preprocessed using a standard direct normalization pipeline in Statistical Parametric Mapping 8 (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK), where fMRI volumes were first aligned to a common orientation, warped to a standardized echo-planar Montreal Neurological Institute template, smoothed using an 8 mm Gaussian kernel, and bandpass filtered to focus on low-frequency (.01 to .10 Hz) neural activity most characteristic of resting state brain activity (Buckner, Andrews-Hanna, & Schacter, 2008). The functional connectivity toolbox was then used to calculate connectivity (r) coefficients, both positive and negative, from the resulting normalized, smoothed, and filtered image time-series using an aCompCor approach (Nieto-Castanon & Whitfield-Gabrieli, 2009).
Behavioral Measures of Emotion Processing
To examine the potential contribution of changes in resting-state functional connectivity to improved social-cognitive outcomes, emotion processing data were collected using the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT; Mayer, Salovey, Caruso, & Sitarenios, 2003). The MSCEIT is a 141-item performance-based measure of the four domains of emotional intelligence outlined by Salovey and Mayer (1990): emotion perception; facilitation, that is, using emotions to facilitate thinking and/or accomplishing tasks; understanding; and regulation. The MSCEIT is performance-based in that participants are asked to solve emotional problems (e.g., identify the emotion best associated with an emotional facial expression) rather than self-report on their abilities. Each domain of emotional intelligence is assessed using two tasks (e.g., viewing pictures of faces and scenery for emotion perception). Items are scored based on consensus norms and scaled with a mean of 100 (SD = 15). The reliability and validity of the MSCEIT for assessments of emotional intelligence has been reported in previous research with both healthy (Mayer et al., 2003) and psychiatric samples (Eack, Greeno, et al., 2010; Nuechterlein et al., 2008). The significant benefits of CET on MSCEIT performance in persons with schizophrenia have been described in previous reports (Eack et al., 2009).
Psychosocial Treatments
Cognitive enhancement therapy
CET is a comprehensive, developmental approach to the remediation of social and non-social cognitive impairments that limit functional recovery in schizophrenia (Hogarty et al., 2004). CET integrates 60 hours of computer-based neurocognitive training with 45 structured 1.5-hour social-cognitive groups. The neurocognitive training centers on attention, memory, and problem solving whereas the social-cognitive groups are designed to facilitate the achievement of adult social milestones (e.g., perspective-taking, social context appraisal, emotion management). Neurocognitive training makes use of computer software from Ben-Yishay, Piasetsky, and Rattok (1985) and Bracy (1994) and is conducted in participant pairs to enhance motivation, support, and socialization. After approximately 3 months of neurocognitive training in attention, three to four participant pairs join together to form a small social-cognitive group. The social-cognitive groups are educational in nature, and use in vivo exercises and experiential learning opportunities to enhance interpersonal success. A deliberately broad array of social-cognitive abilities are covered throughout the group, including perspective-taking, emotion management, gist abstraction, social and emotional cue recognition, and cognitive flexibility. Each group session includes a welcome back, homework presentation based on the previous week, in-group social-cognitive exercise, psychoeducation lecture on a new topic in social cognition, and a homework assignment designed to facilitate generalization. A detailed description of CET has been provided elsewhere (Eack, 2012; Hogarty et al., 2004).
Enriched supportive therapy
EST is an individual support and condition management approach based on the demonstrably effective personal therapy (Hogarty, 2002) for persons with schizophrenia. The treatment is phase-specific based on the level of recovery of the participant, with the first phase focusing primarily on psychoeducation about schizophrenia and the identification of early cues of distress that can contribute to prodromal and psychotic symptoms. The second phase involves a personalized approach to implementing coping strategies designed to reduce stress and support adjustment. In addition, the second phase provides basic relaxation training and guidance on the use of such autoprotective strategies as calming activities, timeouts, and active distraction. During the first phase of the treatment, participants meet individually with a therapist on a weekly basis for 30–60 minutes; these meetings are reduced to biweekly sessions as individuals move to the second phase of the intervention. EST was selected as an active comparison treatment for this trial to provide a more rigorous test of CET effects beyond most commonly implemented usual care controls, and to account for the nonspecific effects of CET (e.g., provision of a skilled empathic therapist, psychoeducation). Given the different nature, purpose, and targets of CET and EST, no attempt was made to match hours of treatment exposure. All study clinicians were at least master’s-level trained, had many years of experience in working with individuals with schizophrenia. Further, all study clinicians provided both interventions with fidelity, which was monitored throughout the study by the treatment developers.
