Summary
Developmentally restricted differentiation antigens or cancer-placental antigens, tastin and bystin, are components of an adhesion molecule that plays a critical role in the implantation of the embryo to the uterus. Cell adhesion molecules have been implicated in the metastasis of carcinomas and could be critical targets for immunotherapy in epithelial ovarian carcinomas (EOCs). Our objectives were to define the expression of tastin and bystin proteins in EOCs. Expression of tastin and bystin mRNA in a panel of human tissues and 70 EOC specimens was investigated using qualitative polymerase chain reaction. Amplification products were confirmed by sequencing. Validation of results was performed using immunohistochemical analysis of tastin and bystin applied on a tissue microarray of 202 EOC tissues. The distribution of tastin and bystin expression and clinicopathologic variables were analyzed. Survival probabilities were estimated using the Kaplan–Meier method and statistical significance was determined by performing the logrank test. Expression of tastin and bystin was restricted to placental and testis tissue by qualitative polymerase chain reaction. Of the 70 EOC specimens tested with polymerase chain reaction, 89% and 94% expressed tastin and bystin, respectively. Immunoexpressions of tastin and bystin protein were observed in 69% and 80 % of the ovarian tumors, respectively. Tastin and bystin expression in Stage I/II disease were 66% and 67% compared with 69% and 81% in Stage III/IV disease, respectively. The tissue-restricted expression of tastin and bystin and their abundant expression in EOCs and advanced-stage disease make these developmentally restricted antigens attractive targets for antigen-specific immunotherapy in EOCs.
Keywords: Tastin, Bystin, Ovarian cancer, PCR, Immunhistochemistry
The discovery of new and innovative treatments for epithelial ovarian carcinomas (EOCs) has led to the identification of proteins or tumor antigens that can be used for targeted immunotherapy. The identification of tumor antigens with restricted expression in normal tissues, but aberrant expression in cancer, has led to rapid progress in the development of antigen-specific immunotherapy for EOC (1). In this regard, clinical trials in EOC patients targeting cancer testis antigens, NY-ESO-1 and LAGE, have demonstrated promising results in patients with recurrent ovarian carcinoma (2–4). Because of the similarities between embryo implantation and the growth of cancer cells, there has been further discovery of new antigens that seem to be expressed only in germ cells and trophoblasts but not in normal tissues. We have termed these proteins “cancer-placental antigens or developmentally restricted differentiation antigens (DRDAGs)” (5). Because these antigens may prove to be markers of “repopulating” cells with stem cell–like characteristics, they are likely to be critical targets for immunotherapy in EOCs.
In the current study, we have focused on 2 DRDAGs, tastin and bystin. In human placental tissue, tastin and bystin are strongly expressed in the trophoblasts and endometrium at the uteroplacental interface during early pregnancy and disappear during the second trimester (6). During embryo implantation, trophoblasts actively proliferate and invade the uterine wall leading to placental formation and embryo implantation. This process is strongly reminiscent of tumor invasion into the surrounding tissues similar to metastasis of tumor (7). The expression and activity of these proteins suggest that these proteins play an important role in attachment of the blastocyts to the uterus and in metastasis of tumor cells.
Our objective was to define the frequency of expression of tastin and bystin and examine their expression with disease outcome in patients with ovarian cancer.
MATERIALS AND METHODS
Patient Population
We searched the archives for patients with EOC who underwent a debulking surgery for their EOCs during a 10-year period from 1995 to 2005. All tissue specimens were collected under an approved protocol from the Institutional Review Board. The medical records of the patients were also retrospectively reviewed under an approved Institutional Review Board protocol. The review included outpatient and inpatient treatment, including surgery and chemotherapy. Study outcomes included overall survival (OS) and time to progression, each measured from the time of definitive surgery. Progression was defined as the objective evidence of recurrence as all therapy was given in the adjuvant setting. The duration of OS was the interval between definitive surgery and death. Observation time was the interval between definitive surgery and last contact (death or last follow-up). Data were censored at the last follow-up for patients with no evidence of recurrence, progression, or death.