Procedures
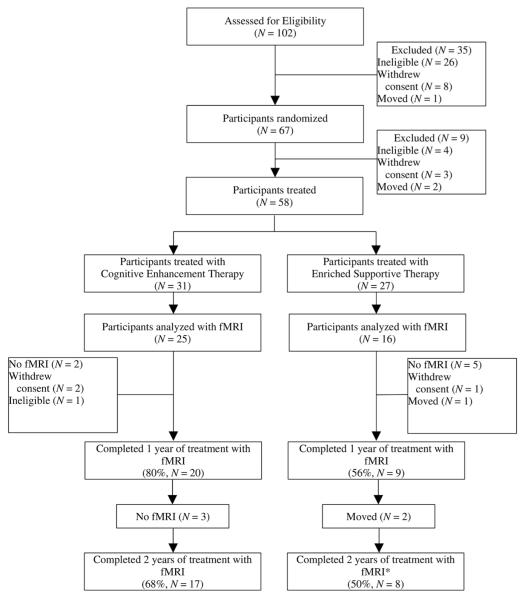
Potential participants were recruited from the Western Psychiatric Institute and Clinic in Pittsburgh, Pennsylvania, and surrounding community clinics. This study was conducted between August 2001 and September 2007 (clinicaltrials.gov registration: NCT00167362). Upon recruitment, participants were screened for eligibility by trained diagnosticians and raters, with final eligibility status determined by consensus. Eligible participants were then randomized, assessed at baseline using fMRI and behavioral measures, and treated for 2 years with either CET or EST. Functional MRI and behavioral data collection was conducted by trained research staff and occurred prior to beginning psychosocial treatment and annually thereafter. Participant’s treatment assignment was not masked to the research staff. Two-year retention was high in the larger study sample in both treatment groups (77% in CET; 82% in EST), with no significant between-group differences in attrition (Eack et al., 2009). However, attrition from fMRI procedures was considerable, given the challenges associated with collecting multi-year neuroimaging data in this population. Specifically, fMRI data were available for 80% of CET participants at Year 1 but dropped to 68% of CET participants at Year 2. For EST participants, Year 1 fMRI data were available for 56% of participants and Year 2 data were available for 50% of EST participants (see Figure 1). No significant differences emerged between treatment groups in attrition of fMRI data, χ2(1, N = 41) = .490, p = .484. In addition, no significant demographic or clinical differences were observed between those with complete data and those without fMRI data, all p > .073. All participants provided written informed consent prior to study participation, and the study protocol was approved and reviewed annually by the University of Pittsburgh Institutional Review Board.
Figure 1.
Participant flow in a 2-year randomized-controlled trial of cognitive enhancement therapy versus enriched supportive therapy for early course schizophrenia.
*One participant in EST had fMRI data available only at Year 2.
Data Analysis
Analyses including all 41 participants with fMRI data who were randomized and received any exposure to their assigned treatment condition were conducted using the functional connectivity toolbox (Nieto-Castanon & Whitfield-Gabrieli, 2009) in SPM8. The posterior cingulate cortex was defined as the seed region on which all other regional correlations were based, consistent with findings that nearly uniformly implicate the posterior cingulate as the central region in the resting state network (Buckner et al., 2008). After preprocessing, seed-to-voxel general linear models were constructed to examine regional correlations with the posterior cingulate over time. The dependent variable in these models was regional correlation values (r). Primary predictors in these models included treatment assignment, time, and their interaction. Age, gender, IQ, illness duration, and medication dose (time-varying) were included as covariates to account for the potential impact on treatment outcome of demographic, clinical, and medication factors. In addition, the scanner model was also included as a confounding covariate to account for inter-scanner variability in the small number of scans completed on the Siemens equipment. Treatment × time interactions were the primary effects of interest, indicating significant differential changes in connectivity values between CET and EST over the course of treatment. To maintain Type I error at acceptable levels, analyses were conducted on fronto-temporal regions of interest including the lateral, orbital, and medial prefrontal cortex, insular cortex, hippocampus, parahippocampal gyrus, amygdala, fusiform gyrus, and superior temporal gyrus with regional definitions provided by Tzourio-Mazoyer and colleagues (2002). In addition, a combined voxel (k = 101) and uncorrected α extent threshold (p = .005) was used maintain the corrected Type I error rates at α = .05 based on 10,000 Monte Carlo simulations using 3dClustSim (Ward, 2000).
Following models to identify differential treatment effects on resting-state connectivity between CET and EST, individual growth curve models were constructed to examine associations between functional connectivity changes and behavioral improvements in emotion processing. These analyses predicted MSCEIT subscale scores from time and time-varying connectivity parameters while adjusting for scanner type and treatment assignment. Significant connectivity effects in these models were then followed up using a mediator-analytic framework for clinical trials (Kraemer, Wilson, Fairburn, & Agras, 2002), to examine the extent to which changes in neural connectivity mediated the previously documented impact of CET on emotion processing outcomes (Eack et al., 2009). The size and significance of the mediation effect was estimated using an asymptotic z′ test for indirect effects (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002).
Missing data were present for 13 (17%) observations in CET and 13 (29%) observations in EST. Analyses addressed the issue of missingness by using the expectation-maximization (EM) algorithm during parameter estimation, which provides maximum-likelihood estimates of model parameters robust to missing data (Dempster, Laird, & Rubin, 1977). This approach is commonly implemented in hierarchical linear modeling and structural equation modeling software, as well as the neuroimaging software used for this study. Moreover, the EM algorithm has been shown to produce parameter estimates that are less biased than complete case or simple (i.e., mean) imputation methods (Schafer & Graham, 2002). The EM algorithm assumes data are missing at random, a theoretical assumption for which there is no available statistical test. Little’s (1988) test indicated that missingness did not meet the more stringent assumption of missing completely at random, χ2(3, Nobs= 123) = 19.53, p < .001, although attrition was found to be unrelated to treatment assignment or demographic and pretreatment clinical variables. Recent research has suggested the EM algorithm is fairly robust to nonrandom missingness, providing more accurate estimates than complete case analysis (Newman, 2003).
Results
Effects of Cognitive Enhancement Therapy on Resting-State Functional Connectivity
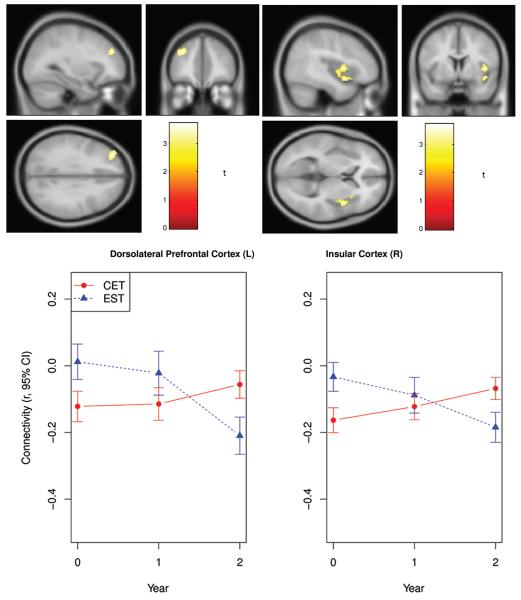
Analysis of the effects of CET on functional connectivity with the resting state network defined by seed voxels in the posterior cingulate cortex revealed two significant treatment x time interactions indicating differential changes in CET compared to EST. As can be seen in Figure 2, the first effect was well-defined and located in a small cluster centered on the left dorsolateral prefrontal cortex (k = 149, x = −34, y = 44, z = 28, df = 1, 88, n = 41, corrected p < .01). The second significant differential effect was larger and primarily included the right insular cortex extending to the superior temporal gyrus (k = 288, x = 38, y = −6, z = −4, df = 1, 88, n = 41, corrected p < .01). These two regional networks support numerous mental processes, and previous studies of schizophrenia have implicated functional abnormalities in these areas with impairments in emotion processing and regulation as well as executive function (Su et al., 2013; Taylor et al., 2012; Weinberger, Berman, & Zec, 1986; Wylie & Tregellas, 2010).
Figure 2.
Fronto-temporal resting state functional connectivity changes during a 2-year trial of cognitive enhancement therapy versus enriched supportive therapy in early course schizophrenia.