Total Tissue RNA Isolation
Tissue RNA was isolated from frozen tumor tissues using the TRI Reagent (Molecular Research Center Inc., Cincinnati, OH) according to manufacturer’s protocol. After isopropanol wash, 75% ethanol treatment, and drying, the RNA was dissolved in RNAase-free water. Resulting RNA concentrations were measured using a spectrophotometer and the quality of RNA was checked by performing electrophoresis on a 1.5% agarose/ethidium bromide gel.
Polymerase Chain Reaction (PCR) Analysis of Tastin and Bystin
Two micrograms of each RNA sample was subjected to cDNA synthesis using the Ready-To-Go TR-PCR beads (GE Healthcare, Buckinghamshire, UK). PCR was subsequently performed to analyze expressions of tastin and bystin (Integrated DNA Technologies, Coralville, IA) in a panel of normal human tissues (Human Total RNA Master Panel II; Clontech, Mountain View, CA) and 70 epithelial ovarian tumor specimens. A 167 bp PCR product was amplified using Tastin 5′-GGA CGA TGA GTG TGC CTT TT-3′ and anti-sense 5′-GGC AGG AGT GGT TGT CTC AT-3′ primers. A 178 bp PCR product was amplified using Bystin sense 5′-CTG GTT CAA AGG GAT CCT GA-3′ and anti-sense 5′-AGT CGC AGG AAG ATG CTG TT-3′ primers. Gylceraldehyde-3-phosphodehydrogenase– specific sense 5′-TCT TCA CCA CCA TGG AGA AG-3′ and anti-sense 5′-CAA AGT TGT CAT GGA TGA CCT TGG-3′ primers were used to obtain a 204 bp PCR product as control. PCR was performed using 35 amplification cycles at an annealing temperature of 60°C. PCR products were then visualized on a 1.5% agarose/ethidium bromide gel. Amplification products were confirmed by sequencing. Validation of results was conducted using immunohistochemical (IHC) analysis of tastin and bystin applied on a tissue microarray (TMA) of 202 EOC tissues.
Histology
Hematoxylin and eosin slides were available for histologic review. The tumor subtypes and grade were rereviewed for confirmation by an experienced pathologist (P.M.-F.). Histologic subtypes were based on the World Health Organization (8). The histologic grade was determined on the basis of the criteria used by the Silverberg grading system (9). In this grading scheme, scores from 1 to 3 were ascertained for each of the predominant architectural pattern, cytologic atypia, and mitotic rate per 10 high-power fields. The total score of 3 to 5 was classified as Grade 1, 6 or 7 as Grade 2, and 8 or 9 as Grade 3.
TMA
Paraffin-embedded tissues obtained from the samples of 202 patients were included in this study. A TMA was constructed as described previously by Kononen and colleagues (10,11). In brief, after carefully choosing the morphologically representative region from the hematoxylin and eosin section, a 0.6-mm cores punched from the individual paraffin-embedded blocks (donor blocks) and transferred to the recipient paraffin-embedded block (recipient block). To overcome tumor heterogeneity, core biopsies were performed from 3 different areas of each tumor. One section was stained with hematoxylin and eosin to confirm the presence of tumor using light microscopy.
IHC
Four-mm-thick sections were deparaffinized and pretreated in citrate buffer, pH 6.0, for 20 minutes using a steamer. Sections were cooled for 20 minutes and were incubated for 10 minutes with 3% H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block (DakoCytomation, Carpinteria, CA) for 30 minutes (A.B. and P.M.-F.). Sections were incubated with tastin and bystin antibodies as follows: tastin (monoclonal, 1:25; Abcam, Cambridge, MA) and bystin (monoclonal, 1:25; Biotechnology Santa Cruz, CA) for 60 minutes at room temperature. Diaminobenzidine tetrahydrochloride was then added for the development of color for 10 minutes, followed by counterstaining with hematoxylin solution. Negative control slides omitting the primary antibody were included in all assays. Evaluation of the IHC slides was performed semiquantitatively by 1 pathologist (P.M.-F.). First, the staining patterns were scored on the basis of the intensity: 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong). The pathologists were blinded to the original histologic diagnosis. The IHC evaluation was performed twice and each review was separated by a 6-week interval. The scores were compared. Whenever a discrepancy between the first and the second readings occurred, the pathologist made the final scoring. Disagreement was not very frequent between the first and the second reading and occurred roughly in around 5% of the specimens. However, for the statistical analysis, the cases were categorized in 2 groups: 0 and 1+ as Group 1 and moderate and strong as Group 2.