Examination of resting-state connectivity parameters over time in these regions indicated that individuals treated with EST showed a continual decrease in functional connectivity between the posterior cingulate and left dorsolateral prefrontal cortex, whereas those treated with CET demonstrated increased connectivity between these regions over time (see Figure 2). The reduction in posterior cingulate-dorsolateral prefrontal connectivity within the EST group was large (d = −1.16, df = 14, n = 16) and significant (p = .015). However, the increase in connectivity in the CET group was not statistically significant (d = .33, df = 35, n = 25, p = .233), suggesting a potential neuroprotective effect against connectivity loss. Investigation of posterior cingulate-insula connectivity changes revealed a similar pattern of results, with EST demonstrating significant reductions in functional connectivity (d = −1.05, df = 14, n = 16, p = .034) and individuals treated with CET showing significant increases in connectivity (d = .81, df = 35, n = 25, p = .005) over time. No significant effects were observed showing differential decreases in resting-state connectivity in CET compared to EST.
Associations Between Functional Connectivity Changes and Improved Emotion Processing
Having found that CET was associated with less decrement in resting state connectivity with the left dorsolateral prefrontal cortex, and significantly increased connectivity in the right insular cortex, we proceeded to conduct a series of individual growth curve analyses to examine the association between these connectivity changes and improvements in emotion processing outcomes assessed using the MSCEIT. No significant associations were observed between connectivity changes in either region and improved emotion facilitation (all p > .194) or emotion understanding (all p > .538). However, as can been seen in Figure 3, increased resting state connectivity with the right insula was associated with improved emotion perception. Further, a nonsignificant trend indicated that increased connectivity with the right dorsolateral prefrontal cortex was associated with improved emotion regulation. Subsequent mediator analyses revealed that increased posterior cingulate-right insula connectivity significantly mediated the previously observed beneficial differential effect of CET on emotion perception, z′ = −1.96, df = 1, n = 41, p = .021. In addition, increased functional connectivity with the left dorsolateral prefrontal cortex also significantly mediated the effect of CET on emotion regulation, z′ = −1.71, df = 1, n = 41, p = .052. Taken together, these findings preliminarily suggest that CET can protect against functional disconnectivity between frontal and temporal brain networks in early course schizophrenia, and that these connectivity changes can serve as active mechanisms for social-cognitive enhancement in the disorder.
Figure 3.
Two-year associations between changes in resting state connectivity and emotion processing outcomes in early course schizophrenia.
Discussion
Schizophrenia is characterized by significant challenges in social and nonsocial information processing that place profound limitations on functional recovery (Fett et al., 2011). Such limitations restrict opportunities for work and the development of meaningful relationships and place considerable burden on family members and provider systems. Cognitive remediation has emerged as an effective set of psychosocial treatments for improving cognitive and functional outcomes in those with schizophrenia, and might achieve its effects through neuroplastic changes in brain function and connectivity (Eack, 2012). There has been considerable interest and speculation on the malleability of neurobiological abnormalities in schizophrenia, yet few studies have investigated this malleability directly within the context of an experimental treatment trial. This study explored the impact of CET (Hogarty & Greenwald, 2006), one of the most promising cognitive remediation interventions for schizophrenia, on resting-state brain connectivity in a group of young individuals in the early course of schizophrenia. Although preliminary, our results revealed that individuals treated with CET demonstrated less reduction of left dorsolateral prefrontal cortical connectivity and significantly increased right insula connectivity with the resting state network. In contrast, over the course of the 2-year treatment, participants treated with EST showed a progressive reduction in functional connectivity between the resting state network and these brain regions. Further, increased connectivity with the right insula and left dorsolateral prefrontal cortex significantly mediated CET effects on improved emotion perception and regulation, respectively.
The results of this preliminary investigation might have significant clinical and pathophysiological implications for the understanding and treatment of schizophrenia, especially if replicated in larger samples. Such findings add to growing support for the beneficial impact of psychosocial treatments on the brain in a disorder that has previously been described as a static encephalopathy. When the results of this research are combined with previous evidence of the positive impact of cognitive remediation on brain function (Subramaniam et al., 2012) and structure (Eack, Hogarty, et al., 2010), it is increasingly clear that the neural abnormalities associated with schizophrenia are not static, but malleable by nonpharmacologic interventions. This observation is consistent with the long-held biopsychosocial perspective in social work of the reciprocal relationship between biology and the environment (Garland & Howard, 2009; Germain & Gitterman, 1980). In addition, that improved resting-state connectivity with the insula and dorsolateral prefrontal cortex mediated CET-related improvements in emotion processing indicates that these neural pathways are critical treatment targets for social-cognitive enhancement in persons with schizophrenia. Most human studies seeking to identify treatment targets are cross-sectional in nature and not embedded within an experimental context. This investigation moves well beyond such approaches to provide longitudinal evidence that not only are resting-state functional connectivity parameters related to social cognition but that these parameters can also be feasibly changed with psychosocial treatment, and once changed, they can result in improved outcomes. Further, these findings underscore the importance of early intervention in schizophrenia because the participants not treated with CET demonstrated significant and large declines in resting-state connectivity over the 2 years of treatment, similar to our neuroanatomical observations (Eack, Hogarty, et al., 2010). This finding is consistent with evidence indicating a potential neurobiological decline early in the course of the disorder (DeLisi, 2008), and the early application of CET might be critical for protecting against this decline and the disabling functional trajectory that is frequently observed in schizophrenia.
Despite the implications of this research, these results need to be understood in the context of several limitations. First, pseudo resting-state data were collected post hoc from an attentional control task, and although recommended steps were taken to avoid task-related effects in the hemodynamic signal (Fair et al., 2007), it is possible that observed functional connectivity changes reflected task-related cognitive demands. A large multisite trial of CET in early schizophrenia is ongoing and will provide pure resting state data outside of a task context to address this limitation.
Second, the sample size for the study was modest, which might have precluded the detection of some effects, particularly effects associated with smaller brain regions. A voxel extent threshold of 101 was required to maintain the Type I error rate at acceptable levels, and the resolution of such a threshold containing millions of neurons is admittedly limited. It will be important for future studies to confirm these findings in larger samples and with refined regions of interest that are capable of detecting connectivity effects with smaller anatomical structures.
Third, attrition from fMRI procedures was considerable, signifying the challenges in longitudinal neuroimaging collection with this population. Although no significant differences emerged between those with and without complete fMRI data in the trial, data were not missing completely at random, and thus parameter estimates need to be interpreted with caution. However, studies have shown that the EM algorithm provides less biased parameter estimates than many other methods of handling missing data, including complete case analysis, even in the presence of nonignorable missingness (Newman, 2003).
Fourth, CET and EST were not matched for hours of therapy exposure, and the greater intensity of CET might have contributed to improved outcomes, although EST did provide an active control for many of the nonspecific therapeutic elements provided by CET.
Last, although the results of this study indicate increased resting-state functional connectivity associated with CET, the pattern of results was largely consistent with a reduction in negative connectivity (less negative or anti-correlations between the right insula/left dorsolateral prefrontal cortex and the resting state network). The quantitative properties of the resting state network in schizophrenia are still being discovered, and it is unclear whether these anti-correlations will become positive over the long-term or whether their reduction represents normalization or compensatory effects. Future studies will need to investigate the long-term impact of cognitive remediation and maintenance of gains in early course samples and incorporate healthy volunteers to answer these questions.
This research suggests that CET, a psychosocial cognitive remediation intervention, may protect against, and in some cases increase, resting state functional connectivity between fronto-temporal brain networks involved in emotion processing and executive function in service of cognitive enhancement in schizophrenia. Despite limitations associated with sample size and attrition, these findings are the first to demonstrate the neuroplastic effects of cognitive remediation on functional brain connectivity at rest in the early course of schizophrenia.
Acknowledgments
This research was supported by NIH grants MH-60902 (Matcheri S. Keshavan) and MH-95783 (Shaun M. Eack).
Contributor Information
Shaun M. Eack, David E. Epperson Associate Professor of Social Work and an associate professor of psychiatry at the University of Pittsburgh.
Christina E. Newhill, professor of social work with a joint appointment to the Clinical and Translational Science Institute at the University of Pittsburgh.
Matcheri S. Keshavan, Stanley Cobb Professor of Psychiatry at Harvard Medical School in Boston, MA.