Statistical Analysis
Significance of the gene expression association with the survival outcome of interest was assessed using the Type 3 Wald P value. If the expression was significant, then the parameter estimates and hazard ratios for that model were shown for further information. Kaplan– Meier plots were also developed to provide a visual comparison of the survival distribution across gene expression level. Logrank P values were included on the plots. Associations between the gene expression and outcomes of clinical response or disease recurrence were tested using logistic regression methods, following similar logic to the survival analysis. Odds ratios and confidence intervals were estimated if the association of interest was significant.
RESULTS
Study Population
The characteristics of the study population are presented in Table 1. The mean age of the study population was 62 years (range, 33–89 yr) and the median duration of follow-up was 45 months (range, 0.2–187 mo). The majority of patients presented with Grade 3 tumors (65%), advanced Stage III/IV (91%), and serous subtype (85%). A complete response to therapy was reached in 99 patients (49%). The median survival for all patients was 40 months (0.5–165 mo).
TABLE 1.
Patient characteristics
| Patient characteristics | |
|---|---|
| Evaluable patients | 202 |
| Age, median (range) | 62 (33–89) |
| Follow-up, median (range) | 45 (0.2–187) |
| FIGO stage | |
| I and II | 18 |
| III and IV | 184 |
| Histology | |
| Papillary serous | 171 |
| Clear cell | 9 |
| Endometrioid | 7 |
| Mucinous | 9 |
| Undifferentiated | 1 |
| Carcinosarcoma | 4 |
| Other | 1 |
| Grade | |
| 1 and 2 | 70 |
| 3 | 132 |
| Optimal debulking (<1 cm) | |
| Yes | 136 |
| No | 65 |
| Current status | |
| Alive, no evidence of disease | 22 |
| Alive with disease | 16 |
| Dead of disease | 164 |
| Tastin | |
| Tastin positive | 139 |
| Bystin | |
| Bystin positive | 162 |
| Tastin and bystin expression | 120 |
Expression of Tastin and Bystin in EOC as Determined by PCR and IHC
Tastin and bystin showed restricted expression in the normal tissue panel with positive control being 6-week placental tissue. Of the 70 EOC specimens tested with PCR, 62/70 (89%) and 66/70 (94%) expressed tastin and bystin, respectively. These results were validated by IHC staining. IHC demonstrated tastin and bystin expressions in 139/202 (68.8%) and 162/202 (80.2%) of specimens on a TMA, respectively. The predominant staining pattern of tastin and bystin were cytoplasmic in EOC tissues (Figs. 1A, B).
FIG. 1.
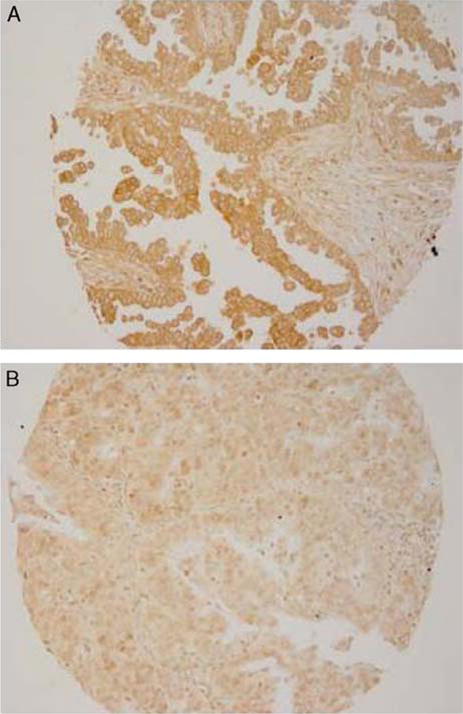
Expression of tastin and bystin in ovarian carcinoma. Immunohistochemical staining was carried out with monoclonal antibody to tastin and bystin antibody. (A) Tastin staining of ovarian tumor specimen. (B) Bystin staining of ovarian tumor specimens.