References
- Ben-Yishay Y, Piasetsky EB, Rattok J. A systematic method for ameliorating disorders in basic attention. In: Meir MJ, Benton AL, Diller L, editors. Neuropsychological rehabilitation. Guilford Press; New York, NY: 1985. pp. 165–181. [Google Scholar]
- Bracy OL. PSSCogRehab [Computer software] Psychological Software Services; Indianapolis, IN: 1994. [Google Scholar]
- Braw Y, Bloch Y, Mendelovich S, Ratzoni G, Gal G, Harari H, Levkovitz Y. Cognition in young schizophrenia outpatients: Comparison of first-episode with multiepisode patients. Schizophrenia Bulletin. 2008;34:544–554. doi: 10.1093/schbul/sbm115. http://dx.doi.org/10.1093/schbul/sbm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. http://dx.doi.org/10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: The CNTRICS initiative. Schizophrenia Bulletin. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. http://dx.doi.org/10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. The concept of progressive brain change in schizophrenia: Implications for understanding schizophrenia. Schizophrenia Bulletin. 2008;34:312–321. doi: 10.1093/schbul/sbm164. http://dx.doi.org/10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society. Series B (Methodological) 1977;39(1):1–38. Retrieved from http://web.mit.edu/6.435/www/Dempster77.pdf. [Google Scholar]
- Eack SM. Cognitive remediation: A new generation of psychosocial interventions for people with schizophrenia. Social Work. 2012;57:235–246. doi: 10.1093/sw/sws008. http://dx.doi.org/10.1093/sw/sws008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Dworakowski D, Montrose DM, Miewald J, Gur RE, Gur RC, Keshavan MS. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia Bulletin. 2010;36:1081–1088. doi: 10.1093/schbul/sbp026. http://dx.doi.org/10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophrenia Bulletin. 2010;36:370–380. doi: 10.1093/schbul/sbn091. http://dx.doi.org/10.1093/schbul/sbn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive enhancement therapy for early-course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60:1468–1476. doi: 10.1176/appi.ps.60.11.1468. http://dx.doi.org/10.1176/ps.2009.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, Keshavan MS. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a two-year randomized controlled trial. Archives of General Psychiatry. 2010;67:674–682. doi: 10.1001/archgenpsychiatry.2010.63. http://dx.doi.org/10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. http://dx.doi.org/10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez MG, Penn DL, Van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. http://dx.doi.org/10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Garland EL, Howard MO. Neuroplasticity, psychosocial genomics, and the biopsychosocial paradigm in the 21st century. Health & Social Work. 2009;34:191–199. doi: 10.1093/hsw/34.3.191. http://dx.doi.org/10.1093/hsw/34.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant default mode functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. http://dx.doi.org/10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Germain CB, Gitterman A. The life model of social work practice. Columbia University Press; New York, NY: 1980. [Google Scholar]
- Green MF, Bearden CE, Cannon TD, Fiske AP, Hellemann GS, Horan WP, Nuechterlein KH. Social cognition in schizophrenia, Part 1: Performance across phase of illness. Schizophrenia Bulletin. 2012;38:854–864. doi: 10.1093/schbul/sbq171. http://dx.doi.org/10.1093/schbul/sbq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. http://dx.doi.org/10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. http://dx.doi.org/10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. http://dx.doi.org/10.1037/0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hogarty GE. Personal therapy for schizophrenia and related disorders: A guide to individualized treatment. Guilford Press; New York, NY: 2002. [Google Scholar]
- Hogarty GE, Greenwald DP. Cognitive enhancement therapy: The training manual. University of Pittsburgh Medical Center: Authors; 2006. Available through www.CognitiveEnhancementTherapy.com. [Google Scholar]
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, Zoretich R. Cognitive enhancement therapy for schizophrenia. Effects of a 2-year randomized trial on cognition and behavior. Archives of General Psychiatry. 2004;61:866–876. doi: 10.1001/archpsyc.61.9.866. http://dx.doi.org/10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. http://dx.doi.org/10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. http://dx.doi.org/10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, the Neurocognitive Working Group Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Archives of General Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. http://dx.doi.org/10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Mehta UM, Padmanabhan JL, Shah JL. Dysplasticity, meta-plasticity, and schizophrenia: Implications for risk, illness, and novel interventions. Development and Psychopathology. 2015;27:615–635. doi: 10.1017/S095457941500019X. http://dx.doi.org/10.1017/S095457941500019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Wilson G, Fairburn CG, Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–884. doi: 10.1001/archpsyc.59.10.877. http://dx.doi.org/10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: A meta-analytic investigation of controlled research. Schizophrenia Bulletin. 2012;38:1092–1104. doi: 10.1093/schbul/sbr036. http://dx.doi.org/10.1093/schbul/sbr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. http://dx.doi.org/10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. http://dx.doi.org/10.1080/01621459.1988.10478722. [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. http://dx.doi.org/10.1037/1082-989X.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and treatment research to improve cognition in schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophrenia Research. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. http://dx.doi.org/10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3:97–105. doi: 10.1037/1528-3542.3.1.97. http://dx.doi.org/10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Newman DA. Longitudinal modeling with randomly and systematically missing data: A simulation of ad hoc, maximum likelihood, and multiple imputation techniques. Organizational Research Methods. 2003;6:328–362. http://dx.doi.org/10.1037/10407-001. [Google Scholar]
- Newman LS. What is social cognition? Four basic approaches and their implications for schizophrenia research. In: Corrigan PW, Penn DL, editors. Social cognition and schizophrenia. American Psychological Association; Washington, DC: 2001. pp. 41–72. http://dx.doi.org/10.1037/10407-001. [Google Scholar]
- Nieto-Castanon A, Whitfield-Gabrieli S. CONN-fMRI functional connectivity toolbox. Massachusetts Institute of Technology; Boston, MA: 2009. [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Marder SR. The MATRICS Consensus Cognitive Battery, Part 1: Test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. http://dx.doi.org/10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, MacDonald AW. Brain correlates of cognitive remediation in schizophrenia: Activation likelihood analysis shows preliminary evidence of neural target engagement. Schizophrenia Bulletin. 2015;41:1276–1284. doi: 10.1093/schbul/sbv025. http://dx.doi.org/10.1093/schbul/sbv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. http://dx.doi.org/10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salovey P, Mayer JD. Emotional intelligence. Imagination, Cognition, and Personality. 1990;9:185–221. http://dx.doi.org/10.2190/DUGG-P24E-52WK-6CDG. [Google Scholar]
- Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: A meta-analysis of the empirical evidence. Schizophrenia Bulletin. 2013;39:979–992. doi: 10.1093/schbul/sbs080. http://dx.doi.org/10.1093/schbul/sbs080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. http://dx.doi.org/10.1037/1082-989X.7.2.147. [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: Functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. http://dx.doi.org/10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Su TW, Lan TH, Hsu TW, Biswal BB, Tsai PJ, Lin WC, Lin CP. Reduced neuro-integration from the dorsolateral prefrontal cortex to the whole brain and executive dysfunction in schizophrenia patients and their relatives. Schizophrenia Research. 2013;148:50–58. doi: 10.1016/j.schres.2013.05.005. http://dx.doi.org/10.1016/j.schres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. http://dx.doi.org/10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, Calhoun VD. In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biological Psychiatry. 2015;78:794–804. doi: 10.1016/j.biopsych.2015.02.017. http://dx.doi.org/10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kang J, Brege IS, Tso IF, Hosanagar A, Johnson TD. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biological Psychiatry. 2012;71:136–145. doi: 10.1016/j.biopsych.2011.09.007. http://dx.doi.org/10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papthanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. http://dx.doi.org/10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Ward DB. Simultaneous inference for fMRI data. Author; Milwaukee, WI: 2000. Retrieved from http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf. [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. http://dx.doi.org/10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. American Journal of Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. http://dx.doi.org/10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- Wykes T, Brammer M, Mellers J, Bray P, Reeder C, Williams C, Corner J. Effects on the brain of a psychological treatment: Cognitive remediation therapy Functional magnetic resonance imaging in schizophrenia. British Journal of Psychiatry. 2002;181(2):144–152. doi: 10.1017/s0007125000161872. Retrieved from http://bjp.rcpsych.org/content/181/2/144.long. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. American Journal of Psychiatry. 2011;168:472–485. doi: 10.1176/appi.ajp.2010.10060855. http://dx.doi.org/10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia Research. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. http://dx.doi.org/10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. http://dx.doi.org/10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]