Correlation of Tastin and Bystin Expression and Clinical Outcomes
Correlating IHC with clinical outcomes, bystin-expressing tumors were more likely to have tastin expression and vice versa (P = 0.001). The presence of bystin and tastin did not have a statistically significant effect on OS. Finally, there were no significant differences in progression-free survival or OS based on tumor expression of tastin and/or bystin (Fig. 2).
FIG. 2.
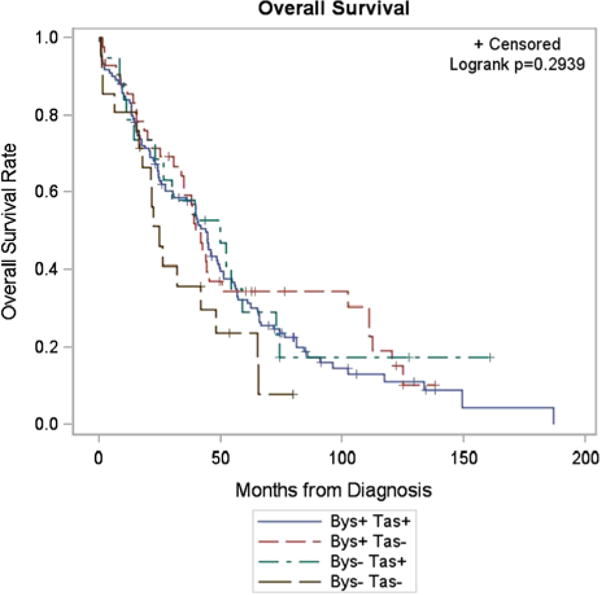
Kaplan–Meier estimates of overall survival in epithelial ovarian carcinoma patients based on tumor expression of tastin and bystin.
DISCUSSION
Identifying novel targets in EOCs is important because of limited treatment options available to patients with recurrent or persistent disease. The detection of tumor-specific antigens is necessary for the advancement of the field of immunotherapy in EOCs. In this study, we sought to identify the expression of cancer-placental antigens that demonstrate a restricted expression in normal tissue and an aberrant expression in EOC. Importantly, these antigens are involved in similar processes involved in embryo implantation and growth and spread of cancer cells and subsequently represent attractive targets for immunotherapy. This study demonstrates the aberrant expression of tastin and bystin proteins in a significant proportion of high-grade and Stage III/IV human EOC.
The restricted expression of tastin and bystin in placental tissue and the overexpression in EOC tissue make tastin and bystin attractive targets for immunotherapy. The lack of correlation between tastin and bystin expression with clinicopathologic characteristics, such as OS, disease-free progression, or response to therapy, may reflect the advanced nature of the disease at diagnosis and small patient numbers. In addition, expression of these antigens does not reflect quantitative protein expression but only reflect qualitative protein expression. Tastin and bystin are constitutively expressed in the human placenta during the first weeks of the first trimester of pregnancy (6). It has previously been demonstrated that these 2 proteins, tastin and bystin, form a complex with trophinin and have a role in blastocystic uterine adhesion during implantation of the embryo (6).
The process of trophoblastic invasion is similar to that of malignant tumor metastasis, with aggressive cell proliferation, angiogenesis, and host cell destruction (7). Hatakeyama et al. (12) demonstrated the upregulation of trophinin, another DRDAG, in testicular germ cell tumors and suggests that it plays a role in tumor metastasis. Understanding the role of tastin and bystin may aid in the understanding of tumor metastasis in ovarian carcinoma. The progression from an early-stage disease to advanced-disease states requires the mobilization of carcinoma cells to migrate and invade to establish secondary tumors at the distant sites. In prostate cancer cells, bystin protein is expressed suggesting a role in cell– cell contact and cell growth that adheres to neurons. Bystin was identified in perineural invasion in prostate cancer (13). This process of invasion and metastasis is a complex process, but many components of this process are similar to embryo implantation identified during early pregnancy.
In a previous report, we examined the expression of 2 DRDAGs, PLAC1, and DPPA2 in human ovarian cancer (5). Expression of PLAC1 and DPPA2 in the EOCs were 21% and 31%, respectively. Similar to tastin and bystin, the presence of PLAC1 and DPPA2 did not have a statistically significant effect on recurrence-free survival and OS. Together, these results suggest that, although DRDAGs may be promising targets for immunotherapy of EOC, their expression does not alter the clinical course of ovarian cancer.
In summary, we have shown the overexpression of 2 DRDAGs in a majority of ovarian cancer patients. We also demonstrated restricted expression of these proteins in a panel of normal tissues. The next step would be to identify the expression of these tumors in primary tumors compared with metastatic lesions to understand whether there is a difference in expression. Further studies regarding the immunogenicity of tastin- and bystin-expressing tumors and characterization of antigen specific T cells are also warranted.
Footnotes
The authors declare no conflict of interest.
References
- 1.Sabbatini P, Odunsi K. Immunologic approaches to ovarian cancer treatment. J Clin Oncol. 2007;25:2884–93. doi: 10.1200/JCO.2007.11.0775. [DOI] [PubMed] [Google Scholar]
- 2.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–83. [PubMed] [Google Scholar]
- 3.Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–42. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odunsi K, Matsuzaki J, Karbach J, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci U S A. 2012;109:5797–802. doi: 10.1073/pnas.1117208109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchabo NE, Mhawech-Fauceglia P, Caballero OL, et al. Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immun. 2009;9:6. [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki M, Tsukagoshi S, Ohwada M, et al. A patient with brain metastasis from ovarian cancer who showed complete remission after multidisciplinary treatment. Gynecol Oncol. 1999;74:483–6. doi: 10.1006/gyno.1999.5476. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda MN, Nozawa S. Trophinin, tastin, and bystin: a complex mediating unique attachment between trophoblastic and endometrial epithelial cells at their respective apical cell membranes. Semin Reprod Endocrinol. 1999;17:229–34. doi: 10.1055/s-2007-1016230. [DOI] [PubMed] [Google Scholar]
- 8.Creasman WT. Announcements: FIGO stages: 1988 revision. Gynecol Oncol. 1989;35:125–7. [Google Scholar]
- 9.Shimizu Y, Kamoi S, Amada S, et al. Toward the development of a universal grading system for ovarian epithelial carcinoma: testing of a proposed system in a series of 461 patients with uniform treatment and follow-up. Cancer. 1998;82:893–901. doi: 10.1002/(sici)1097-0142(19980301)82:5<893::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 10.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 11.Moch H, Kononen T, Kallioniemi OP, et al. Tissue microarrays: what will they bring to molecular and anatomic pathology? Adv Anat Pathol. 2001;8:14–20. doi: 10.1097/00125480-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama S, Ohyama C, Minagawa S, et al. Functional correlation of trophinin expression with the malignancy of testicular germ cell tumor. Cancer Res. 2004;64:4257–62. doi: 10.1158/0008-5472.CAN-04-0732. [DOI] [PubMed] [Google Scholar]
- 13.Ayala GE, Dai H, Li R, et al. Bystin in perineural invastion of prostate cancer. Prostate. 2006;66:266–72. doi: 10.1002/pros.20323. [DOI] [PubMed] [Google Scholar]


